Supplemental Digital Content is available in the text.
Keywords: fibrin, fibrinogen, fibrinolysis, polymerization, systematic review
Objective:
Post-translational modifications of fibrinogen influence the occurrence and progression of thrombotic diseases. In this systematic review, we assessed the current literature on post-translational modifications of fibrinogen and their effects on fibrin formation and clot characteristics.
Approach and Results:
A systematic search of Medline, Embase, Cochrane Library, and Web of Science was performed to find studies reporting post-translational modifications of fibrinogen and the effects on clot formation and structure. Both in vitro studies and ex vivo studies using patient material were included. One hundred five articles were included, describing 11 different modifications of fibrinogen. For the best known and studied modifications, conclusions could be drawn about their effect on clot formation and structure. Oxidation, high levels of nitration, and glycosylation inhibit the rate of polymerization, resulting in dense clots with thinner fibers, while low levels of nitration increase the rate of polymerization. Glycation showed different results for polymerization, but fibrinolysis was found to be decreased, as a consequence of increased density and decreased permeability of clots. Acetylation also decreases the rate of polymerization but results in increased fiber diameters and susceptibility to fibrinolysis. Other modifications were studied less or contrasting results were found. Therefore, substantial gaps in the knowledge about the effect of post-translational modifications remain.
Conclusions:
Overall, post-translational modifications do affect clot formation and characteristics. More studies need to be performed to reveal the effects of all post-translational modifications and the effects on thrombotic diseases. Expanding the knowledge about modifications of fibrinogen can ultimately contribute to optimizing treatments for thrombotic diseases.
Highlights.
Variations in fibrinogen affect the occurrence and progression of thrombotic diseases.
Post-translational modifications of fibrinogen affect clot formation, clot characteristics, and susceptibility to fibrinolysis.
For the best known post-translational modifications of fibrinogen, conclusions regarding their effect on clot characteristics can be drawn.
Additional research is needed to elucidate the effects of all post-translational modifications of fibrinogen on clot characteristics.
The architecture and properties of a thrombus are an important determinant of the disease burden and mortality associated with cardiovascular diseases. One of the main determinants of thrombus characteristics is variations in the fibrinogen molecule, which affect the clotting rate, architecture of the fibrin matrix and its susceptibility to fibrinolysis. Therefore, it is essential to know the effect of variability in the fibrinogen molecule on the coagulation cascade and the clot characteristics. Fibrinogen is a glycoprotein that is synthesized by hepatocytes and circulates in the blood of healthy individuals at concentrations between 2 and 4 g/L. Fibrinogen consists of 2 sets of 3 different polypeptide chains: 2-Aα, 2-Bβ, and 2-γ chains, which are held together by 29 disulfide bridges.1 The genes encoding for these chains (FGA, FGB, and FGG) are found in a cluster of 65 kilobases on the human chromosome 4 (4q23-q32).2 In the fibrinogen molecule, different main structural regions can be identified.3 The central E region contains the amino acid termini of the polypeptide chains and this is also the place where thrombin cleaves. The 2 distal nodules (D regions) contain the carboxyl termini of the Bβ and γ chains and are connected to the E region by 2 α-helical coiled-coil domains. The carboxyl termini of the Aα chains form the αC-regions, which are more flexible and mobile.4
The final step of the coagulation cascade is the cleavage by thrombin between arginine and glycine residues in the Aα and Bβ chain in the E region, which releases fibrinopeptides A and B.5 This results in the formation of fibrin monomers and initiation of polymerization. Thrombin also activates factor XIII, which cross-links γ chains or α chains from neighboring fibrin molecules, thereby enhancing the stability of the fibrin network.6 Fibrinolysis of the fibrin network starts when a tissue-type plasminogen activator converts plasminogen into plasmin, which cleaves fibrin after lysine residues. Susceptibility to fibrinolysis is highly influenced by the structure of the clot.7 Thicker fibers, reduced branching, and larger pores increase the permeability and susceptibility to fibrinolysis, which is considered anti-thrombotic. However, thinner fibers, more branching, and smaller pores make the clot less permeable and more resistant to lysis by plasmin (prothrombotic).8
Fibrinogen Heterogeneity
Fibrinogen heterogeneity is the result of several types of variation: genetic polymorphisms, alternative mRNA processing, proteolytic cleavage, environmental factors, and post-translational modifications of fibrinogen.9–11 The different combinations of these determinants lead to more than a million forms of fibrinogen within a healthy individual.12,13 Post-translational modifications affect the function of fibrinogen, thereby influencing clot formation, the clot structure and susceptibility to clot lysis. These effects have consequences for the occurrence and progression of thrombotic diseases. The post-translational modifications which are discussed in this systematic review will be shortly introduced below. In Figures 1 and 2, an overview of the sites of modifications in the fibrinogen chains and the biochemistry of these modifications are shown.
Figure 1.
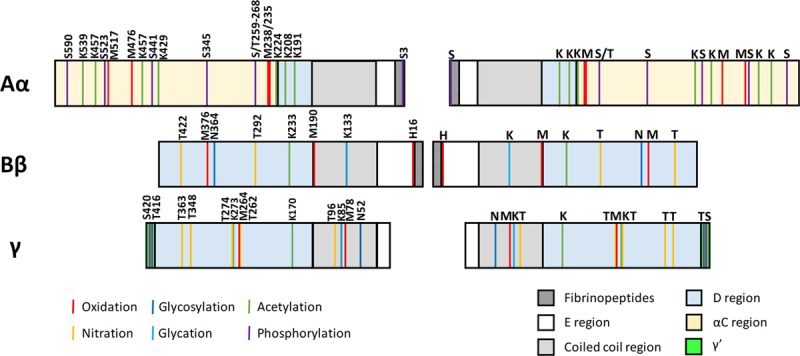
Known sites of post-translational modifications in the fibrinogen chains for the most studied modifications. Schematic representation of the fibrinogen chains with the known sites of the post-translational modifications shown in lines with different colors. The letter and number indicate the type of amino acid which is modified and the position of the amino acid, respectively. The colored blocks correspond to the different regions of the fibrinogen molecule.
Figure 2.
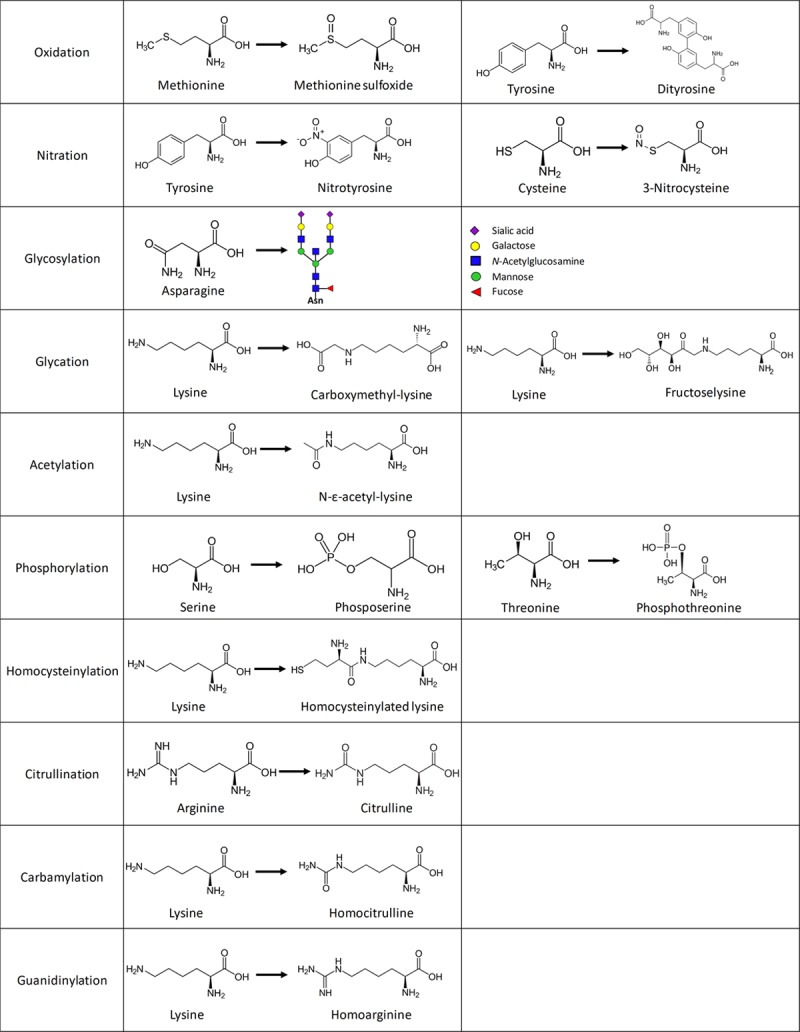
Biochemistry of the post-translational modifications. For each modification discussed in this review, 1 or 2 amino acids with the biochemical changes are depicted.
Post-Translational Modifications
Oxidation
One of the modifications of fibrinogen is oxidation. Oxidative stress occurs in the human body when a high amount of reactive oxygen species are produced (eg, by immune cells, pro-oxidant enzymes, and during oxygen metabolism) which are not sufficiently detoxified by antioxidants.14 Reactive oxygen species can be produced as a consequence of exogenous insults, for example, radiation from UV-light or smoking.15 Among the reactive oxygen species are superoxide anion, hydrogen peroxide, hypochlorous acid, and hydroxyl radical.14 These different reactive oxygen species react with and cause damage to a wide range of components of the cells, for example, proteins and DNA. Consequences of oxidation of proteins are the formation of carbonyl groups and the modification of amino acids (eg, methionine is converted into methionine sulphoxide and tyrosine into dityrosine; Figure 2).16,17 Compared with other plasma proteins, fibrinogen is especially susceptible to oxidation. It was shown that fibrinogen is 20 times more susceptible to oxidation than albumin, the most abundant plasma protein.18 A recent article identified the amino acids in fibrinogen which are oxidized by ozone.19 Previously, other articles have identified amino acids oxidized by photo-oxidation and hypochlorous acid.20 In Figure 1, we show the amino acids which were identified by at least 2 ways of oxidation or which were oxidized above 50% using 50 µmol/L ozone.
Nitration
Oxidative stress also leads to the production of reactive nitrogen species.15 Superoxide anion reacts with nitric oxide to produce the highly reactive peroxynitrite. In addition, the reaction of oxygen with nitric oxide and peroxynitrite results in the formation of other reactive nitrogen species, for example, nitrite and nitronium ion. Nitration of fibrinogen mainly affects tyrosine residues, resulting in the formation of 3-nitrotyrosine.21,22 Also cysteine residues can be affected, resulting in the formation of 3-nitrocysteine (Figure 2).
