Abstract
Objective(s):
One of the common heterogeneous reproductive disorders in women of childbearing age is polycystic ovary syndrome (PCOS). It is characterized by lack of fertility due to anovulatory cycles, hyperandrogenemia, polycystic ovaries, hyperinsulinemia, and obesity. Both reproductive anomalies and metabolic disorders are involved in PCOS pathology. Although the role of increased levels of androgens in initiation of PCOS is almost proven, mechanisms of PCOS pathophysiology are not clear. Here we discuss roles of altered metabolic conditions, obesity, and chronic inflammation in PCOS pathophysiology.
Materials and Methods:
In this review, we attempted to identify genes related to obesity and chronic inflammation aspects of PCOS and their physiological functions to explain the pathways that are regulated by these genes and can be a prominent function in PCOS predisposition. For this purpose, published articles and reviews dealing with genetic evaluation of PCOS in women in peer-reviewed journals in PubMed and Google Scholar databases were included in this review.
Results:
Obesity and chronic inflammation are not prominent diagnostic features of PCOS, but they play an important role in exacerbating metabolic and hyperandrogenic states. ADIPOQ, FTO TGFβ, and DENND1A as the main obesity- and chronic inflammation-related genes have roles in PCOS pathophysiology.
Conclusion:
It seems that genes related to obesity pathology in genomic research association, are related to metabolic aspects and body mass index in PCOS patients. Genomes have roles in chronic inflammation, followed by obesity, in the pathogenesis of PCOS.
Key Words: Chronic inflammation, Gene, Obesity, Pathophysiology, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS), affects 5–15% of reproductive-aged women in the world. It is one of the most prevalent endocrine abnormalities that are characterized by biochemical hyperandrogenemia, chronic anovulation, and polycystic ovaries (1). Besides, PCOS relates to other symptoms among which insulin resistance, abdominal obesity, chronic inflammation, elevated risk of metabolic syndrome, type 2 diabetes, and cardiovascular diseases are more pronounced (1). The genetic basis of PCOS by twin-family based association studies was confirmed, and accordingly, heritability of PCOS is estimated 70% (2). The genome-wide association studies (GWAS) identified 15 susceptible single nucleotide polymorphisms (SNP) in 11 loci (LHCGR, FSHR, THADA, INSR, DENND1A, RAB5B, C9orf3, YAP1, SUMO1P1, TOX3, and HMGA2) in PCOS Chinese women (3, 4). These loci (LHCGR, FSHR, INSR, THADA, DENND1A, and YAP1) are likely more important because they were replicated in European population, too (5-8). Undoubtedly, effects of environmental factors in interacting with genetic agents in creating important features, such as hyperandrogenism and insulin resistance of PCOS cannot be ignored (9).
The lifestyle interacts with genetics in the incidence of weight excess and obesity. For instance, sisters with irregular menses and hyperandrogenism were overweight in contrast with ones who have regular cycles and hyperandrogenism. Abdominal adiposity, excess weight, and obesity are usually common among PCOS patients. Obesity plays an underscored role in pathophysiology of insulin resistance and hyperandrogenism (10). For this reason, diet modification and exercise play an important role in improving PCOS reproductive and metabolic phenotypes (11, 12).
PCOS is also related to elevation of inflammatory indices such as increased levels of c- reactive protein, interleukins, and white blood cell count as well as oxidative stress and endothelial dysfunction, which are components of low-grade chronic inflammation (13). Adipose tissue products can play a role in inflammation creation and its exacerbation. Interleukin 6 (IL-6) secreted from adipocytes, stimulates secretion of hepatic C-reactive protein (CRP) and both of them elevate in PCOS and obese patients (14). Abdominal obesity is associated with dysregulation of sex steroid levels in PCOS, such as androgen overproduction and reduction of sex hormone-binding globulin (SHBG). The problem is that obesity has a confounding effect on the exacerbating of PCOS main traits, hyperandrogenism, and insulin resistance (15).
Obesity influences the development or escalation of PCOS. Mother’s obesity in late pregnancy predisposes her daughter to PCOS in adulthood, but effective pathophysiologic mechanisms are not clear (16). Also, the intrauterine androgen excess may create adiposity in offspring at adulthood. Abdominal obesity can be important in ovarian or adrenal hyperandrogenism in PCOS, although, the elevated levels of androgens can contribute to abdominal fat deposition (10). Apart from the key role of hyperandrogenism in PCOS pathophysiology, obesity, inflammation, and other metabolic factors have the main role in aggravating steroidogenic abnormalities. Furthermore, genetic predisposition underlies both primary steroidogenic disorders and other exacerbating factors (10). Obesity is related to low-grade chronic inflammation, which contributes to insulin resistance by adipocytokines functions such as tumor necrosis factor alpha (TNFα) and adiponectin (17, 18).
In this review, we attempted to identify genes related to obesity and chronic inflammation aspects of PCOS and their physiological functions to explain the pathways that are regulated by these genes and can be a prominent function in PCOS predisposition. Initially, we searched the major databases such as PubMed and Google Scholar based on gene, PCOS, obesity, inflammation, etiology, patient, and human keywords which were taken from the MeSH site. These genes are divided into two groups that are effective in obesity and chronic inflammation, respectively. Then, they are separated from each gene, their actions are described, and thus the involved physiologic pathways identified. Eventually, hypotheses associated with these findings are presented.
Even though the role of increased levels of androgens in the initiation of PCOS is almost documented and approved by most authors, mechanisms of PCOS pathophysiology are not clear. In fact, hyperandrogenism is the common loop of different hypotheses presented on PCOS etiology. These issues are explained in detail in a previous paper about the role of steroid and gonadotropin related genes in PCOS pathophysiology (19). But roles of altered metabolic conditions are prominent.
