Background:
Various surgical techniques exist for lower extremity reconstruction, but limited high-quality data exist to inform treatment strategies. Using multi-institutional data and rigorous matching, the authors evaluated the effectiveness and cost of three common surgical reconstructive modalities.
Methods:
All adult subjects with lower extremity wounds who received bilayer wound matrix, local tissue rearrangement, or free flap reconstruction were retrospectively reviewed (from 2010 to 2017). Cohorts’ comorbidities and wound characteristics were balanced. Graft success at 180 days was the primary outcome; readmissions, reoperations, and costs were secondary outcomes.
Results:
Five hundred one subjects (166 matrix, 190 rearrangement, and 145 free flap patients) were evaluated. Matched subjects (n = 312; 104/group) were analyzed. Reconstruction success at 180 days for matrix, local tissue rearrangement, and free flaps was 69.2 percent, 91.3 percent, and 93.3 percent (p < 0.001), and total costs per subject were $34,877, $35,220, and $53,492 (p < 0.001), respectively. Median length of stay was at least 2 days longer for free flaps (p < 0.0001). Readmissions and reoperations were greater for free flaps. Local tissue rearrangement, if achievable, provided success at low cost. Free flaps were effective with large, traumatic wounds but at higher costs and longer length of stay. Matrices successfully treated older, obese patients without exposed bone.
Conclusions:
Lower extremity reconstruction can be performed effectively using multiple modalities with varying degrees of success and costs. Local tissue rearrangement and free flaps demonstrate success rates greater than 90 percent. Bilayer wound matrix-based reconstruction effectively treats a distinct patient population.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, III.
Lower extremity soft-tissue reconstruction poses a significant surgical challenge, representing a heterogeneous and often complex clinical situation associated with high rates of failure and morbidity. Historically, reconstructive principles of the lower extremity relate to size of the tissue defect and anatomic location, with larger, deeper, and distal wounds often requiring complex autologous reconstruction given a lack of surrounding tissue with which to reconstruct. Common comorbidities, such as diabetes mellitus, peripheral arterial disease, and venous insufficiency, further complicate the ability to heal. Lack of healing often leads to amputation, which is unfortunately fairly common, with rates close to 60 percent at 5 years.1 The costs of any amputations cannot be understated. Below-the-knee or above-the-knee amputations cost hospitals, on average, about $44,000 per patient, with an additional $43,000 to $60,000 in follow-up care.2 Furthermore, annual costs of venous and diabetic wounds alone are $14.9 billion and $9 billion to $13 billion, respectively.3,4 Extremity salvage has a tremendous impact on overall survival, quality of life, function, and cost.
Treatment modalities utilized for lower extremity wound reconstruction, such as local tissue rearrangement, free flaps, and skin substitutes (such as Integra bilayer wound matrix; Integra Lifesciences, Plainsboro, N.J.), have been widely used by surgeons, but little is known about their respective effectiveness in specific clinical scenarios. Bilayer wound matrices, when compared indirectly to autologous tissue reconstruction, demonstrate immediate coverage, minimal morbidity, and shorter operative times. In addition, the ability to reconstruct wounds of various types and locations gives it another competitive advantage.5 While select literature exists highlighting the use of bilayer wound matrices in complex lower extremity soft-tissue reconstruction,6 there is a paucity of direct, comparative data to contrast this modality with conventional, autologous-based tissue transfer. The decision-making process of which treatment to use is convoluted and patient-specific. The complexity and lack of standardization leave surgeons with practice biases when implementing effective treatment strategies. The unmet need for a comparative effectiveness analysis must be addressed.
The ultimate goals in lower extremity reconstruction remain consistent: preservation of life and limb, wound coverage to prevent infection and amputation, and restoration of function.7 While autologous coverage with local tissue rearrangement or free flaps remains the gold standard of lower limb soft-tissue reconstruction, we hypothesized that bilayer wound matrix could be an equally as effective method of lower extremity soft-tissue coverage with low costs. We present a multihospital experience of multidisciplinary reconstructive surgeons utilizing local tissue rearrangement, free flaps, and bilayer wound matrix and evaluated their comparative effectiveness in the treatment of lower extremity soft-tissue wounds after an advanced statistical matching technique, in order to describe and compare postreconstructive outcomes and costs in specific clinical scenarios.
METHODS
An institutional review board–approved and industry-sponsored retrospective study was performed and included all adult patients with lower extremity wounds who underwent bilayer wound matrix, local tissue rearrangement, or free flap reconstruction. Subjects were operated on by physicians in all departments within the University of Pennsylvania Health Systems from May of 2010 to June of 2017. The patient population was derived from a free-text search engine (PennSeek; Penn Medicine, Philadelphia, Pa.) of operative notes using Boolean search terms “lower extremity” and “reconstruction” with either “Integra,” “free flap,” or “tissue rearrangement.” Subjects eligible for study inclusion presented to plastic, orthopedic, or podiatric surgeons with at least one lower extremity wound and were at least 18 years of age. Subjects were excluded if they had prior matrix-based reconstruction of their lower extremity wound or if their defect was a result of a burn. Success for bilayer wound matrix was defined as matrix providing an adequate wound bed for split-thickness skin grafting. Success for local tissue rearrangement and free flaps was defined as not needing an additional coverage procedure.