Modification by Carbohydrates
Glycosylation is the enzymatic process in which sugars (glycans or polysaccharides) are attached to proteins in an ATP (adenosine triphosphate)-dependent manner. Glycosylation is needed at certain sites in a protein to function properly. Sialylation is a form of glycosylation in which sialic acid is bound at the end of a sugar chain of a glycoprotein. Each Bβ and γ chain of fibrinogen has 1 N-glycosylation site, which can contain zero, 1, or 2 sialic acids (Figure 1).23,24 Normally, ≈6 sialic acid residues are present in each fibrinogen molecule.25 Abnormal glycosylation has been related to aging and certain diseases; for example, patients with liver disease show an increased level of sialic acid content of their fibrinogen molecules.26,27 Glucose is known to nonenzymatically bind to proteins, often resulting in an impairment of its functions, in a process called glycation. Glycation occurs in patients with high levels of glucose in their blood, for example, in uncontrolled diabetes mellitus. Normal glucose levels are 5 mmol/L (90 mg/dL), but this can go up to 20 mmol/L (360 mg/dL) in patients with diabetes mellitus.28 Also at normal glucose levels, glycation can occur. This is associated with oxidative stress, for example, observed during inflammation or in age-related diseases.29 In fibrinogen, glycation is found to occur at lysine residues (Figure 1).30
Acetylation
Acetylation is the attachment of an acetyl group to amino acids. In vivo, acetylation occurs at N-termini of polypeptide chains by Nt-acetyltransferases or at the ε-amino group of lysine residues by lysine acetyltransferases (Figure 2).31 Both processes have important physiological functions. Acetylation can also be caused by the intake of aspirin, which is known to exert its beneficial effects in cardiovascular disease by acetylating serine residues in the enzyme platelet cyclooxygenase. In fibrinogen and other coagulation proteins, aspirin can acetylate lysine residues, affecting their functions (Figure 1).30,32
Phosphorylation
Phosphorylation is the attachment of a phosphate group to an amino acid by protein kinases. Phosphorylation and dephosphorylation (by protein phosphatases) of proteins affect the structure and have important consequences for protein function.33 Many enzymes are, for example, activated or deactivated by (de)phosphorylation and in many signaling pathways, phosphorylation plays a crucial role. In fibrinogen, phosphorylation occurs mainly on serine and threonine located in the Aα chain (Figure 1).34,35 It is shown that fetal fibrinogen is phosphorylated to a higher degree than adult fibrinogen, suggesting a functional importance of phosphorylation.34 In addition, phosphorylation of fibrinogen increases after surgery, potentially contributing to prevention of bleeding.36
Other Modifications
High levels of the plasma protein homocysteine results in the nonenzymatic post-translational modification homocysteinylation. Homocysteine has a free sulfhydryl group, with which it forms disulfide bonds with cysteine residues in proteins. Also, lysine residues can be modified, by the metabolite homocysteine thiolactone.37 Homocysteine is produced in the metabolism of methionine, and it is thought that even a small increase in the plasma level is a risk factor for cardiovascular disease.38 Homocysteine levels can be increased as a consequence of renal dysfunction, vitamin deficiencies, or medication affecting homocysteine metabolism.38 Homocysteine levels in plasma higher than 15 μmol/L are considered hyperhomocysteine and levels above 100 μmol/L are classified as homocysteinuria.39 In human fibrinogen, 3 lysine residues (Lys562 in the Aα chain, Lys344 in the Bβ chain, and Lys385 in the γ chain) were shown to be homocysteinylated, both in vitro and in vivo.40
Citrullination is the catalytic conversion of the amino acid arginine into citrulline by the enzyme peptidyl arginine deiminase. This post-translational modification is important in patients with rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune diseases because autoantibodies in these diseases often attack citrullinated proteins.41 Citrullination of fibrinogen potentially plays a role in the increased risk of thrombosis observed in rheumatoid arthritis.42 Using in vitro experiments, multiple arginine residues, mainly in the Aα chain and a few in the Bβ chain, were identified that are citrullinated by 2 different peptidylarginine deiminases.43
Carbamylation of proteins mainly occurs when amino acids interact with isocyanic acid, for example, during chronic kidney disease or atherosclerosis.44 Isocyanic acid forms when urea decomposes into ammonium and cyanate, cyanate subsequently converts into isocyanic acid. Another source of cyanate is the thiocyanate metabolism, in which thiocyanate in the presence of hydrogen peroxide is converted into cyanate by myeloperoxidase.44 Interaction of free amino acids or lysine residues with isocyanic acid results in the formation of α-carbamyl-amino acids or ε-carbamyl-lysine (homocitrulline), respectively.44 Homocitrulline differs from citrulline by only one additional methylene group.
Finally, guanidinylation is the conversion of an amino group into a guanidine group, for example, happening in patients on hemodialysis.45 Mostly, lysine residues are affected, which are converted into homoarginine. These last 2 post-translational modifications are studied less for fibrinogen, but because fibrinogen is a very abundant plasma protein, these modifications will most likely also affect fibrinogen.
It is important to know the effect of these post-translational modifications on clot formation, the clot structure, and susceptibility to fibrinolysis since these clot characteristics affect the development and progression of thrombosis and thromboembolic disease.46,47 With this knowledge, therapies to prevent or treat these diseases by normalizing clot structure or susceptibility to fibrinolysis can be optimized. During the past 70 years, multiple research groups have investigated the effects of these post-translational modifications. However, contradictory results are reported for the effects of certain modifications studied by different research groups. Therefore, the aim of this systematic review was to make an overview of the results of studies investigating the effect of post-translational modifications of fibrinogen on clot formation, clot structure, and susceptibility to fibrinolysis, to be able to draw tentative conclusions or identify the knowledge gaps.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.48
Article Search
We conducted a systemic literature search in the Embase, Medline-Ovid, Cochrane Library, and Web of Science databases on April 18, 2019; and the search was repeated on October 8, 2019. The search strategy included the different post-translational modifications in combination with fibrinogen and the characteristics we were interested in (eg, clotting time, clot structure, density, or permeability; see Methods in the online-only Data Supplement for the full search). No limit was set on the year of publication.
Study Selection
After deduplication, the search yielded 1278 results. Two researchers (J.J. de Vries and C.J.M. Snoek) independently screened these articles. First, relevant articles were included based on title and abstract. The included abstracts were subsequently read full text and articles that did not match the research question were excluded. In addition, conference abstracts, reviews, and articles not available in full text (in English) were excluded. Additional relevant articles identified while reading the full-text articles were also assessed for inclusion and included if there was a match with the research question. In case of disagreement, consensus about the articles was reached through discussion.
Data Extraction
To prevent bias, data were independently extracted from the articles by 2 researchers (J.J. de Vries and C.J.M. Snoek). The following data was collected: first author, publication year, method of experiments (which type of fibrinogen or plasma was used and how it was modified) and effects of the modification compared to control (clottability, cleavage by thrombin, rate of polymerization, initiation of polymerization or lag phase, maximal absorbance, diameter of fibrin fibers, stiffness, permeability, density, cross-linking, plasmin digestion of fibrinogen, and clot lysis).
Results
Study Selection
A total of 1640 articles was found in the literature search. After elimination of duplication, 1278 articles were screened based on title and abstract (Figure 3). From the 183 articles read full text, we included 66 articles. In addition, while reading these articles full text, we found 39 other relevant articles in the references which were also included. The majority of these extra articles which did not show up in our literature search were old studies or did not use the terms for the modification in the title or abstract, which might explain why they did not come up in the search. In total, we identified 105 studies.
Figure 3.
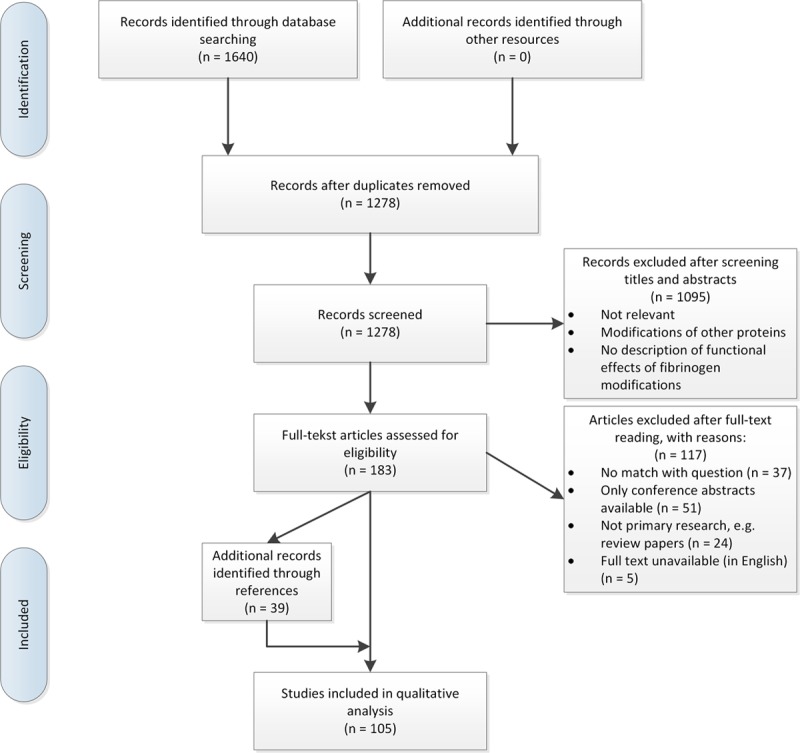
PRISM (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of study selection.
Study Characteristics
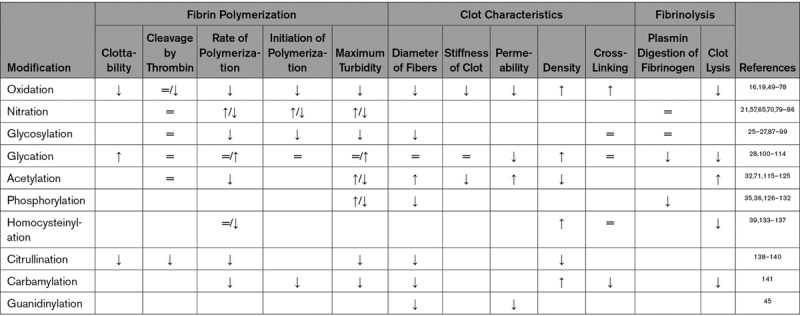
Most of the studies were performed in vitro with commercial purified fibrinogen, however, purified fibrinogen or plasma from patients was also used in a substantial number of studies. Especially studies investigating acetylation used healthy volunteers or patients taking aspirin to study the effect of acetylation on fibrinogen. Also, studies investigating the effect of other modifications used material from patient groups in which the modification was known to be increased (eg, oxidation in myocardial infarction [MI] patients, glycation in diabetes mellitus patients, and sialylation in patients with liver disease). Twelve clot characteristics were studied in this systematic review. Clottability was used to describe the percentage of fibrinogen which is able to clot. Cleavage by thrombin describes the amount or rate of fibrinopeptide release from fibrinogen by thrombin. The rate of polymerization is reported when clotting time or the rate of aggregation was measured. Initiation of polymerization was mostly measured by the length of the lag phase, the time before clotting starts (when lag phase increases, initiation of polymerization is decreasing). Maximum turbidity means the maximum value of absorbance measured in turbidity assays. The diameter of fibrin fibers was determined using the mass-length ratio or measuring thickness of fibers on microscopy images. The stiffness of the clot was measured using rheology or thromboelastography. Permeability describes the permeability of the clot, while density corresponds to the amount of fibers in a certain area. Cross-linking is mainly measured by measuring the amount and rate of formation of γ- or α-dimers. Plasmin digestion of fibrinogen means the breakdown of fibrinogen molecules by plasmin. Finally, clot lysis describes the degree or rate of fibrinolysis of the clot. Of these characteristics, most information was available for the rate of polymerization and maximum turbidity. The findings of all studies per modification are summarized in the Table.
Table.
Overview of the Effects of the Different Modifications
Oxidation of Fibrinogen
Oxidation is the most studied post-translational modification of fibrinogen (31 articles, Table I in the online-only Data Supplement). Most studies used (human) fibrinogen and added a compound or condition that oxidizes fibrinogen (reactive oxygen species, ozone, or illumination). However, the conditions used in vitro show a lot of variation. A few studies used fibrinogen from patients with diseases known to increase oxidative stress, and therefore the level of fibrinogen oxidation is increased49–53 (shown in Table I in the online-only Data Supplement below the thick line). Except for Paton et al,49 the studies which used patient material do not show large differences compared to the in vitro studies.
