Abstract
The role of the munc 13-1 pre-synaptic protein in alcohol-related behaviors has been little-studied, despite being a known site of action for ethanol binding. Munc 13-1 is an active zone protein that plays a vital role in vesicle maturation and the release of neurotransmitter in excitatory neurons. Ethanol binds munc 13-1, decreasing its functionality. In Drosophila, loss of the homologous protein Dunc13 is associated with an increase in ethanol preference, and a resistance to sedation following ethanol exposure. The current study assessed the effects of munc 13-1 heterozygosity on ethanol sensitivity and consumption in mice, as well as on learning and anxiety-like behaviors, which can influence alcohol intake. Wild type and mutant mice underwent 6 cycles of drinking-in-the-dark (DID) as well as rotarod testing following ethanol injection, to probe for differences in ethanol consumption and sensitivity, respectively. We did not detect genotype-based differences in our measures of anxiety, spatial learning, ethanol consumption, or ethanol sensitivity. However, heterozygotes showed increased use of a spatial navigation strategy in a dual solution water maze, as opposed to a stimulus-response strategy. To summarize, although reduction of Dunc13 in flies produces clear effects on ethanol consumption and sensitivity, heterozygosity for munc 13-1 does not, potentially due to compensatory adaptation by other munc 13 isoforms.
Keywords: addiction, tolerance, spatial learning, anxiety, cognition
INTRODUCTION
It has long been known that alcohol acts postsynaptically, affecting numerous ion channels and neurotransmitter systems (Diamond & Gordon, 1997; Harris, 1999), but it has become clear that alcohol exerts presynaptic effects as well. When a neuron is readying itself to release transmitter into the synapse, it recruits a number of proteins to the terminal membrane in order to prepare for exocytosis (Palfreyman & Jorgensen, 2017; Südhof, 2012). These proteins play various roles in getting the vesicles to a sufficiently primed state, and ready to tether to, bind, and fuse with the membrane wall (Andrews-Zwilling, Kawabe, Reim, Varoqueaux, & Brose, 2006), in addition to coordinating the timing of these processes and restricting exocytosis to the synaptic space (Rosenmund, Rettig, & Brose, 2003). Unc-13 is one such protein that was first identified in the nematode Caenorhabditis elegans. C. elegans with mutations in unc-13 showed uncoordinated movement (hence the “unc” designation), which was in stark contrast to the typically smooth muscular movements of this species (Brenner, 1974). In Drosophila melanogaster, the homologous protein Dunc13 has been identified, while in mammals, the homologs are members of the munc-13 family of proteins. We have previously shown that munc 13-1, a presynaptic active zone protein, is a binding site for ethanol, reducing the function of the protein by preventing diacylglycerol (DAG) binding (Das et al., 2013; Xu et al., 2018). In Drosophila, Dunc13 deficient flies display higher ethanol self-administration (Das et al., 2013) and resistance to its sedating effects (Xu et al., 2018).
Given that there is structural similarity between the Dunc13, unc-13, and munc-13 proteins, it seems likely that conservation of function has been achieved across species, and that these proteins serve similar purposes in worms, flies, and mammals (Betz, Okamoto, Benseler, & Brose, 1997) . Indeed, introduction of the rat munc 13-1 gene in Dunc13 deficient flies reversed their increased affinity for ethanol consumption (Das et al., 2013). It is not known, however, whether Munc 13-1 deficiency would influence ethanol consumption or tolerance in mammals. The present study was designed to determine this, using mice in which the gene that codes for munc 13-1 is expressed at ~50% of normal levels (Augustin, Rosenmund, Sudhof, & Brose, 1999). To determine the effect of munc 13-1 reduction on ethanol self-administration, heterozygous and wildtype mice underwent the drinking-in-the-dark (DID) paradigm. We hypothesized that genotype would influence drinking behavior, such that munc 13-1 heterozygotes would consume more ethanol than wildtypes. To determine whether munc 13-1 reduction would alter sensitivity to ethanol intoxication, we examined performance on the accelerating rotarod before and after ethanol injection. We hypothesized that munc 13-1 knockout mice would show less behavioral intoxication (assessed via the Majchrowicz scale and latency to fall on the rotarod) after injection of ethanol.
Because learning and anxiety influence ethanol consumption, we first probed for behavioral differences between wildtype and heterozygous mice. One factor in the initiation and maintenance of ethanol drinking is a high basal anxiety level, in both rodents (Izidio & Ramos, 2007; Pandey, 2003) and humans (Marquenie et al., 2007; Schuckit & Hesselbrock, 2004). As such, we assessed baseline differences in anxiety-like behaviors in the open field. Additionally, high levels of ethanol consumption in rodents can be considered a collection of associatively learned behaviors, given that the taste is naturally aversive to many strains of mouse (Belknap, Crabbe, & Young, 1993; Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008). The rodent comes to associate a number of cues in its environment with the availability of a drug, and the drug with its rewarding effects. The striatum is important for reward-based learning (for review see Lovinger & Alvarez, 2017), and the hippocampus is critical for developing and retrieving contextual and spatial memories (Kennedy & Shapiro, 2004; McNamara & Skelton, 1993; Ross & Slotnick, 2008). The dentate gyrus is necessary for the recall of drug-related contextual memories (Hernandez-Rabaza et al., 2008), and the CA3 subregion is critical for linking context with a reward (Luo, Tahsili-Fahadan, Wise, Lupica, & Aston-Jones, 2011). Thus, prior to assessing alcohol intake and resistance to intoxication, we assessed hippocampal and striatal function in wildtype and heterozygous animals by using a modified version of the Morris Water Maze (MWM), in which the platform location can be learned via either a spatial (hippocampal-based) or stimulus-response (striatal-based) strategy.