Study Covariates, Outcomes, and Costs
Patient variables included demographic information (i.e., age, gender, race, body mass index, smoking history, and medical comorbidities) and perioperative characteristics (i.e., wound location, wound type, exposed deep structures, length of procedure, length of stay, wound size, wound age, and total cost).
Graft success at 180 days served as the primary outcome of interest. Additional, secondary outcomes included 60-day and 120-day success, 180-day amputation rates, readmissions, reoperations, and costs. The investigators derived cost-related data for each patient’s hospital course and subsequent wound-related admissions or reoperations from the Department of Finance at the Hospital of the University of Pennsylvania. Total costs did not account for professional service fees and only reflected costs to the University of Pennsylvania Health System.
Advanced Matching Algorithms
Biases were minimized at an institutional level by specific data abstractors, blocking of the data set, and review by the institutions financial committee. To account for potential confounders, two matching algorithms were implemented to create matched triplets of bilayer wound matrix, local tissue rearrangement, and free flap patients. First, we paired patients between any two treatments in terms of wound size and wound age using a cardinality matching algorithm.8,9 This algorithm finds the largest pair-matched sample that is balanced. In other words, we sacrificed some patients’ data to achieve a better match within wound size and wound age. Second, we took the three pair-matched samples and created a three-way matched sample using an approximate multiple matching algorithm. The second algorithm constructed a matched design with multiple comparison groups.10,11 Using the cardinality matching and approximate multiple matching algorithms, we created 104 triplets of bilayer wound matrix, local tissue rearrangement, and free flap patients. If the standardized difference in means for a covariate after matching was less than 0.1, it indicated that the matching balanced the covariate (Table 1 and Fig. 1).12
Table 1.
Results of Cardinality Matching on Wound Size and Age
| Mean Wound Size (SD) | Mean Wound Age (SD) | |
|---|---|---|
| Before matching | ||
| BWM (B) | 77.5 (131.1) | 308.1 (697.8) |
| LTR (T) | 72.6 (53.4) | 115.9 (170.6) |
| FF (F) | 133.3 (100.3) | 283.7 (1535.5) |
| Standardized difference | ||
| B-F | 0.48 | 0.02 |
| B-T | 0.05 | 0.38 |
| F-T | 0.76 | 0.15 |
| After matching | ||
| BWM (B) | 89.3 (125.9) | 180.4 (353.0) |
| LTR (T) | 81.4 (60.5) | 144.5 (181.0) |
| FF (F) | 83.7 (35.8) | 158.6 (275.1) |
| Standardized difference* | ||
| B-F | 0.05 | 0.02 |
| B-T | 0.08 | 0.07 |
| F-T | 0.03 | 0.01 |
BWM and B, bilayer wound matrix; LTR and T, local tissue rearrangement; FF and F, free flap reconstruction.
*If the standardized difference for a covariate after matching is less than 0.1, the matching balances the covariate.
Fig. 1.
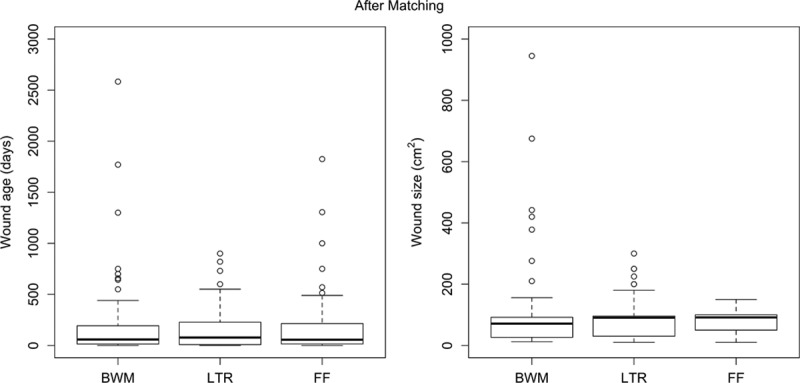
Box plots of wound size and age after matching. BWM, bilayer wound matrix; LTR, local tissue rearrangement; FF, free flaps.
