Abstract
Temozolomide (TMZ) is a drug of choice in glioblastoma treatment. Its therapeutic applications expand also beyond high grade gliomas. However, a significant number of recurrences and resistance to the drug is observed. The key factor in each chemotherapy is to achieve the therapeutic doses of a drug at the pathologic site. Nonetheless, the rate of temozolomide penetration from blood to cerebrospinal fluid is only 20–30%, and even smaller into brain intestinum. That makes a challenge for the therapeutic regimens to obtain effective drug concentrations with minimal toxicity and minor side effects. The aim of our research was to explore a novel epigenetic mechanism of temozolomide action in therapeutic conditions. We analyzed the epigenetic effects of TMZ influence on different glioblastoma cell lines in therapeutically achieved TMZ concentrations through total changes of the level of 5-methylcytosine in DNA, the main epigenetic marker. That was done with classical approach of radioactive nucleotide post-labelling and separation on thin-layer chromatography. In the range of therapeutically achieved temozolomide concentrations we observed total DNA hypomethylation. The significant hypermethylating effect was visible after reaching TMZ concentrations of 10–50 μM (depending on the cell line). Longer exposure time promoted DNA hypomethylation. The demethylated state of the glioblastoma cell lines was overcome by repeated TMZ applications, where dose-dependent increase in DNA 5-methylcytosine contents was observed. Those effects were not seen in non-cancerous cell line. The increase of DNA methylation resulting in global gene silencing and consecutive down regulation of gene expression after TMZ treatment may explain better glioblastoma patients’ survival.
Introduction
The therapeutic potential of temozolomide (TMZ) in the treatment of primary and recurrent glioblastoma (GBM) has been proven [1,2]. Its therapeutic applications expand also beyond high grade gliomas [3–5]. TMZ is most effective in glioblastomas with hypermethylated promotor of the O6-methylguanine-DNA methyltransferase (MGMT) [1,6–8]. However, the questions about its efficacy, patient selection, outcome, and prognosis still remain, and therapy failures are observed in the vast majority of glioblastoma patients. The changing of dosing regimens didn’t fulfill the expectations to increase the treatment effectivity [9].
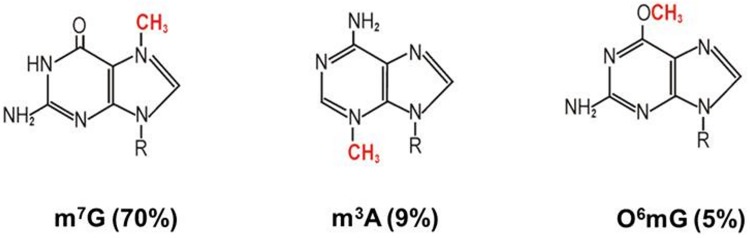
Temozolomide (4-methyl-5-oxo-2,3,4,6,8-pentazabicyclo [4.3.0] nona-2,7,9-triene-9-carboxamide) is a small prodrug, with a molecular weight of 194.15, that undergoes chemical conversion at physiological pH to the active species 5-(3-methyl-1-triazeno)imidazole-4-carboxamide (MTIC), and generates an active methyl group, that reacts with DNA bases [10]. The cytotoxic activity of TMZ manifests mainly through methylation at the O6 position of guanine. Although that methyl adduct comprises less than 5% of the total temozolomide induced DNA modifications [11–13], O6-methylguanine leads to DNA mismatch repair, which results in preservation of the lesion and double-strand breaks, what leads to cell apoptosis [14,15]. Other alkyl adducts, like N7-methylguanine and N3-methyladenine, comprising ca. 80% of the total TMZ methylation products, are generally not cytotoxic, while they are easily repaired by the base excision repair system [12,13] (Fig 1). Therefore it is believed that generation of O6-methylguanine and a functional DNA mismatch repair pathway are both critical to the cytotoxic potential of temozolomide. Consequently, the currently accepted mechanism of resistance to TMZ is the activity of O6-methylguanine DNA methyltransferase (MGMT), the DNA repair enzyme [6]. However, in the light of numerous TMZ effects in the cell, cannot be the only one [16].
Fig 1. The main reaction products of the TMZ reaction with DNA.
7-methylguanosine (m7G) is the most abundant product (60–80%) of TMZ reaction with DNA, followed by 3-methyladenosine (m3A, 10–20%) and O6-methylguanosine (O6mG, 5%). Other products constitute ca. 15% [17].
The site of TMZ action (brain) requires its effective entrance into the central nervous system (CNS) through the blood brain barrier (BBB). That is achieved with the unique chemical structure as well as pharmacokinetic properties [18,19]. Moreover, with oral administration, 100% of the given TMZ dose gets into blood flow [20]. However, the successful chemotherapy requires effective drug concentration in the pathologic site.
The studies on TMZ penetration into CNS done in non-human primate models have shown that the peak levels of the drug were: 104±3 μM in plasma (0.5 hrs after infusion end), and 26±4 μM in the cerebrospinal fluid (CSF) (2.5 hrs after infusion end), therefore the CSF:plasma ratio was 0.33±0.06 [19]. League-Pascual et al. observed that the degrees of CSF drug penetration after intranasal and intravenous administration were 36(32–57) and 22(20–41) %, respectively. The maximum TMZ drug concentration in the CSF was lower after intranasal delivery compared to intravenous administration due to the lower dose administered [21]. In the pharmacokinetic study on TMZ penetration in humans, drug concentration in the CSF was 20% of that of plasma, with an increase of 15% in case of concomitant radiochemotherapy. However, the active metabolite, MTIC, does not effectively penetrate the CNS [22]. With microdialysis method performed in GBM patients an average brain intestinum:plasma ratio was estimated as 17.8%. The mean peak TMZ concentration in brain tissue was 0.6±0.3μg/ml, and the mean time to reach peak level in the brain was 2.0±0.8 hrs [23].
Recently we showed that temozolomide modulates 5-methylcytosine (m5C), the main epigenetic marker, contents in cancer cells’ DNA [17]. An increasing TMZ concentration in the short time induced an increase of total m5C content in DNA (hypermethylation), but at extremely high (1000 μM) TMZ concentration and longer exposition time DNA hypomethylation was observed. DNA hypermethylation can be only explained by activation of DNA methyltransferase (DNMT), because TMZ is not a substrate for DNMT [24]. The other TMZ effect on the cell is chemical and oxidative stress. In such conditions 5-methylcytosine is a target for oxidation, what results with the decrease (demethylation, hypomethylation) of its total genomic contents [25]. Therefore changes in DNA methylation provide an appropriate picture of the disease [26]. In our previous studies we have also shown that m5C is a good marker for the diagnosis of brain tumors, hypertension, and other diseases [27–29]. However, the concentrations of TMZ used (100–1000 μM) in our previous study [17] didn’t correspond well with those reached in regular therapy in brain tissue and cerebrospinal fluid [19,21–23]. Therefore in the present analysis we decided to focus on the concentrations potentially achieved in the brain during TMZ therapy (mean 3 μM±1.5 μM [23]).
The aim of the current project was to evaluate the epigenetic influence of temozolomide treatment explaining its effects in glioblastoma, and other pathologies, in therapeutic concentrations and mimicking the therapeutic schemata.
