Background
Human immunodeficiency virus type 1 (HIV-1) encephalopathy refers to cognitive and motor impairment in individuals, after exclusion of other causes. Chorea may arise from a myriad of insults to the basal ganglia, and although HIV is known to compartmentalise to the brain early in seroconversion as well as affect this region, HIV encephalopathy is thought to be rare in the current era of potent antiretroviral therapy (ART). To our knowledge, this is the first case report of HIV-1 encephalopathy and chorea in a patient with high CD4 T cell counts on an established contemporary ART regimen and low-level viremia.
Case presentation
A 52-year-old Aboriginal female with longstanding HIV-1 infection presented in November 2015 with a 4-month history of choreiform movements. She required increased assistance in activities of daily living and demonstrated dysarthria, confusion and poor oral intake. There were no fevers, focal infective symptoms or loss of weight. Infection with HIV-1 had been diagnosed in August 2003, but adherence to treatment had been erratic until a CD4 T cell count nadir of 96 × 106/L (12%) was reached in 2012 (Table 1). At this time, genotyping had shown an M184V nucleoside reverse transcriptase inhibitor (NRTI) resistance mutation. There were no major resistance mutations to protease inhibitors or non-nucleoside reverse transcriptase inhibitors. The patient underwent subsequent directly-observed therapy with good adherence (Table 1, Figure 1) but still maintained ongoing low-level viremia (87–347 HIV-1 copies/mL).
Table 1.
Clinical events, treatment changes and concurrent HIV-1 viral load and CD4 T cell count
| Date | Viral load (copies/mL) | CD4 count (×106/L) | Key clinical events |
|---|---|---|---|
| September 2003 | >100,000 | 493 (17%) | HIV-1 diagnosis |
| 27 May 2004 | >100,000 | 378 (14%) | ART commenced with EFV+ZDV/3TC |
| 2004–2012 | Multiple medication changes due to intolerance/intermittent adherence to ART. Fluctuating VL and CD4 T-cell count | ||
| 20 August–19 September 2012 | 977 | 96 (12%) | Admission for encephalitis considered secondary to parainfluenzae virus. Directly-observed therapy with ABC/3TC+DRV/r. Persistent low viremia |
| November 2015 | 102 | 529 (23%) | Presented with chorea. Commenced on TBZ with mild improvement. |
| 5–25 January 2016 | 138 | 380 (20%) | Readmission with ongoing chorea and progressive functional decline. TBZ stopped. ART switched to EVG/c/TDF/FTC |
| 3 March–
27 April 2016 |
288
48 |
Third admission with persistent symptoms. Switch to DTG/ABC/3TC (8 March 2016), sodium valproate commenced for chorea and TBZ re-introduced. Significant improvement in symptoms and reduction in VL on discharge | |
| June 2016 | <40 | 540 (20%) | Ongoing clinical improvement and undetectable VL |
| October 2016 | Resolution of chorea and improvement in memory. Persistent behavioural issues with little insight | ||
| February 2017 | <40 | 567 (27%) | No recurrence of chorea but ongoing cerebellar signs, behavioural issues and dysthymia |
3TC: lamivudine; ART: antiretroviral therapy; ABC: abacavir; c: cobicistat; DRV/r: boosted darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; TDF: tenofovir; VL: viral load. TBZ: tetrabenazine; ZDV: zidovudine.
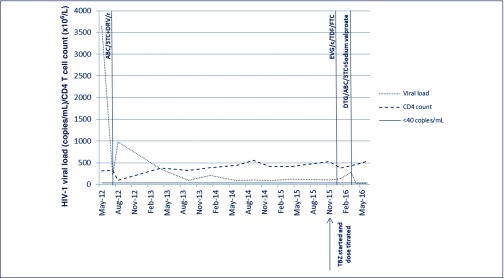
Figure 1.
Antiretroviral regimens and impact on HIV-1 viral load and CD4 T cell count.
ABC: abacavir; c: cobicistat; DRV/r: boosted darunavir; DTG: dolutegravir; EVG: elvitegravir; FTC: emtricitabine; TDF: tenofovir; TBZ = tetrabenazine; 3TC: lamivudine.
Medical history included rheumatic fever at age 14 years with no witnessed chorea, ischaemic heart disease, dyslipidaemia and depression. Interestingly, she had been diagnosed with meningoencephalitis secondary to parainfluenza virus in 2012 when she presented with confusion and coryzal symptoms. Cerebrospinal fluid (CSF) biochemistry was consistent with viral meningitis, but the sample was inadequate to perform parainfluenza and HIV1 PCR testing. The diagnosis of parainfluenza meningoencephalitis was made as concurrent serum parainfluenza titre was significantly elevated at titre >320 (<80). Other medications included aspirin, atenolol, mirtazapine, rosuvastatin and thiamine. She was a smoker of 10 cigarettes/day and, despite a history of alcohol excess, no longer used alcohol.