Obesity-related genes in etiology of polycystic ovary syndrome
Since the genetic nature of obesity is well known (20), environmental factors such as lifestyle, nutrition, and exercise also contribute to obesity. Obesity leads to insulin resistance, followed by other events that ultimately result in the occurrence of PCOS. Obesity can result from the reduced lipolytic effect of insulin in PCOS, in turn, by increased serum inflammatory mediators such as TNFα and high-sensitivity CRP (hs-CRP), leads to beta cell dysfunction and insulin resistance of PCOS. Obesity can intensify the hyperandrogenic state in PCOS because abdominal obesity alters fat-soluble androgen clearance and deposition and also exacerbates hyperandrogenism by reduction of SHBG levels.
Obesity is associated with PCOS, and between 38–88% of PCOS patients are overweight or obese (21). Obesity is accompanied by other PCOS metabolic attributes, such as insulin resistance (22). Obesity is related to insulin resistance and compensatory hyperinsulinemia (23). Central adiposity and hyperandrogenemia by reduction of natural insulin sensitizer adipokines such as adiponectin lead to the development of insulin resistance in PCOS (24). Also, both obesity and insulin resistance elevate the risk of cardiovascular and type 2 diabetes mellitus diseases (25), which are considered metabolic features of PCOS. Body mass index (BMI) of women with PCOS is usually higher than normal women (26). Several growth factors and inflammatory factors were increased in obesity and could promote ovarian androgen overproduction (23). Thus, obesity can develop insulin resistance, androgen excess, and inflammation in PCOS women.
Dyslipidemia is prevalent in almost 70% of women with PCOS (27). In addition to obesity, dyslipidemia causes insulin resistance; in contrast, insulin resistance also affects lipid metabolism and serum lipid parameters, all of which are characteristics of PCOS (28). Dyslipidemia and metabolic syndrome have a pivotal role in PCOS development but not obesity and insulin resistance (25).
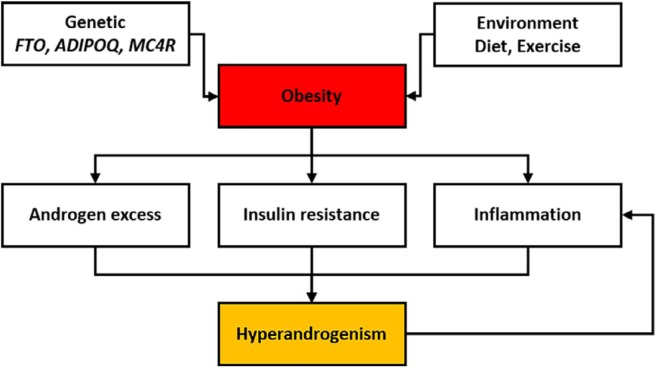
It seems that factors of genetic and lifestyle, both or alone lead to obesity in humans. Moreover, obesity has adverse effects on the physiological pathways in the body, which eventually result in PCOS metabolic parameters (Figure 1). Obesity is related to anovulation, loss of pregnancy, and occurrence of pregnancy complications (pre-eclampsia and gestational diabetes); as well as, delayed or failed responses to therapeutic strategies such as clomiphene citrate and gonadotropins. A five percent weight loss in women increased the rate of normal and spontaneous ovulation and pregnancy (25). Thus it seems the alteration of environmental factors such as exercise and diet is useful in the treatment of PCOS. But the effects of obesity-related genetic variants cannot be forgotten. Below, a number of these genes are described and are summarized in Table 1.
Figure 1.
Causes of obesity and its adverse effects are expressed. Obesity mainly can occur due to genetic background and inappropriate lifestyle. Effect of abdominal obesity in exacerbating complications of PCOS is more than peripheral obesity. Abdominal obesity by alteration of metabolic clearance of androgens, increased levels of inflammatory mediators, and elevated beta cells mass in the pancreas is involved in hyperandrogenism, insulin resistance, and inflammation, respectively. The relationship between inflammation and hyperandrogenism is reciprocal, and androgens can create inflammation state
Table1.
Candidate genes involved in etiology of polycystic ovary syndrome related to obesity and dyslipidemia
| Gene | Genetic marker(s) | Type of study | Physiologic function | Studied population | Type of polymorphism | References |
|---|---|---|---|---|---|---|
| ADIPOQ | T45G G276T |
Meta-analysis | Glucose regulation and fatty acid oxidation | Different population | G276T | (29) |
| ADIPOQ | rs2241766 rs1501299 |
Family-based analysis | Glucose regulation and fatty acid oxidation | Chinese Han | rs1501299 | (30) |
| ADIPOQ | SNPs at position −11377 of the ADIPOQ gene | Case-control | Glucose regulation and fatty acid oxidation | Japanese | ND | (31) |
| ADIPOQ | 45T/G 276G/T |
Meta-analysis | Glucose regulation and fatty acid oxidation | Different population | 45T/G 276G/T |
(32) |
| ADIPOQ | C45G15G(T/G) C276(G/T) |
Case-control | Glucose regulation and fatty acid oxidation | Chinese Han | +45G15G(T/G) +276(G/T) |
(33) |
| ADIPOQ | +45G15G(T/G) +276(G/T) |
Case-control | Glucose regulation and fatty acid oxidation | Korean | +276(G/T) | (34) |
| FTO | rs9939609 | Case-control | Energy homeostasis regulation | British/Irish | rs9939609 | (35) |
| FTO | Five SNPs | Family-based and case-control | Energy homeostasis regulation | White American | rs1421085 | (36) |
| FTO | rs9939609 | Case-control | Energy homeostasis regulation | Australian | rs9939609 | (37) |
| FTO | rs1421085 rs17817449 rs8050136 |
Case-control | Energy homeostasis regulation | Korean | rs1421085 rs17817449 rs8050136 |
(38) |
| MC4R | rs17782313 (T/C) | Case-control | Regulator of melanocortin neuronal pathways | Chinese | rs17782313 | (39) |
| MC4R | rs12970134 | Case-control | Regulator of melanocortin neuronal pathways | Czech | rs12970134 | (40) |
| ADIPOQ | rs2241766 | Case-control | Metabolic features of PCOS | Iranian | rs2241766 “TT” | (41) |
| SREBP-2 LXRa | rs2228314 rs11039155 |
Case-control | Lipid metabolism | Chinese Han | rs2228314 G to C rs11039155 G to A |
(28) |
ADIPOQ: Adiponectin; FTO: Fat mass, and obesity-associated; MC4R: Melanocortin 4 receptor; SREBP-2: Sterol Regulatory Element Binding Protein-2; LXRa: Liver X Receptor a; ND: No data
ADIPOQ
It is well known that insulin resistance is a prominent risk factor for obesity. The adipocyte cells are one of the target cells of insulin and secrete several adipokines such as adiponectin (34). About six polymorphisms of the adiponectin gene have been detected in various racial populations and with different environmental factors associated with metabolic abnormalities of PCOS. Among them, SNPs +45(T/G) and +276(G/T) are highly associated with obesity, insulin resistance, and type 2 diabetes mellitus in Asian populations (34). Also, in a family-based study of the Chinese Han population, two SNPs of the ADIPOQ gene are associated with PCOS (30). Generally, adiponectin is related to metabolic syndrome (insulin resistance, obesity, and dyslipidemia) in PCOS (42), which reflects the role of obesity in the mediation of adiponectin in PCOS. Because the genetic variants of the ADIPOQ gene in different racial populations were associated with PCOS (Table 1), this gene may be a risk factor for obesity and insulin resistance of PCOS.