Statistical Data Analyses
Categorical variables were summarized in frequencies and percentages and compared using Pearson chi-square or Fisher’s exact tests. Continuous variables were summarized in means and standard deviations or in medians and interquartile ranges, and compared using analysis of variance or Kruskal-Wallis tests. Using the matched data, the graft success and amputation rates were compared using the conditional logistic regression models with matched triplets in each stratum; the readmission and reoperation rates were compared using the generalized estimating equations models with a Poisson distribution for count data and an independent correlation structure for matched triplets; and costs, length of stay, and procedure length were compared using Friedman’s rank sum tests.13 Using the matched data, the predicted probability of success at 180 days was based on a conditional logistic regression model adjusting for age, body mass index, gender, history of diabetes, smoking status, wound location, wound type, and exposure of bone and/or tendon, with interactions between treatment modality and age, gender, history of diabetes, and smoking status. The predicted probabilities of success at 180 days were provided in clinical scenarios where each treatment modality was most effective. All analyses were performed using R version 3.5.2.14 A significance level of 0.05 was used for all analyses.
RESULTS
Patient and Wound Characteristics
A total of 501 subjects with 547 wounds underwent lower extremity reconstruction with one of the three aforementioned modalities (166 bilayer wound matrix patients, 190 local tissue rearrangement patients, and 145 free flap patients). The average age of the entire cohort was 55.9 years, and the average body mass index was 29.3 kg/m2 (Table 2). Regarding differences among the groups, the free flap cohort had the lowest percentage of female patients (28.3 percent, p = 0.002). The matrix group had the highest portion of subjects with a history of diabetes (51.2 percent, p < 0.001), whereas smoking history showed no significant differences among the three groups (Table 1). The median wound sizes in the matrix, tissue rearrangement, and free flap groups were 29.5 cm2, 30.0 cm2, and 120.0 cm2, respectively (p < 0.001). The median wound ages also differed significantly, with matrix wounds (55 days) being much older than local tissue rearrangement (30 days) and free flap (42 days) wounds (p = 0.007), respectively. The majority of wounds in the matrix group were located on the foot (45.2 percent, p < 0.001), and 28 patients (16.9 percent) had multiple defects. The wounds in the local tissue rearrangement and free flap groups were mostly on the ankle to knee, 43.2 percent and 70.3 percent, respectively, and very few patients (2.1 percent and 0.7 percent, respectively) had multiple defects. Regarding the wound type of the entire cohort, surgical site (28.5 percent) and traumatic (27.9 percent) wounds were the most common. Within each group, diabetic and vascular ulcers (42.2 percent) made up the majority of wounds in the matrix group, surgical site wounds (36.8 percent) were more common in the local tissue rearrangement cohort, and traumatic wounds (61.4 percent) were more common in the free flap group (p < 0.001). Of note, oncologic wounds only comprised 9.4 percent of the entire cohort. Thirty-four patients received either preoperative chemotherapy or radiation, with the highest number (n = 20, 13.8 percent) occurring in the free flap group (p < 0.001). In terms of wounds having exposed bone or tendon, the free flap group had significantly more patients (n = 118, 81.4 percent) than the other two groups (p < 0.001).
Table 2.
Baseline Characteristics of the Three Cohorts before Matching*
| Total (n = 501) | BWM (n = 166) | LTR (n = 190) | Free Flap (n = 145) | p | |
|---|---|---|---|---|---|
| Mean age (SD), yr | 55.9 (17.0) | 59.8 (16.5) | 57.4 (16.1) | 49.4 (17.0) | <0.001† |
| Female, no. (%) | 192 (38.3) | 62 (37.3) | 89 (46.8) | 41 (28.3) | 0.002† |
| Mean BMI (SD), kg/m2 | 29.3 (7.4) | 30.1 (7.7) | 29.3 (8.1) | 28.3 (5.9) | 0.082 |
| History of diabetes, no. (%) | 183 (36.5) | 85 (51.2) | 72 (37.9) | 26 (17.9) | <0.001† |
| Smoking, no. (%) | 0.860 | ||||
| Never smoked | 261 (52.3) | 89 (53.9) | 98 (51.9) | 74 (51.0) | |
| Former smoker | 99 (19.8) | 48 (29.1) | 51 (27.0) | 40 (27.6) | |