Materials and methods
Chemicals
Temozolomide (Sigma-Aldrich/Merck) was freshly dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich/Merck) at a concentration of 0.103 M. TMZ stock solution was used to prepare the required concentration with the complete medium.
Cell lines’ source and identification
Human glioblastoma cell lines (T98G, U138, U118) and human keratinocyte (non-neoplastic) cell line (HaCaT) were purchased from ATCC (American Tissue Culture Collection, US; Cat. No. ATCC® CRL-1690™, ATCC® HTB-16™, ATCC® HTB-15™, and ATCC® CRL-2404™ respectively). ATCC’s recommended basic benchmark verification tests were implemented.
Cell culture conditions
T98G and U138 cell lines were cultured in EMEM medium (ATCC), U118 in DMEM (ATCC), HaCaT in EMEM (Sigma-Aldrich/Merck). Each medium was supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco) and antibiotics (ATCC, penicillin 10 U/ml, streptomycin 10 μg/ml, amphotericin B 25 ng/ml). Cells were cultured at 37°C with 5% CO2 in humidified air. Cell lines were seeded at a density of 5x105 cells per well in 6-well plates containing 1 ml appropriated medium. Cells with 90–95% confluence, after ~24 hrs, were washed with phosphate-buffered saline (PBS, Sigma-Aldrich/Merck), placed in fresh medium and underwent treatment with TMZ as described below.
Safety procedures were used with cell lines to avoid contamination from other cell lines and microbes. Low-passage (up to 25) cell lines were used. Plasmocin™ Prophylactic (InvivoGen), a broad-spectrum anti-mycoplasma reagent, was used at 5 μg/ml in liquid media to prevent mycoplasma contamination. It is also active on a broad range of Gram-positive bacteria, such as Staphylococcus species, and Gram-negative bacteria. Medium with fresh Plasmocin was changed every 3 days. Plasmocin™ Prophylactic (5 μg/ml) exhibits no toxicity in eukaryotic cells. Cell cultures were monitored with PlasmoTest™ (InvivoGen). Mycoplasma was not detected in our cell cultures.
Cell lines’ treatment with TMZ
The TMZ stock solution was added directly to culture medium according to designed concentrations:
TMZ at 0.5, 1, 3, 5, 10, 20, 30, 50 and 100 μM for 3, 12, 24 and 48 hrs.
TMZ at 0.5, 1, 3, 5, 10 μM for 5 days, wherein after 24, 48, 72 and 96 hrs cells were washed with PBS and placed in fresh medium with indicated TMZ concentrations.
TMZ at 0.5, 1, 3, 5, 10 μM for 24, 48, 72, 96, 120 and 168 hrs.
The control cells were treated with H2O or DMSO only. After appropriated (indicated above) time with TMZ, the cells were washed with PBS, trypsinized and collected by centrifugation at 3500 rpm for 5 min. The cellular pellets were quickly frozen and stored at—20°C for DNA isolation.
MTT assay for TMZ was carried out during our previous experiments [17]. It was shown that TMZ treatment in the range of 1–2000 μM triggers cell death apoptosis independent.
DNA isolation from cell cultures
DNA from tissue samples was extracted with Genomic Mini kit (A&A Biotechnology, Poland). Shortly, tissue samples were incubated with RNase A, and then with proteinase K. After centrifugation (15000 rpm for 3 min), the supernatant was applied to mini column and DNA bound to the column was eluted with Tris-buffer pH 8.5 and stored at -20°C for further analysis. The purity and concentration of DNA preparations was checked by measuring of UV absorbance at 260 and 280 nm. The A260/A280 ratio was 2.0–2.1.
DNA hydrolysis, labelling and TLC chromatography
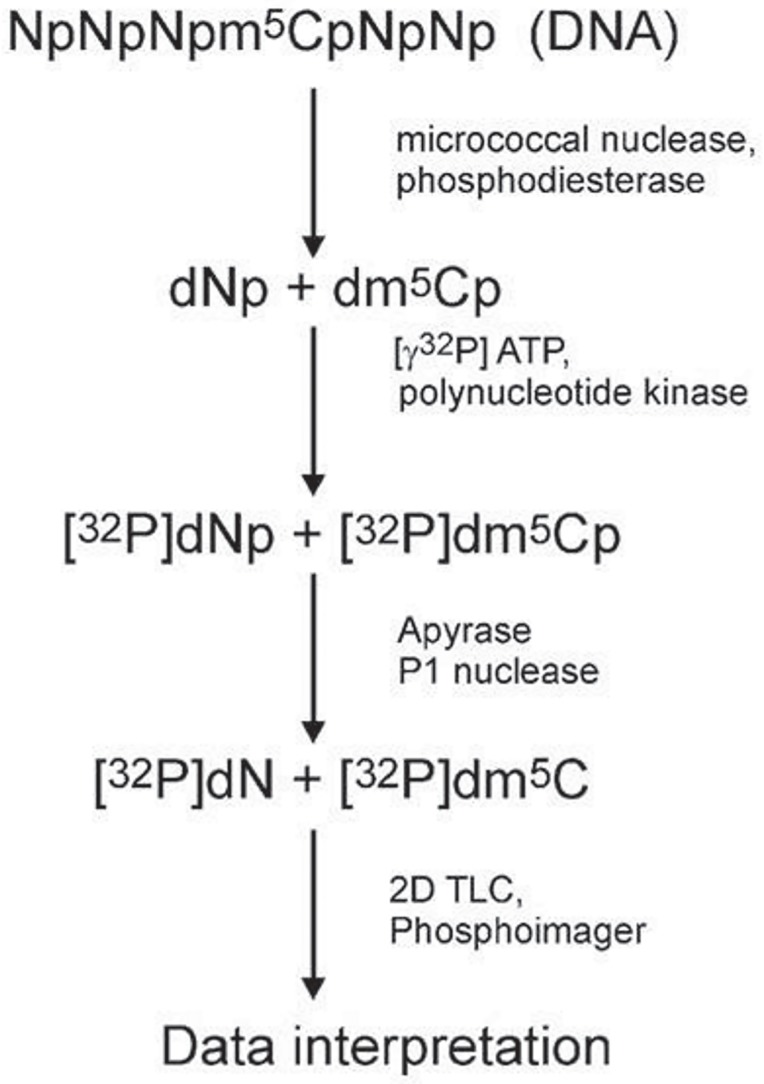
1 μg of dried DNA was dissolved in a succinate buffer (pH 6.0) containing 10 mM CaCl2 and digested with 0.001 units of spleen phosphodiesterase II and 0.02 units of micrococcal nuclease in 3.5 μl total volume for 5 h at 37°C. 0.17 μg of DNA digest was labeled with 1 μCi [γ-32P]ATP (6000 Ci/mM; Hartmann Analytic GmbH) and 1.5 units of T4 polynucleotide kinase in 3 μl of 10 mM bicine-NaOH pH 9.7 buffer containing 10 mM MgCl2, 10 mM DTT, and 1 mM spermidine. After 0.5 h at 37°C 3 μl of apyrase (10 units/ml) in the same buffer were added and incubated for another 0.5 h. The 3’ nucleotide phosphate (from [32P]dNp) was cleaved off with 0.2 μg RNase P1 in 500 mM ammonium acetate buffer, pH 4.5. Identification of [32P]dN was performed with a two-dimensional thin-layer chromatography on cellulose plates (Merck, Germany) using solvent system: isobutyric acid:NH4OH:H2O (66:1:17 v/v) in the first dimension and 0.1 M sodium phosphate (pH 6.8)-ammonium sulfate-n-propyl alcohol (100 ml/60 g/1.5 ml) in the second dimension. Radioactive spot analysis was done with the Phosphoimager Typhoon Screen (Pharmacia, Sweden) and ImageQuant Software (GE Healthcare, USA). For precise calculation we used the amount of material in spots corresponding to m5dC (5-methylcytosine), dC (cytosine) and dT (thymine). The total m5C contents was calculated as R = (m5dC/(m5dC+dC+dT))×100 (Fig 2).