FTO
Fat mass and obesity-associated (FTO) gene is a large gene that encodes 2-oxoglutarate-dependent nucleic acid demethylase and is mainly expressed in hypothalamic nuclei regulating feed intake. FTO has also been shown in other tissues such as liver, pancreas, muscles, and adipose tissue (43). Generally, the FTO gene is associated with type 2 diabetes mellitus and obesity (38). In studies evaluating the association of FTO and PCOS, FTO was mainly associated with BMI and anthropometric parameters in women of various races with PCOS (37-39, 44) (Table 1). Increasing evidence suggests the association of variants of FTO with hyperandrogenemia in PCOS patients (37, 38). The relationship between FTO variants and impaired glucose tolerance, insulin resistance, and hyperandrogenism, that are prominent features of PCOS, are mediated via obesity and BMI (38). So, FTO can be a main genetic factor in predisposing to PCOS, primarily via an effective role in obesity and BMI, and secondarily with influencing the metabolic parameters and hyperandrogenemia.
MC4R
Melanocortin-4 receptor (MC4R) gene encodes the G-protein coupled receptor that is dominantly expressed in the brain and mediates the signaling pathway of melanocortin (45). MC4R has an important role in energy homeostasis and appetite and is the main genetic cause of obesity in humans (45, 46) and animals (47-49). Due to the critical role of MC4R in obesity pathology, the association between different SNPs of the MC4R gene and BMI and obesity in PCOS patients were demonstrated (36, 39, 40). According to the evidence, the MC4R gene via a causal effect on obesity contributes to PCOS etiology. The same evidence has been shown in PCOS animal models, too (50).
SREBP-2 and LXR
Given that insulin resistance disrupts lipid metabolism and serum lipid parameters, central transcription factors in metabolic pathways appear to be likely candidates for PCOS abnormalities (28). The central transcription factors of lipid metabolism, are liver X receptor (NR1H3, LXRa), and sterol regulatory element binding protein-2 (SREBP-2) (51-53). These two transcription factors regulate the expression of effective genes on lipoprotein metabolism, cholesterol homeostasis, and lipogenesis. LXRα transcription factor also plays a key action in insulin secretion of pancreatic beta cells (53). The SREBP-2, which is encoded by a single gene on human chromosome 22, also plays an important role in maintaining lipid homeostasis (54). In a case-control study on Chinese Han women, variants of these two transcription factors were associated with PCOS (28). Although more research is needed to prove this association.
Chronic inflammation-related genes in etiology of polycystic ovary syndrome
Inflammation can be the key marker of endothelial dysfunction, atherosclerosis and cardiovascular diseases, and also metabolic disruptions of PCOS. It seems inflammatory reactions are more often secondary pathways in PCOS etiology and are affected by obesity and hyperglycemia. Glucose-induced nuclear factor-κB (NF-κB) activation from mononuclear cells eventually leads to beta cell dysfunction and insulin secretion irregularities in PCOS (55). Adipokines derived adipocytes contribute to inflammation and insulin resistance development. For instance, decreased adiponectin and increased TNFα and IL-6 are contributed to insulin resistance development.
PCOS is an inflammatory state, and chronic inflammatory-related genes may be effective in the incidence of PCOS through mediating role in hyperandrogenism, obesity, insulin resistance, and anovulation (56). Chronic inflammation is involved in the development of PCOS. Dietary glucose-induced oxidative stress by the particular molecular signaling pathway leads to increased production of pro-inflammatory cytokines from mononuclear cells (MNC).
Hyperandrogenism may be the progenitor of low-grade chronic inflammation in PCOS and via increase of MNC sensitivity, stimulates glucose-induced inflammation (57). On the other hand, the pro-inflammatory cytokines, TNFα, can elevate the production of androgens with upregulation of steroidogenic enzymes and stimulation of proliferation of theca cells. Also, TNFα is a mediator of insulin resistance, thus it is likely dietary-induced inflammation, is the base of insulin resistance in PCOS (57). Exposure of androgen excess prone the adipocytes to hypertrophy and hypertrophic adipocytes were observed in PCOS women. Hypertrophic adipocytes were more predisposed to inflammation (58). Adipose tissue adipocytes in an autocrine/paracrine manner by secretion of products, some of which are inflammation factors, contributed to low-grade inflammation related to PCOS (13). The low-grade chronic inflammation in PCOS may be related to hyperandrogenism and hypertrophic adipocyte (58).