The clottability of oxidized fibrinogen was decreased after oxidation in 2 studies.54,55 When light, radiation, or peroxynitrite was used to oxidize fibrinogen, no difference in cleavage by thrombin was found (3 studies).55–57 However, when fibrinogen was oxidized by reactive carbonyl compounds or ascorbate and iron, the cleavage by thrombin was decreased (2 studies).56,58 This suggests that the manner of oxidation influences the effects, probably by affecting different sites in fibrinogen. The rate of polymerization of fibrinogen after oxidation was mostly found to be significantly decreased compared to nonoxidized fibrinogen (in 25 of the 34 experiments). The in vitro studies reporting an increased rate of polymerization used relatively high concentrations of oxidative compounds, which might explain the discrepancy.59,60 In almost all experiments, the initiation of polymerization was significantly delayed (6 out of 9) and the maximum absorbance measured in turbidity assays was significantly decreased (15 out of 19). In correspondence with this, the diameter of the fibrin fibers was found to be significantly smaller in 8 of the 12 experiments. Only a few studies report contradictory results: a shorter lag phase and increased maximum absorbance or diameter.49,61,62 The study done by Rosenfeld et al61 found a significantly shorter lag phase and thicker fibrin fibers after oxidation of fibrinogen with ozone. The thicker fibers are also reported in 62, where the same researchers used a similar method of oxidation. It is possible that oxidation by ozone differently affects the fibrinogen molecule than other methods of oxidation. The final study which shows contradictory results compared to the majority of the studies used purified fibrinogen from MI patients 24 to 96 hours after the heart attack and compared clot characteristics between patients in different quartiles of plasma protein carbonyl values (representative of oxidative modifications and correlated to fibrinogen carbonyl content).49 The authors themselves describe that the fibrinogen in other studies is probably more highly oxidized than their plasma samples, which would suggest that a low level of oxidation increases the ability of fibrinogen to clot. However, there is quite a broad range in concentrations of reactive compounds used in the other 30 studies, which gives a broad range of oxidation and most studies show a decreased ability of clotting. In addition, it can be appreciated in Table I in the online-only Data Supplement that incubating fibrinogen with relatively high concentrations of oxidative compounds shows an increased rate of polymerization, which would suggest the opposite: fibrinogen from the MI patients is oxidized to a higher degree instead of a lower degree. The other study that used fibrinogen from MI patients used blood drawn from patients 6 months after the event,50 which is very different from 24 to 96 hours after MI. Although comparable levels of fibrinogen carbonyl content were reported in both studies, this difference in time after MI can be one of the reasons for the discrepancy between the 2 studies.
Other properties of the fibrin clot are studied in fewer articles. In general, a decreased stiffness (6 out of 7), lower permeability (4 out of 5), and higher density of fibrin clots (4 out of 8) were found after oxidation. Two studies found an increase in cross-linking after oxidation,63,64 while another study reported no difference.16 Digestion of fibrinogen by plasmin was found to be decreased after oxidation by concentrations of peroxynitrite higher than 100 μmol/L, whereas lower concentrations did not show significant differences.65 Fibrinolysis was found to be significantly decreased by most studies (6 out of 9),16,50,51,66,67 but also no difference (one study) or an increase (2 studies) in fibrinolysis was reported.53,68,69
Overall, oxidation seems to decrease the rate of clotting and results in more dense fibrin clots with thinner fibers which are less permeable. No conclusions can be drawn about the degree of cross-linking, digestion of fibrinogen or fibrinolysis, since only a few studies are done which all report different results. However, since most studies report a more dense fibrin clot with lower permeability, fibrinolysis is most likely decreased after oxidation.
Nitration of Fibrinogen
A post-translational modification that is relatively similar to oxidation and often studied in combination with oxidation is nitration of fibrinogen. We identified 12 studies on nitration of fibrinogen (Table II in the online-only Data Supplement), of which 3 are also included in the oxidation table (65 and 57 use peroxynitrite to study oxidation, which is also a nitrating agent and70 performed both oxidating and nitrating experiments). In Table II in the online-only Data Supplement, the upper part shows the in vitro studies, arranged in order of increasing concentrations per nitrating compound used. Four studies also included experiments with fibrinogen from coronary artery disease patients,70,79 smokers,80 or healthy volunteers taking lipopolysacharides,81 all resulting in increased levels of nitration of fibrinogen (shown in the lower part of Table II in the online-only Data Supplement).
No significant effect of nitration on cleavage by thrombin was found in 2 studies.57,70 The rate of polymerization is generally found to be increased for nitrated fibrinogen from patients (5 out of 5 studies with patients) or fibrinogen nitrated by low levels of nitrifying agents. Except for one study, all experiments using high concentrations (>10 μmol/L peroxynitrite or 100 μmol/L nitronium fluoroborate) to nitrate fibrinogen showed a decreased rate of polymerization. Ding et al82 used a concentration of peroxynitrite slightly lower than 10 μmol/L but also added increasing concentrations of manganese, which is known to increase fibrinogen nitration, explaining why they also find a decreased rate of polymerization. Helms et al83 used 5 μmol/L ProliNONOate to nitrate fibrinogen by the action of nitric oxide and found a decreased rate of polymerization, although not significant. There is one study that shows an increased rate of polymerization, although a high concentration of peroxynitrite (1 mmol/L) was used.21 This might be caused by a relatively low level of nitration found in this study; only 1.13 nitrotyrosine residues per fibrinogen molecule formed in contrast to levels up to 8 nitrotyrosine residues per fibrinogen molecule in other studies where high concentrations of peroxynitrite were used. Initiation of polymerization and maximum absorbance of turbidity measurements correspond to the results found for rate of polymerization; if the rate of polymerization was found to be increased, initiation of polymerization and maximum turbidity were also increased. The diameter of the fibrin fibers was found to be thinner after nitration with a high concentration of peroxynitrite84 or in fibrinogen from coronary artery disease patients,70 while another study with fibrinogen from smokers reported no difference80 and the study in which fibrinogen was nitrated by nitric oxide showed thicker fibers.83 Also the stiffness of the clot showed all different results (decrease, no difference or increase in stiffness).70,80,83 Permeability and cross-linking were investigated by only one study, which showed both characteristics to be increased.70 Density is shown to be higher in one study84 and lower in another83 after nitration in vivo. Digestion of fibrinogen or lysis of the fibrin clot by plasmin is shown to be similar65,70 or decreased after nitration of fibrinogen.65,80
Overall, a low level of nitration increases the rate of fibrinogen polymerization, while increased nitration of fibrinogen decreases the rate of polymerization. The few studies studying the other clot characteristics find opposing results, probably also caused by different degrees of nitration or by the presence of other oxidative modifications that can occur upon treating fibrinogen with peroxynitrite.
Modification of Fibrinogen by Carbohydrates
We identified only 3 studies on the effect of glycosylation on fibrinogen (Table III in the online-only Data Supplement). One ex vivo study used fibrinogen from subjects of varying age, since aging increases the glycosylation of fibrinogen.27 However, there were no differences in clot characteristics between older people (with a high level of glycosylation) and younger people.27 Two other in vitro studies used another approach to study the effect of glycosylation of fibrinogen: the glycosylation of fibrinogen was inhibited during the production of fibrinogen in rabbit hepatocytes87 or sugars were removed from fibrinogen using peptide-N-(N-acetyl-β-glucosaminyl)asparagine amidase.88 The first approach resulted in no difference in rate of polymerization, while the second study showed an increased rate of polymerization and maximum turbidity, thicker fibers and a more permeable clot, while cross-linking and fibrinogen digestion by plasmin were not affected by removal of glycosylation. Another in vitro study (not included in Table III in the online-only Data Supplement) was found in which fibrinogen was incubated with different sugars, investigating the lectin activity of fibrinogen. In addition, the effect of incubating fibrinogen with these different sugars was studied. A decreased rate of polymerization, decreased maximum turbidity and diameter of the fibers and a decreased cross-linking were found after incubation with different sugars.142
A specific form of glycosylation, sialylation, was studied by 13 articles. Four studies with fibrinogen from patients with specific diseases which are shown to increase the levels of sialic acid all presented a decreased rate of polymerization and initiation of polymerization.26,89–91 In other experiments, a different approach was used, namely removing sialic acid from fibrinogen. In addition, one article used fibrinogen from pregnant women, having a lower degree of sialylation.91 This desialylation resulted in increased rates of polymerization in 5 out of 6 studies, confirming the inhibitory role of sialic acid on polymerization.
Overall, glycosylation of fibrinogen results in decreased rates of polymerization and thinner fibers. There is not enough evidence to say something about the effect of glycosylation on the other characteristics of the fibrin clots.
Glycation of fibrinogen occurs in conditions with a high glucose concentration. We identified 16 studies investigating the effect of glucose binding on fibrinogen (Table IV in the online-only Data Supplement). Fibrinogen was incubated with glucose in vitro, or fibrinogen or plasma from patients with diabetes mellitus was used to assess the effects of glycation. Table IV in the online-only Data Supplement is sorted on glucose concentration used in vitro in the upper part of the table. Below the thick line, the studies using fibrinogen or plasma from patients with diabetes mellitus are sorted on the use of fibrinogen or plasma and type of patients with diabetes mellitus used.
After glycation, the clottability of fibrinogen was found to be similar (in one study) or increased (in 3 studies) compared to control fibrinogen. The 4 in vitro studies investigating the cleavage by thrombin reported no difference between glycated and control fibrinogen. However, fibrinogen from patients with type 2 diabetes mellitus showed an increased cleavage of fibrinopeptide B compared to control subjects.100 The studies show contradictory results regarding the rate of polymerization, initiation of polymerization, and maximal turbidity. Different concentrations of glucose were used to glycate control fibrinogen, or fibrinogen or plasma from both patients with type 1 and type 2 diabetes mellitus were used, which might explain these differences. The diameter of the fibers did not change after glycation, but this was only investigated by 2 studies.100,101 The stiffness of the clot was also found to be similar for fibrinogen or plasma from patients with diabetes mellitus and controls in 2 studies.101,102 Three out of 4 studies measuring permeability found a decreased permeability after glycation and density is mostly shown to be increased (in 3 out of 4 studies). Most studies (7 out of 9) found no difference in cross-linking between glycated and control fibrinogen or between fibrinogen from patients with diabetes mellitus and control subjects. Finally, the majority of the results showed a decreased digestion of fibrinogen (3 out of 4) and fibrinolysis of the clot (4 out of 5) after glycation.
Overall, studies investigating glycation show different results regarding the polymerization, probably caused by the different conditions of modification used. The few studies which are performed regarding the other characteristics of fibrin clots from glycated fibrinogen show a decreased permeability, increased density, no detectable effect on thickness of fibers, stiffness or cross-linking and decreased fibrinolysis of the clot after glycation.
Acetylation of Fibrinogen
Another modification is the acetylation of fibrinogen, which is mostly studied in the context of aspirin intake. We identified 13 studies, of which 6 did experiments with fibrinogen or plasma from patients or healthy volunteers who took aspirin (below the thick line in Table V in the online-only Data Supplement). The clottability and cleavage by thrombin were shown to be similar for control fibrinogen and fibrinogen acetylated in vitro.115–117 The rate of polymerization and maximum turbidity was mostly found to be significantly decreased after fibrinogen acetylation or ingestion of aspirin compared to nonacetylated fibrinogen (5 out of 9 studies). Only plasma from healthy volunteers taking a high dose of aspirin twice daily showed an increased polymerization.32 The other 3 studies showed no effects of acetylation on the rate of polymerization. Most experiments show a significantly increased diameter of the fibrin fibers after acetylation (7 out of 9). In correspondence with this, all studies agree that the permeability is increased (8 studies), the density is lower (3 studies) and susceptibility to clot lysis is increased (6 studies). It becomes clear that taking a low dose of aspirin is more beneficial than a higher dose since the high dose does not affect the fiber thickness and permeability of clots in 2 studies testing both doses.118,119 It is suggested that a high dose of aspirin can result in the formation of salicyclic acid molecules, which block acetylation of fibrinogen and, therefore, inhibit the beneficial effects of aspirin on clot structure.120 In conclusion, the acetylation of fibrinogen decreases fibrin polymerization and increases permeability and susceptibility to fibrinolysis.