| Current smoker | 139 (27.9) | 28 (17.0) | 40 (21.2) | 31 (21.4) | |
| Median wound size, cm2 (IQR) | 45 (21–120) | 29.5 (15–74.25) | 30 (10–82.5) | 120 (60–200) | <0.001† |
| Median wound age, days (IQR) | 42 (10–156) | 55 (14.5–189.5) | 30 (2.5–150) | 42 (12.5–102) | 0.007† |
| Wound location, no. (%) | <0.001† | ||||
| Foot | 180 (35.9) | 85 (51.2) | 64 (33.7) | 31 (21.4) | |
| Ankle to knee | 266 (53.1) | 77 (46.4) | 86 (45.3) | 103 (71.0) | |
| Knee to hip | 55 (11.0) | 4 (2.4) | 40 (21.1) | 11 (7.6) | |
| Wound type, no. (%) | <0.001† | ||||
| Traumatic | 140 (27.9) | 19 (11.4) | 32 (16.8) | 89 (61.4) | |
| Pressure ulcer | 14 (2.8) | 11 (6.6) | 3 (1.6) | 0 (0.0) | |
| Vascular ulcer | 41 (8.2) | 32 (19.3) | 9 (4.7) | 0 (0.0) | |
| Diabetic ulcer | 57 (11.4) | 38 (22.9) | 18 (9.5) | 1 (0.7) | |
| Cellulitic | 22 (4.4) | 12 (7.2) | 10 (5.3) | 0 (0.0) | |
| Surgical site | 143 (28.5) | 43 (25.9) | 70 (36.8) | 30 (20.7) | |
| Oncologic | 47 (9.4) | 3 (1.8) | 22 (11.6) | 22 (15.2) | |
| AKA site | 6 (1.2) | 0 (0.0) | 6 (3.2) | 0 (0.0) | |
| BKA site | 13 (2.6) | 2 (1.2) | 11 (5.8) | 0 (0.0) | |
| TMA site | 11 (2.2) | 4 (2.4) | 4 (2.1) | 3 (2.1) | |
| Unknown | 7 (1.4) | 2 (1.2) | 5 (2.6) | 0 (0.0) | |
| Preoperative chemotherapy or XRT, no. (%) | 34 (6.8) | 5 (3.0) | 9 (4.7) | 20 (13.8) | <0.001† |
| Exposed bone or tendon, no. (%) | 279 (55.7) | 86 (51.8) | 78 (41.1) | 118 (81.4) | <0.001† |
BWM, bilayer wound matrix; LT, local tissue rearrangement; BMI, body mass index; AKA, above-knee amputation; BKA, below-knee amputation; TMA, transmetatarsal amputation.
*Continuous variables are summarized as mean (standard deviation), and categorical variables are summarized as frequency (percent).
Success and Costs
After applying the cardinality matching and approximate multiple matching algorithms, 104 matched triplets were analyzed. The overall graft success rate at 180 days for bilayer wound matrix, local tissue rearrangement, and free flaps was 69.2 percent, 91.3 percent, and 93.3 percent (p < 0.001), respectively, as can be seen in Table 3. Free flap reconstructions tended to take longer (408 minutes versus 50 and 85 minutes for matrix and tissue rearrangement, respectively, p < 0.001), and patients had a longer length of stay in the hospital (7 days versus 2 and 5 days for matrix and tissue rearrangement, respectively, p < 0.001). In addition, the costs for the free flap group were significantly more expensive, with total costs of $53,492 per patient (p < 0.001) compared with $34,877 for matrix patients and $35,220 for local tissue rearrangement patients. Amputation rates were highest in the matrix group (14.4 percent), followed by the local tissue rearrangement group (5.8 percent) and the free flap group (3.8 percent) (p = 0.017).
Table 3.
Outcomes of Matched Patients
| BWM (n = 104) | LTR (n = 104) | FF (n = 104) | p | |
|---|---|---|---|---|
| Success, no. (%) | ||||
| 60-day rate | 83 (79.8) | 100 (96.2) | 99 (95.2) | <0.001 |
| 120-day rate | 74 (71.2) | 98 (94.2) | 97 (93.3) | <0.001 |
| 180-day rate | 72 (69.2) | 95 (91.3) | 97 (93.3) | <0.001 |
| Total direct costs (range) | $23,853 ($0–249,312) | $22,968 ($0– 211,666) | $34,566 ($0–131,634) | <0.001 |
| Total costs (range) | $34,877 ($0–355,316) | $35,220 ($0– 326,666) | $53,492 ($0–200,694) | <0.001 |
| Charges (range) | $168,789 ($0– 1,870,772) | $184,221 ($0– 1,766,476) | $276,736 ($0–983,540) | <0.001 |
| Mean length of stay (range), days | 2 (0–6) | 5 (1–6) | 7 (5–8) | <0.001 |
| Mean procedure time (range), min | 50 (25–85) | 85 (60–119) | 408 (318–490) | <0.001 |
| Amputation rates, no. (%) | 15 (14.4) | 6 (5.8) | 4 (3.8) | 0.017 |
| Readmissions, no. | 45 | 30 | 68 | |
| Total costs of readmission (range) | $7,044 ($0–59,476) | $5,349 ($0–59,926) | $7,724 ($0–74,446) | 0.033 |
| Rate ratios (95% CI)* | Ref | 0.75 (0.45–1.24) | 1.58 (0.95–2.61) | |
| Reoperations, no. | 69 | 58 | 122 | |
| Total costs of reoperations (range) | $118 ($0–4,684) | $808 ($0–49,746) | $1,565 ($0–42,736) | <0.001 |
| Rate ratios (95% CI)* | ref | 1.00 (0.63–1.59) | 1.46 (1.00–2.15) |
BWM, bilayer wound matrix; LTR, local tissue rearrangement; FF, free flaps.
*Rate ratios were estimated using the generalized estimating equations models, with a Poisson distribution for count data and an independent correlation structure for matched triplets.
