Abstract
Objectives
The aim of this study was to assess soluble CD30 (sCD30), a protein that colocalises with HIV-1 RNA and DNA in lymphoid cells and tissues, in cerebrospinal fluid (CSF) as a marker of HIV-1 infection in the central nervous system (CNS).
Methods
This was a cross-sectional study using archived samples from two clinical cohorts. Soluble CD30 concentrations were measured in paired CSF and plasma from untreated viraemic individuals (n=52), individuals on suppressive antiretroviral therapy (ART) (n=33), HIV-1 controllers (n=10), participants with CSF HIV-1 ‘escape’ (n=11) and controls without HIV-1 infection (n=16). Nonparametric tests were used to compare levels across groups and evaluate correlations with HIV-1 RNA, CSF neurofilament light chain protein (NFL) and neopterin.
Results
Compared with controls (median 30 ng/mL, interquartile range [IRQ] 23–50), plasma sCD30 levels were elevated in viraemic participants (75 ng/mL, 52–116; P<0.001), but not in those on suppressive ART (38 ng/mL, 32–62). In contrast, CSF sCD30 levels were elevated in ART-suppressed individuals (34 ng/mL, 19–46; P=0.001) and in those with CSF ‘escape’ (33 ng/mL, 27–40; P=0.004) compared with controls (18 ng/mL, 11–23), but not in untreated viraemic individuals. No association was observed between CSF sCD30 and plasma HIV-1 RNA, concurrent or nadir CD4+ T cell count, duration of infection or plasma sCD30. CSF sCD30 correlated with CSF NFL (r=0.34, P=0.001).
Conclusions
In contrast to plasma, sCD30 levels are elevated in the CSF of individuals with HIV-1 infection who are on suppressive ART. Elevated levels of sCD30 in the CSF may be an indicator of persistent CNS HIV-1 infection, although the mechanism underlying this elevation warrants further investigation.
Keywords: HIV-1, reservoir, central nervous system (CNS), cerebrospinal fluid (CSF), CD30
Background
Although antiretroviral therapy (ART) has substantially reduced the morbidity and mortality rates of HIV-1 infection, the persistence of viral reservoirs continues to prevent the elimination of HIV-1 from all tissue compartments [1,2]. Consequently, attention has turned to detecting cells harbouring either latent or transcriptionally active HIV-1 in order to better characterise the HIV-1 reservoir and identify targets for viral eradication strategies.
CD30 is a member of the tumour necrosis factor (TNF) receptor superfamily [3–5] that is rarely expressed in healthy individuals [5–8]. Although CD30 is primarily expressed on lymphocytes, there is evidence that myeloid cells are also capable of expressing CD30 in vitro and in vivo [9–12]. Infection with viral pathogens including HIV-1, human T cell lymphotropic virus and Epstein–Barr virus may increase CD30 surface expression [4,13–15]. In some individuals with HIV-1, CD4+ T lymphocyte-associated HIV-1 RNA and DNA are highly enriched in cells expressing CD30 and surface expression colocalises with HIV-1 transcriptional activity in gut-associated lymphoid tissues [16]. In individuals on ART, depletion of cells expressing CD30 using a cytotoxic antibody–drug conjugate appears to reduce HIV-1 DNA and RNA in peripheral blood mononuclear cells (PBMCs) [17]. Taken together, these findings suggest that CD30 could be a marker of cells that harbour HIV-1 infection.
Surface CD30 is cleaved by metalloproteases such as TNF-α converting enzyme and released in a soluble form (sCD30) [18]. Plasma sCD30 concentrations are elevated in individuals with untreated HIV-1 infection but normal in those on suppressive ART [3,16,19–21]. This result suggests that increased sCD30 levels may be due to persistent viral replication or T cell activation within the peripheral blood compartment.
Beyond the long-lived CD4+ T cell reservoir [22], the central nervous system (CNS) may serve as an additional source of persistent infection in people living with HIV-1 [23–25]. Lymphoid cells, the primary target of HIV-1 infection, can travel into the CNS compartment [26]. HIV-1 can also infect CNS-resident myeloid cells including macrophages and microglia [27,28]. The extent to which cells of lymphoid or myeloid origin contribute to persistent HIV-1 in the setting of suppressive therapy and whether persistent CNS infection underlies continued low-level CNS inflammation and neurocognitive impairment despite ‘undetectable’ CSF HIV-1 RNA [24,27–30] remains unclear.
Direct access to CNS tissue in individuals living with HIV-1 is generally not feasible [25]. As an initial approach to evaluating sCD30 expression in CNS HIV-1 infection, we measured CSF sCD30 concentrations across a clinically relevant spectrum of individuals and examined their correlation with plasma sCD30 levels, measures of HIV-1 replication and progression, and CSF biomarkers of inflammation and axonal injury. We hypothesised that the dynamics of sCD30 concentrations in the CSF would parallel those previously described in plasma, with viraemic individuals having elevated sCD30 levels and ART-suppressed individuals having sCD30 levels similar to uninfected controls [16,17]. We found this not to be the case.
