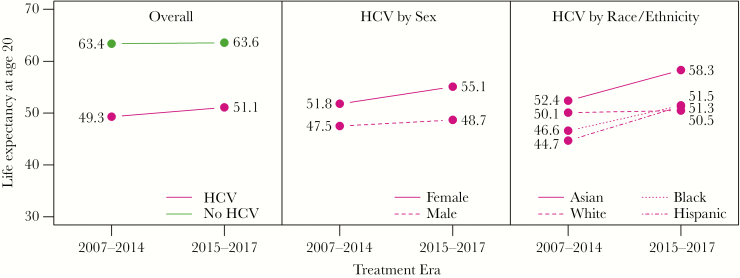Abstract
Among 25 291 and 4 921 830 people with and without hepatitis C, life expectancy at age 20 increased 1.8 years and 0.3 years from the interferon to interferon-free era, respectively. Increases were highest for racial and/or ethnic minority groups with hepatitis C.
Keywords: direct-acting antiviral agents, hepatitis C virus (HCV), mortality, survival, treatment
Hepatitis C virus (HCV) infection affects 4 million individuals in the United States, with HCV-associated deaths surpassing 60 other nationally notifiable infectious diseases combined during 2003–2013 [1]. Interferon-free treatment with direct-acting antiviral agents (DAAs) has substantially increased the number of people with HCV infection achieving sustained virologic response (SVR). Although a systematic review of randomized trials found no effect of DAAs on mortality [2], these findings were controversial, in part because the included trials did not have sufficient follow-up to detect effects on mortality [3]. In contrast, several observational studies with a longer duration of follow-up have suggested lower mortality among individuals with versus without SVR [4] and with versus without exposure to DAAs [5]. However, it remains unclear whether the emergence of interferon-free treatment has been associated with a population-level improvement in life expectancy for people with HCV.
METHODS
The study population included all adult (aged ≥20) members of Kaiser Permanente Northern California (KPNC) during 2007–2017. Kaiser Permanente Northern California is a large healthcare system providing comprehensive medical services to more than 30% of insured Californians [6]. Interferon-free treatment using DAAs became widely used at KPNC in 2015 [7]. Kaiser Permanente Northern California recommended prioritization of patients with advanced liver fibrosis for DAA initiation but did not place restrictions on use for patients in early stages of disease.
We defined HCV treatment eras as interferon (2007–2014) and interferon-free (2015–2017). Although some DAAs were used before 2015, they were typically prescribed in combination with interferon; beginning in 2015, almost all treatment regimens were interferon-free. For each era, we defined members as having HCV infection if they had a positive HCV ribonucleic acid (RNA) test or results indicating HCV genotype before that era, with no evidence of treatment at KPNC in the interim, or an initial positive HCV RNA test or results indicating HCV genotype during that era. Members were allowed to contribute person-time to both eras. We collected demographic and clinical data from the KPNC electronic health record, including pharmacy fill data for anti-HCV medications, and we additionally collected dates of death from California death certificates and Social Security Administration datasets.
We estimated life expectancy using previously described methods [8, 9]. In brief, we first estimated the number of deaths and person-years for people with and without HCV for each calendar year, with person-years based on mid-year membership. We then constructed abridged life tables from age-specific mortality rates using 5-year age intervals starting from age 20, with a final open interval for age ≥85 years. The life tables described the experience that hypothetical cohorts of people with and without HCV would have had if they were subject to the observed age-specific mortality rates from age 20 until death. We estimated the average number of years of life remaining for those surviving to age 20 by HCV status within each treatment era. For people with HCV, we also estimated life expectancy at age 20 by sex and race and/or ethnicity. We generated 95% confidence interval (CIs) using variance and standard error formulas provided by Chiang [8], with z tests to obtain P values. Analyses were conducted in SAS 9.4 (Cary, NC) and Microsoft Excel 2013. The institutional review board at KPNC approved this study with a waiver of written informed consent.
RESULTS
The study population included 25 291 people with HCV and 4 921 830 people without HCV during 2007–2017. Among those with HCV, mean age was 54.4 years, 38.1% were female, 53.6% were non-Hispanic white, 16.1% were Hispanic, 15.1% were non-Hispanic Black, 6.5% were Asian, and 8.7% were of other or unknown race/ethnicity. Among those without HCV, mean age was 42.1 years, 50.9% were female, 43.2% were non-Hispanic white, 17.7% were Hispanic, 6.2% were non-Hispanic Black, 16.6% were Asian, and 16.3% were of other or unknown race/ethnicity.
During 2007–2017, there were 3135 and 228 742 deaths among people with and without HCV, with mortality rates of 2355 and 817 per 100 000 person-years, respectively. Figure 1 shows life expectancy at age 20 (1) for people with and without HCV by treatment era and (2) for people with HCV by sex and race/ethnicity. For people without HCV, there was a slight increase in life expectancy at age 20 from the interferon era to the interferon-free era, from 63.4 to 63.6 years (change +0.3 years; 95% CI, 0.2 to 0.3; P < .001). For people with HCV overall, life expectancy at age 20 increased 1.8 years from the interferon era to the interferon-free era, from 49.3 and 51.1 years, but this increase was not statistically significant (change +1.8 years; 95% CI, −1.0 to 4.7; P = .20). In the interferon and interferon-free eras, the gap in life expectancy between people with and without HCV was 14.1 and 12.5 years, respectively.
Figure 1.
Life expectancy at age 20 for people with and without hepatitis C virus (HCV) by treatment era and for people with HCV by sex and race/ethnicity.