Readmissions and Reoperations
There were 45 readmissions in the bilayer wound matrix group, leading to a rate of 0.45 per 220 patient-days. Using this number as the reference point, local tissue rearrangement (30 readmissions; adjusted rate ratio, 0.75; 95 percent CI, 0.45 to 1.24) and free flaps (68 readmissions; adjusted rate ratio, 1.58; 95 percent CI, 0.95 to 2.61) were analyzed but no significant difference was shown. The total costs associated with readmissions was greatest for free flaps at $11,102 (range, $0 to $97,644), compared with $10,811 (range, $0 to $90,367) for the matrix group and $7,953 (range, $0 to $90,690) for the local tissue rearrangement group (p = 0.033). Similarly, reoperations were analyzed under the same generalized estimating equations models. Sixty-nine reoperations occurred in the matrix group not including split-thickness skin grafting. Three of the 69 reoperations were due to failure leading to subsequent free flap reconstruction. With regard to the entire cohort, the majority of reoperations were due to debridement (bilayer wound matrix, n = 35; local tissue rearrangement, n = 20; and free flaps, n = 23). The matrix group had a rate of 0.66 per 220 patient-days. Using this value as the reference point, local tissue rearrangement (58 reoperations; adjusted rate ratio, 1.00; 95% CI, 0.63 to 1.59) and free flaps (122 reoperations; adjusted rate ratio, 1.46; 95% CI, 1.00 to 2.15) were examined and demonstrated significantly more reoperations for the free flap group.
Comparative Analysis
The predictive probability model as described in the Methods section was created to support the clinical scenarios in which each treatment modality was most effective. Variables included in the model were patient age (three levels), body mass index (three), gender (two), history of diabetes (two), smoking status (two), wound location (three), wound type (two), and exposure of bone and/or tendon (two), leading to 864 possible clinical scenarios (3 × 3 × 2 × 2 × 2 × 3 × 2 × 2 = 864). The area under the receiver operating characteristic or c statistic for the predictive probability model was 0.79 (95% CI, 0.74 to 0.85). Wound size and wound age were controlled via the cardinality matching algorithms. The model predicted that bilayer wound matrix was not as effective a treatment as local tissue rearrangement and free flaps but did show greater than 90 percent success rates for 61 conditions of the possible 864. As seen in Table 4, these simulated patients were older (72.1 percent greater than 65 years old), obese (55.7 percent with a body mass index greater than 35 kg/m2), male (65.6 percent), nondiabetic (98.4 percent), nonsmokers (68.9 percent) with traumatic wounds (57.4 percent) located on the foot (55.7 percent) without exposed bone or tendon (95.1 percent).
Table 4.
Characteristics for Predicted Probability of Success for Bilayer Wound Matrix
| <70% BWM (n = 751) | 70% to 80% BWM (n = 25) | 80% to 90% BWM (n = 27) | >90% BWM (n = 61) | |
|---|---|---|---|---|
| Age | ||||
| <45 years | 259 (34.5) | 8 (32.0) | 7 (25.9) | 14 (23.0) |
| 45-65 years | 276 (36.8) | 4 (16.0) | 5 (18.5) | 3 (4.9) |
| >65 years | 216 (28.8) | 13 (52.0) | 15 (55.6) | 44 (72.1) |
| Body mass index | ||||
| <25 kg/m2 | 272 (36.2) | 3 (12.0) | 5 (18.5) | 8 (13.1) |
| 25-35 kg/m2 | 251 (33.4) | 9 (36.0) | 9 (33.3) | 19 (31.2) |
| >35 kg/m2 | 228 (30.4) | 13 (52.) | 13 (48.2) | 34 (55.7) |
| Gender | ||||
| Female | 359 (47.8) | 11 (44.0) | 8 (29.6) | 21 (34.4) |
| Male | 392 (52.2) | 14 (56.0) | 19 (70.4) | 40 (65.6) |
| History of diabetes | ||||
| No | 327 (43.5) | 22 (88.0) | 23 (85.2) | 60 (98.4) |
| Yes | 424 (56.5) | 3 (12.0) | 4 (14.8) | 1 (1.6) |
| Smoking status | ||||
| Current/former | 357 (47.5) | 10 (40.0) | 9 (33.3) | 19 (31.2) |
| Never | 394 (52.5) | 15 (60.0) | 18 (66.7) | 42 (68.9) |
| Wound location | ||||
| Foot | 229 (30.5) | 12 (48.0) | 13 (48.2) | 34 (55.7) |
| Ankle to knee | 239 (31.8) | 12 (40.0) | 13 (48.2) | 26 (42.6) |
| Knee to hip | 283 (37.7) | 3 (12.0) | 1 (3.7) | 1 (1.6) |
| Wound type | ||||
| Nontraumatic | 384 (51.1) | 11 (44.0) | 11 (40.7) | 26 (42.6) |
| Traumatic | 367 (48.9) | 14 (56.0) | 16 (59.3) | 35 (57.4) |
| Bone or tendon exposure | ||||
| No | 333 (44.3) | 19 (76.0) | 22 (81.5) | 58 (95.1) |
| Yes | 418 (55.7) | 6 (24.0) | 5 (18.5) | 3 (4.9) |
DISCUSSION
Data presented in this large, multi-institutional, multidisciplinary series highlight the relative clinical benefits of a customized surgical approach to lower extremity reconstruction based on wound size, wound type, wound location, patient health conditions, etiology, and presence of exposed vital structures. We demonstrate that local tissue rearrangement, if achievable, provides great success at low cost. Free flap reconstruction was quite effective with large, traumatic wounds but required high costs, increased length of stay, and long operating times. Bilayer wound matrix was effective in about 70 percent of patients but successfully treated older, obese patients without exposed bone at minimal expense. Understanding treatment successes maximizes clinical impact while reducing costs. This study advances the science of lower extremity reconstruction by presenting how different modalities can be effectively utilized with varying degrees of success and costs. Specific findings merit further discussion.