Methods
Study design and sample
This was a cross-sectional study using archived CSF and plasma samples from a total of 134 individuals from two clinical centres, one in Gothenburg, Sweden (n=62), and the other in San Francisco, California (n=72). All participants had been enrolled in study protocols collecting CSF and plasma samples by lumbar puncture and phlebotomy using standard procedures. The samples were stored at −80°C [31,32]. We selected matching CSF and plasma samples from controls without HIV-1 infection and several clinically relevant groups with HIV-1 infection, including (1) untreated viraemic individuals prior to initiation of ART stratified by CD4+ T lymphocyte count; (2) ART-treated individuals who had been on suppressive therapy for at least 1 year and achieved virological suppression (plasma and CSF HIV-1 RNA below the limit of quantitation on standard clinical assays); (3) HIV-1 controllers exhibiting spontaneous viraemic (plasma viral load <500 copies/mL) or ‘elite’ control (plasma viral load below the limit of quantitation on standard clinical assays); and (4) individuals with both symptomatic and asymptomatic CSF HIV-1 ‘escape’, [33,34] defined as CSF HIV-1 RNA >50 copies/mL in the presence of plasma HIV-1 RNA <50 copies/mL (if suppressed) or CSF HIV-1 RNA greater than plasma HIV-1 RNA (if not fully suppressed) [35]. We excluded individuals with concomitant CNS infections, including neurosyphilis.
Specimen sampling, processing and analysis
Soluble CD30 levels were measured in CSF and plasma samples using the Human sCD30 Platinum ELISA Kit (Affymetrix eBioscience) according to the manufacturer's instructions. Because this kit was designed for plasma or serum, we tested CSF specimens in duplicate, and values were averaged to obtain an adjusted CSF sCD30 value for each specimen. Quantifiable results were within the linear range of the assay. When variation between duplicates on the assay exceeded 20% (20/134 samples), the test was repeated in duplicate and the new values, if concordant, were used. If variation continued to exceed 20%, the adjusted CSF sCD30 value was calculated as the mean of the four values. Plasma sCD30 was also tested in duplicate.
Data on standard HIV-1 parameters (CD4+ T lymphocyte count, plasma HIV-1 RNA) and CSF parameters (white blood cell [WBC] count, albumin ratio, CSF HIV-1 RNA) from clinical testing were available for all individuals from paired time points. The limit of quantification of the plasma and CSF HIV-1 RNA assay was 40 copies/mL for the San Francisco site and 20 copies/mL for the Gothenburg site; we used 40 copies/mL as the limit of quantification in our analyses. Correlative data for CSF neurofilament light chain protein (NFL) were determined by immunoassay using published methods [32]. Because CSF NFL varies with age, we used a standard age correction to compare across groups and designate a consistent threshold for abnormality as previously described [36]. Neopterin concentrations were measured using a commercially available immunoassay (NEOPT-SCR.EIA 384 Det., Thermo Fisher Scientific – BRAHMS GmbH, Henningsdorf, Germany) [37].
Antibody staining and flow cytometry
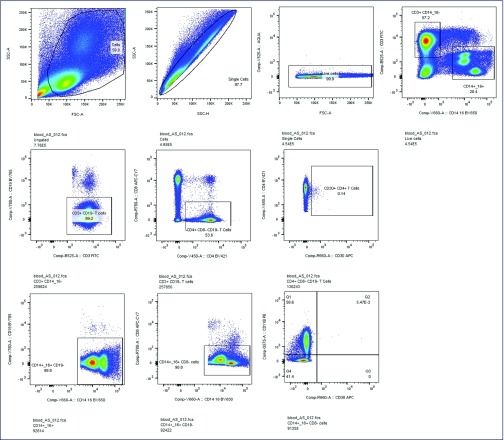
Five participants were willing to provide fresh CSF and matched PBMCs for flow cytometric analyses. These samples were analysed by a multiparameter flow cytometric panel that included CD30 expression on CD4+ T cells. Full panels and antibodies used for staining are described in Supplementary Table 1. The flow cytometric panel allowed for the measurement of CD30 expression on CD4+ T lymphocytes as well as cells of myeloid origin (expressing CD14 or CD16). Samples stained with all but one fluorescent antibody were used in addition to unstained controls to adequately gate on the low-frequency cell populations while accounting for spectral overlap, even after rigorous compensation. Cells were analysed on a BD LSR II (BD Biosciences), and data were analysed in FlowJo V10 (Tree Star). Single stained beads (Life Technologies) were used for compensation. Sample gating is shown in Supplemental Figure 1. Because of the low frequency of CD30 expression and the relatively few numbers of cells that can be obtained from CSF, further characterisation of CD30+ lymphocyte or myeloid cell populations was limited.
Supplementary Table 1.
Flow cytometric antibodies
| Antibody | Host Species | Clone | Fluorochrome | Supplier |
| CD30 expression panel | ||||
| CD4 | Mouse | OKT4 | BV421 | BioLegend |
| CD8 | Mouse | HIT8a | APC-Cy7 | BioLegend |
| CD3 | Mouse | UCHT1 | FITC | BioLegend |
| CD14* | Mouse | M5E2 | BV650 | BioLegend |
| CD16* | Mouse | 3G8 | BV650 | BioLegend |
| CD19 | Mouse | HIB19 | BV785 | BioLegend |
| CD11B | Mouse | ICRF44 | PE | BD Biosciences |
| CD30 | Mouse | BerH8 | APC | BD Biosciences |
| LIVE/DEAD™ Stain | Aqua | Invitrogen | ||
| Brilliant Stain Buffer | BD Biosciences |
The same channel was utilised for CD14 and CD16.
Supplemental Figure 1.
Sample gating strategy for surface CD30 measurement.