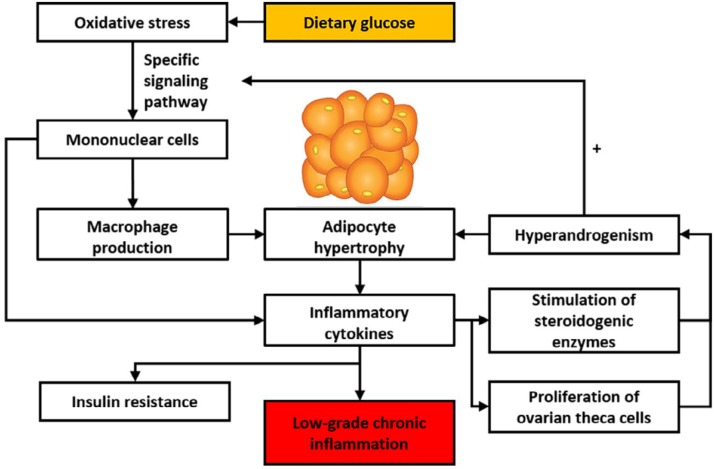
The correlation between the incidence of PCOS and chronic inflammation has increased attention to the inflammatory factors coding genes and their association with PCOS (59). The imbalance between pro-inflammatory and anti-inflammatory cytokines and cytokine genes polymorphisms may be involved in etiology of PCOS (60). Therefore, inflammatory reactions act as mediators and contribute to the development and aggravation of metabolic properties of PCOS (Figure 2). The genes related to chronic inflammation are presented in Table 2.
Figure 2.
Pathophysiological pathways contribute to low-grade inflammation in polycystic ovary syndrome (PCOS). Glucose-induced oxidative stress by production of inflammatory cytokines leads to insulin resistance. Cytokines via effects on theca cells eventually create hyperandrogenism. Macrophage derived mononuclear cells resulted in adipocytes hypertrophy and in turn by secretion of inflammatory cytokine create low-grade chronic inflammation
Table 2.
Candidate genes involved in etiology of polycystic ovary syndrome related to cell proliferation and signaling and chronic inflammation
| Gene | Genetic marker(s) | Type of study | Physiologic function | Studied population | Type of polymorphism | Reference |
|---|---|---|---|---|---|---|
| DENND1A | rs2479106 | Case control | Clathrin-mediated endocytosis | Caucasian | rs2479106 G | (61) |
| DENND1A | rs10818854 rs2479106 rs10986105 |
Meta-analysis | Clathrin-mediated endocytosis | Asian and European | rs10818854 rs10986105 |
(62) |
| DENND1A | rs2479106 rs10818854 |
Meta-analysis | Clathrin-mediated endocytosis | Chinese Han | rs10818854 | (63) |
| DENND1A | rs10818854 rs2479106 rs10986105 |
Retrospective case-control study. | Clathrin-mediated endocytosis | Tunisian | 0818854 rs10986105 |
(64) |
| AQP8 | rs7198838 rs1076973 rs1076974 rs2287797 rs2287798 rs2287796 |
case control | Water channel protein | Chinese Han | rs2287798 | (65) |
| YAP1 | rs11225138 rs11225161 rs1122516 |
Replication study | Transcriptional regulator | Chinese Han | rs11225161 (A/G) | (66) |
| TNF | rs1799964 rs1799724 |
Family association | Pro-inflammatory cytokine | Chinese Han | rs1799964 | (67) |
| TGF-β1 | rs4803457C/T rs11466313 deletion/AGG rs2217130C/T rs1800469C/T rs1800470C/T |
GWAS | Low-grade chronic inflammation | Chinese Han | rs4803457C/T | (68) |
|
TNFα
IL-6 IL-10 |
−308 G/A −174 G/C −1082 G/A |
case-control | Inflammatory cytokines | Turkish | IL-6 promoter region polymorphism | (60) |
| TNFRSF1B | M196R (676 T→G) variant in exon 6 | GWAS | TNF signaling | Spanish Italian |
M196R (676 T→G) | (69) |
| IL-6 | -174 G/C | Case- control | Inflammatory cytokine | Indian | −174 G/C SNP | (70) |
|
TNFα
IL-6 |
(−308 G/A), (−174 G/C) |
meta-analysis | Chronic low-grade inflammation | Different populations | No association | (71) |
DENND1A: DENN domain containing 1A; AQP8: Aquaporin 8; YAP1: yeast associated protein 1; TNF: Tumor necrosis factor; TGF-β1: transforming growth factor β1; TNFRSF1B: TNF receptor 2
TGF-β1
The beta-transforming growth factor-β (TGF-β1) is a component of multifunctional cytokines family and mediates wound healing, tissue fibrosis, and embryonic development (68). In recent years, pathogenic immunity factors have been widely considered in PCOS, which shows PCOS is a low-grade chronic inflammatory condition (68). PCOS patients showed higher levels of lymphocytes, monocytes, and eosinophils, plus CRPs, TNFα, and IL-6 in serum, all of which are peripheral inflammatory factors (72, 73). Moreover, PCOS ovaries showed chronic inflammation and higher numbers of inflammatory cells compared to normal conditions. Cytokines seem to be important for folliculogenesis and ovulation and also participate in the process of follicular atresia and corpus luteum regression by activating immune cells (68, 74). Polymorphism of rs4803457C/T in TGFβ gene was associated with susceptibility to PCOS and was demonstrated as the main constituent of PCOS development in Chinese women (68).