Fig 2. A flow chart of the total DNA methylation (m5C) analysis.
Isolated DNA is hydrolyzed to 3’-mononucleotides (Np: A/adenosine, G/guanosine, C/cytidine, T/thymidine). The hydrolysate is labelled with [γ-32P] ATP, dephosphorylated (detachment of 3’ phosphate) and separated with two-dimensional TLC. The chromatogram is then evaluated with phosphoimager and the spots’ intensities are measured. Those values are used for the calculation of the R coefficient according to the given equation [27].
Statistical analysis
Microsoft Excel 2010 software with Data Analysis package was used for statistical analysis of all data. The data are the result of three independent experiments. Descriptive statistics function was used to generate mean and SD (S1–S3 Tables). One-tailed t-test was used to calculate significant differences of R values for tested samples as compared with control in experiments 1–3.
Results
We analyzed changes in 5-methylcytosine (epigenetic marker) contents in DNA of three types of human glioblastoma cell lines (T98G, U138, U118), and one human keratinocyte cell line (HaCaT) after treatment with 0.5–100 μM of TMZ in the time range between 3 hrs and 7 days in different treatment regimens. These TMZ concentrations were chosen to cover the possible concentration of TMZ in the brain during chemotherapy (0.6±0.3μg/ml, corresponding to 3±1.5 μM) [19,21–23], and perform the observation after reaching the peak concentration in the brain (2.0±0.8 hrs) [23], as well as standard chemotherapy course time [1].
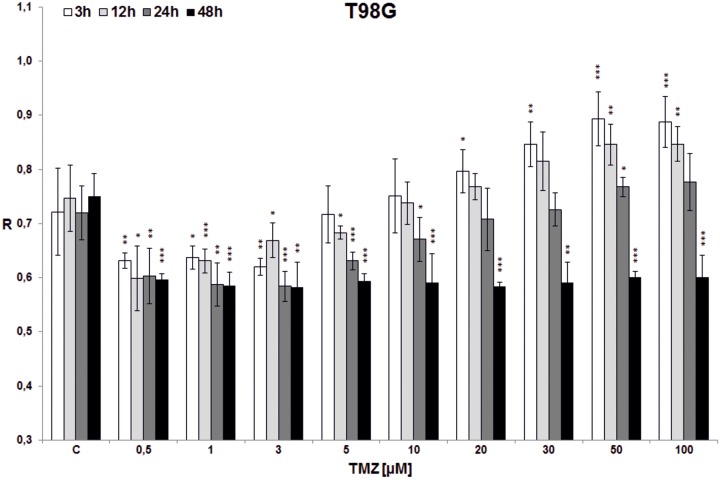
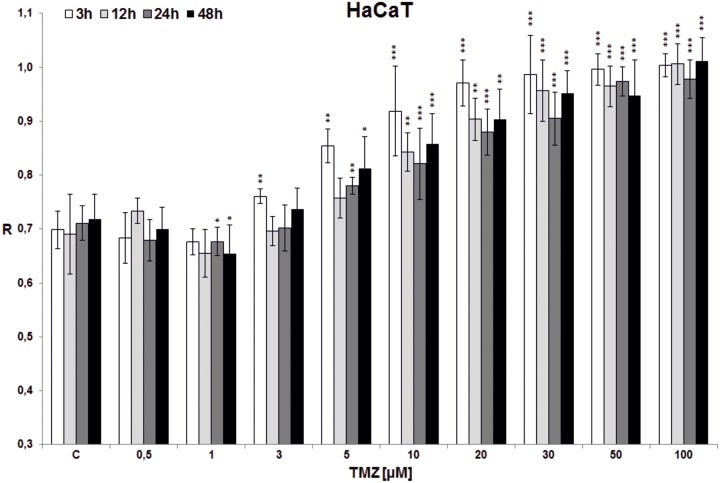
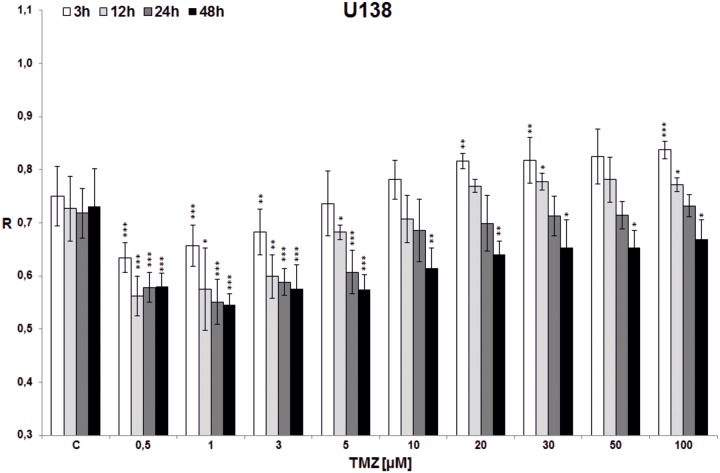
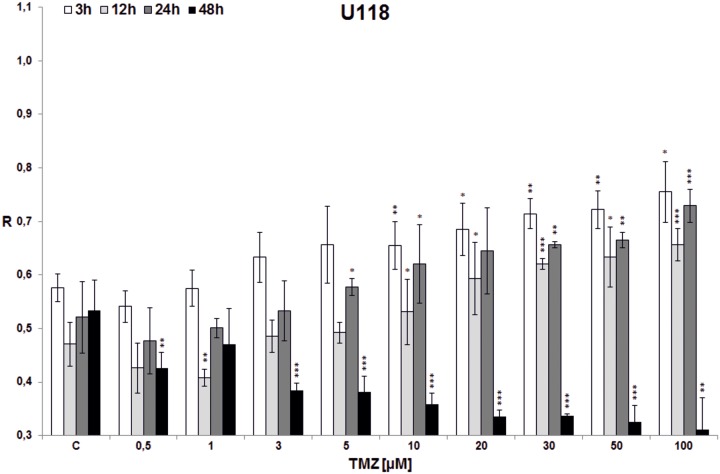
In the first experiment total DNA methylation in cell lines (T98G, U138, U118, HaCaT) was estimated after treatment with single doses of TMZ (0.5, 1, 3, 5, 10, 20, 30, 50, 100 μM) and incubation for 3, 12, 24 and 48 hrs (Figs 3–6). Analyzing the time factor (in every set of bars of the same TMZ concentration) in glioblastoma cell lines one can clearly see DNA demethylation in all glioblastoma cell lines increased with prolonging time, and evident extreme at 48 hrs. That effect is little influenced by increasing TMZ dose to a degree depending on the line (more in U118 and T98G). Taking into account the concentration factor we observe initial DNA hypomethylation (in comparison to control) in glioblastoma cell lines in concentration range 0.5–10 μM (depending on the cell line, p values are presented in S1 Table). That effect is particularly interesting because that range covers the concentrations that are most probably reached in the brain during TMZ treatment. After reaching the concentration of 10 μM (3 μM in case of U118) dose dependent DNA hypermethylation is observed. The dependence on dose increase in all cell lines was statistically significant (***p<0.001), whereas dependence on time was observed at significant level up from doses: 3*/5**/10***μM in T98G (Fig 3), 1***μM in U138 (Fig 4), 0.5*/1**/3***μM in U118 (Fig 5), and only for TMZ 10*μM in HaCaT (Fig 6). Both phenomena were not seen in HaCaT cell line, where in general hypermethylation is detected. The results of the first experiment show that therapeutically achieved TMZ concentrations can be too low to result in hypermethylation, which is regarded as a prognostically better state [30].