Statistical methods
The primary outcome variable was the concentration of sCD30 in CSF specimens in the different clinical groups. Descriptive statistics (median, interquartile range [IQR]) were derived for each group and compared using Mann–Whitney or Kruskal–Wallis tests with Dunn's pairwise comparisons where appropriate. Correlations between CSF sCD30 and other variables used nonparametric Spearman statistics. For most analyses, we evaluated correlations in the cohort as a whole and within the discrete clinical subgroups to explore relationships that might provide mechanistic clues between correlates in specific clinical scenarios. We used Stata release 15.1SE (StataCorp, LLC) to conduct all analyses and GraphPad Prism version 8.1.2 (GraphPad Software) to display results.
Study approval
The samples and related background data were all obtained between 2000 and 2018 within the context of research protocols approved by the institutional review boards of the two study sites. Informed consent was obtained from all subjects.
Results
Characteristics of participants
Table 1 describes the demographic, HIV-1 and CSF parameters of the study participants by group. There were no site-based differences in gender or race demographics, plasma or CSF HIV-1 RNA, or CD4+ T cell count; participants from San Francisco were statistically, but not substantially, older (median 48 vs 42 years, P<0.001). The majority of participants in all groups were male, and age and race demographics were similar across groups. All participants in the ART-suppressed group had been on suppressive therapy for at least 1 year, although information regarding the composition of the ART regimen and the duration of suppression was not available. In addition to higher median plasma HIV-1 RNA, untreated viraemic individuals had higher CSF HIV-1 RNA levels, higher CSF WBC counts and lower CD4+ T lymphocyte counts at the time of the study. CSF:blood albumin ratio, an index of blood–brain barrier permeability, did not differ between groups. CSF neopterin and NFL were both higher in the untreated viraemic group and in the CSF ‘escape’ group.
Table 1.
Characteristics of study participants. Values reported as median (interquartile range) unless otherwise indicated
| Characteristics | HIV-uninfected | Untreated viraemic | ART-suppressed | Controller | CSF escape | P-value* |
|---|---|---|---|---|---|---|
| n=16 | n=52 | n=33 | n=10 | n=11 | ||
| Age (years) | 46.2 (36.8–54.8) | 43.2 (35.7–50.0) | 47.6 (42.4–53.2) | 37.4 (30.5–44.2) | 48.2 (35.6–51.4) | 0.17 |
| Male sex (%) | 15 (94) | 44 (85) | 28 (85) | 7 (70) | 7 (64) | 0.19 |
| Ethnicity (%) | 0.27 | |||||
| White | 7 (44) | 30 (58) | 22 (67) | 3 (30) | 5 (45) | |
| Black | 7 (44) | 17 (33) | 6 (18) | 6 (60) | 4 (36) | |
| Asian | 0 (0) | 3 (6) | 4 (12) | 1 (10) | 2 (18) | |
| Other/unknown | 2 (12.5) | 2 (4) | 1 (3) | 0 (0) | 0 (0) | |
| Nadir CD4+ T cell count (cells/mm3) | n/a | 230 (60–360) | 207 (61–310) | 435 (320–624) | 130 (110–170) | 0.03 |
| Current CD4+ T cell count (cells/mm3) | 827 (764–940) | 284 (97–423) | 601 (507–702) | 568 (397–1100) | 400 (290–480) | <0.001 |
| Plasma HIV-1 RNA (copies/mL) | n/a | 41,050 (9240–186,500) | <40 (<40 to <40) | 183 (43–367) | <40 (<40 to <40) | <0.001 |
| CSF parameters | ||||||
| White blood cells (cells/mm3) | 1 (1–3) | 5 (0–10) | 2 (0–3) | 1 (0–3) | 10 (2–28) | 0.001 |
| Albumin ratio | 6.0 (4.6-8.7) | 5.2 (3.6–7.0) | 4.3 (3.4–6.6) | 4.6 (2.9–5.9) | 5.2 (3.7–7.3) | 0.29 |
| HIV-1 RNA (copies/mL) | n/a | 1368 (370–7515) | <40 (<40 to <40) | <40 (<40 to <40) | 605 (134–860) | <0.001 |
| Neopterin (nmol/L) | 6.3 (4.3–8.6) | 16.7 (9.5–24.3) | 6.3 (4.9–9.8) | 6.3 (5.5–9.6) | 40.5 (22.5–80.2) | <0.001 |
| Neurofilament (age-adjusted; ng/L) | 511 (443–639) | 527 (359–834) | 475 (357–632) | 405 (328–511) | 794 (544–1323) | 0.02 |
P-values calculated using Kruskal–Wallis test evaluating whether all subgroups derive from same distribution. ART: antiretroviral therapy; CSF: cerebrospinal fluid; n/a: not applicable.