TNFα
It is reported that the TNFα cytokine is secreted by adipose tissue and plays an essential role in mediating insulin resistance and as a result of obesity (75). The TNFα pro-inflammatory cytokine is known as a mediator of insulin resistance in PCOS (57). Androgen excess promotes the release of TNFα from MNCs in vivo and in vitro (76). TNFα plays a cardinal role in oxidative stress and inflammation; in PCOS, production of TNFα is induced by hyperglycemia and hyperandrogenemia (77). Serine phosphorylation of Insulin receptor substrate 1 (IRS-1) seems to be a mechanism for insulin resistance by mediating TNFα (78). Overexpression of TNFα in peripheral tissues such as muscle and adipose, by reducing the tyrosine kinase activity in the insulin signaling pathway, is one of the mechanisms for insulin resistance development mediated by TNFα (78). Also, TNFα directly effects ovary and adrenal function (10) and then increases steroidogenesis in theca cells in vitro (79). Despite the finding that TNFα is the mediator of insulin resistance, a significant association of TNFα gene with PCOS was not observed.
TNFRS1β
The TNF receptor superfamily 1β is a member of TNFα receptors that are said to localize on the surface of all normal and tumor cells (80). Type 2 TNF receptor mediates most of the metabolic effects of TNFα. Its serum levels increase in obese subjects correlating with insulin resistance indices (81). The M196R variant in exon 6 of the TNF receptor superfamily 1β (TNFRS1β) gene was associated with hyperandrogenism and PCOS in the study of hyperandrogenic and PCOS Spanish women (82). It has also been suggested that TNFα cytokines were able to induce proliferation of theca internal cells in porcine ovaries (80).
IL-6
IL-6 is a pleiotropic cytokine and is secreted by numerous cells including lymphocytes, monocytes, and endothelial cells. Interleukin-6 is involved in reproductive physiologic processes, such as regulating of ovarian steroid production, follicular maturation, fertilization, implantation, and modulating of ovarian development and functions (70). Concentrations of the IL-6 and CRP increase in obese women, but not in PCOS patients (14). It is shown that the polymorphism of the promoter region of the IL-6 gene could be associated with the occurrence of metabolic abnormalities in Turkish PCOS women (60). Also, IL-6 may have a direct effect on ovaries and adrenal cells (10). In vitro studies have shown that IL-6 induced the function of human adrenal cells, in turn, elevated steroidogenesis in adrenals (83). The results of an investigation of IL-6 gene polymorphisms are very inconsistent (Table 2), which is possibly related to racial background, genetic variants, and epigenetic environmental factors among different populations (70).
DENND1A
Differentially expressed in normal and neoplastic (cell) domain containing 1A (DENND1A) is a member of connecdenn proteins family. These proteins have differential expression in normal and neoplastic domains of cells (84). The DENND1A gene encodes the connecdenn-1 protein, which has a clathrin-binding domain and facilitates receptor-mediated endocytosis and participates in endosomes trafficking (84, 85). Overexpression of variant 2 DENND1A, alternative splicing of DENND1A, in PCOS theca cells was observed, which can contribute to androgen overproduction in these cells (86). High expression of variant 2 DENND1A leads to over mRNA expression of cytochrome P450 17A1 (CYP17A1) and hyperactivity of CYP11A1 and CYP17A1, which in turn increases the level of androgen production in theca cells (86). So, DENND1A can be a mediator for hyperandrogenism of PCOS, and maybe through this pathway connect to hyperandrogenism, the common loop of all hypotheses of PCOS. Previous studies reported DENND1A as a susceptibility locus in PCOS, but there are racial differences about this association (64). Even though the DENND1A locus in various GWAS among different populations is identified as a risk locus in PCOS and maybe a valuable gene in PCOS pathogenesis and diagnosis, it is not applicable to all populations.
Conclusion
To sum up, identifying the root cause of PCOS as a heterogeneous disorder is hard and at the same time, it provides the basis of many investigations. So far, the role of hyperandrogenism and its pre- and post-pathways in PCOS development is better explained and confirmed; various backgrounds including genetic, environmental factors, and developmental origins can interfere in its creation. Hyperandrogenism can also be caused by insulin resistance. While the roles of hyperandrogenism and hyperinsulinemia are the major reasons for PCOS development, but abdominal obesity and low-grade inflammation can play a significant role through the mediation of some pathways leading to insulin resistance and hyperandrogenism. As foregoing, chronic inflammation and obesity are not necessarily primary factors, and sometimes their role exacerbates the syndrome or even themselves as a consequence of the syndrome. It seems that genes related to obesity pathology (FTO, ADIPOQ, and MC4R), in genomic research association, are related to metabolic aspects and BMI in PCOS patients. These findings suggest that obesity, especially abdominal phenotype, completes and exacerbates the phenotypic picture of PCOS. Inflammation also occurs, followed by obesity and has the same role in PCOS. It should be kept in mind that these hypotheses are better evaluated by clinical and experimental research such as animal models and therapeutic approaches.
Acknowledgment
The results presented in this paper were part of a student thesis. This review paper was financially supported by the Department of Animal Science, College of Agriculture, Shiraz University, Shiraz, Iran.