Fig 3. The effect of TMZ on total DNA methylation of T98G cell line.
The analysis was performed after 3 (white bars), 12 (light grey bars), 24 (dark grey bars) and 48 (black bars) hrs of incubation in a given TMZ concentration (0.5–100 μM). Control cells (C) were treated with DMSO only. The R values are means from three experiments ±SD. Asterisks indicate a significant difference (*p < 0,05, **p < 0,01, ***p < 0,001) from the control (DMSO) value. Striking DNA hypomethylation happens in therapeutically achieved concentrations (less than 5 μM). Significant DNA hypermethylation is achieved at minimal TMZ concentrations of 20–50 μM in 3–24 hrs incubation time. Clear demethylating tendency is seen with 48 hrs incubation time in all TMZ concentrations.
Fig 6. The effect of TMZ on total DNA methylation of HaCaT cell line.
The analysis was performed after 3 (white bars), 12 (light grey bars), 24 (dark grey bars) and 48 (black bars) hrs of incubation in a given TMZ concentration (0.5–100 μM). Control cells (C) were treated with DMSO only. The R values are means from three experiments ±SD. Asterisks indicate a significant difference (*p < 0,05, **p < 0,01, ***p < 0,001) from the control (DMSO) value. In all incubation times the hypermethylation effect is seen over TMZ concentration of 5 μM. No hypomethylation in lower concentrations was detected.
Fig 4. The effect of TMZ on total DNA methylation of U138 cell line.
The analysis was performed after 3 (white bars), 12 (light grey bars), 24 (dark grey bars) and 48 (black bars) hrs of incubation in a given TMZ concentration (0.5–100 μM). Control cells (C) were treated with DMSO only. The R values are means from three experiments ±SD. Asterisks indicate a significant difference (*p < 0,05, **p < 0,01, ***p < 0,001) from the control (DMSO) value. A very little DNA hypermethylating effect is observed (for incubation times 3 and 12 hrs, over concentrations 20 and 30 μM respectively). Very clear initial hypomethylation is seen in TMZ concentrations up to 100 μM for incubation times of 24 and 48 hrs.
Fig 5. The effect of TMZ on total DNA methylation of U118 cell line.
The analysis was performed after 3 (white bars), 12 (light grey bars), 24 (dark grey bars) and 48 (black bars) hrs of incubation in a given TMZ concentration (0.5–100 μM). Control cells (C) were treated with DMSO only. The R values are means from three experiments ±SD. Asterisks indicate a significant difference (*p < 0,05, **p < 0,01, ***p < 0,001) from the control (DMSO) value. Significant demethylating tendency is seen with 48 hrs incubation time in all TMZ concentrations. The TMZ amount needed to reach hypermethylating effect depends on incubation time and is within the range 1–10 μM.
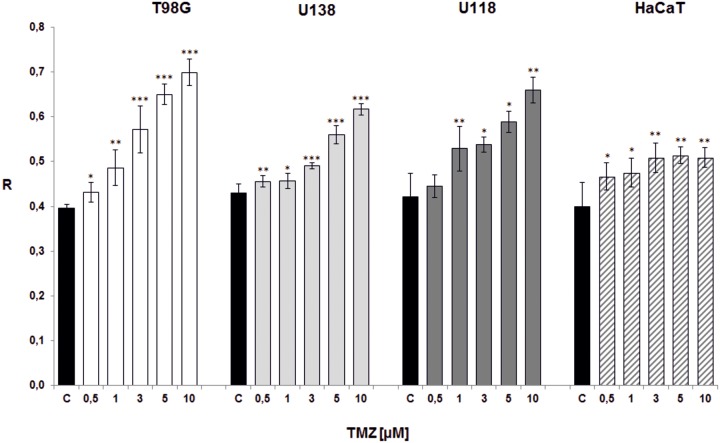
The next experiment was intended to partially mimic the Stupp scheme for adjuvant TMZ therapy [1], and therefore to explore the effect of repetitive TMZ doses on DNA methylation. TMZ was administered to cell lines (T98G, U138, U118, HaCaT) at 0.5, 1, 3, 5, 10 μM concentrations for 5 days, wherein after 24, 48, 72 and 96 hrs cells were washed with PBS and placed in fresh medium with indicated TMZ concentrations. The total DNA methylation was estimated after 5th day of treatment (Fig 7). The general TMZ dose-dependent hypermethylation is observed. Even the lowest concentration (0.5 μM) resulted with the increase of DNA methylation. That is not the case with HaCaT cell line, where rise in R level is scarce, and not clearly related to TMZ dose. One can see that repeated dosing of TMZ (every day) allows bypassing the hypomethylation effect that occurred after single therapeutically achieved TMZ doses in previous experiment (Figs 3–6). The results (S2 Table) are statistically significant for all cell lines (***p<0.001).
Fig 7. The effect of repetitive doses of TMZ on total DNA methylation.
Total amount of m5C in DNA (R) changes during Stupp-mimicking TMZ-therapy. TMZ was administered to glioblastoma (T98G –white bars, U138 –light grey bars, U118 –dark grey bars) and keratinocyte (HaCaT–diagonally striped bars) cell lines at 0.5, 1, 3, 5, 10 μM concentrations for 5 days. Each day cells were washed with PBS and placed in fresh medium with indicated TMZ concentrations. Control (C) cells were treated with DMSO only. The total DNA methylation was estimated after 5th day of treatment. The R values are means from three experiments ±SD. Asterisks indicate a significant difference (*p < 0,05, **p < 0,01, ***p < 0,001) from the control (DMSO) value.