METHOD
Animals
For this study, we used mice that express a knockdown of the gene that codes for the munc 13-1 protein. The mice were heterozygous for munc 13-1 deletion, because homozygotes die shortly after birth (Augustin, Rosenmund, et al., 1999). We crossed C57BL/6J males that were heterozygous for munc 13-1 KO allele (a generous gift from Dr. Thomas Südhof) with C57BL/6J females (Harlan) or 129S1/ SvImJ females (Jackson Labs). Unlike many other mouse strains, the C57BL/6J mouse willingly consumes enough ethanol to reach intoxicating levels, whereas the 129S1/SvImJ mouse does not typically consume much alcohol (Rhodes et al., 2007). The progeny of these crosses were used for subsequent behavioral testing. The progeny from both sets of crosses were either heterozygous for the munc13-1 KO allele or wildtype for munc13-1. Siblings that were wild type for munc13-1 were used as genetic background controls. The progeny from the cross with 129S1/SvImJ were used only to assess alcohol consumption, in order to increase the possibility of detecting a small effect of munc 13-1 heterozygosity. C57BL/6J derived progeny were used for behavioral testing measures of ethanol consumption and to examine the effect of munc 13-1 heterozygosity on ethanol tolerance. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Houston. All mice used in the study were bred in-house and acclimated to a reverse light/dark cycle (lights off at 9:00/on at 21:00). Mice were weaned at 21 days of age, and group housed with litter mates until measurement of ethanol consumption commenced, at which point they were singly housed. Mice were between eight and twelve weeks of age at the beginning of the study, and had ad libitum access to mouse chow for the duration of the experiment. Water was continuously available except for the DID animals for 2 to 4 hours per day once testing began (described in Drinking in the Dark below). All procedures were performed blind to genotype.
Open-field test for anxiety
Munc13-1KO/C57 mice and their wild type controls (N = 36) were individually placed into the center of a square open-field arena (17” x 17”). Infrared beams detected the ambulation of each mouse and where it was located throughout the 5-minute trial. This exploration behavior was tracked by the automated Activity Monitor system (MED Associates). Typical rodents in control conditions will spend a significantly greater amount of time exploring the periphery of the arena, usually in contact with the walls rather than the center area (also known as thigmotaxis). Mice that spend significantly more time exploring the unprotected center area are considered less anxious.
Morris Water Maze
Acquisition
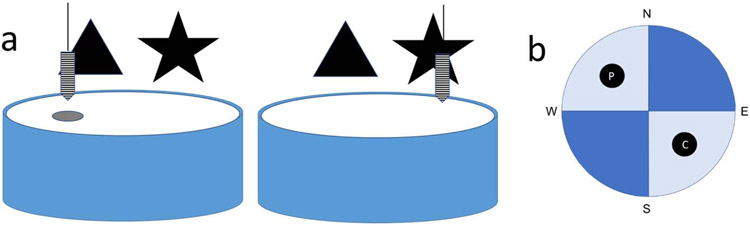
Munc13-1KO/C57 mice, along with their wild type controls (N = 64), were tested in a dual-solution paradigm to examine spatial versus cued navigation (McDonald & White, 1994). The pool (120 cm diameter x 81 cm high) was filled with water made opaque with non-toxic white paint, and during the acquisition phase a circular escape platform (10 cm diameter, submerged until the top was 1 cm below the water surface) was placed in the center of the NW quadrant (see Figure 1). We hung large black simple shapes (50-100 cm wide) on white shower curtains that surrounded the pool to serve as distal, extramaze cues. In addition, an intramaze cue was suspended ~5 cm above the hidden platform for all 6 days of training/acquisition. This cue was made from a 50 mL Falcon tube (Snyder, Cahill, & Frankland, 2017). Mice could therefore navigate to the hidden platform using the distal cues to orient themselves in space (a hippocampal-based, spatial strategy), or they could simply swim to the hanging cue that marked the platform location (a striatal-based, stimulus-response strategy) (McDonald & White, 1994; Packard & McGaugh, 1992). Mice received 4 trials per day (for 6 consecutive days) with a 5-minute inter-trial interval. Each trial had a maximum length of 60 seconds; if a mouse did not escape within 60 seconds, it was placed on the platform for 10 seconds before being returned to the home cage. Mice were released from the N, S, W, and E points of the pool in a pseudorandom fashion. Activity of each mouse within the pool was recorded and latency to platform, swim speed and path distance calculated (EthoVision, Noldus).
Figure 1. Visual representation of the dual-solution water maze.
a) The setup of the maze during 6 days of acquisition (left). The extramaze cues are the large simple figures in the background, while the intramaze cue is the striped tube hanging over the hidden platform. The setup of the maze during the probe trial on Day 7 (right). The intramaze cue has been moved to the opposite side of the pool, but the extramaze cues do not change location. The hidden platform has been removed. b) Quadrants and zones of the maze. The pool is divided into four quadrants based on the cardinal directions, colored in light and dark blue. The platform (P) and cue (C) zones occupy a smaller space within the NW and SE quadrants, and are colored in black. The platform zone is where the platform and the intramaze cue are located for all 6 days of acquisition. The cue zone is where the intramaze cue is moved for the probe trial. To assess use of a spatial strategy versus a stimulus-response strategy, time spent in the NW and SE quadrants during the probe trial were compared.
Probe Trial
To assess which strategy the mice used during acquisition, on the 7th day, each mouse was given one 60-second probe trial. For the probe trial, the hidden platform was removed and the intramaze cue was moved to the quadrant (SE) opposite where it had been during the acquisition phase (Figure 1). The extramaze cues were not moved. All mice were released from the SW point of the pool. Measures of interest were time spent near the former platform location, which indicates the mice were using a spatial strategy, and time spent near the new cue location, which would indicate the mice were using a stimulus-response strategy. We also determined spatial bias score, which is a measure of the tendency to spend time in the platform quadrant, indicating that a spatial strategy was employed during acquisition (as opposed to a stimulus-response strategy). This difference score was calculated as the percent time spent in the platform quadrant minus the percent time spent in the cue quadrant. For these difference scores, values above 0 reflect more time spent in the platform location, while values below 0 reflect more time in the cue location.
Interlimb coordination
A rotarod (Ugo Basile, Italy) was used to assess motor coordination. The rotarod had a rotating bar (3 cm diameter), which was machined with grooves to provide traction, and five lanes (each 5.7 cm wide), enabling testing of five mice simultaneously. The fall distance was 16 cm. Methods were adapted from (Deacon, 2013). The starting speed was set to 10 rotations per minute (rpm), and the rod accelerated to a maximum of 45 rpm over the course of 180 seconds. Beneath each lane was a stainless steel trip-box, which automatically recorded the latency to fall. If the mouse fell off before 5 seconds had passed, this was considered to be erroneous placement by the researcher, and the mouse was placed again on the rotating bar. Mice underwent six trials in total, over the course of two consecutive days.