References
- 1.Azziz R. PCOS in 2015: New insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol. 2016;12:74. doi: 10.1038/nrendo.2015.230. [DOI] [PubMed] [Google Scholar]
- 2.Vink J, Sadrzadeh S, Lambalk C, Boomsma D. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z-J, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16 2p21 and 9q33 3. Nat Genet. 2011;43 doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 5.Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen Y-DI, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49:90–95. doi: 10.1136/jmedgenet-2011-100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welt CK, Styrkarsdottir U, Ehrmann DA, Thorleifsson G, Arason G, Gudmundsson JA, et al. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab. 2012;97:E1342–E1347. doi: 10.1210/jc.2011-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutharasan P, Galdones E, Peñalver Bernabé B, Garcia OA, Jafari N, Shea LD, et al. Evidence for chromosome 2p16 3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. 2013;98:E185–E190. doi: 10.1210/jc.2012-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brower MA, Jones MR, Rotter JI, Krauss RM, Legro RS, Azziz R, et al. Further investigation in europeans of susceptibility variants for polycystic ovary syndrome discovered in genome-wide association studies of Chinese individuals. J Clin Endocrinol Metab. 2015;100:E182–E186. doi: 10.1210/jc.2014-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631–643. doi: 10.1093/humupd/dmi025. [DOI] [PubMed] [Google Scholar]
- 10.Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18:266–272. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Huber-Buchholz M-M, Carey D, Norman R. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 12.van Dam EW, Roelfsema F, Veldhuis JD, Hogendoorn S, Westenberg J, Helmerhorst FM, et al. Retention of estradiol negative feedback relationship to LH predicts ovulation in response to caloric restriction and weight loss in obese patients with polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2004;286:E615–E620. doi: 10.1152/ajpendo.00377.2003. [DOI] [PubMed] [Google Scholar]
- 13.Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97:7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escobar-Morreale H, Villuendas G, Botella-Carretero J, Sancho J, San Millan J. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46:625–633. doi: 10.1007/s00125-003-1090-z. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali R, Gambineri A. Androgen Excess Disorders in Women. Springer; 2006. The Endocrine Impact of Obesity and Body Habitus in the Polycystic Ovary Syndrome; pp. 283–291. [Google Scholar]
- 16.Cresswell J, Barker D, Osmond C, Egger P, Phillips D, Fraser R. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997;350:1131–1135. doi: 10.1016/s0140-6736(97)06062-5. [DOI] [PubMed] [Google Scholar]
- 17.Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res. 2009;50:S395–S399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaaban Z, Khoradmehr A, Jafarzadeh Shirazi MR, Tamadon A. Pathophysiological mechanisms of gonadotropins–and steroid hormones–related genes in etiology of polycystic ovary syndrome. Iran J Basic Med Sci. 2019;22:3–16. doi: 10.22038/ijbms.2018.31776.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YS. Consequences of childhood obesity. Ann Acad Med Singapore. 2009;38:75–77. [PubMed] [Google Scholar]
- 21.Barber TM, Franks S. Polycystic Ovary Syndrome. Karger Publishers; 2013. Genetics of polycystic ovary syndrome; pp. 28–39. [DOI] [PubMed] [Google Scholar]
- 22.Fenichel P, Rougier C, Hieronimus S, Chevalier N, editors Which origin for polycystic ovaries syndrome: genetic, environmental or both? Ann Endocrinol. 2017:Elsevier. doi: 10.1016/j.ando.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. NIH Public Access; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luque-Ramírez M, San Millán JL, Escobar-Morreale HF. Genomic variants in polycystic ovary syndrome. Clin Chim Acta. 2006;366:14–26. doi: 10.1016/j.cca.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Beatriz Motta A. The role of obesity in the development of polycystic ovary syndrome. Curr Pharm Des. 2012;18:2482–2491. doi: 10.2174/13816128112092482. [DOI] [PubMed] [Google Scholar]
- 26.Xiong W, Lin Y, Xu L, Tamadon A, Zou S, Tian F, et al. Circulatory microRNA 23a and microRNA 23b and polycystic ovary syndrome (PCOS): the effects of body mass index and sex hormones in an Eastern Han Chinese population. J Ovarian Resh. 2017;10 doi: 10.1186/s13048-016-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, Hu Z, Cai L, Liu L, Jiang X, Wu L, et al. Association between single nucleotide polymorphisms of sterol regulatory element binding protein-2 and liver X receptor α gene and risk of polycystic ovary syndrome in a Chinese Han population. Cell Biochem Biophys. 2014;70:1421–1426. doi: 10.1007/s12013-014-0075-5. [DOI] [PubMed] [Google Scholar]
- 29.Jia H, Yu L, Guo X, Gao W, Jiang Z. Associations of adiponectin gene polymorphisms with polycystic ovary syndrome: a meta-analysis. Endocrine. 2012;42:299–306. doi: 10.1007/s12020-012-9605-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Wei D, Sun X, Li J, Yu X, Shi Y, et al. Family-based analysis of adiponectin gene polymorphisms in Chinese Han polycystic ovary syndrome. Fertil Steril. 2014;101:1419–1423. doi: 10.1016/j.fertnstert.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Baba T, Endo T, Sata F, Nagasawa K, Honnma H, Kitajima Y, et al. The contributions of resistin and adiponectin gene single nucleotide polymorphisms to the genetic risk for polycystic ovary syndrome in a Japanese population. Gynecol Endocrinol. 2009;25:498–503. doi: 10.1080/09513590902972042. [DOI] [PubMed] [Google Scholar]