Phosphorylation of Fibrinogen
The effect of phosphorylation or dephosphorylation of fibrinogen on clotting was studied by 9 research articles (Table VI in the online-only Data Supplement). These are all studies performed >20 years ago, no recent data were available. In most studies, fibrinogen was phosphorylated by incubating fibrinogen with kinases, although there is one study that uses fibrinogen from 5 patients after a hip surgery, who have elevated phosphorylation levels of fibrinogen.36 It appears that phosphorylation by protein kinase A or C reduces the maximum turbidity and thickness of fibers, while phosphorylation by casein kinase II increases maximum turbidity and the fiber diameter. The patients after hip surgery also showed an increased maximum turbidity and fiber diameter, which would suggest casein kinase II is the enzyme responsible for phosphorylation of fibrinogen in vivo.36 However, only 5 patients were used, and more research is needed to confirm this hypothesis. The digestion of fibrinogen by plasmin was shown to be decreased in all studies, it made no difference which kinase was used. Another strategy to study the role of phosphorylation on fibrinogen was to remove the phosphate group from normal fibrinogen by alkaline phosphatase. It was found that dephosphorylation increases the maximum turbidity and diameter of fibrin fibers in 3 studies, while plasmin digestion was not affected (only one study). Finally, also the reversibility of these effects was investigated by dephosphorylating phosphorylated fibrinogen. It was shown that dephosphorylation increases maximum turbidity and diameter of fibrin fibers (and therefore normalizes back to normal after phosphorylation by protein kinase C and higher than normal in the case of casein kinase-phosphorylated fibrinogen).36,126,127 However, plasmin digestion of fibrinogen stayed reduced and is therefore not reversible.36,127,128
In conclusion, the effect of phosphorylation on polymerization and the diameter of fibrin fibers depends on the kinase used to phosphorylate fibrinogen in vitro. Ex vivo, it was shown that increased phosphorylation of fibrinogen results in increased clot turbidity and fiber diameters, although this was only investigated by one study using a limited amount of patients.
Other Modifications of Fibrinogen
Six studies were identified in which the effect of homocysteinylation of fibrinogen was studied (Table VII in the online-only Data Supplement). Plasma from a rabbit model of homocystinuria showed an increased rate of polymerization and decreased fiber diameter,133 while human fibrinogen and plasma incubated with homocysteine showed a decrease in rate of polymerization, decreased maximum turbidity, and no change in fiber diameter.39 However, when plasma was incubated with higher levels of homocysteine (500 μmol/L), an increase in maximum turbidity and fiber diameter was observed.134 These differences are probably the result of different concentrations of homocysteine, the use of fibrinogen or plasma and the difference between in vitro and in vivo situations. Clot density is observed to be increased by 3 studies and also agreement is reached over a decreased susceptibility to fibrinolysis after homocysteinylation in 5 studies. Stiffness of the clot was found to be significantly increased after incubation of plasma with 50 to 500 μmol/L homocysteine.135
Two studies on citrullination of fibrinogen reported an inability of fibrinogen to clot because thrombin is not able to cleave off the fibrinopeptides of citrullinated fibrinogen and, therefore, no polymerization occurs138,139 (Table VII in the online-only Data Supplement). A more recent article showed clotting after citrullination of fibrinogen and observed a decreased rate of polymerization, turbidity, fiber diameter, and density.140
Carbamylation was studied in only one article in which carbamylation was found to decrease the ability of fibrinogen to clot, resulting in fibrin clots with thinner fibers, a higher density, less cross-linking, and decreased susceptibility to fibrinolysis.141
Finally, one article was found in which fibrinogen was modified by guanidinylation. The level of guanidinylation is increased in patients on hemodialysis.45 Both clots formed from plasma from these patients and fibrinogen to which o-methylisourea-bisulfate solution was added to guanidinylate fibrinogen showed clots with thinner fibers.45 Also, the permeability of clots formed from plasma from patients on hemodialysis was decreased. The kinetics of polymerization were not investigated in this study.
Discussion
In this systematic review, 105 research articles were identified that investigated the effects of post-translational modifications of fibrinogen on polymerization, clot structure, and fibrinolysis.
For oxidation, most research reported a decreased rate of polymerization, which results in more dense fibrin clots with thinner fibers and lower permeability. This results in clots that are more resistant to fibrinolysis, and therefore, prothrombotic. The mechanism by which oxidation affects clotting is thought to be oxidation of methionine residues in the αC region, which is involved in lateral aggregation. The αC region is proven to be most vulnerable to oxidation by ozone and hypochlorite.19,143 Weigandt et al16 identified methionine residue at position 476 in the αC region to be oxidized by hypochlorite. Conversion of Met476 into methionine sulfoxide was shown to impair dimerization of αC domains, thereby inhibiting lateral aggregation.144
An interesting finding by Wang et al64 was the observation that the stiffness of the clot measured at low frequencies is higher for oxidized fibrinogen compared to the control, while increasing the frequency at which stiffness is measured results in a lower value for oxidized fibrinogen. This difference implicates that the effect of oxidation on the stiffness can change corresponding to the conditions used in the measurement. This is an interesting finding, since blood flow and shear stress in the human body can also be variable. Therefore, it would be interesting to perform more experiments studying the effect of oxidation of clot stiffness measured during different degrees of shear stress.
It is hard to distinguish between nitration and oxidation, but in this systematic review, we separated these modifications. However, it should be noted that nitration and oxidation often occur simultaneously. In general, nitration of fibrinogen using low concentrations of nitrating agents (low level of nitration) results in an increased rate of polymerization and higher maximum turbidity. The same results are found when fibrinogen nitrated in vivo is compared to control fibrinogen without nitration. However, high levels of nitration in vitro decrease the rate of polymerization and maximum turbidity. This shows that we need to be careful translating in vitro results to the in vivo situation. The other clot characteristics are less well studied for the effect of nitration. It is shown that nitration of fibrinogen results in the formation of nitrotyrosines in the part of the Bβ chain which is involved in lateral aggregation.80 It is suggested that a low degree of nitration results in a gain of function of fibrinogen, having an increased rate of polymerization. When fibrinogen is nitrated with high concentrations of nitrating compounds, there might be too much nitrotyrosine formation which blocks lateral aggregation.
Glycosylation, and specifically sialylation, inhibits the rate of fibrin polymerization and leads to thinner fibers, which was shown both by increasing glycosylation and deglycosylation of fibrinogen. It is suggested that sialic acid inhibits polymerization by electrostatic repulsion between fibrin monomers.92 In normal conditions, calcium binds these sialic acid residues, thereby neutralizing this inhibitory effect. However, during liver disease, sialylation levels are increased, leading to insufficient neutralization of sialic acid by calcium and therefore inhibition of polymerization and thinner fibrin fibers.92 There has not been done enough research to draw conclusions about the effect of glycosylation on the other clot characteristics, such as stiffness of the clot, cross-linking, and permeability.
Glycation of fibrinogen showed contradictory effects on the polymerization, which might be caused by the different conditions in which this modification was tested (in vitro or fibrinogen or plasma from type 1 or type 2 diabetes mellitus patients). Therefore, more research is needed to determine the effect of glycation on fibrin polymerization and to evaluate if this effect is similar in diabetic patients. Few studies investigated other characteristics of fibrin clots and showed an increased density and decreased permeability and fibrinolysis of the clot after glycation, which corresponds to the resistance to clot lysis seen in diabetic patients.145 In this systematic review, only studies are included which specifically investigated the glycation of fibrinogen (either by using purified fibrinogen or measuring fibrinogen glycation when plasma was used). However, there are multiple studies that used plasma from patients with diabetes mellitus to study clot characteristics, without quantifying fibrinogen glycation. These studies also show a decreased permeability and impaired fibrinolysis in clots from diabetic patients.146,147 It is known that the risk to develop major thrombotic diseases, for example, MI, stroke, or deep vein thrombosis, is increased in diabetes mellitus, due to the inflammatory and prothrombotic environment, of which the latter can partly be ascribed to the glycation of fibrinogen.148 Besides the increased density and decreased permeability, another reason for resistance to fibrinolysis might be the glycation of specific lysine residues in plasmin-sensitive coiled-coil regions of fibrinogen, thereby occupying the binding sites of plasmin.30
Acetylation was shown by most studies to decrease the rate of fibrin polymerization and increase permeability and susceptibility to fibrinolysis. This is beneficial in the prevention and treatment of thrombosis and thromboembolic diseases, since a more permeable clot increases the susceptibility to fibrinolytic treatment. Acetylation as a consequence of aspirin intake is shown to target lysine residues, thereby disturbing the charge distribution of the fibrinogen molecule and affecting polymerization, resulting in clots with thicker fibers and increased permeability.120 In addition, acetylation of lysine residues involved in cross-linking might be the cause of the decreased cross-linking seen in 121, which further contributes to the increased permeability of fibrin clots.30
It has been hypothesized that there is a competition between acetylation of amino acids by aspirin and glycation in diabetes mellitus, resulting in aspirin resistance in patients with diabetes mellitus.149 However, identification of the lysine residues affected by both modifications showed no overlap between these sites.30 Another study describes that taking aspirin can prevent the disadvantageous effects of oxidation. It was shown that acetylation of lysine residues in fibrinogen prevents the effects of oxidation of fibrinogen.71 The question whether this is due to competition remains to be answered.
The effect of in vitro phosphorylation of fibrinogen was different for the specific kinases used. Phosphorylation by casein kinase II showed similar results (increased maximum turbidity and thicker fibers after phosphorylation) as fibrinogen with an increased phosphorylation purified from patients, which suggests this kinase plays a role in fibrinogen phosphorylation in vivo. Also, the sequence specificity of type II casein kinases corresponds to the sequences around the phosphorylated amino acids.34 It was reported that casein kinase II phosphorylates serine and threonine residues in the Aα and γ’ chain.35 Although protein kinase C also phosphorylates serine residues in the carboxyl-terminal part of the Aα chain,150 the effects of phosphorylation are different (decrease in maximum turbidity and fiber thickness), which suggests that amino acids at different positions are involved. To determine the effects of phosphorylation of fibrinogen, further studies are required which investigate which kinase is responsible for in vivo phosphorylation of fibrinogen, to use this information in in vitro experiments. In addition, fibrinogen phosphorylated in vivo can be used to investigate the effect of phosphorylation on clot characteristics. For example, no information is available on the effect of phosphorylation on clot stiffness, permeability, and susceptibility to fibrinolysis.
Studies on homocysteinylation of fibrinogen did not find consistent results in the polymerization, probably because different (mainly nonphysiological) concentrations of homocysteine were used with either fibrinogen or plasma. It would be recommended in further studies to use physiological homocysteine levels for homocysteinylation of fibrinogen to be certain about the effects on polymerization and fiber diameter. However, the studies did all show an increase in density and decrease in fibrinolysis after homocysteinylation. This can be explained by the finding that homocysteinylation of fibrinogen occurs mostly on lysines in the Aα chain which are involved in plasmin binding and cleavage.136,137
In 2 studies on citrullination of fibrinogen, no clots could be formed due to the citrullination of arginine residues in the N-terminus of the Aα and Bβ chain.138,139 Since arginine residues are the cleavage site of thrombin, this modification inhibits the cleavage of fibrinopeptides by thrombin and formation of fibrin monomers, which explains the absence of clot formation. However, it is interesting to note that patients with rheumatoid arthritis (high levels of citrullinated fibrinogen) have an increased risk of thrombosis and do not have an increased risk of bleeding. This suggests that there is (increased) clot formation when fibrinogen is citrullinated in vivo. It is possible that the degree of citrullination in the performed in vitro studies is too high and, therefore, not relevant for the in vivo situation. A more recent study was indeed able to form clots with citrullinated fibrinogen and observed a decreased rate of polymerization, decreased turbidity, thinner fibers, and a decreased density when clots were formed from citrullinated fibrinogen.140 Since this is only one study, these results need to be confirmed. In addition, ex vivo research with fibrinogen from rheumatoid arthritis patients would also be interesting.