Voluntary Ethanol Consumption
Adult munc13-1KO/C57 mice (n = 85) and munc13-1KO/129S1 mice (n = 74), along with their wild type controls, were exposed to 20% ethanol using the four-day Drinking in the Dark (DID) paradigm (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Rhodes et al., 2007) for six consecutive weeks. First, mice were habituated to being singly-housed and drinking water from a metal-tipped bottle for 7 days. Then, the four-day procedure began. For the first 3 days, the water bottle was temporarily removed from the animal’s cage and replaced with an identical bottle containing a solution of 20% ethanol in water. This occurred at 3 hours after lights-off. The ethanol solution was left in the cage for 2 hours, after which it was removed and replaced with the water bottle again. The amount of ethanol solution consumed by each animal was recorded. On the fourth day of DID, the same 2 hours of exposure occurred, and the amount of intake was recorded. However, on this day, the bottle containing ethanol was given to the animals for an additional 2 hours, giving the mice 4 total hours of ethanol exposure for the day. After the second round of exposure, the amount of ethanol solution consumed was once again recorded. The mice then had three ethanol-free days before the four-day procedure started again. The mean intake was expressed as grams of ethanol per kilogram of body weight consumed (g/kg), per 4-hour session. At the end of the last 4-hour ethanol session (week 6, end of day 4), blood was collected from the saphenous vein for determination of blood ethanol content (BEC). Blood was collected in heparinized glass capillary tubes, and then centrifuged at 10,000 rpm for 5 minutes in 1.5 mL micro-centrifuge tubes. The blood plasma that collected on the top of the sample was withdrawn and stored at −20°C for later analysis. Samples were then processed using an Analox AM-1 model alcohol analyzer (Analox Instruments, Lunenburg, MA) and BEC expressed as mg/dL.
Measure of Ethanol Sensitivity
In order to determine the doses of ethanol we would use for this experiment, we first performed pilot testing with a separate group of male mice (n = 13). Mice were dosed with ethanol via intraperitoneal (i.p.) injection at doses of either 0.5, 1.0, 1.5, 2.25, or 3.0 g/kg and given one trial on the rotarod at 10 minutes post-injection. Although other studies have used high ethanol doses (Blednov et al., 2017; Rustay, Wahlsten, & Crabbe, 2003), we found that mice injected with 2.25 and 3.0 g/kg were too intoxicated and/or ataxic to walk on the rotating bar, so those doses were eliminated. For the remaining three doses, mice were able to walk on the rotating bar, so 0.5 and 1.5 g/kg were chosen as low and high doses, respectively.
Performance baseline was established on the first day of testing, when male mice were administered an i.p. injection of isotonic saline, and latency to fall from the rotating rod recorded 10, 30 and 60 minutes later. On the second day of testing, mice were randomly assigned to the 0.5 or 1.5 g/kg group. Injections were prepared by diluting 190 proof ethanol in 0.9% saline to form a 20% ethanol solution, which was filtered and administered by i.p. injection in a volume of 0.2 ml per 10 g of body weight. At 10, 30 and 60 minutes post-injection, mice were rated on the Majchrowicz 6-point behavioral intoxication scale (Majchrowicz, 1975), and also given a trial on the rotarod. Blood was collected after the last trial and BEC determined as described above. Sixty minutes was chosen because this is when mice show peak BEC levels after i.p. injection of ethanol (Livy, Parnell, & West, 2003). Additionally, this ensured that needle puncture of the saphenous vein did not hinder performance.
Statistical Analyses
Two-way ANOVA (Sex x Genotype) was used to analyze open field and probe trial data, as well as BEC, followed by t tests where appropriate. For water maze acquisition, interlimb coordination and DID data, mixed model ANOVA was used, with Time (Day, Trial or Week, respectively) as the repeated measure. Paired-samples t tests were used where appropriate. For the rotarod, mixed model ANOVA was used (Trial x Genotype x Dose). The Majchrowicz assessment of behavioral intoxication was measured on a scale from 0-5; given the ordinal nature of the data, the median was chosen as the measure of central tendency (Manikandan, 2011), and independent samples Mann-Whitney U tests were used to compare the medians. All statistics were run using the statistical software SPSS, and for all analyses, a p value of less than 0.05 was deemed significant.
RESULTS
Open Field
Two-way ANOVA (Sex x Genotype) revealed no significant differences for any of the open field outcome measures, means for which are depicted in Table 1.
Table 1.
Descriptive Statistics for the Open Field measures.
| Female | Male | |||
|---|---|---|---|---|
| Munc13-1KO (n=10) |
Wild type (n=8) |
Munc13-1KO (n=9) |
Wild type (n=9) |
|
| Mean Velocity | 7.25±1.79 | 8.56±1.35 | 7.57±1.55 | 8.33±1.45 |
| Distance Travelled (cm) | 2148.16±529.99 | 2554.27±404.98 | 2252.21±468.38 | 2474.11±423.31 |
| Time in Perimeter (s) | 186.52±55.53 | 210.62±67.35 | 210.46±41.70 | 193.53±46.99 |
| % Time in Perimeter | 62.77±18.16 | 70.37±22.14 | 70.69±13.67 | 64.98±15.49 |
| Frequency of Perimeter Entries | 55.90±22.67 | 48.50±14.97 | 50.33±13.69 | 60.44±18.04 |
| Mean Bout Length in Perimeter (s) | 4.32±3.14 | 5.12±2.93 | 4.60±1.84 | 3.66±1.78 |
| Time in Interior (s) | 74.87±24.26 | 65.46±26.15 | 72.49±16.47 | 85.71±16.94 |
| % Time in Interior | 25.30±8.32 | 21.98±8.95 | 24.39±5.58 | 28.84±5.79 |
| Frequency of Interior Entries | 40.70±16.62 | 42.63±11.26 | 43.33±9.62 | 50.89±7.94 |
| Mean Bout Length in Interior (s) | 2.02±0.86 | 1.57±0.67 | 1.74±0.51 | 1.71±0.34 |
Results displayed as Mean ± SD.