Heterogeneity of Cohorts
Lower extremity wounds represent a common clinical problem with heterogeneous etiologies. Our granular data demonstrate such heterogeneity, with 11 unique wound types from three different locations with varying degrees of size and age. The majority of wounds in the bilayer wound matrix cohort were of an ulcer type, accounting for 48.8 percent, whereas the free flap group revealed wounds that were mostly of traumatic origin and local tissue rearrangement demonstrated most commonly surgical site wounds. Lower extremity ulcers, alone, have an estimated prevalence of 1 percent to 2 percent among U.S. adults, with venous leg and diabetic foot ulcers accounting for the majority.15 Traumatic lower extremity wounds similarly are quite common and morbid, with about 278,100 occurring in 2012 according to the National Trauma Data Bank.16 A comparison of such unique wounds and cases is difficult to achieve. We implemented cardinality matching and an approximate multiple matching technique, both advanced forms of matching algorithms, that balanced wound size and age in addition to covariate adjustments. We initially started with 699 patients and collected and analyzed data from 501 patients based on exclusion criteria. However, we only compared data between 312 patients to ensure similar populations, minimize the complexity of comparing uniquely different wounds, and allow for the best match among three treatment arms, which until now has never been described in the literature.
Success and Costs after Matching
Given diverse patient populations and the aforementioned heterogeneity of wound defects, surgeons are left with a diversity of treatment options but without adequate high-quality, comparative data, leading to practices biases. Our goal was to show the relative comparative success of each group using a novel matching technique. We demonstrated a 180-day success rate of 69.2 percent with median length of stay of 2 days with bilayer wound matrix. Local tissue rearrangements had a 91.3 percent success rate with median length of stay of 5 days, while free flaps had the greatest success (93.3 percent) but with longest length of stay (7 days). Furthermore, free flap cases took significantly longer than the other two treatments (408 minutes versus 50 and 80 minutes, p < 0.001).
Bilayer wound matrices such as Integra have historically been utilized in burn patients but are now used in other clinical settings,17 demonstrating success in 70 percent to 80 percent of cases.18,19 Shakir et al.20 recently published a cohort study on bilayer wound matrix showing multiple factors leading to reconstructive failure and large discrepancies in outcomes of healed versus nonhealed wounds regarding length of stay and costs. Local tissue rearrangement for lower extremity salvage is not well studied but can provide adequate coverage for elective and traumatic defects; however, it often requires subsequent debulking procedures and lack the aesthetic match that patients seek.20 The local tissue rearrangement group, as indicated by our data, demonstrated smaller, younger wounds of nondiabetic, nonvascular, and atraumatic origin, to suggest a cohort more likely to succeed. Free flap reconstruction historically has been the gold standard, with success rates ranging from 93 percent to 95 percent, but it requires expertise in microsurgery.21,22 Our data also support that free flap reconstruction is indeed the gold standard for graft success, but it can be costly, as depicted by a large, significant cost discrepancy between free flap reconstruction and the other two groups. Each treatment modality has its associated advantages and disadvantages (Fig. 2).
Fig. 2.
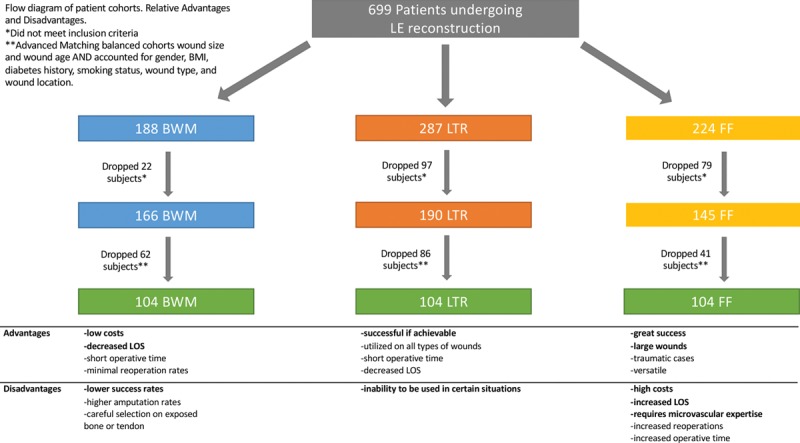
Relative advantages and disadvantages of treatment modalities. LE, lower extremity; BMI, body mass index; BWM, bilayer wound matrix; LTR, local tissue rearrangement; FF, free flap; LOS, length of stay.