- 32.Xian L, He W, Pang F, Hu Y. ADIPOQ gene polymorphisms and susceptibility to polycystic ovary syndrome: a HuGE survey and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012;161:117–124. doi: 10.1016/j.ejogrb.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhang N, Shi Y-H, Hao C-F, Gu HF, Li Y, Zhao Y-R, et al. Association of+ 45G15G (T/G) and+ 276 (G/T) polymorphisms in the ADIPOQ gene with polycystic ovary syndrome among Han Chinese women. Eur J Endocrinol. 2008;158:255–260. doi: 10.1530/EJE-07-0576. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Yun J-H, Lee J-H, Song S, Choi B-C, Baek K-H. Association study of+ 45G15G (T/G) and+ 276 (G/T) polymorphisms in the adiponectin gene in patients with polycystic ovary syndrome. Int J Mol Med. 2011;27:283–287. doi: 10.3892/ijmm.2010.565. [DOI] [PubMed] [Google Scholar]
- 35.Barber T, Bennett A, Groves C, Sovio U, Ruokonen A, Martikainen H, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia. 2008;51:1153–1158. doi: 10.1007/s00125-008-1028-6. [DOI] [PubMed] [Google Scholar]
- 36.Ewens KG, Jones MR, Ankener W, Stewart DR, Urbanek M, Dunaif A, et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS ONE. 2011;6:e16390. doi: 10.1371/journal.pone.0016390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehr E, Schweighofer N, Möller R, Giuliani A, Pieber TR, Obermayer-Pietsch B. Association of FTO gene with hyperandrogenemia and metabolic parameters in women with polycystic ovary syndrome. Metabolism. 2010;59:575–580. doi: 10.1016/j.metabol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Song DK, Lee H, Oh J-Y, Hong YS, Sung Y-A. FTO gene variants are associated with PCOS susceptibility and hyperandrogenemia in young Korean women. Diabetes Metab J. 2014;38:302–310. doi: 10.4093/dmj.2014.38.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Zhu G, Wang F, Wang X, Guo H, Shen M. Interaction between common variants of FTO and MC4R is associated with risk of PCOS. Reprod Biol Endocrinol. 2015;13 doi: 10.1186/s12958-015-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradnová O, Vejražková D, Vaňková M, Lukášová P, Včelák J, Stanická S, et al. Metabolic and hormonal consequencies of the obesity risk” MC4R variant (rs12970134) in Czech women. Physiol Res. 2015;64:S187–S195. doi: 10.33549/physiolres.933119. [DOI] [PubMed] [Google Scholar]
- 41.Ranjzad F, Mahmoudi T, Shemirani AI, Mahban A, Nikzamir A, Vahedi M, et al. A common variant in the adiponectin gene and polycystic ovary syndrome risk. Mol Biol Rep. 2012;39:2313–2319. doi: 10.1007/s11033-011-0981-1. [DOI] [PubMed] [Google Scholar]
- 42.Zaki M, Kholoussi S, Ismail S, Raouf HA, Helwa I, Hassan N, et al. Metabolic abnormalities in young Egyptian women with polycystic ovary syndrome and their relation to ADIPOQ gene variants and body fat phenotype. Egyptian J Med Hum Gen. 2015;16:367–374. [Google Scholar]
- 43.Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1185–R1196. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attaoua R, El Mkadem SA, Radian S, Fica S, Hanzu F, Albu A, et al. FTO gene associates to metabolic syndrome in women with polycystic ovary syndrome. Biochem Biophys Res Commun. 2008;373:230–234. doi: 10.1016/j.bbrc.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 45.Grant SF, Bradfield JP, Zhang H, Wang K, Kim CE, Annaiah K, et al. Investigation of the locus near MC4R with childhood obesity in Americans of European and African ancestry. Obesity. 2009;17:1461–1465. doi: 10.1038/oby.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y-k, Dickinson CJ, Zeng Q, Li J-Y, Thompson DA, Gantz I. Contribution of melanocortin receptor exoloops to Agouti-related protein binding. J Biol Chem. 1999;274:14100–14106. doi: 10.1074/jbc.274.20.14100. [DOI] [PubMed] [Google Scholar]
- 47.Sarvestani FS, Tamadon A, Hematzadeh A, Jahanara M, Shirazi MRJ, Moghadam A, et al. Expression of melanocortin-4 receptor and agouti-related peptide mRNAs in arcuate nucleus during long term malnutrition of female ovariectomized rats. Iran J Basic Med Sci. 2015;18:104–107. [PMC free article] [PubMed] [Google Scholar]
- 48.Zandi MR, Jafarzadeh Shirazi MR, Tamadon A, Akhlaghi A, Salehi MS, Niazi A, et al. Hypothalamic expression of melanocortin-4 receptor and agouti-related peptide mRNAs during the estrous cycle of rats. Int J Mol Cell Med. 2014;3:183–189. [PMC free article] [PubMed] [Google Scholar]
- 49.Asadi-Yousefabad S-L, Sabet Sarvestani F, Tamadon A, Jafarzadeh Shirazi MR, Ahmadloo S, Moghadam A, et al. Agouti-related peptide and melanocortin-4 receptor mRNAs expressions in arcuate nucleus during the pregnancy and lactation of rats. Vet Arh. 2015;85:689–700. [Google Scholar]
- 50.Nooranizadeh MH, Rahmanifar F, Ahmadloo S, Shaaban Z, Shirazi MRJ, Tamadon A. Enhancement of melanocortin-4 receptor (MC4R) and constancy of Kiss1 mRNAs expression in the hypothalamic arcuate nucleus in a model of polycystic ovary syndrome rat. Galen Med J. 2018;7:e1070. doi: 10.22086/gmj.v0i0.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65–73. doi: 10.1016/j.tem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–1079. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 54.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malin SK, Kirwan JP, Sia CL, González F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic β-cell dysfunction in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:322–329. doi: 10.1210/jc.2013-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojeda-Ojeda M, Murri M, Insenser M, F Escobar-Morreale H. Mediators of low-grade chronic inflammation in polycystic ovary syndrome (PCOS) Curr Pharm Des. 2013;19:5775–5791. doi: 10.2174/1381612811319320012. [DOI] [PubMed] [Google Scholar]
- 57.González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77:300–305. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149:R219–R227. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 59.Deligeoroglou E, Kouskouti C, Christopoulos P. The role of genes in the polycystic ovary syndrome: predisposition and mechanisms. Gynecol Endocrinol. 2009;25:603–609. doi: 10.1080/09513590903015619. [DOI] [PubMed] [Google Scholar]