Morris Water Maze
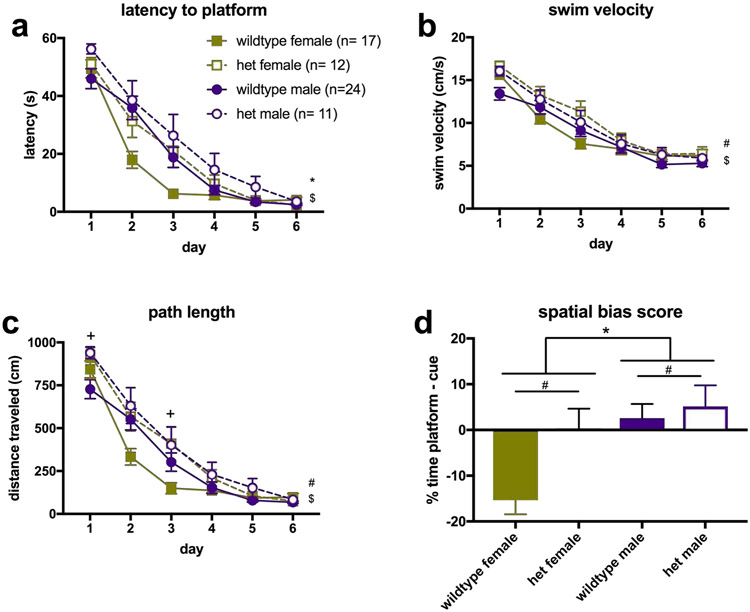
For latency to reach the platform during acquisition, the Day x Genotype x Sex interaction was not significant, [F(3.151, 113.429) = 673, p = .577]. Mauchly’s test indicated that the assumption of sphericity had been violated, [χ2(14) = 56.312, p < .001], therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .630). The Genotype x Sex interaction was not significant, [F(1, 36) = 1.509, p = .22], and neither was the Sex x Day interaction [F(3.151, 113.429) = 2.217, p = .087)] or Genotype x Day, [(F3.151, 113.429) = 2.006, p = .114]. The main effect of genotype was not significant [F(1, 36) = 3.192, p = .082], however, there were significant main effects of Day [F(3.151, 113.429) = 76.036, p < .001)], reflecting the overall learning curve shown by all groups and Sex [F(1, 36) = 5.199, p =.029] such that males were slower to reach the platform (see Figure 2a).
Figure 2. Performance in the dual-solution water maze.
a) Latency to find the hidden platform across 6 days of acquisition. All mice learned the location of the platform by Day 6, as indicated by the main effect of Day as well as latencies of <10 seconds. Females found the platform significantly faster as indicated by the main effect of Sex. b) Swim velocity of mice in the water maze. Overall, heterozygotes swam faster than wildtypes, and swim speed for all animals decreased over time. c) Swim path length of mice in the water maze. Heterozygotes travelled further than wildtype mice, and swim paths of all mice shortened over time. d) Spatial bias scores across sex and genotype, based on quadrant. Values above 0 indicate bias toward a spatial strategy, while values below 0 indicate bias toward a stimulus-response strategy. There was no interaction, but there were main effects of Sex (p = .004) and Genotype (p = .023), such that males and heterozygotes showed higher scores (more spatial bias) when compared to females and wildtypes. *p < 0.05 main effect of Sex; #p < 0.05 main effect of Genotype; $p < 0.05 main effect of Day; +p < 0.05 heterozygotes significantly different from wildtype
For swim velocity, the interaction of Day x Genotype x Sex was not significant [F(3.722, 223.318) = 1.713, p = .153]. Mauchly’s test indicated that the assumption of sphericity had been violated, [χ2(14) = 47.922, p < .001], therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .744). The interaction of Genotype x Sex on swim velocity was not significant [F(1, 60)= .149, p = .701], and neither was the interaction of Sex x Day [F(3.722, 223.318) = 1.089, p = .367] or Genotype x Day [F(3.722, 223.318) = 1.538 , p = .196]. The main effect of Sex was not significant [F(1, 60) = .520, p = .473]. However, there was a main effect of Day [F(3.722, 223.318) = 136.007, p < .001)], indicating that all animals swam slower over time and a main effect of Genotype [F(1, 60) = 7.224, p = .009] such that heterozygotes swam faster than wildtypes (see Figure 2b).
For swim path length, the interaction of Day x Genotype x Sex was not significant, [F(3, 180.012) = 1.829, p = .143]. Mauchly’s test indicated that the assumption of sphericity had been violated, [χ2(14) = 131.956, p < .001], therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .6). The interaction of Genotype x Sex on latency to reach the platform was not significant, [F(1, 60) = .039, p = .845], and neither was the interaction of Sex x Day [F(3, 180.012)= 2.347, p = .074]. However, the interaction of Genotype x Day was significant, [F(3, 180.012)= 2.759 , p = .044]. The main effect of Sex was not significant, [F(1, 60) = .934, p = .338]. However, there was a main effect of Day [F(3, 180.012)= 186.179, p < .001], such that path length decreased with time, and a main effect of Genotype [F(1, 60) = 9.103, p = .004] such that heterozygotes had a longer path length than wildtypes. Following up on the interaction of Genotype x Day, independent samples t-tests revealed that heterozygotes swam for a greater distance than wildtypes on Day 1 (t61.753 = −3.354, p = .001) and Day 3 (t33.699 = −2.136, p = .04) (see Figure 2c). Levene’s test indicated that the variances for path length were not equal in the t-test comparisons, so degrees of freedom have been adjusted using the Welch-Satterthwaite method.