Plasma sCD30 levels are elevated in viraemic individuals and normal in participants on ART
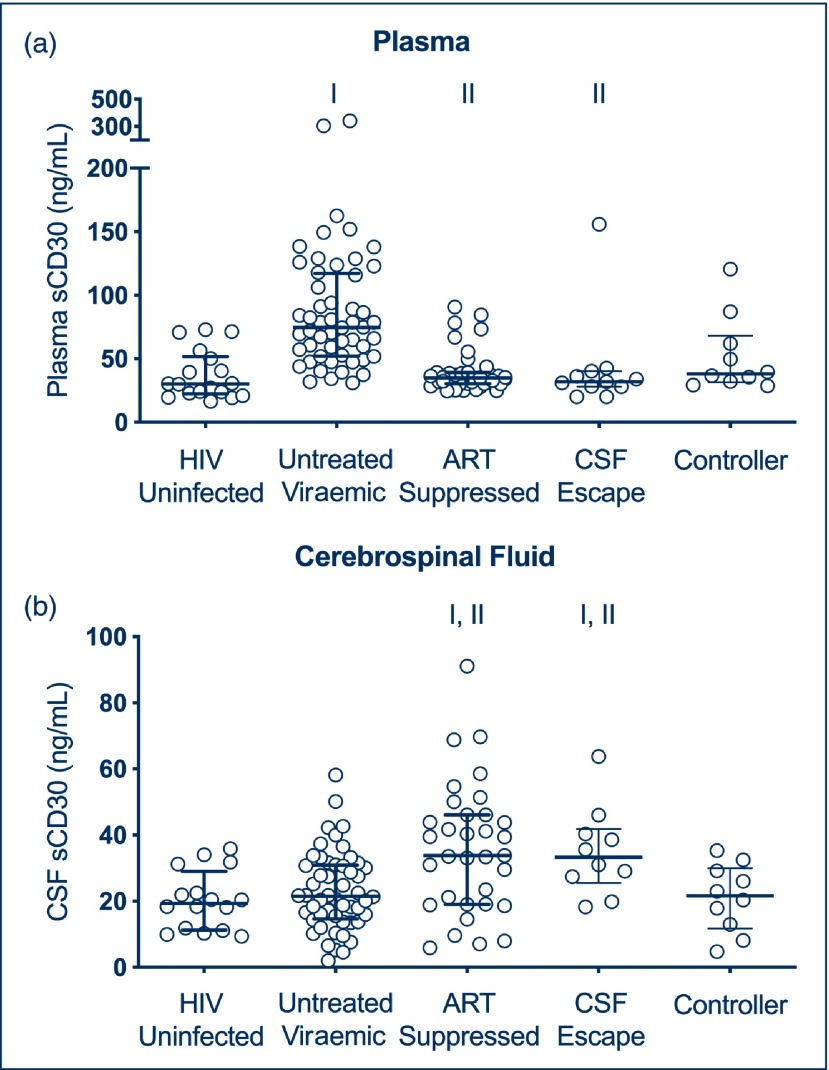
Figure 1a shows plasma sCD30 levels in the various subgroups. Consistent with prior studies [3,16], plasma sCD30 levels were significantly elevated in viraemic participants (median 74.6 ng/mL, IQR 52.2–116.8) than in controls without HIV-1 infection (median 30.4 ng/mL, 22.5–48.5; P<0.001). Individuals on suppressive ART (median 38.1 ng/mL, IQR 32.1–62.3) had lower levels of plasma sCD30 compared with viraemic participants (P<0.001), and no significant difference was detected between this group and controls without HIV-1 infection. Individuals with CSF ‘escape’ and controllers did not have elevated plasma sCD30 levels.
Figure 1.
(a) Plasma sCD30 in HIV-uninfected, untreated viraemic, ART-suppressed, CSF ‘escape’ and controller subgroups. (b) CSF sCD30 in HIV-uninfected, untreated viraemic, ART-suppressed, CSF ‘escape’ and controller subgroups. I indicates statistically significant comparison between group of interest and HIV-1 uninfected subgroup. II indicates statistically significant comparison between group of interest and viraemic subgroup. ART: antiretroviral therapy; CSF: cerebrospinal fluid
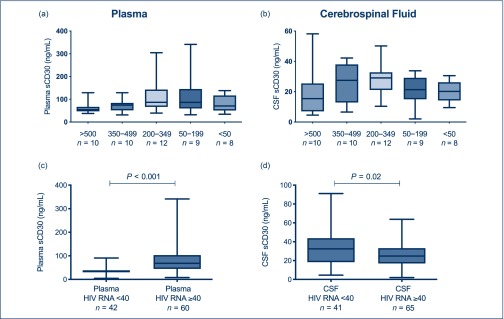
Among untreated viraemic individuals, plasma sCD30 correlated with plasma HIV-1 RNA (r=0.32, P=0.019) and CD4+ T lymphocyte count (r=−0.26, P=0.05). Plasma sCD30 levels by CD4+ T cell count subgroup are summarised in Figure 2a.
Figure 2.
(a) Plasma sCD30 within viraemic individuals, stratified by CD4+ T lymphocyte count at the time of sampling. (b) CSF sCD30 within viraemic individuals, stratified by CD4+ T lymphocyte count at the time of sampling. (c) Plasma sCD30 in individuals with quantifiable plasma HIV-1 RNA (≥40 copies/mL) compared with unquantifiable plasma HIV-1 RNA (<40 copies/mL). (d) CSF sCD30 in individuals with quantifiable CSF HIV-1 RNA compared with unquantifiable CSF HIV-1 RNA. CSF: cerebrospinal fluid.
CSF sCD30 levels are normal in viraemic individuals and elevated in participants on ART
Figure 1b shows CSF sCD30 levels in the various subgroups. Unexpectedly, we did not detect an elevation in CSF sCD30 in untreated viraemic participants compared with uninfected controls. Three individuals in the untreated viraemic subgroup with HIV-1-associated dementia (HAD) had relatively low CSF sCD30 levels (18.5, 28.7, 16.1 ng/mL, respectively) despite high levels of CSF HIV-1 RNA.
Unlike in plasma, and despite a lack of elevation in viraemic participants, CSF sCD30 levels were elevated in ART-suppressed individuals (median 33.8 ng/mL, IQR 19.1–46.1) compared with uninfected controls (median 19.4 ng/mL, IQR 11.4–26.9; P=0.001). A difference was also detected between ART-suppressed and untreated viraemic individuals (P=0.004).
In a sub-analysis of 10 individuals exhibiting CSF HIV-1 ‘escape’, CSF sCD30 levels were elevated (median 33.3 ng/mL, IQR 27–40). This elevation was significant when compared with controls without HIV-1 infection (P=0.004), but not with those on suppressive ART.