Both for carbamylation and guanidinylation only one study was found, suggesting these modifications result in more dense clots with thinner fibers which are less susceptible to fibrinolysis. This corresponds to the in vivo situation seen in diseases in which these modifications occur (kidney disease or atherosclerosis).
Most studies included in this review are performed in vitro, sometimes with high concentrations of chemicals to induce the post-translational modifications. This makes it challenging to translate these findings to the in vivo situation. However, for most modifications, also in vivo work has been done, which mostly shows the same effects as found in vitro (except for nitration, as described above). Another source of variation between the studies is the variation in techniques used to study certain clot characteristics. Especially older articles used different methods to determine, for example, clotting time and clot lysis time (by visual inspection), while in more recent studies turbidity assays are used. Another example is the assessment of the diameter of the fibrin fibers, techniques used are, for example, the turbidity assay to calculate the mass-length ratio or electron microscopy. However, we conclude that the different techniques used to measure the clot characteristics did not cause different observed effects of the post-translational modifications.
Although reviews are written about the effect of oxidative or other post-translational modifications, no recent systematic review is performed in which the effects of modifications are systematically shown as in our systematic review. A quantitative meta-analysis on the effect of post-translational modifications on clot characteristics was not possible, since there is too much variation in the way of modification and measurements of the different effects. Another limitation of this review is that studies might be missed in our literature search. However, we think that by identifying additional articles while reading our articles in full text, we covered a sufficient part of articles available on this subject.
Conclusions
In conclusion, there is knowledge about the effects of most post-translational modifications of fibrinogen on clot characteristics, especially about the best known post-translational modifications (oxidation, glycosylation, and acetylation). There are still many unanswered questions which deserve attention in upcoming research. For example, nitration shows different effects on fibrin polymerization and clot structure, potentially caused by the degree of nitration, which needs to be elucidated. Current research on glycation also shows contradictory effects on fibrin polymerization. For phosphorylation, in vivo studies could provide more information on which kinase is used in the body to phosphorylate fibrinogen and what the effect of this modification is on polymerization and clot structure. To investigate the effect of other (less known) modifications, for example, homocysteinylation and citrullination, only a few studies have been performed, which need confirmation. Expanding the knowledge about these modifications of fibrinogen can ultimately contribute to optimizing treatments for thrombotic diseases.
Acknowledgments
We thank librarian S. Gunput for her excellent help with the literature search used in this systematic review.
Sources of Funding
None.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- MI
- myocardial infarction
For Sources of Funding and Disclosures, see page 565.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.119.313626.
References
- 1.Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- 2.Espitia Jaimes C, Fish RJ, Neerman-Arbez M. Local chromatin interactions contribute to expression of the fibrinogen gene cluster. J Thromb Haemost. 2018;16:2070–2082. doi: 10.1111/jth.14248. doi: 10.1111/jth.14248. [DOI] [PubMed] [Google Scholar]
- 3.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 4.Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal structure of human fibrinogen. Biochemistry. 2009;48:3877–3886. doi: 10.1021/bi802205g. doi: 10.1021/bi802205g. [DOI] [PubMed] [Google Scholar]
- 5.Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem. 2017;82:405–456. doi: 10.1007/978-3-319-49674-0_13. doi: 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duval C, Allan P, Connell SD, Ridger VC, Philippou H, Ariëns RA. Roles of fibrin α- and γ-chain specific cross-linking by FXIIIa in fibrin structure and function. Thromb Haemost. 2014;111:842–850. doi: 10.1160/TH13-10-0855. doi: 10.1160/TH13-10-0855. [DOI] [PubMed] [Google Scholar]
- 7.Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–1361. doi: 10.1161/01.atv.20.5.1354. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 8.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–142. doi: 10.1016/j.blre.2006.11.001. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.de Vries PS, Chasman DI, Sabater-Lleal M, Chen MH, Huffman JE, Steri M, Tang W, Teumer A, Marioni RE, Grossmann V, et al. A meta-analysis of 120 246 individuals identifies 18 new loci for fibrinogen concentration. Hum Mol Genet. 2016;25:358–370. doi: 10.1093/hmg/ddv454. doi: 10.1093/hmg/ddv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronjé HT, Nienaber-Rousseau C, Zandberg L, de Lange Z, Green FR, Pieters M. Fibrinogen and clot-related phenotypes determined by fibrinogen polymorphisms: Independent and IL-6-interactive associations. PLoS One. 2017;12:e0187712. doi: 10.1371/journal.pone.0187712. doi: 10.1371/journal.pone.0187712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Maat MP, Verschuur M. Fibrinogen heterogeneity: inherited and noninherited. Curr Opin Hematol. 2005;12:377–383. doi: 10.1097/01.moh.0000169287.51594.3b. doi: 10.1097/01.moh.0000169287.51594.3b. [DOI] [PubMed] [Google Scholar]
- 12.Henschen AH. Human fibrinogen–structural variants and functional sites. Thromb Haemost. 1993;70:42–47. [PubMed] [Google Scholar]
- 13.Henschen-Edman AH. On the identification of beneficial and detrimental molecular forms of fibrinogen. Haemostasis. 1999;29:179–186. doi: 10.1159/000022498. doi: 10.1159/000022498. [DOI] [PubMed] [Google Scholar]
- 14.Moslen MT. Reactive oxygen species in normal physiology, cell injury and phagocytosis. Adv Exp Med Biol. 1994;366:17–27. doi: 10.1007/978-1-4615-1833-4_2. doi: 10.1007/978-1-4615-1833-4_2. [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A American Heart Association Council on Basic Cardiovascular Sciences. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res. 2016;119:e39–e75. doi: 10.1161/RES.0000000000000110. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigandt KM, White N, Chung D, Ellingson E, Wang Y, Fu X, Pozzo DC. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103:2399–2407. doi: 10.1016/j.bpj.2012.10.036. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinecke JW, Li W, Daehnke HL, 3rd, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993;268:4069–4077. [PubMed] [Google Scholar]
- 18.Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 19.Yurina L, Vasilyeva A, Indeykina M, Bugrova A, Biryukova M, Kononikhin A, Nikolaev E, Rosenfeld M. Ozone-induced damage of fibrinogen molecules: identification of oxidation sites by high-resolution mass spectrometry. Free Radic Res. 2019;53:430–455. doi: 10.1080/10715762.2019.1600686. doi: 10.1080/10715762.2019.1600686. [DOI] [PubMed] [Google Scholar]
- 20.Martinez M, Weisel JW, Ischiropoulos H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic Biol Med. 2013;65:411–418. doi: 10.1016/j.freeradbiomed.2013.06.039. doi: 10.1016/j.freeradbiomed.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;278:L961–L967. doi: 10.1152/ajplung.2000.278.5.L961. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Shi J, Li J. Peroxynitrite induced fibrinogen site identification. Biomed Mater Eng. 2015;26(suppl 1):S2241–S2248. doi: 10.3233/BME-151530. doi: 10.3233/BME-151530. [DOI] [PubMed] [Google Scholar]
- 23.Clerc F, Reiding KR, Jansen BC, Kammeijer GS, Bondt A, Wuhrer M. Human plasma protein N-glycosylation. Glycoconj J. 2016;33:309–343. doi: 10.1007/s10719-015-9626-2. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Townsend RR, Hilliker E, Li YT, Laine RA, Bell WR, Lee YC. Carbohydrate structure of human fibrinogen. Use of 300-MHz 1H-NMR to characterize glycosidase-treated glycopeptides. J Biol Chem. 1982;257:9704–9710. [PubMed] [Google Scholar]
- 25.Martinez J, Palascak J, Peters C. Functional and metabolic properties of human asialofibrinogen. J Lab Clin Med. 1977;89:367–377. [PubMed] [Google Scholar]
- 26.Martinez J, MacDonald KA, Palascak JE. The role of sialic acid in the dysfibrinogenemia associated with liver disease: distribution of sialic acid on the constituent chains. Blood. 1983;61:1196–1202. [PubMed] [Google Scholar]
- 27.Gligorijević N, Zámorová Križáková M, Penezić A, Katrlík J, Nedić O. Structural and functional changes of fibrinogen due to aging. Int J Biol Macromol. 2018;108:1028–1034. doi: 10.1016/j.ijbiomac.2017.11.016. doi: 10.1016/j.ijbiomac.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Ney KA, Pasqua JJ, Colley KJ, Guthrow CE, Pizzo SV. In vitro preparation of nonenzymatically glucosylated human transferrin, alpha 2-macroglobulin, and fibrinogen with preservation of function. Diabetes. 1985;34:462–470. doi: 10.2337/diab.34.5.462. doi: 10.2337/diab.34.5.462. [DOI] [PubMed] [Google Scholar]
- 29.Simm A, Müller B, Nass N, Hofmann B, Bushnaq H, Silber RE, Bartling B. Protein glycation -between tissue aging and protection. Exp Gerontol. 2015;68:71–75. doi: 10.1016/j.exger.2014.12.013. doi: 10.1016/j.exger.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Svensson J, Bergman AC, Adamson U, Blombäck M, Wallén H, Jörneskog G. Acetylation and glycation of fibrinogen in vitro occur at specific lysine residues in a concentration dependent manner: a mass spectrometric and isotope labeling study. Biochem Biophys Res Commun. 2012;421:335–342. doi: 10.1016/j.bbrc.2012.03.154. doi: 10.1016/j.bbrc.2012.03.154. [DOI] [PubMed] [Google Scholar]