For the probe trial, there was no significant Sex x Genotype interaction for time spent in the platform zone [F(1, 58) = 3.51, p = .066], nor was there a significant main effect of Sex [F(1, 58) = .093, p = .762] or Genotype [F(1, 58) = 3.404, p = .070]. For time spent in the cue zone, there was no significant Sex x Genotype interaction [F(1, 60) = 3.13, p = .082], however, the main effect of Sex was significant [F(1, 60) = 12.609, p = .001], such that females spent more time in this zone when compared to males. The main effect of Genotype was not significant [F(1, 60) = 3.094, p = .084]. For time spent in the platform quadrant, there was no significant Sex x Genotype interaction [F(1, 60) = 2.617, p = .111], and no significant main effect of Genotype [F(1,60) = 2.977, p = .090]. There was a significant main effect of Sex [F(1,60) = 5.470, p = .023] as males spent more time there. For time spent in the cue quadrant, the Sex x Genotype interaction was not significant [F(1,60) = 1.719, p = .195], however, the main effects of Sex [F(1,60) = 8.463, p = .005] and Genotype [F(1,60) = 5.636, p = .021] were significant, such that wildtypes spent more time in this quadrant than did heterozygotes, and females spent more time in this quadrant than males. For latency to enter the platform zone, the Sex x Genotype interaction was not significant [F(1,58) = .349, p = .557], but there was a significant main effect of Genotype [F(1, 58) = 4.168, p = .046] such that wildtypes took longer. There was no significant main effect of Sex [F(1, 58) = 1.454, p = .233]. For latency to enter the cue zone, the Sex x Genotype interaction was not significant [F(1,60) = .084, p = .773]. There was a significant main effect of Sex [F(1, 60) = 4.863, p = .031], as males took longer to enter. There was no main effect of Genotype [F(1, 60) = .008, p = .930]. For latency to enter the platform quadrant, there was no significant Sex x Genotype interaction [F(1,60) = .084, p = .773], nor were there main effects of Sex [F(1,60) = 3.437, p = .069] or Genotype [F(1,60) = 1.113, p = .296]. For latency to enter the cue quadrant, there was no significant interaction of Sex x Genotype [F(1,60) = .092, p = .763], nor were there main effects of Sex [F(1,60) = 1.922, p = .171] or Genotype [F(1,60) = 3.845, p = .055]. For spatial bias score based on time spent in the cue vs platform quadrants, there was no significant Sex x Genotype interaction [F(1, 60) = 2.809, p = .099], however the main effects for both Sex [F(1, 60) = 8.968, p = .004] and Genotype [F(1, 60) = 5.463, p = .023] were significant, such that wildtypes and females showed a lower spatial bias score (see Figure 2d).
Interlimb coordination
The Sex x Genotype x Trial interaction was not significant [F(3.310, 188.667) = .857, p = .474], and neither was Sex x Trial [F(3.310, 188.667) = .537, p = .675] or Genotype x Trial [F(3.310, 188.667) = .277, p = .860]. Mauchly’s test indicated that the assumption of sphericity had been violated, [χ2(14) = 94.122, p < .001], therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .662). There was not a significant interaction for Sex x Genotype [F(1, 57) = .055, p = .815] nor Sex [F(1, 57) = .700, p = .406] or Genotype [F(1, 57) = .729, p = .397] alone. There was, however, a main effect of Trial on rotarod fall latency [F(3.310, 188.667) = 28.419, p < .001]. To follow up, within each of the two days, we compared the first and third trials to look for performance improvement over time. Paired samples t-tests indicated that fall latency on trial 3 was significantly higher than on trial 1, for both the first day [t(60) = −8.313, p < .001] and the second [t(60) = −6.296, p < .001], indicating that all animals improved performance across trials.
Ethanol Consumption
C57BL Mice
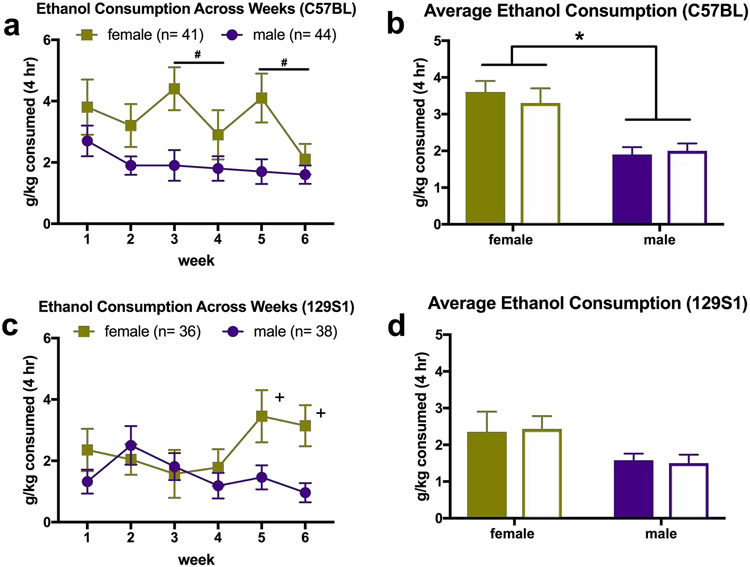
The Sex x Genotype x Week interaction was not significant, [F(4.194, 335.527) = 1.20, p = .310]. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(14) = 38.49, p < .001, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .84). The interactions of Sex x Week [F(4.194, 335.527)= 1.654, p = .157], Genotype x Week [F(4.194, 335.527) = .503, p = .742], and Sex x Genotype [F(1, 80) = .379, p = .540] were also not significant. There was no main effect of Genotype [F(1, 80) = .007, p = .934). However, there were significant main effects of Week, [F(4.19, 335.53) = 2.886, p = .014], and Sex, [F(1, 80) = 26.973, p < .001], such that females drank more than males did (see Figure 3A,B). Following up on the main effect of week, paired samples t tests indicated that overall consumption on Week 4 was lower than on Week 3 [t(84) = 2.240, p = .028], and consumption on Week 6 was lower than on Week 5 (t(84) = 2.345, p = .021). For BEC, two-way ANOVA showed no Sex x Genotype interaction [F(1, 73) = .014, p = .907] and no significant effects of Sex [F(1, 73) = .591, p = .445) or Genotype [F(1, 73) = .000, p = .983].