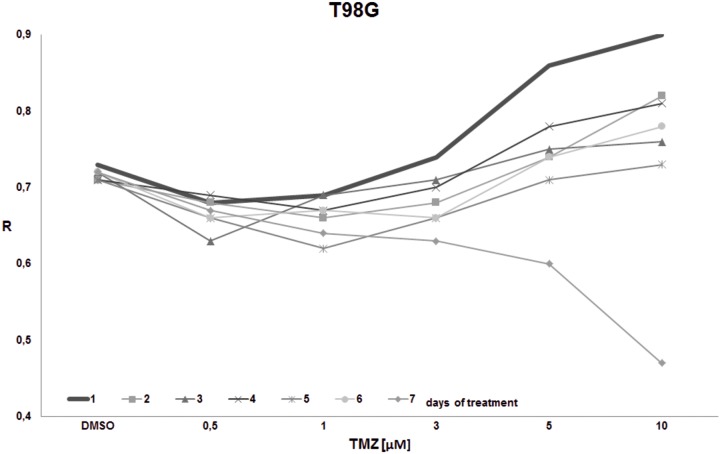
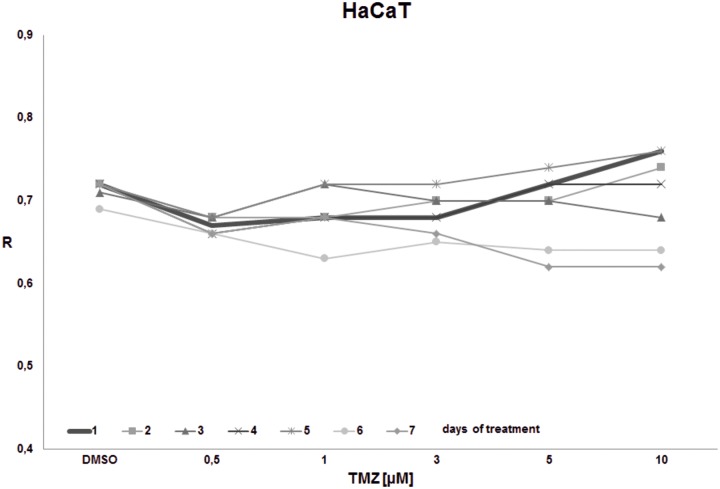
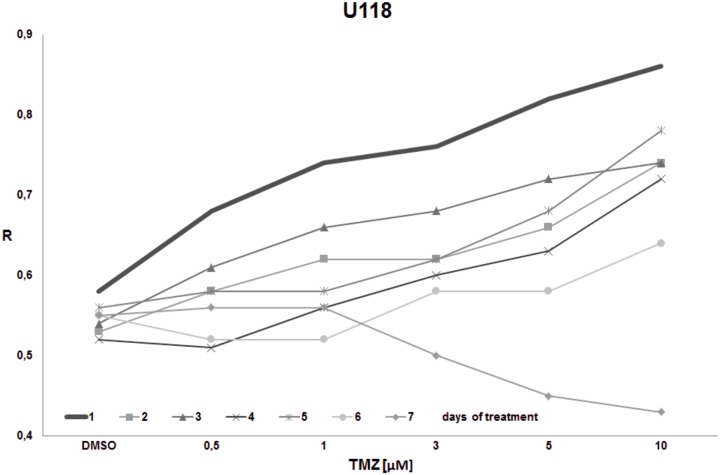
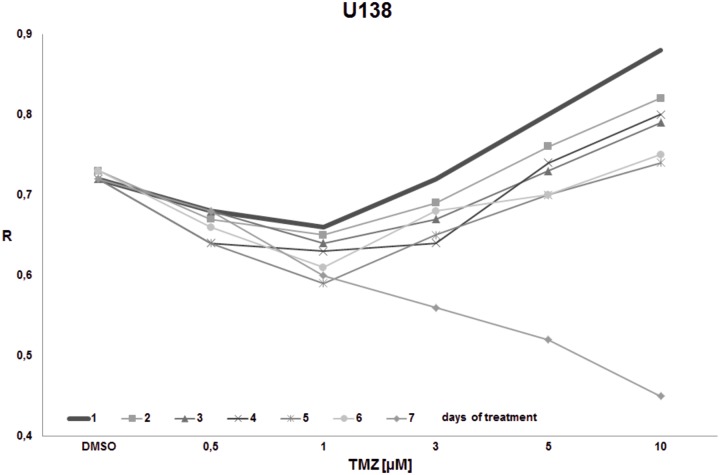
The goal of the final experiment was to evaluate the time effect on total DNA methylation during prolonged TMZ treatment within concentrations reachable in the brain interstitium. Cell cultures (T98G, U138, U118, HaCaT) were treated with the single dose of TMZ (0.5, 1, 3, 5, 10 μM) and were left for 1–7 days. The global DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th day of incubation (Figs 8–11). For glioblastoma cell lines we observed general hypomethylation (when compared with day 1) tendency during time with some stabilization of R level between days 2–6, and clear drop down at day 7. The hypomethylating effect is overcome with increasing TMZ dose. In line U118 (Fig 10) all TMZ concentrations induced hypermethylation over control condition (DMSO treatment), while in T98G (Fig 8) and U138 (Fig 9) that was the case only in highest concentrations (5 and 10 μM). That effect lasted until day 6 at maximum. At day 7 general loss of DNA methylation below the control level was observed. The results of that experiment suggest time limitation of pause in chemotherapy to 4–5 days (depending on the line). The effect of time and initial TMZ dose on HaCaT (Fig 11) culture is scarce, the R values change in a very small range. The numeric results of that experiment are presented in S3 Table.
Fig 8. The impact of TMZ exposure period on DNA methylation in T98G cell line.
T98G cell culture was treated with the given single dose of TMZ (0.5, 1, 3, 5, 10 μM) and left for 1–7 days. The global DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, and 7th day of incubation. The highest methylation (R) is seen on day 1 in all concentrations. In concentrations over 3 μM we observe increasing DNA methylation. On day 7 a hypomethylating trend is observed potentiated by increasing concentration.
Fig 11. The impact of TMZ exposure period on DNA methylation in HaCaT cell line.
HaCaT cell culture was treated with the given single dose of TMZ (0.5, 1, 3, 5, 10 μM) and left for 1–7 days. The global DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, and 7th day of incubation. No significant change in DNA methylation is observed during time.
Fig 10. The impact of TMZ exposure period on DNA methylation in U118 cell line.
U118 cell culture was treated with the given single dose of TMZ (0.5, 1, 3, 5, 10 μM) and left for 1–7 days. The global DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, and 7th day of incubation. A general hypermethylation is observed, with no initial hypomethylating effect in lower concentration range. Hypomethylation after 7 days starts with concentrations over 1 μM.
Fig 9. The impact of TMZ exposure period on DNA methylation in U138 cell line.
U138 cell culture was treated with the given single dose of TMZ (0.5, 1, 3, 5, 10 μM) and left for 1–7 days. The global DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, and 7th day of incubation. The hypomethylation at prolonged time (7 days) is clearly seen. The hypermethylation is achieved in shorter incubation times in concentrations over 5 μM.