- 31.Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta. 2016;1864:1372–1401. doi: 10.1016/j.bbapap.2016.06.007. doi: 10.1016/j.bbapap.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Bjornsson TD, Schneider DE, Berger H., Jr. Aspirin acetylates fibrinogen and enhances fibrinolysis. Fibrinolytic effect is independent of changes in plasminogen activator levels. J Pharmacol Exp Ther. 1989;250:154–161. [PubMed] [Google Scholar]
- 33.Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–E130. doi: 10.1038/ncb0502-e127. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 34.Seydewitz HH, Kaiser C, Rothweiler H, Witt I. The location of a second in vivo phosphorylation site in the A alpha-chain of human fibrinogen. Thromb Res. 1984;33:487–498. doi: 10.1016/0049-3848(84)90014-8. doi: 10.1016/0049-3848(84)90014-8. [DOI] [PubMed] [Google Scholar]
- 35.Heldin P. Phosphorylation in vitro of human fibrinogen with casein kinase TS and characterization of phosphorylated sites. Arch Biochem Biophys. 1987;257:269–275. doi: 10.1016/0003-9861(87)90566-2. doi: 10.1016/0003-9861(87)90566-2. [DOI] [PubMed] [Google Scholar]
- 36.Martin SC, Ekman P, Forsberg PO, Ersmark H. Increased phosphate content of fibrinogen in vivo correlates with alteration in fibrinogen behaviour. Thromb Res. 1992;68:467–473. doi: 10.1016/0049-3848(92)90059-j. doi: 10.1016/0049-3848(92)90059-j. [DOI] [PubMed] [Google Scholar]
- 37.Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr. 2000;130(2S)(suppl):377S–381S. doi: 10.1093/jn/130.2.377S. doi: 10.1093/jn/130.2.377S. [DOI] [PubMed] [Google Scholar]
- 38.Welch GN, Upchurch GR, Jr, Loscalzo J. Homocysteine, oxidative stress, and vascular disease. Hosp Pract (1995) 1997;32:81–82, 85, 8892. doi: 10.1080/21548331.1997.11443510. doi: 10.1080/21548331.1997.11443510. [DOI] [PubMed] [Google Scholar]
- 39.Marchi R, Carvajal Z, Weisel JW. Comparison of the effect of different homocysteine concentrations on clot formation using human plasma and purified fibrinogen. Thromb Haemost. 2008;99:451–452. doi: 10.1160/TH07-06-0404. doi: 10.1160/TH07-06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikora M, Marczak Ł, Kubalska J, Graban A, Jakubowski H. Identification of N-homocysteinylation sites in plasma proteins. Amino Acids. 2014;46:235–244. doi: 10.1007/s00726-013-1617-7. doi: 10.1007/s00726-013-1617-7. [DOI] [PubMed] [Google Scholar]
- 41.Derksen VFAM, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol. 2017;39:437–446. doi: 10.1007/s00281-017-0627-z. doi: 10.1007/s00281-017-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama-Hamada M, Suzuki A, Kubota K, Takazawa T, Ohsaka M, Kawaida R, Ono M, Kasuya A, Furukawa H, Yamada R, et al. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun. 2005;327:192–200. doi: 10.1016/j.bbrc.2004.11.152. doi: 10.1016/j.bbrc.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 44.Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem. 2011;57:1499–1505. doi: 10.1373/clinchem.2011.163188. doi: 10.1373/clinchem.2011.163188. [DOI] [PubMed] [Google Scholar]
- 45.Schuett K, Savvaidis A, Maxeiner S, Lysaja K, Jankowski V, Schirmer SH, Dimkovic N, Boor P, Kaesler N, Dekker FW, et al. Clot structure: a potent mortality risk factor in patients on hemodialysis. J Am Soc Nephrol. 2017;28:1622–1630. doi: 10.1681/ASN.2016030336. doi: 10.1681/ASN.2016030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–e99. doi: 10.1161/ATVBAHA.111.230631. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 47.Bridge KI, Philippou H, Ariëns R. Clot properties and cardiovascular disease. Thromb Haemost. 2014;112:901–908. doi: 10.1160/TH14-02-0184. doi: 10.1160/TH14-02-0184. [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paton LN, Mocatta TJ, Richards AM, Winterbourn CC. Increased thrombin-induced polymerization of fibrinogen associated with high protein carbonyl levels in plasma from patients post myocardial infarction. Free Radic Biol Med. 2010;48:223–229. doi: 10.1016/j.freeradbiomed.2009.10.044. doi: 10.1016/j.freeradbiomed.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 50.Becatti M, Marcucci R, Bruschi G, Taddei N, Bani D, Gori AM, Giusti B, Gensini GF, Abbate R, Fiorillo C. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34:1355–1361. doi: 10.1161/ATVBAHA.114.303785. doi: 10.1161/ATVBAHA.114.303785. [DOI] [PubMed] [Google Scholar]
- 51.Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, Vaglio A, Taddei N, Abbate R, Emmi L, et al. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in behçet disease. Circulation. 2016;133:302–311. doi: 10.1161/CIRCULATIONAHA.115.017738. doi: 10.1161/CIRCULATIONAHA.115.017738. [DOI] [PubMed] [Google Scholar]
- 52.Hugenholtz GC, Macrae F, Adelmeijer J, Dulfer S, Porte RJ, Lisman T, Ariëns RA. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054–1066. doi: 10.1111/jth.13278. doi: 10.1111/jth.13278. [DOI] [PubMed] [Google Scholar]
- 53.White NJ, Wang Y, Fu X, Cardenas JC, Martin EJ, Brophy DF, Wade CE, Wang X, St John AE, Lim EB, et al. Post-translational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury. Free Radic Biol Med. 2016;96:181–189. doi: 10.1016/j.freeradbiomed.2016.04.023. doi: 10.1016/j.freeradbiomed.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sizer IW, Wagley PF. The action of tyrosinase on thrombin, fibrinogen, and fibrin. J Biol Chem. 1951;192:213–221. [PubMed] [Google Scholar]
- 55.Inada Y, Hessel B, Blombäck B. Photooxidation of fibrinogen in the presence of methylene blue and its effect on polymerization. Biochim Biophys Acta. 1978;532:161–170. doi: 10.1016/0005-2795(78)90459-2. doi: 10.1016/0005-2795(78)90459-2. [DOI] [PubMed] [Google Scholar]
- 56.Shacter E, Williams JA, Levine RL. Oxidative modification of fibrinogen inhibits thrombin-catalyzed clot formation. Free Radic Biol Med. 1995;18:815–821. doi: 10.1016/0891-5849(95)93872-4. doi: 10.1016/0891-5849(95)93872-4. [DOI] [PubMed] [Google Scholar]
- 57.Lupidi G, Angeletti M, Eleuteri AM, Tacconi L, Coletta M, Fioretti E. Peroxynitrite-mediated oxidation of fibrinogen inhibits clot formation. FEBS Lett. 1999;462:236–240. doi: 10.1016/s0014-5793(99)01500-8. doi: 10.1016/s0014-5793(99)01500-8. [DOI] [PubMed] [Google Scholar]
- 58.Xu YJ, Qiang M, Zhang JL, Liu Y, He RQ. Reactive carbonyl compounds (RCCs) cause aggregation and dysfunction of fibrinogen. Protein Cell. 2012;3:627–640. doi: 10.1007/s13238-012-2057-y. doi: 10.1007/s13238-012-2057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torbitz VD, Bochi GV, de Carvalho JA, de Almeida Vaucher R, da Silva JE, Moresco RN. In vitro oxidation of fibrinogen promotes functional alterations and formation of advanced oxidation protein products, an inflammation mediator. Inflammation. 2015;38:1201–1206. doi: 10.1007/s10753-014-0085-x. doi: 10.1007/s10753-014-0085-x. [DOI] [PubMed] [Google Scholar]
- 60.Harutyunyan HA. Prothrombin and fibrinogen carbonylation: How that can affect the blood clotting. Redox Rep. 2017;22:160–165. doi: 10.1080/13510002.2016.1200289. doi: 10.1080/13510002.2016.1200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenfeld MA, Leonova VB, Konstantinova ML, Razumovskii SD. Self-assembly of fibrin monomers and fibrinogen aggregation during ozone oxidation. Biochemistry (Mosc) 2009;74:41–46. doi: 10.1134/s0006297909010064. doi: 10.1134/s0006297909010064. [DOI] [PubMed] [Google Scholar]
- 62.Rosenfeld MA, Shchegolikhin AN, Bychkova AV, Leonova VB, Biryukova MI, Kostanova EA, Konstantinova ML. Ozone-induced oxidative modification of fibrinogen molecules. Biochemistry (Mosc) 2013;78:1171–1179. doi: 10.1134/S000629791310012X. doi: 10.1134/S000629791310012X. [DOI] [PubMed] [Google Scholar]
- 63.Rosenfeld MA, Shchegolikhin AN, Bychkova AV, Leonova VB, Biryukova MI, Kostanova EA. Ozone-induced oxidative modification of fibrinogen: role of the D regions. Free Radic Biol Med. 2014;77:106–120. doi: 10.1016/j.freeradbiomed.2014.08.018. doi: 10.1016/j.freeradbiomed.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Cui C, Li R, Xu S, Li H, Li L, Liu J. Study on the oxidation of fibrinogen using Fe3O4 magnetic nanoparticles and its influence to the formation of fibrin. J Inorg Biochem. 2018;189:58–68. doi: 10.1016/j.jinorgbio.2018.09.008. doi: 10.1016/j.jinorgbio.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Nowak P, Zbikowska HM, Ponczek M, Kolodziejczyk J, Wachowicz B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: functional consequences. Thromb Res. 2007;121:163–174. doi: 10.1016/j.thromres.2007.03.017. doi: 10.1016/j.thromres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Andrades ME, Lorenzi R, Berger M, Guimarães JA, Moreira JC, Dal-Pizzol F. Glycolaldehyde induces fibrinogen post-translational modification, delay in clotting and resistance to enzymatic digestion. Chem Biol Interact. 2009;180:478–484. doi: 10.1016/j.cbi.2009.04.005. doi: 10.1016/j.cbi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Tetik S, Kaya K, Yardimci T. Effect of oxidized fibrinogen on hemostatic system: in vitro study. Clin Appl Thromb Hemost. 2011;17:259–263. doi: 10.1177/1076029610363129. doi: 10.1177/1076029610363129. [DOI] [PubMed] [Google Scholar]
- 68.Roitman EV, Azizova OA, Morozov YA, Aseichev AV. Effect of oxidized fibrinogens on blood coagulation. Bull Exp Biol Med. 2004;138:245–247. doi: 10.1007/s10517-005-0011-1. doi: 10.1007/s10517-005-0011-1. [DOI] [PubMed] [Google Scholar]
- 69.Piryazev AP, Aseichev AV, Azizova OA. Effect of oxidation-modified fibrinogen on the formation and lysis of fibrin clot in the plasma. Bull Exp Biol Med. 2009;148:881–885. doi: 10.1007/s10517-010-0841-3. doi: 10.1007/s10517-010-0841-3. [DOI] [PubMed] [Google Scholar]
- 70.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 71.Upchurch GR, Jr, Ramdev N, Walsh MT, Loscalzo J. Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. J Thromb Thrombolysis. 1998;5:9–14. doi: 10.1023/a:1008859729045. doi: 10.1023/a:1008859729045. [DOI] [PubMed] [Google Scholar]
- 72.Zieve PD, Solomon HM. Effect of hematoporphyrin and light on human fibrinogen. Am J Physiol. 1966;210:1391–1395. doi: 10.1152/ajplegacy.1966.210.6.1391. doi: 10.1152/ajplegacy.1966.210.6.1391. [DOI] [PubMed] [Google Scholar]
- 73.Krugelis EJ, Sizer IW. The effects of tyrosinase on certain components of the blood clotting system. Blood. 1954;9:513–519. [PubMed] [Google Scholar]