The controllers included in this study were a heterogeneous group that included individuals without exposure to ART who were naturally aviraemic (n=2) or exhibited low-level plasma viraemia (n=8). CSF sCD30 levels were not elevated in the combined population of controllers when compared with controls without HIV-1 infection.
Correlates of CSF sCD30
No correlation was detected between plasma and CSF sCD30 levels in the cohort or in any of the subgroups.
CSF sCD30 did not correlate with duration of infection, nadir CD4+ T lymphocyte count, plasma HIV-1 RNA, or concurrent CD4+ T lymphocyte count in the cohort or in any of the subgroups. When untreated viraemic participants were stratified by CD4+ T lymphocyte count, CSF sCD30 levels appeared to peak at moderate (200–499 cells/uL) levels of immunocompromise (Figure 2b), although the differences between groups were not statistically significant.
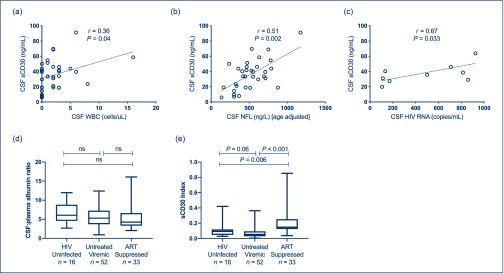
Within the ART-suppressed group, CSF sCD30 correlated with CSF WBCs (r=0.36, P=0.04; Figure 3a), although this association was heavily influenced by one data point and was not detected in the other cohorts. CSF sCD30 correlated with age-adjusted CSF NFL, a marker of axonal injury [36], within both the total cohort (r=0.34, P=0.001) and the ART-suppressed subgroup (r=0.51, P=0.0024; Figure 3b); notably, most values of NFL were within the normal range.
Figure 3.
(a) CSF sCD30 correlates with CSF WBC in the ART-suppressed subgroup. (b) CSF sCD30 correlates with age-adjusted CSF NFL in the ART-suppressed subgroup. (c) CSF sCD30 correlates with CSF HIV-1 RNA in individuals with CSF HIV-1 ‘escape’. (d) CSF:plasma albumin ratio in HIV-uninfected, untreated viraemic and ART-suppressed individuals. (e) sCD30 index in HIV-uninfected, untreated viraemic and ART-suppressed individuals. Note: r and P values represent nonparametric calculations; lines represent best-fit linear regression. ART: antiretroviral therapy; CSF: cerebrospinal fluid; NFL: neurofilament light chain protein.
We did not detect a relationship between sCD30 and CSF neopterin, a marker of myeloid cell activation in the overall cohort or in the untreated viraemic or ART-suppressed subgroups. There was a nonsignificant association between CSF sCD30 and neopterin in the CSF ‘escape’ group (r=0.59, P=0.058).
No clear pattern emerged in a subset of five ART-suppressed individuals with two CSF samples 1 year apart (two had stable CSF sCD30 values, two had values that decreased, and one had values that increased).
CSF sCD30 associates with low-level CSF HIV-1 RNA in individuals on ART
We conducted a secondary analysis stratifying the cohort by HIV-1 RNA above or below the limit of quantification (40 copies/mL). In the combined study cohort, individuals with CSF HIV-1 RNA below the limit of quantification had higher CSF sCD30 compared with those with higher RNA levels (33.7 vs 25.9 ng/mL, P=0.02). This is the opposite of the relationship seen between plasma HIV-1 RNA and plasma sCD30 (Figure 2c and 2d).
No association was detected between CSF HIV-1 RNA and CSF sCD30 in the viraemic subgroup. Within the ART-suppressed subgroup, a single individual with very low CSF HIV-1 RNA at 38 copies/mL (not meeting our definition of CSF ‘escape’) had a disproportionately high CSF sCD30 level (54.7 ng/mL). In the CSF ‘escape’ subgroup, in which all individuals had CSF HIV-1 RNA between 50 and 1000 copies/mL, a positive correlation was detected between CSF HIV-1 RNA and CSF sCD30 (r=0.67, P=0.033) Figure 3c.
An sCD30:albumin index suggests sCD30 production in the CNS compartment
There was no significant difference between groups in the CSF:plasma albumin index, a marker of blood–brain barrier permeability (Figure 3d). To assess a possible contribution of altered blood–brain barrier function to CSF sCD30 concentrations, we calculated a ‘CSF sCD30:albumin index’ ([CSF/plasma sCD30 ÷ CSF/plasma albumin]; Figure 3e). This index did not alter the basic findings of higher CSF sCD30 in the ART suppressed group, consistent with local production within the CNS or CSF space independent of the plasma concentrations.
CD30 surface expression may be present on CSF myeloid cells
Five volunteers were willing to provide fresh PBMC and CSF specimens for analysis. Analysis of fresh PBMCs demonstrated the presence of a small population of CD30+ CD4+ T lymphocytes in the peripheral blood, consistent with previous work [16]. As seen in prior analyses of individuals with HIV-1 [16], CD30 expression on PBMCs was primarily on CD4+ T cells (0.19%, 0.13%–0.45%) rather than on myeloid cells (0.008%, 0.001%–0.13%). In contrast, although rare in the CSF, a higher proportion of CD30+ expressing cells were of myeloid origin (0.07%, 0%–0.47%) than CD4+ T lymphocytes (0.02%, 0.001%–0.03%). We were unable to perform further phenotyping of either lymphocyte or myeloid cell populations in the CSF given the rarity of CD30 expression and relatively few cells from CSF collection (8508, 7938 to 39,154 total live cells).