Discussion
The aim of our work was to evaluate the effects of temozolomide action on the total contents of m5C in DNA of glioblastoma cell lines in conditions mimicking clinical treatment situations. The choice of the research area was driven by the fact that epigenetic modifications, particularly DNA methylation, are very sensitive to environmental (chemotherapy) changes and react faster than genetic ones, therefore that level of observation seems to be most promising in such an etiologically complex phenomenon as cancer [31,32]. In cancer, global hypomethylation and local hypermethylation are observed [33]. Global hypomethylation is regarded as a result of unspecific, broad oxidative DNA damage (all bases including m5C), which is associated with cancer formation and progression by many different pathways, whereas local hypermethylation is regarded as a carcinogenic event through repression of promoters of tumor suppressor genes, thereby facilitating cancer formation [34–37]. Nevertheless, globally decreased DNA methylation, which appears to correlate with carcinogenesis, is an adverse phenomenon in cancer treatment [35,37].
Decreasing amount of m5C in DNA of cells treated TMZ can be explained straightforward by oxidative removal of methyl group from m5C (demethylation) [38,39] or by demethylating activity of DNA Methyltransferases (DNMTs) in redox state, which can be induced by cancer chemotherapeutics [40,41]. Although there are data that the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine can be done with oxo-reductases (TET enzymes), the mechanism of cellular DNA damage by induction of reactive oxygen species (ROS) is also plausible, The proof for that is the synthesis and presence of 8-oxo-deoxyguanosine in DNA. Newly published data provide evidence, that also in the case of loss of TET function, general DNA demethylation occurs [42]. Recently we showed fluctuations in m5C level in DNA and parallel 8-oxo-guanosine synthesis, which is the product of hydroxyl radical reaction with guanosine [25]. Therefore, a general conclusion is that TMZ induced genomic demethylation (hypomethylation) is a result of global non-specific oxidative stress and damage of DNA components. The level of hypomethylation depends on time and dose of treatment.
5-methylcytosine is a product of enzymatic reaction catalyzed by DNA Methyltransferases, where the only methyl donor is SAM (S-adenosyl methionine) [24]. Neither TMZ, nor TMZ degradation products are the substrates for DNMT. Therefore, the only mechanism through which a drug can increase m5C contents can be through the induction of DNMTs activation. The induction of DNA hypermethylation by drugs, hormones, and other biological compounds was already observed [43–45].
In our experiments we showed TMZ dose-dependent total DNA hypo- and hypermethylation, as well as repeating-dose-dependent hypermethylation. That can argue with the fact that several drugs that inhibit DNA methylation have been studied for the reactivation of tumor suppressor genes (from silencing with promoter hypermethylation) and repression of cancer cell growth [46], and also hypomethylation is regarded as an event helping with the chemotherapy effect. The problem is, the drugs don’t act selectively on hypermethylated promoters only, but on the entire DNA, resulting in global DNA hypomethylation, the hallmark of cancer, aging, and other pathologic processes [29,33,37,47,48], as well as result with mutations in epigenetic enzymes (e.g. DNMTs), that are important for response to hypomethylating treatment [49]. Moreover, DNA damage response that is activated after treatment with radiation and different classes of chemotherapeutic drugs can result both in hypersensitivity or resistance of tumors to therapy and can be exploited for improvement of cancer treatment [50]. The concept of hypomethylation as the goal of treatment has to be probably reevaluated, especially when some results show better survival in globally hypermethylated gliomas [30]. What was also observed, even if MGMT promoter methylation was stable, LINE1 (global methylation surrogate) methylation status was not, resulting with decreased overall survival and GBMs post-treatment evolution with newly reprogrammed epigenetic status [51].
Besides our previous study [17] currently there are no data exploring the subject on total DNA methylation changes during TMZ therapy. In a study analyzing the impact of temozolomide in concentrations 0–200 μM during 24–72 hrs on T98G cell line Jakubowicz-Gil et al. showed that significant level of apoptosis is observed over TMZ concentration of 50 μM. That effect was potentiated with increasing incubation time, and accompanied in higher concentrations with necrosis [52]. That is in concordant with our previous results [17], and supports the results of the present study–with no significant impact on cell apoptosis, necrosis, and autophagy in the concentrations under 50 μM, the total DNA hypomethylation is observed. Similar results were showed on U118 cell line [53].
In the experiment partially mimicking the Stupp scheme (only chemotherapy factor) we observed dose-dependent increase of total DNA methylation (m5C), however never up to the level previously estimated to that of less malignant tumors or healthy individuals [29]. Exploring the prolonged time effect we showed that any hypermethylating treatment effect of TMZ in our experimental cell lines lasted over 5–6 days after drug intake, then having a definite demethylating trend. In the last decades a new concept in TMZ administration emerged–metronomic chemotherapy–involving low doses administered on a frequent schedule, without taking a prolonged break. Such approach appeared to be effective for primary [54] and recurrent glioblastoma [55–57]. Also prolonged (> 6 cycles) standard TMZ therapy was proven to be successful [58,59]. There are data showing that this result does not depend on MGMT promoter methylation [55,60], but other indicate such connection [8]. However, there are also trials that present no significant difference in overall and progression free survival after implementation of a dose-dense protocol for primary glioblastoma [60]. The positive therapeutic effect of dose-dense treatment was shown on animal models [61], however the best results were achieved for daily doses approximately 4x higher (recalculated for human conditions) than those given in a standard TMZ therapy in humans [62,63].
The question concerning a real TMZ concentration achieved in the target tissue (brain) is only partially answered [19,21–23]. The doses we tested in our experiments (0.5–10 μM), being the result of previous studies measuring real TMZ contents in the brain [19,22–23], showed DNA hypomethylation tendency compared to control. That effect was only overcome by repetitive TMZ doses. Even if not analyzed on the epigenetic background before, that effect (inadequate drug amount in target tissue) can be the reason for progression of the disease / chemotherapy resistance.
Many strategies are taken to increase the temozolomide concentration in the CNS, overcoming the boundaries of BBB [64–68]. What was shown earlier, simple implementation of radiotherapy, which is the damaging factor of BBB [69], improves the efficacy of TMZ [70]. That clinical action supports our results that increased TMZ dose results in total hypermethylation [present work, 17], and on clinical level effects in longer progression free and overall survival, as well as less general drug toxicity (because of better penetration of the given dose without the need to increase the intake).
Conclusions
We observed dose-dependent DNA hypermethylation in glioblastoma cell lines and lack of that effect in non-cancerous cell line. A high TMZ concentration induced a significant increase of m5C contents in DNA even in the short time. Therefore an increase of DNA methylation, resulting in genes’ silencing and their expression down regulation, can be the reason for better glioblastoma patients’ survival. However, in the range of concentrations achieved in target tissue during standard treatment, loss of total DNA methylation is observed, what can explain the resistance to TMZ therapy and tumor recurrence. That effect is somehow overcome by repeated TMZ doses in short periods of time.
Our results show the epigenetic response to the therapeutic doses and regimens of temozolomide chemotherapy in glioblastoma. They provide a new insight into the molecular background of the treatment effects, and possible therapy modifications.