- 74.PALOS LA. Oxidation of the coagulation factors. Nature. 1949;164:926. doi: 10.1038/164926b0. doi: 10.1038/164926b0. [DOI] [PubMed] [Google Scholar]
- 75.Ishida Y, Takiuchi H, Matsushima A, Inada Y. Functional consequences of tryptophan modification in human fibrinogen. Biochim Biophys Acta. 1978;536:70–77. doi: 10.1016/0005-2795(78)90052-1. doi: 10.1016/0005-2795(78)90052-1. [DOI] [PubMed] [Google Scholar]
- 76.Azizova OA, Piryazev AP, Aseychev AV, Shvachko AG. Oxidative modification of fibrinogen inhibits its transformation into fibrin under the effect of thrombin. Bull Exp Biol Med. 2009;147:201–203. doi: 10.1007/s10517-009-0474-6. doi: 10.1007/s10517-009-0474-6. [DOI] [PubMed] [Google Scholar]
- 77.Štikarová J, Kotlín R, Riedel T, Suttnar J, Pimková K, Chrastinová L, Dyr JE. The effect of reagents mimicking oxidative stress on fibrinogen function. ScientificWorldJournal. 2013;2013:359621. doi: 10.1155/2013/359621. doi: 10.1155/2013/359621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yurina LV, Vasilyeva AD, Bugrova AE, Indeykina MI, Kononikhin AS, Nikolaev EN, Rosenfeld MA. Hypochlorite-induced oxidative modification of fibrinogen. Dokl Biochem Biophys. 2019;484:37–41. doi: 10.1134/S1607672919010101. doi: 10.1134/S1607672919010101. [DOI] [PubMed] [Google Scholar]
- 79.Parastatidis I, Thomson L, Fries DM, Moore RE, Tohyama J, Fu X, Hazen SL, Heijnen HF, Dennehy MK, Liebler DC, et al. Increased protein nitration burden in the atherosclerotic lesions and plasma of apolipoprotein A-I deficient mice. Circ Res. 2007;101:368–376. doi: 10.1161/CIRCRESAHA.107.157537. doi: 10.1161/CIRCRESAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 80.Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, et al. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J Biol Chem. 2008;283:33846–33853. doi: 10.1074/jbc.M805522200. doi: 10.1074/jbc.M805522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heffron SP, Parastatidis I, Cuchel M, Wolfe ML, Tadesse MG, Mohler ER, 3rd, Ischiropoulos H, Rader DJ, Reilly MP. Inflammation induces fibrinogen nitration in experimental human endotoxemia. Free Radic Biol Med. 2009;47:1140–1146. doi: 10.1016/j.freeradbiomed.2009.07.025. doi: 10.1016/j.freeradbiomed.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding Y, Luo Y, Fu J. Effects of Mn (II) on peroxynitrite nitrifying fibrinogen. Biomed Mater Eng. 2014;24:901–907. doi: 10.3233/BME-130884. doi: 10.3233/BME-130884. [DOI] [PubMed] [Google Scholar]
- 83.Helms CC, Kapadia S, Gilmore AC, Lu Z, Basu S, Kim-Shapiro DB. Exposure of fibrinogen and thrombin to nitric oxide donor ProliNONOate affects fibrin clot properties. Blood Coagul Fibrinolysis. 2017;28:356–364. doi: 10.1097/MBC.0000000000000602. doi: 10.1097/MBC.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bijak M, Nowak P, Borowiecka M, Ponczek MB, Żbikowska HM, Wachowicz B. Protective effects of (-)-epicatechin against nitrative modifications of fibrinogen. Thromb Res. 2012;130:e123–e128. doi: 10.1016/j.thromres.2012.03.017. doi: 10.1016/j.thromres.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Bijak M, Saluk J, Antosik A, Ponczek MB, Żbikowska HM, Borowiecka M, Nowak P. Aronia melanocarpa as a protector against nitration of fibrinogen. Int J Biol Macromol. 2013;55:264–268. doi: 10.1016/j.ijbiomac.2013.01.019. doi: 10.1016/j.ijbiomac.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 86.Ponczek MB, Nowak P, Wachowicz B. The effects of nitronium ion on nitration, carbonylation and coagulation of human fibrinogen. Gen Physiol Biophys. 2008;27:55–58. [PubMed] [Google Scholar]
- 87.Gilman PB, Keane P, Martinez J. The role of the carbohydrate moiety in the biologic properties of fibrinogen. J Biol Chem. 1984;259:3248–3253. [PubMed] [Google Scholar]
- 88.Langer BG, Weisel JW, Dinauer PA, Nagaswami C, Bell WR. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J Biol Chem. 1988;263:15056–15063. [PubMed] [Google Scholar]
- 89.Gralnick HR, Givelber H, Abrams E. Dysfibrinogenemia associated with hepatoma. Increased carbohydrate content of the fibrinogen molecule. N Engl J Med. 1978;299:221–226. doi: 10.1056/NEJM197808032990503. doi: 10.1056/NEJM197808032990503. [DOI] [PubMed] [Google Scholar]
- 90.Martinez J, Palascak JE, Kwasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61:535–538. doi: 10.1172/JCI108964. doi: 10.1172/JCI108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maghzal GJ, Brennan SO, George PM. The sialic acid content of fibrinogen decreases during pregnancy and increases in response to fibrate therapy. Thromb Res. 2005;115:293–299. doi: 10.1016/j.thromres.2004.08.013. doi: 10.1016/j.thromres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 92.Dang CV, Shin CK, Bell WR, Nagaswami C, Weisel JW. Fibrinogen sialic acid residues are low affinity calcium-binding sites that influence fibrin assembly. J Biol Chem. 1989;264:15104–15108. [PubMed] [Google Scholar]
- 93.Diaz-Mauriño T, Castro C, Albert A. Desialylation of fibrinogen with neuraminidase. Kinetic and clotting studies. Thromb Res. 1982;27:397–403. doi: 10.1016/0049-3848(82)90057-3. doi: 10.1016/0049-3848(82)90057-3. [DOI] [PubMed] [Google Scholar]
- 94.Diaz-Mauriño T, Albert A. Interaction of fibrinogen and asialofibrinogen with concanavalin A and clotting properties of the complexes. Biochim Biophys Acta. 1981;677:381–389. doi: 10.1016/0304-4165(81)90250-6. doi: 10.1016/0304-4165(81)90250-6. [DOI] [PubMed] [Google Scholar]
- 95.Gentry PA, Alexander B. Human fibrinogen and asialo-fibrinogen: a comparison of coagulation parameters. Arch Biochem Biophys. 1976;173:50–57. doi: 10.1016/0003-9861(76)90233-2. doi: 10.1016/0003-9861(76)90233-2. [DOI] [PubMed] [Google Scholar]
- 96.Laki K, Mester L. The role of the carbohydrate moiety in bovine fibrinogen. Biochim Biophys Acta. 1962;57:152–154. doi: 10.1016/0006-3002(62)91094-6. doi: 10.1016/0006-3002(62)91094-6. [DOI] [PubMed] [Google Scholar]
- 97.Chandrasekhar N, Warren L, Osbahr AJ, Laki K. Role of sialic acid in fibrinogen. Biochim Biophys Acta. 1962;63:337–339. doi: 10.1016/0006-3002(62)90688-1. doi: 10.1016/0006-3002(62)90688-1. [DOI] [PubMed] [Google Scholar]
- 98.Okude M, Yamanaka A, Morimoto Y, Akihama S. Sialic acid in fibrinogen: effects of sialic acid on fibrinogen-fibrin conversion by thrombin and properties of asialofibrin clot. Biol Pharm Bull. 1993;16:448–452. doi: 10.1248/bpb.16.448. doi: 10.1248/bpb.16.448. [DOI] [PubMed] [Google Scholar]
- 99.El-Fasakhany FM, Ichihara-Tanaka K, Uchimura K, Muramatsu T. N-acetylglucosamine-6-O-sulfotransferase-1: production in the baculovirus system and its applications to the synthesis of a sulfated oligosaccharide and to the modification of oligosaccharides in fibrinogen. J Biochem. 2003;133:287–293. doi: 10.1093/jb/mvg039. doi: 10.1093/jb/mvg039. [DOI] [PubMed] [Google Scholar]
- 100.Dunn EJ, Ariëns RA, Grant PJ. The influence of type 2 diabetes on fibrin structure and function. Diabetologia. 2005;48:1198–1206. doi: 10.1007/s00125-005-1742-2. doi: 10.1007/s00125-005-1742-2. [DOI] [PubMed] [Google Scholar]
- 101.Pieters M, Covic N, van der Westhuizen FH, Nagaswami C, Baras Y, Toit Loots D, Jerling JC, Elgar D, Edmondson KS, van Zyl DG, et al. Glycaemic control improves fibrin network characteristics in type 2 diabetes - a purified fibrinogen model. Thromb Haemost. 2008;99:691–700. doi: 10.1160/TH07-11-0699. doi: 10.1160/TH07-11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W, Sigley J, Pieters M, Helms CC, Nagaswami C, Weisel JW, Guthold M. Fibrin fiber stiffness is strongly affected by fiber diameter, but not by fibrinogen glycation. Biophys J. 2016;110:1400–1410. doi: 10.1016/j.bpj.2016.02.021. doi: 10.1016/j.bpj.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hood JE, Yesudasan S, Averett RD. Glucose concentration affects fibrin clot structure and morphology as evidenced by fluorescence imaging and molecular simulations. Clin Appl Thromb Hemost. 2018 doi: 10.1177/1076029618792304. doi: 10.1177/1076029618792304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norton DG, Fan NK, Goudie MJ, Handa H, Platt MO, Averett RD. Computational imaging analysis of glycated fibrin gels reveals aggregated and anisotropic structures. J Biomed Mater Res A. 2017;105:2191–2198. doi: 10.1002/jbm.a.36074. doi: 10.1002/jbm.a.36074. [DOI] [PubMed] [Google Scholar]
- 105.Mirmiranpour H, Bathaie SZ, Khaghani S, Nakhjavani M, Kebriaeezadeh A. Investigation of the mechanism(s) involved in decreasing increased fibrinogen activity in hyperglycemic conditions using L-lysine supplementation. Thromb Res. 2012;130:e13–e19. doi: 10.1016/j.thromres.2012.04.010. doi: 10.1016/j.thromres.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 106.Kanska U, Omar MS, Budzynska R, Nevozhay D, Jagiello M, Opolski A, Boratynski J. Antileukemic activity of glycated fibrinogen-methotrexate conjugates. Anticancer Res. 2005;25:2229–2234. [PubMed] [Google Scholar]
- 107.Mirshahi M, Soria J, Soria C, Bertrand O, Mirshahi M, Basdevant A. Glycosylation of human fibrinogen and fibrin in vitro. Its consequences on the properties of fibrin(ogen). Thromb Res. 1987;48:279–289. doi: 10.1016/0049-3848(87)90440-3. doi: 10.1016/0049-3848(87)90440-3. [DOI] [PubMed] [Google Scholar]
- 108.Krantz S, Lober M, Thiele M, Teuscher E. Properties of in vitro nonenzymatically glycated plasma fibrinogens. Exp Clin Endocrinol. 1987;90:37–45. doi: 10.1055/s-0029-1210670. doi: 10.1055/s-0029-1210670. [DOI] [PubMed] [Google Scholar]
- 109.McVerry BA, Thorpe S, Joe F, Gaffney P, Huehns ER. Non-enzymatic glucosylation of fibrinogen. Haemostasis. 1981;10:261–270. doi: 10.1159/000214409. doi: 10.1159/000214409. [DOI] [PubMed] [Google Scholar]
- 110.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation reduces the susceptibility of fibrin to degradation by plasmin. Diabetes. 1983;32:680–684. doi: 10.2337/diab.32.7.680. doi: 10.2337/diab.32.7.680. [DOI] [PubMed] [Google Scholar]
- 111.Robinson PW, Jury DR, Langdon AG. The relative clotting activity of glycated and non-glycated forms of fibrinogen. Ann Clin Biochem. 1991;28(pt 6):618–619. doi: 10.1177/000456329102800613. doi: 10.1177/000456329102800613. [DOI] [PubMed] [Google Scholar]