Figure 3. Ethanol consumption in the DID paradigm.
a) Consumption in C57BL mice over 6 weekly cycles of DID. Ethanol consumption decreased over time, as indicated by the main effect of Week. b) Consumption of C57BL mice averaged over 6 weeks. Overall, female mice drank more than males. c) Consumption in 129S1 mice over 6 weekly cycles of DID. Females drank more than males did at Week 5 (p = .013) and Week 6 (p = .008), but not on average, as shown in d). *p < 0.05 main effect of Sex; $p < 0.05 main effect of Week; +p < 0.05 significant pairwise comparisons
129S1 Mice
The Sex x Genotype x Week interaction was not significant, [F(4.325, 324.365) = 0.62, p = .66]. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(14) = 26.84, p = .02, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .87). The Sex x Week interaction was significant, [F(4.325, 324.365) = 4.733, p = .001], but the interactions of Genotype x Week [F(4.325, 324.365) = .722, p = .588], and Sex x Genotype [F(1, 75) = .191, p = .663] were not. The main effects of Week (F4.325, 324.365 = 1.787, p = .126), Genotype (F1, 75 = .708, p = .403) and Sex (F1,75 = .519, p = .474) were not significant. Following up on the interaction of Sex x Week, independent samples t-tests revealed that females drank more than males on Week 5 (t65.146 = −2.556, p = .013) and Week 6 (t76.390 = − 2.713, p = .008) (see Figure 3C,D). Levene’s test indicated that the variances for ethanol consumption were not equal in the t-test comparisons, so degrees of freedom have been adjusted using the Welch-Satterthwaite method. For BEC, the interaction of Sex x Genotype was not significant, according to a two-way ANOVA, [F(1, 60) = 1.685, p = .199], and there were no main effects of Sex [F(1, 60) = 1.401, p = .241) or Genotype [F(1, 60) = .003, p = .955).
Independent samples t test verified that there was a significant difference in ethanol consumption between the high-drinking (C57BL) and low-drinking (129S1) strains, with C57BL mice consuming more when intake was averaged across weeks [t(162) = 2.052, p = .042]. C57BL mice also had significantly higher average BEC, compared to the 129S1 mice [t(139) = 2.111, p = .037].
Measure of Ethanol Sensitivity
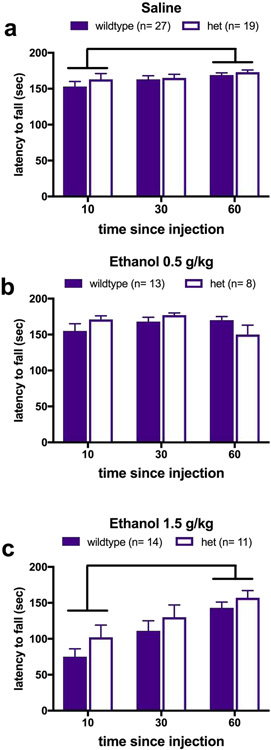
The Dose x Genotype x Trial interaction was not significant, [F(3.147, 132.153) = .461, p = .719]. However, as shown in Figure 4, the Trial x Dose interaction was significant [F(3.147, 132.153) = 13.836, p < .001], as were the main effects of Trial [F(3.147, 132.153) = 16.847, p < .001], and Dose [F(1, 42) = 15.501, p < .001]. The effect of Genotype was not significant [F(1, 42) = 1.356, p = .251]. To follow up on the Trial x Dose interaction, we used paired-samples t-tests to examine each dose separately, and found improvement from Trial 1 to Trial 3 for the saline dose [t(45) = −2.45, p = .018] and the 1.5 g/kg dose [t(24) = −9.94, p < .001]. Behavioral intoxication score was ordinally scaled; thus, independent samples Mann-Whitney U tests were used to compare the medians. Collapsing across dose, there were no differences in Majchrowicz scores between Genotypes, at either 10 mins (U = 246, p = .787), 30 minutes (U = 237, p = .581), or at 60 minutes (U = 249.5, p = .789). To determine if there were differences in BEC after ethanol injection, we ran a two-way ANOVA for Genotype x Dose, which was not significant, [F(1, 33) = .159, p = .693], nor was the main effect of Genotype [F(1, 33) = .042, p = .839]. However, the main effect of Dose was significant, [F(1, 33) = 213.676, p < .001], as mice that received the 1.5 g/kg dose had higher BECs than mice that received 0.5 g/kg.
Figure 4. Rotarod performance following injection of ethanol or saline.
a) Rotarod latency across trials after saline injection (a 0.0 g/kg “dose” of ethanol). Mice significantly improved performance from Trial 1 to Trial 3 (p = .018). b) Rotarod latency across trials after injection of a 0.5 g/kg dose of ethanol. c) Rotarod latency across trials after injection of a 1.5 g/kg dose of ethanol. Mice significantly improved performance from Trial 1 to Trial 3 (p < .001). All values are presented as mean ± standard error.
DISCUSSION
The purpose of the current study was to compare ethanol consumption and sensitivity in munc 13-1 heterozygous and wildtype mice. Drosophila melanogaster with reduced Dunc13 activity self-administer ethanol at a higher rate compared to wildtype flies (Das et al., 2013). These flies also display a robust resistance to ethanol-induced sedation (Xu et al., 2018). We therefore tested whether the same would be true in a mammalian model system, using mice heterozygous for munc 13-1 deletion. Because baseline differences in anxiety, activity, motor coordination or cognition could influence ethanol behaviors, we first performed a behavioral analysis in munc 13-1 heterozygotes and wildtype mice. To probe for differences in motor activity and anxiety-like behavior, we tested mice in the open field. We found no genotype differences in any of the open field measures. We next assessed motor coordination using the rotarod task. Mice received 6 trials over 2 days and while there was steady improvement across trials, there was no effect of genotype on latency to fall from the rotating rod, indicating that munc 13-1 heterozygotes were as coordinated as wildtypes.