Supporting information
Numeric data (R) are followed with SD and p values (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
Glioblastoma (U138, T98G, U118) and HaCaT cell lines were treated for 5 days with daily repeated TMZ doses [μM]. After 24, 48, 72 and 96 hrs cells were washed with PBS and placed in fresh medium with indicated TMZ concentrations. The total DNA methylation was estimated after 5th day of treatment. Numeric data (R) are followed with SD and p value (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
The total DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th day of incubation. Numeric data (R) are followed with SD and p values (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Nam JY, de Groot JF. Treatment of Glioblastoma. J Oncol Pract. 2017;13:629–638. 10.1200/JOP.2017.025536 [DOI] [PubMed] [Google Scholar]
- 3.Bady P, Kurscheid S, Delorenzi M, Gorlia T, van den Bent MJ, Hoang-Xuan K, et al. The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol. 2018;135:601–615. 10.1007/s00401-018-1810-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bei R, Marzocchella L, Turriziani M. The use of temozolomide for the treatment of malignant tumors: clinical evidence and molecular mechanisms of action. Recent Pat Anticancer Drug Discov 2010;5:172–187. 10.2174/157489210791760526 [DOI] [PubMed] [Google Scholar]
- 5.Li RH, Hou XY, Yang CS, Liu WL, Tang JQ, Liu YQ, et al. Temozolomide for Treating Malignant Melanoma. J Coll Physicians Surg Pak. 2015;25:680–688 [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 8.Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res. 2015;21:2057–2064. 10.1158/1078-0432.CCR-14-2737 [DOI] [PubMed] [Google Scholar]
- 9.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69–79. 10.1215/15228517-2008-078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny BJ, Wheelhouse RT, Stevens MF, Tsang LL, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33:9045–9051. 10.1021/bi00197a003 [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Taverna P, Whitacre CM, Chatterjee S, Gerson SL. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin Cancer Res. 1999;5:2908–2917. [PubMed] [Google Scholar]
- 12.Tentori L, Graziani G. Pharmacological strategies to increase the antitumor activity of methylating agents. Curr Med Chem. 2002;9:1285–1301. 10.2174/0929867023369916 [DOI] [PubMed] [Google Scholar]
- 13.Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005;65:6394–6400. 10.1158/0008-5472.CAN-05-0715 [DOI] [PubMed] [Google Scholar]
- 14.Bignami M, O’Driscoll M, Aquilina G, Karran P. Unmasking a killer: DNA O(6)-methylguanine and the cytotoxicity of methylating agents. Mutat Res. 2000;462:71–82. 10.1016/s1383-5742(00)00016-8 [DOI] [PubMed] [Google Scholar]
- 15.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. 10.1038/sj.onc.1209785 [DOI] [PubMed] [Google Scholar]
- 16.Strobel H, Baisch T, Fitzel R, Schilberg K, Siegelin MD, Karpel-Massler G, et al. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines. 2019. September 9 10.3390/biomedicines7030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barciszewska AM, Gurda D, Głodowicz P, Nowak S, Naskręt-Barciszewska MZ. A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PLoS One. 2015;10:e0136669 10.1371/journal.pone.0136669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61. 10.1016/s0305-7372(97)90019-0 [DOI] [PubMed] [Google Scholar]
- 19.Patel M, McCully C, Godwin K, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol. 2003;61:203–207. 10.1023/a:1022592913323 [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis GV, Kouloulias V, Zacharias G, Papadimitriou C, Pantelakos P, Maravelis G, et al. Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules. 2009;14:1561–1577. 10.3390/molecules14041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.League-Pascual JC, Lester-McCully CM, Shandilya S, Ronner L, Rodgers L, Cruz R, et al. Plasma and cerebrospinal fluid pharmacokinetics of select chemotherapeutic agents following intranasal delivery in a non-human primate model. J Neurooncol. 2017;132:401–407. 10.1007/s11060-017-2388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. 10.1158/1078-0432.CCR-03-0807 [DOI] [PubMed] [Google Scholar]
- 23.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. 10.1158/1078-0432.CCR-09-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. 10.1038/nrg1655 [DOI] [PubMed] [Google Scholar]
- 25.Barciszewska AM, Giel-Pietraszuk M, Perrigue PM, Naskręt-Barciszewska M. Total DNA Methylation Changes Reflect Random Oxidative DNA Damage in Gliomas. Cells. 2019. September 11 10.3390/cells8091065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. 10.1016/j.cell.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 27.Żukiel R, Nowak S, Barciszewska AM, Gawrońska I, Keith G, Barciszewska MZ. A simple epigenetic method for the diagnosis and classification of brain tumors. Mol Cancer Res. 2004;2:196–202. [PubMed] [Google Scholar]
- 28.Smolarek I, Wyszko E, Barciszewska AM, Nowak S, Gawronska I, Jablecka A, et al. Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Monit. 2010;16:CR149-55. [PubMed] [Google Scholar]
- 29.Barciszewska AM, Nowak S, Naskret-Barciszewska MZ. The degree of global DNA hypomethylation in peripheral blood correlates with that in matched tumor tissue in several neoplasia. PLoS One. 2014;9:e92599 10.1371/journal.pone.0092599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Bent MJ, Gravendeel LA, Gorlia T, Kros JM, Lapre L, Wesseling et al. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin Cancer Res. 2011;17:7148–7155. 10.1158/1078-0432.CCR-11-1274 [DOI] [PubMed] [Google Scholar]
- 31.Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Philos Trans R Soc Lond B Biol Sci. 2009;364:3403–3418. 10.1098/rstb.2009.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vineis P, Chatziioannou A, Cunliffe VT, Flanagan JM, Hanson M, Kirsch-Volders M, et al. Epigenetic memory in response to environmental stressors. FASEB J. 2017;31:2241–2251. 10.1096/fj.201601059RR [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich M, Lacey M.DNA hypomethylation and hemimethylation in cancer. Adv Exp Med Biol. 2013;754:31–56. 10.1007/978-1-4419-9967-2_2 [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer GP. Defining Driver DNA Methylation Changes in Human Cancer. Int J Mol Sci. 2018. April 12 10.3390/ijms19041166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Good CR, Panjarian S, Kelly AD, Madzo J, Patel B, Jelinek J, et al. TET1-Mediated Hypomethylation Activates Oncogenic Signaling in Triple-Negative Breast Cancer. Cancer Res. 2018;78:4126–4137. 10.1158/0008-5472.CAN-17-2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter BL, Liu J, Qi L, Wang C, O’Connor KL. Integrin α6β4 Upregulates Amphiregulin and Epiregulin through Base Excision Repair-Mediated DNA Demethylation and Promotes Genome-wide DNA Hypomethylation. Sci Rep. 2017;7:6174 10.1038/s41598-017-06351-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Tongelen A, Loriot A, De Smet C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017;396:130–137. 10.1016/j.canlet.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 38.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. 10.1016/j.canlet.2008.02.026 [DOI] [PubMed] [Google Scholar]
- 39.Wachsman JT. DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutat Res. 1997;375:1–8. 10.1016/s0027-5107(97)00003-1 [DOI] [PubMed] [Google Scholar]