Discussion
This study examined soluble CD30 in the CSF across clinically relevant groups of individuals with HIV-1. In the peripheral blood compartment, sCD30 is known to be associated with persistent viral replication and immune activation [3,16,19–21]. We hypothesised that the CSF dynamics of this sCD30 marker would mirror those in the peripheral blood and that levels of CSF sCD30 would normalise in individuals on ART [16]. Instead, we found that the dynamics of sCD30 differ between the CNS and peripheral blood compartments. Despite suppressive ART, CSF sCD30 may represent ongoing HIV-1 transcriptional activity (and potentially viral replication) or bystander cell activation associated with myeloid or lymphoid cells in the CNS. These findings support the need for additional studies to delineate the location of the CNS cell type(s) responsible for producing CD30 and to determine whether these cells harbour HIV-1 genetic material.
Our study suggests a source of CSF sCD30 within the CNS. The lack of association between plasma and CSF sCD30 and the sCD30:albumin index support the likelihood of sCD30 production by a cell present within the CNS compartment and argue against diffusion of this protein across an inflamed blood–brain barrier. Metalloproteases like TNF-α converting enzyme have been identified in the CSF of individuals with cognitive impairment, suggesting that these enzymes are available in the CNS compartment to cleave surface-expressed CD30 [38].
This study had two unexpected findings. The first was the elevation of CSF sCD30 in individuals on suppressive ART. In the peripheral blood compartment, suppressive ART is associated with decreased levels of plasma sCD30 [16], which was also the case in this study cohort. The presence of elevated CSF sCD30 concentrations in the ART-suppressed group suggests that certain HIV-1-specific phenomena, such as persistent activation of infected cells or potentially low-level viral replication (i.e. below the limit of quantification), may be present within the CNS compartment despite suppressive ART. Whether ART is mitigating the cytopathic effects of HIV-1 on CD30+ cells is unknown, but such a mechanism would be compatible with the CSF sCD30 elevations observed in individuals with low-level HIV-1 RNA replication described earlier. However, ongoing CNS replication during suppressive ART is contradicted by several previous findings. There is no evidence of viral evolution or escape with resistant virus from the CNS, which would be expected after long-term treatment if low-level replication were present. Furthermore, treatment intensification studies have not shown any effect on CSF residual viral levels or inflammation [39,40].
The second unexpected finding of this study was the lack of CSF sCD30 elevations in ART-naive viraemic individuals, including participants with HIV-1-associated dementia with known high viral burden in the brain. Plasma sCD30 was elevated in the untreated viraemic group and correlated with plasma HIV-1 RNA, consistent with prior studies [3,19]. In contrast, CSF sCD30 was not elevated in viraemic individuals, and the association between CSF sCD30 and HIV-1 RNA was strongest in those with low levels of CSF HIV-1 RNA (i.e. CSF ‘escape’). Individuals with the greatest neurological morbidity (HAD) and highest levels of CSF HIV-1 RNA had comparatively low CSF sCD30. This pattern could be seen if greater levels of HIV-1 replication resulted in higher turnover of a CD30-producing cell population owing to cytopathic effects or chemotaxis out of the CNS compartment. Alternative possibilities include decreased CD30 cleaving by metalloproteases or active trafficking of the sCD30 product out of the CNS in the setting of productive infection. Further investigation of these specific mechanisms is warranted.
Although not statistically significant, the pattern of an apparent CSF sCD30 peak at a moderate level of immune dysfunction is consistent with earlier work. A similar dynamic has been suggested in prior work looking at T cell responses across levels of plasma viraemia [41] and in unrelated studies of CSF sCD30 in multiple sclerosis, which have found that CSF sCD30 levels may be elevated with the moderate levels of immune activation characterising remission rather than the extreme levels of immune activation associated with relapse [42,43].
Within the ART-suppressed individuals, a relatively strong association was detected between CSF sCD30 and NFL, an established marker of axonal injury in HIV-1 infection [36,44,45]. This association suggests that some process, perhaps HIV-1-mediated T cell activation within the CNS compartment, could underlie neuronal damage. General immune activation may also lead to axonal injury [46].
Because the cell type responsible for the production of sCD30 in the CNS remains unclear, we conducted a proof-of-concept study to identify CD30+ cells in the CSF. In blood, the CD30+ CD4+ lymphocytes outnumbered the CD30+ myeloid cells; in the CSF, however, we detected CD30 on both lymphocytes and cells of myeloid origin. Although we observed more CD30 positivity on myeloid cells, our studies were limited by the small number of participants for which fresh CSF cells were available and the limited number of cells available within each CSF sample; further characterisation of the lymphocyte and myeloid cell populations was therefore not possible. Nonetheless, these data support the need for further in vivo and in vitro study of cellular sources of CD30 in the CNS. Of note, earlier experimental studies using cells from individuals without HIV-1 have shown the capacity for monocytes and microglia to express CD30 [9–12]. Further work exploring CD30 in CNS tissue is needed to identify the cellular source of this soluble marker. In vivo studies are challenging given the very few numbers of cells that can be obtained from CSF and the inability to directly sample parenchymal brain tissue in living subjects.