We assessed cognition using a version of the water maze task that can be solved with either a spatial or stimulus-response strategy. During the acquisition phase, there were no differences between heterozygote and wildtype mice in latency to locate the hidden platform, although there was a sex difference, such that females found the platform faster than males. Previous studies in rats have found similar results, with females escaping the water faster than males when given a choice of strategy (Kanit et al., 1998; Kanit et al., 2000). We found a significant difference in swim velocity between heterozygotes and wildtypes, such that heterozygotes swam faster. They also, however, had longer path length to the platform. Thus, although munc 13-1 heterozygosity did not affect latency to the platform, these mice swam faster and further, demonstrating decreased efficiency at finding the platform compared to wildtypes. Ultimately, however, all groups achieved the same performance by the final day of acquisition. For this dual-solution version of the water maze, the probe trial indicates which strategy each animal was using during the acquisition phase – spatial or stimulus-response. Our probe trial data indicated that females (regardless of genotype) were faster to approach and spent more time in the vicinity of the cue, indicating use of a stimulus-response strategy. In contrast, males (regardless of genotype) spent significantly more time in the quadrant where the platform had formerly been located, indicating use of a spatial strategy. Spatial bias scores supported this idea as females, particularly wildtype females, had a strongly negative score, indicative of use of a stimulus-response strategy. This is in line with previous dual-solution water maze studies that show that females exhibit a bias toward use of a cue (Blokland, Rutten, & Prickaerts, 2006; Daniel & Lee, 2004; Hawley, Grissom, Barratt, Conrad, & Dohanich, 2012), and by a large body of research highlighting a male spatial advantage/bias (Blokland et al., 2006; Hawley et al., 2012; Perrot-Sinal, Kostenuik, Ossenkopp, & Kavaliers, 1996).
Although our water maze results suggested that heterozygotes were initially less efficient than wildtypes at finding the platform, all groups ultimately achieved the same performance. Therefore, we had no reason to believe that inherent activity, anxiety, motor or cognitive impairments were present in the munc 13-1 heterozygotes that would influence their behaviors in the presence of alcohol. Therefore we proceeded to assess ethanol consumption, using the DID paradigm, which encourages binge-like drinking during limited exposure to a relatively high concentration of ethanol (Thiele & Navarro, 2014). Our original hypothesis that munc 13-1 heterozygous mice would consume more ethanol than wildtypes was not supported. Mice underwent 6 cycles of DID, during which female mice consumed more than males, consistent with previous findings (Yoneyama et al., 2008) and C57BL mice consumed more than 129S1, also consistent with previous findings (Rhodes et al., 2007). We found no effect of genotype on ethanol consumption however, on any given cycle or across time, or in either strain of mouse.
In Drosophila, Dunc13 deficient flies are resistant to the sedative effects of ethanol (Xu, et al. 2018), so we tested whether munc 13-1 heterozygote mice would also be behaviorally resistant. To assess this, we tested heterozygote and wildtype mice on the rotarod task before and after ethanol injection. There was no difference in fall latency between heterozygotes and wildtype controls after a saline injection. Moreover, both groups improved performance across trials. Contrary to our hypothesis, genotype did not influence performance after either the 0.5 g/kg or 1.5 g/kg dose, as again, there was no significant difference in fall latency. Ethanol did impair performance on the rotarod (compared to saline injection) and this was more noticeable at the 1.5 g/kg dose versus the 0.5 g/kg dose. In addition, we found no effect of genotype on acute functional tolerance, as heterozygotes and wildtypes showed similar performance on trial 3, 60 minutes after ethanol injection. Finally, there was no effect of genotype on Majchrowicz score or BEC following ethanol injection.
The heterozygotes in our study had been experiencing an inherent reduction in munc 13-1 for their entire lives, and neuroadaptation may explain why we were unable to replicate in this mouse model what has previously been shown in flies. It is possible that the pool of readily releasable glutamate is reduced in munc 13-1 heterozygous mice, so pre-synaptic upregulation of potassium/calcium channels may have occurred that normalized the pattern of action potential firing. For instance, the opening of BK (big potassium) channels allow a neuron to fire more often, by speeding up the process by which a cell becomes repolarized after an action potential (Hermann, Sitdikova, & Weiger, 2015; Raffaelli, Saviane, Mohajerani, Pedarzani, & Cherubini, 2004). Increasing the number of these channels could be one method of compensation by which heterozygotes are able to maintain normal function. It is also possible that compensatory upregulation or increased activity of munc 13-2 occurred, or increases in synapses that utilize it. There is likely redundancy among the multiple isoforms of the munc protein (−1, −2, and −3), such that if one is depleted, another can compensate and rescue function in a subpopulation of neurons. It has been established that munc 13-1 and 13-2 are co-expressed in the rodent hippocampus, and the appearance of munc 13-3 is mostly concentrated in the caudal regions of the brain (Augustin, Betz, Herrmann, Jo, & Brose, 1999). For this reason, it seems most likely that munc 13-2, out of the available proteins of this family, would compensate when 13-1 is reduced.
Conclusions
Although reduction of Dunc13 in flies produces clear effects on ethanol consumption and tolerance, heterozygosity for the mammalian homolog, munc 13-1KO allele, does not. Overall, munc 13-1KO/+ heterozygotes are very similar to wildtype mice in the presence or absence of ethanol. This is may be due to compensatory adaptations to munc 13-1 reduction in the mammalian brain.
Highlights:
Munc 13-1 heterozygosity does not impair spatial navigation or increase anxiety-like behaviors
Munc 13-1 heterozygosity does not increase ethanol consumption in the drinking-in-the-dark paradigm
Munc 13-1 heterozygosity does not influence ethanol intoxication behaviors
Acknowledgements:
Thank you to Dr. Therese Kosten for the use of her open field and rotarod equipment.