- 40.Chen CC, Wang KY, Shen CK. DNA 5-methylcytosine demethylation activities of the mammalian DNA methyltransferases. J Biol Chem. 2013;288:9084–9091. 10.1074/jbc.M112.445585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milkovic L, Siems W, Siems R, Zarkovic N. Oxidative stress and antioxidants in carcinogenesis and integrative therapy of cancer. Curr Pharm Des. 2014;20:6529–6542. 10.2174/1381612820666140826152822 [DOI] [PubMed] [Google Scholar]
- 42.López-Moyado IF, Tsagaratou A, Yuita H, Seo H, Delatte B, Heinz S, et al. Paradoxical association of TET loss of function with genome-wide DNA hypomethylation. Proc Natl Acad Sci U S A. 2019;116:16933–16942. 10.1073/pnas.1903059116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyce JW. Drug-induced DNA hypermethylation: a potential mediator of acquired drug resistance during cancer chemotherapy. Mutat Res. 1997;386:153–161. 10.1016/s1383-5742(96)00051-8 [DOI] [PubMed] [Google Scholar]
- 44.García-Carpizo V, Ruiz-Llorente L, Fraga M, Aranda A. The growing role of gene methylation on endocrine function. J Mol Endocrinol. 2011;47:R75–89. 10.1530/JME-11-0059 [DOI] [PubMed] [Google Scholar]
- 45.Fukui T, Soda K, Takao K, Rikiyama T. Extracellular Spermine Activates DNA Methyltransferase 3A and 3B. Int J Mol Sci. 2019. March 12 10.3390/ijms20051254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perri F, Longo F, Giuliano M, Sabbatino F, Favia G, Ionna F, et al. Epigenetic control of gene expression: Potential implications for cancer treatment. Crit Rev Oncol Hematol. 2017;111:166–172. 10.1016/j.critrevonc.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 47.Barciszewska MZ, Barciszewska A-M, Rattan SIS. TLC-based detection of methylated cytosine: application to aging epigenetics. Biogerontology. 2007;8:673–678. 10.1007/s10522-007-9109-3 [DOI] [PubMed] [Google Scholar]
- 48.Unnikrishnan A, Hadad N, Masser DR, Jackson J, Freeman WM, Richardson A. Revisiting the genomic hypomethylation hypothesis of aging. Ann N Y Acad Sci. 2018;1418:69–79. 10.1111/nyas.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voso MT, Santini V, Fabiani E, Fianchi L, Criscuolo M, Falconi G, et al. Why methylation is not a marker predictive of response to hypomethylating agents. Haematologica. 2014;99:613–619. 10.3324/haematol.2013.099549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstein M, Kastan MB. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. 10.1146/annurev-med-081313-121208 [DOI] [PubMed] [Google Scholar]
- 51.O’Regan CJ, Kearney H, Beausang A, Farrell MA, Brett FM, Cryan JB, et al. Temporal stability of MGMT promoter methylation in glioblastoma patients undergoing STUPP protocol. J Neurooncol. 2018;137:233–240. 10.1007/s11060-017-2722-3 [DOI] [PubMed] [Google Scholar]
- 52.Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34:2367–78. 10.1007/s13277-013-0785-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmo A, Carvalheiro H, Crespo I, Nunes I, Lopes MC. Effect of temozolomide on the U-118 glioma cell line. Oncol Lett. 2011;2:1165–1170. 10.3892/ol.2011.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nachbichler SB, Schupp G, Ballhausen H, Niyazi M, Belka C. Temozolomide during radiotherapy of glioblastoma multiforme: Daily administration improves survival. Strahlenther Onkol. 2017;193:890–896. 10.1007/s00066-017-1110-4 [DOI] [PubMed] [Google Scholar]
- 55.Kong DS, Lee JI, Kim JH, Kim ST, Kim WS, Suh YL, et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010;12:289–296. 10.1093/neuonc/nop030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15:242–250. 10.1093/neuonc/nos295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Kong X, Guo Y, Wang R, Ma W. Continuous dose-intense temozolomide and cisplatin in recurrent glioblastoma patients. Medicine (Baltimore). 2017;96:e6261 10.1097/MD.0000000000006261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skardelly M, Dangel E, Gohde J, Noell S, Behling F, Lepski G, et al. Prolonged Temozolomide Maintenance Therapy in Newly Diagnosed Glioblastoma. Oncologist. 2017;22:570–575. 10.1634/theoncologist.2016-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh SY, Chan DT, Kam MK, Loong HH, Tsang WK, Poon DM, et al. Feasibility and safety of extended adjuvant temozolomide beyond six cycles for patients with glioblastoma. Hong Kong Med J. 2017;23:594–598. 10.12809/hkmj165002 [DOI] [PubMed] [Google Scholar]
- 60.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. 10.1200/JCO.2013.49.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q, Guo P, Wang X, Nuthalapati S, Gallo JM. Preclinical pharmacokinetic and pharmacodynamic evaluation of metronomic and conventional temozolomide dosing regimens. J Pharmacol Exp Ther. 2007;321:265–275. 10.1124/jpet.106.118265 [DOI] [PubMed] [Google Scholar]
- 62.Ferrer-Font L, Arias-Ramos N, Lope-Piedrafita S, Julià-Sapé M, Pumarola M, Arús C, et al. Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide. NMR Biomed. 2017;30 10.1002/nbm.3748 [DOI] [PubMed] [Google Scholar]
- 63.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rai R, Banerjee M, Wong DH, McCullagh E, Gupta A, Tripathi S, et al. Temozolomide analogs with improved brain/plasma ratios—Exploring the possibility of enhancing the therapeutic index of temozolomide. Bioorg Med Chem Lett. 2016;26:5103–5109. 10.1016/j.bmcl.2016.08.064 [DOI] [PubMed] [Google Scholar]
- 65.Nordling-David MM, Yaffe R, Guez D, Meirow H, Last D, Grad E, et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J Control Release. 2017;261:138–146. 10.1016/j.jconrel.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 66.Harder BG, Blomquist MR, Wang J, Kim AJ, Woodworth GF, Winkles JA, et al. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front Oncol. 2018;8:462 10.3389/fonc.2018.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu L, Wang A, Ni L, Yan X, Song Y, Zhao M, et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018;25:1634–1641. 10.1080/10717544.2018.1494226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci Rep. 2019;9:321 10.1038/s41598-018-36340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng F, Tsien CI, Lawrence TS, Cao Y. Blood-tumor barrier opening changes in brain metastases from pre to one-month post radiation therapy. Radiother Oncol. 2017;125:89–93. 10.1016/j.radonc.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 70.Feng E, Sui C, Wang T, Sun G. Temozolomide with or without Radiotherapy in Patients with Newly Diagnosed Glioblastoma Multiforme: A Meta-Analysis. Eur Neurol. 2017;77:201–210. 10.1159/000455842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numeric data (R) are followed with SD and p values (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
Glioblastoma (U138, T98G, U118) and HaCaT cell lines were treated for 5 days with daily repeated TMZ doses [μM]. After 24, 48, 72 and 96 hrs cells were washed with PBS and placed in fresh medium with indicated TMZ concentrations. The total DNA methylation was estimated after 5th day of treatment. Numeric data (R) are followed with SD and p value (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
The total DNA methylation was estimated after 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th day of incubation. Numeric data (R) are followed with SD and p values (one-tailed t-test, comparison with the control–DMSO, *p < 0,05, **p < 0,01, ***p < 0,001).
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.