- 60.Vural P, Değirmencioğlu S, Saral NY, Akgül C. Tumor necrosis factor α (− 308), interleukin-6 (− 174) and interleukin-10 (− 1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;150:61–65. doi: 10.1016/j.ejogrb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Eriksen MB, Brusgaard K, Andersen M, Tan Q, Altinok ML, Gaster M, et al. Association of polycystic ovary syndrome susceptibility single nucleotide polymorphism rs2479106 and PCOS in Caucasian patients with PCOS or hirsutism as referral diagnosis. Eur J Obstet Gynecol Reprod Biol. 2012;163:39–42. doi: 10.1016/j.ejogrb.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Gao J, Xue J-D, Li Z-C, Zhou L, Chen C. The association of DENND1A gene polymorphisms and polycystic ovary syndrome risk: a systematic review and meta-analysis. Arch Gynecol Obstet. 2016;294:1073–1080. doi: 10.1007/s00404-016-4159-x. [DOI] [PubMed] [Google Scholar]
- 63.Bao S, Cai J-H, Yang S-Y, Ren Y, Feng T, Jin T, et al. Association of DENND1A gene polymorphisms with polycystic ovary syndrome: a meta-analysis. J Clin Res Pediatr Endocrinol. 2016;8:135. doi: 10.4274/jcrpe.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dallel M, Sarray S, Douma Z, Hachani F, Al-Ansari AK, Letaifa DB, et al. Differential association of DENND1A genetic variants with polycystic ovary syndrome in Tunisian but not Bahraini Arab women. Gene. 2018;647:79–84. doi: 10.1016/j.gene.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Liu H, Zhao H, Xu C, Zhao Y, Ma J, et al. Association of AQP8 in women with PCOS. Reprod Biomed Online. 2013;27:419–422. doi: 10.1016/j.rbmo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Li T, Zhao H, Zhao X, Zhang B, Cui L, Shi Y, et al. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49:254–257. doi: 10.1136/jmedgenet-2011-100727. [DOI] [PubMed] [Google Scholar]
- 67.Diao X, Han T, Zhang Y, Ma J, Shi Y, Chen Z-J. Family association study between tumour necrosis factor a gene polymorphisms and polycystic ovary syndrome in Han Chinese. Reprod Biomed Online. 2014;29:581–587. doi: 10.1016/j.rbmo.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Zhong T, Xiao G, Chen Y, Liu J, Xia C, et al. Polymorphisms and haplotypes of the TGF-β1 gene are associated with risk of polycystic ovary syndrome in Chinese Han women. Eur J Obstet Gynecol Reprod Biol. 2015;186:1–7. doi: 10.1016/j.ejogrb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Peral Bn, San Millán JL, Castello R, Moghetti P, Escobar-Morreale HcF. The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab. 2002;87:3977–3983. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- 70.Tumu VR, Govatati S, Guruvaiah P, Deenadayal M, Shivaji S, Bhanoori M. An interleukin-6 gene promoter polymorphism is associated with polycystic ovary syndrome in South Indian women. J Assist Reprod Genet. 2013;30:1541–1546. doi: 10.1007/s10815-013-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo R, Zheng Y, Yang J, Zheng N. Association of TNF-alpha, IL-6 and IL-1beta gene polymorphisms with polycystic ovary syndrome: a meta-analysis. BMC Genetics. 2015;16:5. doi: 10.1186/s12863-015-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deligeoroglou E, Vrachnis N, Athanasopoulos N, Iliodromiti Z, Sifakis S, Iliodromiti S, et al. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol. 2012;28:974–978. doi: 10.3109/09513590.2012.683082. [DOI] [PubMed] [Google Scholar]
- 73.Xiong Y-l, Liang X-y, Yang X, Li Y, Wei L-n. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159:148–150. doi: 10.1016/j.ejogrb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Kim YY, Tamadon A, Ku S-Y. Potential use of antiapoptotic proteins and noncoding RNAs for efficient in vitro follicular maturation and ovarian bioengineering. Tissue Eng Part B Rev. 2017;23:142–158. doi: 10.1089/ten.TEB.2016.0156. [DOI] [PubMed] [Google Scholar]
- 75.Hotamisligil G. The role of TNFα and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 76.González F, Sia CL, Bearson DM, Blair HE. Hyperandrogenism induces a proinflammatory TNFα response to glucose ingestion in a receptor-dependent fashion. J Clin Endocrinol Metab. 2014;99:E848–E854. doi: 10.1210/jc.2013-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szczuko M, Zapałowska-Chwyć M, Maciejewska D, Drozd A, Starczewski A, Stachowska E. High glycemic index diet in PCOS patients The analysis of IGF I and TNF-α pathways in metabolic disorders. Med Hypotheses. 2016;96:42–47. doi: 10.1016/j.mehy.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α-and obesity-induced insulin resistance. Science. 1996;271:665–670. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 79.Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-α stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–998. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- 80.Hong L, Zhang Y, Wang Q, Han Y, Teng X. Effects of interleukin 6 and tumor necrosis factor-α on the proliferation of porcine theca interna cells: Possible role of these cytokines in the pathogenesis of polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2016;55:183–187. doi: 10.1016/j.tjog.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Fernández-Real JM, Gutiérrez C, Ricart W, Castiñeira Ma-J, Vendrell J, Richart C. Plasma levels of the soluble fraction of tumor necrosis factor receptors 1 and 2 are independent determinants of plasma cholesterol and LDL-cholesterol concentrations in healthy subjects. Atherosclerosis. 1999;146:321–327. doi: 10.1016/s0021-9150(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 82.Peral B, San Millán JL, Castello R, Moghetti P, Escobar-Morreale HcF. The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab. 2002;87:3977–3983. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- 83.Päth Gn, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82:2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- 84.Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem. 2011;286:13791–13800. doi: 10.1074/jbc.R110.217067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, et al. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016;434:25–35. doi: 10.1016/j.mce.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014:201400574. doi: 10.1073/pnas.1400574111. [DOI] [PMC free article] [PubMed] [Google Scholar]