This study has several limitations. First, although our sample size was relatively large for studies of this nature, the individual subgroups were small and the selection for individuals distributed across these groups could have introduced unknown biases. Further studies of sCD30 in more homogeneous populations will help to confirm the patterns observed here. Second, detailed HIV-1 treatment history including ART duration and regimen composition was not widely available. This limited our ability to evaluate a relationship between sCD30 and duration of ART, although no clear pattern emerged in the five individuals with two CSF samples. Further studies should evaluate how the composition or duration of different ART regimens might impact this marker. Third, it is possible that other unmeasured infectious processes within the CNS, such as Epstein–Barr virus reactivation, may have led to increased sCD30 production. However, elevations would be expected within individuals with lower CD4+ T cell counts and active viraemia, which were not observed. Fourth, because our study was meant to generate hypotheses for further investigation, we did not control for multiple statistical comparisons. Finally, the CSF flow cytometry studies were limited by the number of participants willing to provide samples and the number of cells that we could obtain. Evaluation of brain tissue was beyond the scope of this study. In the future, a longitudinal study evaluating individuals with prolonged suppression or with very low-level CSF HIV-1 RNA would further elucidate the dynamics and role of sCD30.
This study of sCD30 in the CSF of individuals with HIV-1 demonstrated that individuals on suppressive ART may have a persistent CNS source of sCD30. It also revealed important differences in the dynamics of sCD30 between the CNS and peripheral blood and suggested several potential mechanisms by which this could occur. Further work to confirm these findings and investigate the potential cellular source of sCD30 is warranted. Such studies have the potential to provide information on the presence of persistent foci of HIV-1 in the setting of suppressive ART and to help direct interventions aimed at inducing long-term suppression of HIV-1.
Acknowledgements
We are grateful to the participants in this research. This work was supported by the following grants: NIH/NIMH R21 MH116716 (PI: Henrich); NIH/NINDS R01 NS094067 (PI: Price); NIH/NIAID T32 AI60530-12; the Swedish State support for Clinical Research; and ALFGBG-717531 (PI: Gisslén).
Contributions
TJH conceptualised the study. MJP, MG, RWP and TJH designed the project and obtained funding. VMA, SS, MG, SGD, and RWP managed the clinical cohorts and obtained participant samples. MJP, CT, CAP, LEH, PJN, CD, DF, HZ, MG, RWP and TJH performed or oversaw the laboratory assays. MJP, SS, PJN, CD, DF, HZ, MG, RWP and TJH analysed the data. MJP, RWP and TJH drafted the manuscript, which was reviewed and edited by all authors.
Conflicts of interest
MJP, CT, LEH, CP, VMA, SS, CD, PJN and DF have no conflicts of interest to declare. HZ has served on scientific advisory boards for Roche Diagnostics, Samumed, CogRx and Wave. HZ is also a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. MG served on the scientific advisory board of Gilead Sciences, BMS, Janssen, MSD and GSK/ViiV Healthcare; received speaker honoraria from Gilead, BMS and Janssen; and received research support from Gilead and Janssen. SGD has received grant support from Gilead, Merck & Co. and ViiV. He has consulted for AbbVie, Janssen and Shionogi. SGD is also a member of the scientific advisory boards for Enochian Biosciences and BryoLogyx. RWP received research support from the National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse and National Institute on Mental Health in obtaining the samples used in the study. TJH provides consulting for Merck and receives grant support from Gilead.
References
- 1. Deeks SG, Lewin SR, Ross AL et al. . International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22 ( 8): 839– 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goulder P, Deeks SG. HIV control: is getting there the same as staying there? PLoS Pathog 2018; 14 ( 11): e1007222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biswas P, Cozzi-Lepri A, Delfanti F et al. . Significant link between sCD30 changes and HIV viremia in patients treated with HAART. J Med Virol 2006; 78 ( 12): 1513– 1519. [DOI] [PubMed] [Google Scholar]
- 4. Biswas P, Mantelli B, Delfanti F et al. . CD30 ligation differentially affects CXCR4-dependent HIV-1 replication and soluble CD30 secretion in non-Hodgkin cell lines and in gamma delta T lymphocytes. Eur J Immunol 2003; 33 ( 11): 3136– 3145. [DOI] [PubMed] [Google Scholar]
- 5. Falini B, Pileri S, Pizzolo G et al. . CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995; 85 ( 1): 1– 14. [PubMed] [Google Scholar]
- 6. Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol 1996; 157 ( 8): 3229– 3234. [PubMed] [Google Scholar]
- 7. Chiarle R, Podda A, Prolla G et al. . CD30 in normal and neoplastic cells. Clin Immunol 1999; 90 ( 2): 157– 164. [DOI] [PubMed] [Google Scholar]
- 8. Durkop H, Foss HD, Eitelbach F et al. . Expression of the CD30 antigen in non-lymphoid tissues and cells. J Pathol 2000; 190 ( 5): 613– 618. [DOI] [PubMed] [Google Scholar]
- 9. Andreesen R, Brugger W, Lohr GW et al. . Human macrophages can express the Hodgkin's cell-associated antigen Ki-1 (CD30). Am J Pathol 1989; 134 ( 1): 187– 192. [PMC free article] [PubMed] [Google Scholar]