Amputations, Readmissions, and Reoperations
Amputation rates were significantly greater in the bilayer wound matrix group compared with local tissue rearrangement and free flap groups, which may be a product of wound type, where 19.3 percent and 22.9 percent of cases were vascular and diabetic wounds, respectively. Patients with diabetes carry a 15-fold increased risk for lower extremity amputation, and 45 percent of all amputations occur in diabetic patients.23 In addition, peripheral vascular disease in combination with diabetes exacerbates these outcomes.24 Traumatic wounds are also quite common, with an estimated 3700 major amputations occurring annually as a result of falls, work-related accidents, and motor vehicle crashes.16 The cost of amputations is remarkable, especially when compared to limb salvage. One study estimated savings of $480,000 over a 60-year period after reconstruction versus amputation.25 The free flap group had the lowest amputation rate but also had the highest number of traumatic wound patients. Trauma surgeons may be more cognizant of limb salvage because of the associated costs and patient population, as patients are relatively young, diabetes rates are low, and longstanding peripheral vascular disease is unlikely.
Reoperation rates were highest in the free flap group (n = 122), compared with 69 reoperations in the matrix group and 58 in the local tissue rearrangement group. Of note, only 10 matrix patients received reoperations due to a cause other than split-thickness skin grafting. To compare between groups, bilayer wound matrix was used as the reference group; free flaps had significantly more reoperations (n = 122, adjusted RR, 1.46; 95 percent CI, 1.00 to 2.15), depicting a patient and wound group that is quite complex compared with the other cohorts.
Predicted Probabilities of Treatments
An advanced predicted probability of success model reviewed 864 possible clinical scenarios and the predicted graft successes of each treatment arm. The model overall demonstrated that local tissue rearrangement and free flaps were quite successful. Bilayer wound matrix had success rates close to 70 percent but often was used on patients with higher rates of comorbidities who were not candidates for autologous tissue coverage and therefore only had bilayer wound matrix as a potential coverage option. While we expect last-resort wound therapies to demonstrate low success rates, given the often severe nature of the responsible disease process, our detailed analysis revealed certain clinical scenarios where underlying patient and wound characteristics led to matrix success rates of 90 percent or better. These patients tended to be older, obese male patients who were nonsmokers and nondiabetic. Their wounds tended to be on the foot with an atraumatic origin and without exposed bones. Although bilayer wound matrix may be the only option for a large group of high-comorbidity patients with lower extremity wounds, our findings demonstrate that in certain patients it can be a very successful and cost-effective option.
Limitations
This study is not without limitations that deserve further consideration. These data were collected in a retrospective manner, capturing multiple, granular data points over an extended period of time. We understand that accuracy in the collection may have been compromised, but to mitigate this we had several data abstractors investigate collected data to review and correct errors. We attempted to standardize patient cohorts and wound descriptors with our advanced matching technique, which was created by an unbiased statistician from the Center for Clinical Epidemiology and Biostatistics, but we understand that missing data points led to an imperfect match. We also attempted to employ cardinality matching to wound type as well, understanding that, for instance, a vasculopathic patient with a venous stasis ulcer is much different than a young traumatic, nonvasculopathic patient with normal venous circulation. However, we would have sacrificed too many patients, leading to a less robust data analysis. The decision-making process is multifactorial, and we were not able to account for patient wishes and surgeon preferences. Furthermore, conflicts of interests were minimized at an institutional level by specific data abstractors, blocking of the data set, and review by the institution’s financial committee.
CONCLUSIONS
Data presented in this large, multi-institutional study highlight the relative clinical benefits to specific lower extremity reconstruction based on patient and wound characteristics. We effectively compare three treatment modalities using an advanced matching technique. We demonstrated that free flap reconstruction is a successful reconstructive option; however, it leads to longer length of stay, increased numbers of readmissions and reoperations, and high costs. Local autologous tissue rearrangement, if achievable, provides successful coverage at minimal costs and decreased readmissions and reoperations. Bilayer wound matrix can be effectively used in certain patient populations while reducing costs and decreasing length of stay.
ACKNOWLEDGMENTS
The authors would like to thank Integra LifeScience and the University of Pennsylvania Center for Human Appearance for its generous support in the completion of this study. They would like to express their gratitude to all surgeons at the University of Pennsylvania Health System that treated and cared for the patients included in this study.