- 112.Murakami T, Egawa H, Komiyama Y, Masuda M, Murata K. Increased accumulation of nonenzymatically glycated fibrinogen in the renal cortex in rats. Thromb Res. 1990;58:23–33. doi: 10.1016/0049-3848(90)90240-d. doi: 10.1016/0049-3848(90)90240-d. [DOI] [PubMed] [Google Scholar]
- 113.Lütjens A, Jonkhoff-Slok TW, Sandkuijl C, vd Veen EA, vd Meer J. Polymerisation and crosslinking of fibrin monomers in diabetes mellitus. Diabetologia. 1988;31:825–830. doi: 10.1007/BF00277485. doi: 10.1007/bf00277485. [DOI] [PubMed] [Google Scholar]
- 114.Pieters M, Covic N, Loots du T, van der Westhuizen FH, van Zyl DG, Rheeder P, Jerling JC, Weisel JW. The effect of glycaemic control on fibrin network structure of type 2 diabetic subjects. Thromb Haemost. 2006;96:623–629. [PubMed] [Google Scholar]
- 115.CASPARY EA. Studies on the acetylation of human fibrinogen. Biochem J. 1956;62:507–512. doi: 10.1042/bj0620507. doi: 10.1042/bj0620507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phillips HM, York JL. Bovine fibrinogen. II. Effects of tyrosine modification on fibrin monomer aggregation. Biochemistry. 1973;12:3642–3647. doi: 10.1021/bi00743a012. doi: 10.1021/bi00743a012. [DOI] [PubMed] [Google Scholar]
- 117.Wegrzynowicz Z, Kloczewiak M, Kopec M. Bovine fibrinogen modified with 3H-acetic anhydride (3H-AcOAc). J Lab Clin Med. 1975;86:360–368. [PubMed] [Google Scholar]
- 118.Williams S, Fatah K, Hjemdahl P, Blombäck M. Better increase in fibrin gel porosity by low dose than intermediate dose acetylsalicylic acid. Eur Heart J. 1998;19:1666–1672. doi: 10.1053/euhj.1998.1088. doi: 10.1053/euhj.1998.1088. [DOI] [PubMed] [Google Scholar]
- 119.Antovic A, Perneby C, Ekman GJ, Wallen HN, Hjemdahl P, Blombäck M, He S. Marked increase of fibrin gel permeability with very low dose ASA treatment. Thromb Res. 2005;116:509–517. doi: 10.1016/j.thromres.2005.02.007. doi: 10.1016/j.thromres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 120.He S, Bark N, Wang H, Svensson J, Blombäck M. Effects of acetylsalicylic acid on increase of fibrin network porosity and the consequent upregulation of fibrinolysis. J Cardiovasc Pharmacol. 2009;53:24–29. doi: 10.1097/FJC.0b013e3181953e0f. doi: 10.1097/FJC.0b013e3181953e0f. [DOI] [PubMed] [Google Scholar]
- 121.He S, Blombäck M, Yoo G, Sinha R, Henschen-Edman AH. Modified clotting properties of fibrinogen in the presence of acetylsalicylic acid in a purified system. Ann N Y Acad Sci. 2001;936:531–535. doi: 10.1111/j.1749-6632.2001.tb03540.x. doi: 10.1111/j.1749-6632.2001.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 122.Ajjan RA, Standeven KF, Khanbhai M, Phoenix F, Gersh KC, Weisel JW, Kearney MT, Ariëns RA, Grant PJ. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol. 2009;29:712–717. doi: 10.1161/ATVBAHA.109.183707. doi: 10.1161/ATVBAHA.109.183707. [DOI] [PubMed] [Google Scholar]
- 123.Williams S, Fatah K, Ivert T, Blombäck M. The effect of acetylsalicylic acid on fibrin gel lysis by tissue plasminogen activator. Blood Coagul Fibrinolysis. 1995;6:718–725. doi: 10.1097/00001721-199512000-00004. doi: 10.1097/00001721-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Fatah K, Beving H, Albåge A, Ivert T, Blombäck M. Acetylsalicylic acid may protect the patient by increasing fibrin gel porosity. Is withdrawing of treatment harmful to the patient? Eur Heart J. 1996;17:1362–1366. doi: 10.1093/oxfordjournals.eurheartj.a015070. doi: 10.1093/oxfordjournals.eurheartj.a015070. [DOI] [PubMed] [Google Scholar]
- 125.Jörneskog G, Fatah K, Blombäck M. Fibrin gel structure in diabetic patients before and during treatment with acetylsalicylic acid: a pilot study. Fibrinolysis and Proteolysis. 1998;12:360–365. [Google Scholar]
- 126.Forsberg PO. Dephosphorylation of human fibrinogen, previously phosphorylated in vitro by protein kinase C, by whole blood or intestinal alkaline phosphatase. Effects on thrombin-induced gelation of in vitro dephosphorylated human fibrinogen. Thromb Res. 1989;53:1–9. doi: 10.1016/0049-3848(89)90110-2. doi: 10.1016/0049-3848(89)90110-2. [DOI] [PubMed] [Google Scholar]
- 127.Martin SC, Forsberg PO, Eriksson SD. The effects of in vitro phosphorylation and dephosphorylation on the thrombin-induced gelation and plasmin degradation of fibrinogen. Thromb Res. 1991;61:243–252. doi: 10.1016/0049-3848(91)90100-b. doi: 10.1016/0049-3848(91)90100-b. [DOI] [PubMed] [Google Scholar]
- 128.Forsberg PO, Martin SC. Plasmin digestion of human fibrinogen previously phosphorylated by protein kinase C or dephosphorylated by alkaline phosphatase in vitro. Thromb Res. 1990;58:119–127. doi: 10.1016/0049-3848(90)90169-d. doi: 10.1016/0049-3848(90)90169-d. [DOI] [PubMed] [Google Scholar]
- 129.Hanna LS, Scheraga HA, Francis CW, Marder VJ. Comparison of structures of various human fibrinogens and a derivative thereof by a study of the kinetics of release of fibrinopeptides. Biochemistry. 1984;23:4681–4687. doi: 10.1021/bi00315a025. doi: 10.1021/bi00315a025. [DOI] [PubMed] [Google Scholar]
- 130.Suk K, Lee JY, Kim SH. Synergistic stimulation of fibrinogen gelation by casein kinase II and polycationic compounds. Biochem Mol Biol Int. 1997;42:487–495. doi: 10.1080/15216549700202891. doi: 10.1080/15216549700202891. [DOI] [PubMed] [Google Scholar]
- 131.Heldin P, Hessel B, Humble E, Blombäck B, Engström L. Effect of phosphorylation in vitro of human fibrinogen with protein kinase C on thrombin-induced gelation. Thromb Res. 1987;47:93–99. doi: 10.1016/0049-3848(87)90244-1. doi: 10.1016/0049-3848(87)90244-1. [DOI] [PubMed] [Google Scholar]
- 132.Blomback B, Blomback M, Searle J. On the occurrence of phosphorus in fibrinogen. Biochim Biophys Acta. 1963;74:148–151. doi: 10.1016/0006-3002(63)91345-3. doi: 10.1016/0006-3002(63)91345-3. [DOI] [PubMed] [Google Scholar]
- 133.Sauls DL, Wolberg AS, Hoffman M. Elevated plasma homocysteine leads to alterations in fibrin clot structure and stability: implications for the mechanism of thrombosis in hyperhomocysteinemia. J Thromb Haemost. 2003;1:300–306. doi: 10.1046/j.1538-7836.2003.00053.x. doi: 10.1046/j.1538-7836.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 134.Lauricella AM, Quintana I, Castañon M, Sassetti B, Kordich L. Influence of homocysteine on fibrin network lysis. Blood Coagul Fibrinolysis. 2006;17:181–186. doi: 10.1097/01.mbc.0000220238.99843.45. doi: 10.1097/01.mbc.0000220238.99843.45. [DOI] [PubMed] [Google Scholar]
- 135.Rojas AM, Kordich L, Lauricella AM. Homocysteine modifies fibrin clot deformability: another possible explanation of harm. Biorheology. 2009;46:379–387. doi: 10.3233/BIR-2009-0459. doi: 10.3233/BIR-2009-0459. [DOI] [PubMed] [Google Scholar]
- 136.Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry. 2006;45:2480–2487. doi: 10.1021/bi052076j. doi: 10.1021/bi052076j. [DOI] [PubMed] [Google Scholar]
- 137.Sauls DL, Warren M, Hoffman M. Homocysteinylated fibrinogen forms disulfide-linked complexes with albumin. Thromb Res. 2011;127:576–581. doi: 10.1016/j.thromres.2011.01.009. doi: 10.1016/j.thromres.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 138.Okumura N, Haneishi A, Terasawa F. Citrullinated fibrinogen shows defects in FPA and FPB release and fibrin polymerization catalyzed by thrombin. Clin Chim Acta. 2009;401:119–123. doi: 10.1016/j.cca.2008.12.002. doi: 10.1016/j.cca.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 139.Nakayama-Hamada M, Suzuki A, Furukawa H, Yamada R, Yamamoto K. Citrullinated fibrinogen inhibits thrombin-catalysed fibrin polymerization. J Biochem. 2008;144:393–398. doi: 10.1093/jb/mvn079. doi: 10.1093/jb/mvn079. [DOI] [PubMed] [Google Scholar]
- 140.Damiana T, Damgaard D, Sidelmann JJ, Nielsen CH, de Maat MPM, Münster AB, Palarasah Y. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin Chim Acta. 2019;501:6–11. doi: 10.1016/j.cca.2019.10.033. doi: 10.1016/j.cca.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 141.Binder V, Bergum B, Jaisson S, Gillery P, Scavenius C, Spriet E, Nyhaug AK, Roberts HM, Chapple ILC, Hellvard A, et al. Impact of fibrinogen carbamylation on fibrin clot formation and stability. Thromb Haemost. 2017;117:899–910. doi: 10.1160/TH16-09-0704. doi: 10.1160/TH16-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Date K, Ohyama M, Ogawa H. Carbohydrate-binding activities of coagulation factors fibrinogen and fibrin. Glycoconj J. 2015;32:385–392. doi: 10.1007/s10719-015-9603-9. doi: 10.1007/s10719-015-9603-9. [DOI] [PubMed] [Google Scholar]
- 143.Bychkova AV, Vasilyeva AD, Bugrova AE, Indeykina MI, Kononikhin AS, Nikolaev EN, Konstantinova ML, Rosenfeld MA. Oxidation-induced modification of the fibrinogen polypeptide chains. Dokl Biochem Biophys. 2017;474:173–177. doi: 10.1134/S1607672917030115. doi: 10.1134/S1607672917030115. [DOI] [PubMed] [Google Scholar]
- 144.Pederson EN, Interlandi G. Oxidation-induced destabilization of the fibrinogen αC-domain dimer investigated by molecular dynamics simulations. Proteins. 2019;87:826–836. doi: 10.1002/prot.25746. doi: 10.1002/prot.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol. 2017;16:34. doi: 10.1186/s12933-017-0515-9. doi: 10.1186/s12933-017-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nair CH, Azhar A, Wilson JD, Dhall DP. Studies on fibrin network structure in human plasma. Part II–Clinical application: diabetes and antidiabetic drugs. Thromb Res. 1991;64:477–485. doi: 10.1016/0049-3848(91)90347-y. doi: 10.1016/0049-3848(91)90347-y. [DOI] [PubMed] [Google Scholar]
- 147.Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res. 2010;7:260–273. doi: 10.1177/1479164110383723. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]
- 148.King RJ, Grant PJ. Diabetes and cardiovascular disease: pathophysiology of a life-threatening epidemic. Herz. 2016;41:184–192. doi: 10.1007/s00059-016-4414-8. doi: 10.1007/s00059-016-4414-8. [DOI] [PubMed] [Google Scholar]
- 149.Ajjan R, Storey RF, Grant PJ. Aspirin resistance and diabetes mellitus. Diabetologia. 2008;51:385–390. doi: 10.1007/s00125-007-0898-3. doi: 10.1007/s00125-007-0898-3. [DOI] [PubMed] [Google Scholar]
- 150.Heldin P, Humble E. Phosphorylation of human fibrinogen in vitro with protein kinase C: characterization of the phosphorylated sites. Arch Biochem Biophys. 1987;252:49–59. doi: 10.1016/0003-9861(87)90007-5. doi: 10.1016/0003-9861(87)90007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