- 10. Epstein ML, Windebank KP, Burt AD et al. . CD30 expression by peripheral blood monocytes and hepatic macrophages in a child with miliary tuberculosis. J Clin Pathol 1992; 45 ( 7): 638– 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu SM, Ho YS, Hsu PL. Lymphomas of true histiocytic origin. Expression of different phenotypes in so-called true histiocytic lymphoma and malignant histiocytosis. Am J Pathol 1991; 138 ( 6): 1389– 1404. [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng W, Medeiros LJ, Hu Y et al. . CD30 expression in high-risk acute myeloid leukemia and myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk 2013; 13 ( 3): 307– 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higuchi M, Matsuda T, Mori N et al. . Elevated expression of CD30 in adult T-cell leukemia cell lines: possible role in constitutive NF-kappaB activation. Retrovirology 2005; 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makino M, Yashiki S, Fujiyoshi T et al. . An expression of anaplastic large cell lymphoma-associated antigens on HTLV-I-infected CD4+ T cells. Ann Hematol 1998; 76 ( 1): 31– 35. [DOI] [PubMed] [Google Scholar]
- 15. Haque T, Chaggar T, Schafers J et al. . Soluble CD30: a serum marker for Epstein-Barr virus-associated lymphoproliferative diseases. J Med Virol 2011; 83 ( 2): 311– 316. [DOI] [PubMed] [Google Scholar]
- 16. Hogan LE, Vasquez J, Hobbs KS et al. . Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS Pathog 2018; 14 ( 2): e1006856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang CC, Thanh C, Gibson EA et al. . Transient loss of detectable HIV-1 RNA following brentuximab vedotin anti-CD30 therapy for Hodgkin lymphoma. Blood Adv 2018; 2 ( 23): 3479– 3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansen HP, Matthey B, Barth S et al. . Inhibition of metalloproteinases enhances the internalization of anti-CD30 antibody Ki-3 and the cytotoxic activity of Ki-3 immunotoxin. Int J Cancer 2002; 98 ( 2): 210– 215. [DOI] [PubMed] [Google Scholar]
- 19. Rizzardi GP, Barcellini W, Tambussi G et al. . Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS 1996; 10 ( 13): F45– F50. [DOI] [PubMed] [Google Scholar]
- 20. Pizzolo G, Vinante F, Morosato L et al. . High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS 1994; 8 ( 6): 741– 745. [DOI] [PubMed] [Google Scholar]
- 21. Pizzolo G, Vinante F, Nadali G et al. . High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol 1997; 108 ( 2): 251– 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finzi D, Blankson J, Siliciano JD et al. . Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999; 5 ( 5): 512– 517. [DOI] [PubMed] [Google Scholar]
- 23. Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad 2015; 1 ( 2): 67– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph SB, Arrildt KT, Sturdevant CB et al. . HIV-1 target cells in the CNS. J Neurovirol 2015; 21 ( 3): 276– 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Churchill MJ, Deeks SG, Margolis DM et al. . HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 2016; 14 ( 1): 55– 60. [DOI] [PubMed] [Google Scholar]
- 26. Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol 2017; 17 ( 3): 179– 194. [DOI] [PubMed] [Google Scholar]
- 27. Castellano P, Prevedel L, Eugenin EA. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci Rep 2017; 7 ( 1): 12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong JK, Yukl SA. Tissue reservoirs of HIV. Curr Opin HIV AIDS 2016; 11 ( 4): 362– 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 2008; 111 ( 9): 4660– 4663. [DOI] [PubMed] [Google Scholar]
- 30. Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012; 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staprans S, Marlowe N, Glidden D et al. . Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS 1999; 13 ( 9): 1051– 1061. [DOI] [PubMed] [Google Scholar]
- 32. Peterson J, Gisslen M, Zetterberg H et al. . Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS ONE 2014; 9 ( 12): e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferretti F, Gisslen M, Cinque P et al. . Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep 2015; 12 ( 2): 280– 288. [DOI] [PubMed] [Google Scholar]
- 34. Peluso MJ, Ferretti F, Peterson J et al. . Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012; 26 ( 14): 1765– 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winston A, Antinori A, Cinque P et al. . Defining cerebrospinal fluid HIV RNA escape. AIDS 2019; 33( Supple 2): S107– S111. [DOI] [PubMed] [Google Scholar]
- 36. Yilmaz A, Blennow K, Hagberg L et al. . Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn 2017; 17 ( 8): 761– 770. [DOI] [PubMed] [Google Scholar]
- 37. Hagberg L, Cinque P, Gisslen M et al. . Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang H, Hampel H, Prvulovic D et al. . Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer's disease. Mol Neurodegener 2011; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dahl V, Lee E, Peterson J et al. . Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis 2011; 204 ( 12): 1936– 1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yilmaz A, Verhofstede C, D'Avolio A et al. . Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 2010; 55 ( 5): 590– 596. [DOI] [PubMed] [Google Scholar]
- 41. Deeks SG, Martin JN, Sinclair E et al. . Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J Infect Dis 2004; 189 ( 2): 312– 321. [DOI] [PubMed] [Google Scholar]
- 42. McMillan SA, McDonnell GV, Douglas JP et al. . Elevated serum and CSF levels of soluble CD30 during clinical remission in multiple sclerosis. Neurology 1998; 51 ( 4): 1156– 1160. [DOI] [PubMed] [Google Scholar]
- 43. Navikas V, Martin C, Matusevicius D et al. . Soluble CD30 levels in plasma and cerebrospinal fluid in multiple sclerosis, HIV infection and other nervous system diseases. Acta Neurol Scand 1997; 95 ( 2): 99– 102. [DOI] [PubMed] [Google Scholar]
- 44. Abdulle S, Mellgren A, Brew BJ et al. . CSF neurofilament protein (NFL) – a marker of active HIV-related neurodegeneration. J Neurol 2007; 254 ( 8): 1026– 1032. [DOI] [PubMed] [Google Scholar]
- 45. Peluso MJ, Meyerhoff DJ, Price RW et al. . Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013; 207 ( 11): 1703– 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jessen Krut J, Mellberg T, Price RW et al. . Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS ONE 2014; 9 ( 2): e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]