Role of the funding source: Funding was provided by NIAAA R01 AA022414-01. The funding source had no role in study design, collection, analysis or interpretation of data or in the decision to submit the manuscript for publication.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, & Brose N (2006). Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J Biol Chem, 281(28), 19720–19731. doi: 10.1074/jbc.M601421200 [DOI] [PubMed] [Google Scholar]
- Augustin I, Betz A, Herrmann C, Jo T, & Brose N (1999). Differential expression of two novel Munc13 proteins in rat brain. Biochem J, 337 (Pt 3), 363–371. [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, & Brose N (1999). Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature, 400(6743), 457–461. doi: 10.1038/22768 [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, & Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl), 112(4), 503–510. [DOI] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, & Brose N (1997). Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem, 272(4), 2520–2526. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, Ruiz CI, Cullins MA, Da Costa A, Osterndorff-Kahanek EA, … Harris RA (2017). Mutation of the inhibitory ethanol site in GABAA rho1 receptors promotes tolerance to ethanol-induced motor incoordination. Neuropharmacology, 123, 201–209. doi: 10.1016/j.neuropharm.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A, Rutten K, & Prickaerts J (2006). Analysis of spatial orientation strategies of male and female Wistar rats in a Morris water escape task. Behav Brain Res, 171(2), 216–224. doi: 10.1016/j.bbr.2006.03.033 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, & Lee CD (2004). Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem, 82(2), 142–149. doi: 10.1016/j.nlm.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Das J, Xu S, Pany S, Guillory A, Shah V, & Roman GW (2013). The pre-synaptic Munc13-1 binds alcohol and modulates alcohol self-administration in Drosophila. J Neurochem, 126(6), 715–726. doi: 10.1111/jnc.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM (2013). Measuring motor coordination in mice. J Vis Exp(75), e2609. doi: 10.3791/2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, & Gordon AS (1997). Cellular and molecular neuroscience of alcoholism. Physiol Rev, 77(1), 1–20. doi: 10.1152/physrev.1997.77.1.1 [DOI] [PubMed] [Google Scholar]
- Harris RA (1999). Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res, 23(10), 1563–1570. [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Barratt HE, Conrad TS, & Dohanich GP (2012). The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav, 105(4), 1014–1020. doi: 10.1016/j.physbeh.2011.11.021 [DOI] [PubMed] [Google Scholar]
- Hermann A, Sitdikova GF, & Weiger TM (2015). Oxidative Stress and Maxi Calcium-Activated Potassium (BK) Channels. Biomolecules, 5(3), 1870–1911. doi: 10.3390/biom5031870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A, … Canales JJ (2008). The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem, 90(3), 553–559. doi: 10.1016/j.nlm.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Izidio GS, & Ramos A (2007). Positive association between ethanol consumption and anxiety-related behaviors in two selected rat lines. Alcohol, 41(7), 517–524. doi: 10.1016/j.alcohol.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Furedy JJ, Kulali B, McDonald R, & Pogun S (1998). Nicotine interacts with sex in affecting rat choice between "look-out" and "navigational" cognitive styles in the Morris water maze place learning task. Brain Res Bull, 46(5), 441–445. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgoren S, Furedy JJ, & Pogun S (2000). Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull, 52(4), 243–248. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, & Shapiro ML (2004). Retrieving memories via internal context requires the hippocampus. J Neurosci, 24(31), 6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, & West JR (2003). Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol, 29(3), 165–171. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, & Alvarez VA (2017). Alcohol and basal ganglia circuitry: Animal models. Neuropharmacology, 122, 46–55. doi: 10.1016/j.neuropharm.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, & Aston-Jones G (2011). Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science, 333(6040), 353–357. doi: 10.1126/science.1204622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E (1975). Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia, 43(3), 245–254. [DOI] [PubMed] [Google Scholar]
- Manikandan S (2011). Measures of central tendency: Median and mode. Journal of Pharmacology and Pharmacotherapeutics, 2(3), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquenie LA, Schade A, van Balkom AJ, Comijs HC, de Graaf R, Vollebergh W, … van den Brink W (2007). Origin of the comorbidity of anxiety disorders and alcohol dependence: findings of a general population study. Eur Addict Res, 13(1), 39–49. doi: 10.1159/000095814 [DOI] [PubMed] [Google Scholar]
- McDonald RJ, & White NM (1994). Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol, 61(3), 260–270. [DOI] [PubMed] [Google Scholar]
- McNamara RK, & Skelton RW (1993). The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev, 18(1), 33–49. [DOI] [PubMed] [Google Scholar]
- Packard MG, & McGaugh JL (1992). Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci, 106(3), 439–446. [DOI] [PubMed] [Google Scholar]
- Palfreyman MT, & Jorgensen EM (2017). Unc13 Aligns SNAREs and Superprimes Synaptic Vesicles. Neuron, 95(3), 473–475. doi: 10.1016/j.neuron.2017.07.017 [DOI] [PubMed] [Google Scholar]
- Pandey SC (2003). Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci, 24(9), 456–460. doi: 10.1016/S0165-6147(03)00226-8 [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, & Kavaliers M (1996). Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci, 110(6), 1309–1320. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, & Cherubini E (2004). BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol, 557(Pt 1), 147–157. doi: 10.1113/jphysiol.2004.062661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav, 84(1), 53–63. doi: 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr., & Crabbe JC (2007). Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav, 6(1), 1–18. doi: 10.1111/j.1601-183X.2006.00210.x [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Rettig J, & Brose N (2003). Molecular mechanisms of active zone function. Curr Opin Neurobiol, 13(5), 509–519. [DOI] [PubMed] [Google Scholar]
- Ross RS, & Slotnick SD (2008). The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci, 20(3), 432–446. doi: 10.1162/jocn.2008.20035 [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, & Crabbe JC (2003). Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A, 100(5), 2917–2922. doi: 10.1073/pnas.0437273100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, & Hesselbrock V (2004). Alcohol dependence and anxiety disorders: what is the relationship? Focus, 151(3), 1723–1453. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Cahill SP, & Frankland PW (2017). Running promotes spatial bias independently of adult neurogenesis. Hippocampus, 27(8), 871–882. doi: 10.1002/hipo.22737 [DOI] [PubMed] [Google Scholar]
- Südhof Thomas C. (2012). The Presynaptic Active Zone. Neuron, 75(1), 11–25. doi: 10.1016/j.neuron.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). "Drinking in the dark" (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48(3), 235–241. doi: 10.1016/j.alcohol.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Pany S, Benny K, Tarique K, Al-Hatem O, Gajewski K, … Roman G (2018). Ethanol Regulates Presynaptic Activity and Sedation through Presynaptic Unc13 Proteins in Drosophila. eNeuro, 5(3). doi: 10.1523/ENEURO.0125-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, & Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol, 42(3), 149–160. doi: 10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]