Footnotes
Presented at the 30th European Association of Plastic Surgeons Annual Meeting, in Helsinki, Finland, May 23 through 25, 2019, and the 10th Congress of the World Society of Reconstructive Microsurgery, in Bologna, Italy, June 12 through 15, 2019. Accepted for oral presentation at the American College of Surgeons Clinical Congress 2019, in San Francisco, California, October 27 through 31, 2019, and Plastic Surgery The Meeting 2019: The 88th Annual Meeting of the American Society of Plastic Surgeons, in San Diego, California, September 20 through 23, 2019.
Disclosure: Dr. Fischer has received consultant payments from Becton Dickinson, Integra Life Sciences, Gore, and Allergan. Dr. Kovach has received consultant payments from Becton Dickinson and Integra LifeSciences. The remaining authors do not have any financial disclosures to report. This research received financial support from Integra LifeSciences (investigator initiated) and the University of Pennsylvania Center for Human Appearance.
REFERENCES
- 1.Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: A 5-year follow-up study. Diabetes Care 2004;27:1598–1604. [DOI] [PubMed] [Google Scholar]
- 2.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: The economic case for the limb salvage team. J Vasc Surg. 2010;52(3 Suppl):17S–22S. [DOI] [PubMed] [Google Scholar]
- 3.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17:347–356. [DOI] [PubMed] [Google Scholar]
- 4.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care 2014;37:651–658. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MB, Wong AK. Integra-based reconstruction of large scalp wounds: A case report and systematic review of the literature. Plast Reconstr Surg Glob Open 2016;4:e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim PJ, Attinger CE, Steinberg JS, Evans KK. Integra bilayer wound matrix application for complex lower extremity soft tissue reconstruction. Surg Technol Int. 2014;24:65–73. [PubMed] [Google Scholar]
- 7.Jordan DJ, Malahias M, Hindocha S, Juma A. Flap decisions and options in soft tissue coverage of the lower limb. Open Orthop J. 2014;8:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visconti G, Zubizarreta JR. Handling limited overlap in observational studies with cardinality matching. 2017Columbia University; Unpublished manuscript. [Google Scholar]
- 9.Zubizarreta JR, Kilcioglu C, Vielma JP. Matched samples that are balanced and representative by design. R package version 03. Package Designmatch, June 18, 2018. [Google Scholar]
- 10.Karmakar B, Small DS, Rosenbaum PR. Using approximation algorithms to build evidence factors and related designs for observational studies. J Comput Graph Stat. 2019;28:1–34. [Google Scholar]
- 11.Karmakar B. approxmatch: Approximately optimal fine balance matching with multiple groups. R package version 10. Package Approxmatch, November 5, 2017. [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 13.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 14.Team RC. R: A language and environment for statistical computing. 2018. Vienna, Austria: R Foundation for Statistical Computing, Available at: https://www.R-project.org/. [Google Scholar]
- 15.Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med. 2017;377:1559–1567. [DOI] [PubMed] [Google Scholar]
- 16.Nance ML. National Trauma Data Bank 2013 Annual Report. 2013Chicago, Ill: American College of Surgeons. [Google Scholar]
- 17.Burke JF, Yannas IV, Quinby WC, Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham GP, Helmer SD, Haan JM, Khandelwal A. The use of Integra Dermal Regeneration Template in the reconstruction of traumatic degloving injuries. J Burn Care Res. 2013;34:261–266. [DOI] [PubMed] [Google Scholar]
- 19.Yao M, Attalla K, Ren Y, French MA, Driver VR. Ease of use, safety, and efficacy of Integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: A prospective pilot study. J Am Podiatr Med Assoc. 2013;103:274–280. [DOI] [PubMed] [Google Scholar]
- 20.Shakir S, Messa CA, Broach RB, et al. Indications and limitations of bilayer wound matrix-based lower extremity reconstruction: A multidisciplinary case-control study of 191 wounds. Plast Reconstr Surg. 2019;145:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bigdeli AK, Gazyakan E, Schmidt VJ, et al. Long-term outcome after successful lower extremity free flap salvage. J Reconstr Microsurg. 2019;35:263–269. E-published ahead of print October 16, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, DeFazio MV, Lakhiani C, et al. Limb salvage and functional outcomes following free tissue transfer for the treatment of recalcitrant diabetic foot ulcers. J Reconstr Microsurg. 2019;35:117–123. [DOI] [PubMed] [Google Scholar]
- 23.Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care 1983;6:87–91. [DOI] [PubMed] [Google Scholar]
- 24.Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care 2001;24:1433–1437. [DOI] [PubMed] [Google Scholar]
- 25.Chung KC, Saddawi-Konefka D, Haase SC, Kaul G. A cost-utility analysis of amputation versus salvage for Gustilo type IIIB and IIIC open tibial fractures. Plast Reconstr Surg. 2009;124:1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