Changes in life expectancy varied across demographic subgroups among people with HCV. Compared with the interferon era, life expectancy at age 20 in the interferon-free era increased 3.3 years (95% CI, −0.6 to 7.3; P = .10) for females and 1.2 years (95% CI, 3.0 to 5.4; P = .58) for males. Among racial/ethnic subgroups of people with HCV, life expectancy at age 20 increased 6.6 years (95% CI, 2.3 to 10.9; P = .003) for Hispanic individuals, 5.9 years (95% CI, 2.7 to 14.4; P = .18) for Asian individuals, 4.8 years (95% CI, 3.3 to 13.1; P = .25) for non-Hispanic black individuals, and 0.4 years (95% CI, −3.8 to 4.7; P = .84) for non-Hispanic white individuals from the interferon era to the interferon-free era.
Discussion
In this large insured population, life expectancy at age 20 increased 1.8 years for people with HCV between the interferon and interferon-free treatment eras, compared with an increase of only 0.3 years among people without HCV, although the increase for people with HCV was not statistically significant. Given that 45% of people with HCV in the interferon-free era had not yet been treated, people with HCV may experience a greater population-level improvement in life expectancy with broader use of DAAs. Life expectancy for people with HCV may also increase with implementation of the US Preventive Services Task Force grade B recommendation for universal HCV screening among adults aged 18 to 79 years [10], which will facilitate diagnosis, treatment, and achievement of SVR.
Among people with HCV, we observed larger gains in life expectancy for females compared with males and for racial/ethnic minority groups compared with non-Hispanic whites, with Hispanics experiencing a gain in life expectancy of approximately 7 years between treatment eras compared with a gain of less than half a year among non-Hispanic whites. Although racial/ethnic disparities in HCV treatment initiation persist in the interferon-free era [7], our findings may indicate a narrowing of these disparities compared with the interferon era. The emergence of DAAs has also mitigated the poor treatment outcomes that were observed for black individuals in the interferon era [11]. Finally, lack of improvement in life expectancy for white individuals with HCV may reflect the impact of the opioid epidemic, which is concentrated in this population [12].
Our study had several limitations. First, despite our large sample size, estimates among people with HCV had wide CIs. Second, people who spontaneously cleared HCV or were treated outside of KPNC may have been misclassified as having HCV, whereas those who had HCV but were untested may have been misclassified as not having HCV. However, misclassification was likely nondifferential by treatment era. Third, our study was ecological and therefore not designed to make inferences about the causal effect of DAAs or SVR on mortality. Strengths of our study included its long duration of follow-up and internal comparison group of people without HCV who had equal access to care. Moreover, because the KPNC membership mirrors the age, sex, and race/ethnicity distributions of the surrounding population [6], our results are likely to be generalizable to the broader insured population.
Conclusions
In summary, we observed an increase in life expectancy of 1.8 years for people with HCV from the interferon era to the interferon-free era. Although this increase was not statistically significant, gains were greater in racial/ethnic minority subgroups, with Hispanics experiencing a gain in life expectancy of approximately 7 years between treatment eras compared with a gain of less than half a year for non-Hispanic whites. Our results suggest that life expectancy may improve at a population level for people with HCV with implementation of universal HCV screening among adults, which will facilitate increases in HCV diagnosis, use of DAAs, and achievement of SVR.
Acknowledgments
Financial support. This work was funded by the Kaiser Permanente Delivery Science Research Program and the National Institute of Allergy and Infectious Diseases (K01 AI122853; to J. L. M.).
Potential conflicts of interest. J. L. M. has consulted for Kaiser Permanente Northern California on a research grant from Gilead Sciences outside the submitted work. M. J. S. and C. P. Q. report research grant support from Merck outside the submitted work. All other authors report no potential conflicts.
References
- 1. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jakobsen JC, Nielsen EE, Feinberg J, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev 2017; 9:CD012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powderly WG, Naggie S, Kim AY, et al. IDSA/AASLD response to cochrane review on direct-acting antivirals for hepatitis C. Clin Infect Dis 2017; 65:1773–5. [DOI] [PubMed] [Google Scholar]
- 4. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology 2018; 68:827–38. [DOI] [PubMed] [Google Scholar]
- 5. McGlynn EA, Adams JL, Kramer J, et al. Assessing the safety of direct-acting antiviral agents for hepatitis C. JAMA Netw Open 2019; 2:e194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey Available at: https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf. Accessed 17 January 2019
- 7. Marcus JL, Hurley LB, Chamberland S, et al. Disparities in initiation of direct-acting antiviral agents for hepatitis C virus infection in an insured population. Public Health Rep 2018; 133:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiang CL. The Life Table and Its Construction. Introduction to Stochastic Processes in Biostatistics. New York: John Wiley and Sons; 1983: pp 189–214. [Google Scholar]
- 9. Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep 2017; 66:1–64. [PubMed] [Google Scholar]
- 10. U.S. Preventive Services Task Force. Draft Recommendation Statement. Hepatitis C Virus Infection in Adolescents and Adults: Screening Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/hepatitis-c-screening1. Accessed 23 January 2020.
- 11. Struble K, Chan-Tack K, Qi K, et al. Sustained virological response rates with direct-acting antivirals in black subjects with HCV genotype 1 infection: systematic analysis of clinical trials. J Virus Erad 2019; 5:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powell D, Alpert A, Pacula RL. A transitioning epidemic: how the opioid crisis is driving the rise in hepatitis C. Health Aff (Millwood) 2019; 38:287–94. [DOI] [PubMed] [Google Scholar]