At presentation, she appeared disorientated and had choreiform movements involving her head, tongue and upper limbs with unsteady gait, left-sided dysdiadochokinesis and past pointing. Reflexes and power were normal. Cardiorespiratory examination was unremarkable.
Investigations
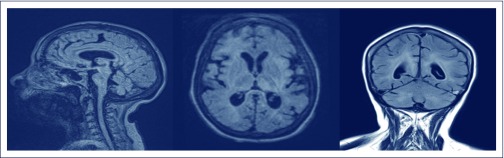
Blood testing showed an HIV-1 VL of 102 copies/mL, CD4 T cell count at 529 ×106/L, erythrocyte sedimentation rate at 60 mm/hour (1–20 mm/hour), mildly elevated anti-streptolysin O titre at 213 KU/L (<200 KU/L) and anti-DNAse B at 251 KU/L (<187 KU/L), likely in keeping with chronic elevation secondary to prior infection. Brain magnetic resonance imaging (MRI) demonstrated volume loss since 2012 and diffuse, bilateral, symmetrical white matter hyper-intensity involving the basal ganglia, thalamus, midbrain, pons and cerebellum (Figure 2). The CSF examination showed glucose at 2.7 mmol/L, protein at 1.19 g/L (0.15– 0.45 g/L), β2-microglobulin at 6.21 mg/L (<0.40 mg/L) and 61 ×106/L leucocytes; however, cellular differentiation was not possible. Viremia in CSF was at 219 copies/mL. Genotyping was not performed on this sample owing to the low viral copy number.
Figure 2.
T2 images illustrating cortical and brainstem volume loss with widespread white matter hyper-intensity
Diagnosis, treatment and outcome
The patient's s clinical presentations over three admissions from November 2015 to April 2016 are summarised in Table 1 with trends in VL and CD4 T cell counts illustrated on Figure 1. Electroencephalogram had excluded non-convulsive status epilepticus and serial MRI had shown no new pathology. The diagnosis of chorea secondary to HIV-1 encephalopathy was established after extensive investigations to exclude other potential causes. There was evidence of multifocal brain involvement with active CNS inflammation. Initial changes to her ART regimens and tetrabenazine dosing made negligible difference to symptoms and viremia. It was a switch to dolutegravir (DTG)/abacavir (ABC)/lamivudine (3TC) in combination with sodium valproate which was associated with a decline in plasma HIV-1 VL and notable improvement in neurological symptoms.
Discussion
Despite dramatic advances in ART and decline in HIV-1-related morbidity and mortality, our case highlights that neurological complications such as chorea may still occur. In addition to its direct deleterious impact on the immune system, HIV-1 crosses the blood–brain barrier early in the course of the infection and settles in perivascular macrophages, microglia and astrocytes causing neuroinflammation and neurotoxicity [1–4]. It may persist and be detected in the CSF for decades without causing symptoms [1]. However, it may also cause neuronal damage soon after infection through a number of potential mechanisms. Firstly, neuroinflammation caused by the virus promotes the influx of proinflammatory molecules to the central nervous system (CNS) as well as chemokine and cytokine release that perpetuates the damage [3]. Secondly, Tat, a transactivating HIV-1 protein, can promote apoptosis, thereby reducing numbers of uninfected CD4+ T lymphocytes [3]. Thirdly, HIV-1 infection of astrocytes restricts their significant immunomodulatory effects [2,3].
As a hyperkinetic movement disorder, chorea can arise from a myriad of insults to the basal ganglia in the setting of HIV-1, including opportunistic infections, neoplasms or cerebrovascular disease [5]. The virus itself is known to cause injury to the dopaminergic system of basal ganglia [6], as evidenced by low CSF dopamine and neuronal loss in the globus pallidus [5]. HIV-1 infection with movement disorders of any cause, accounted for 3% of presentations at the height of the acquired immune deficiency syndrome epidemic [5,6]. Such presentations are now only rarely described in the context of effective therapy.
Recent studies support the concept of compartmentalisation and viral reservoir in the CNS [2–5,7,8]. Once early CNS HIV-1 dissemination has occurred, viral quasispecies develop into genetically distinct populations from blood which becomes ‘compartmentalised’ [3]. This can occur within the first year of infection but holds especially true in late infection [4]. The CSF viral load is typically 10-fold lower than the concurrent plasma VL; however, this ratio can vary [2,7]. Both active replication and ongoing viral transport from the periphery are believed to be responsible for sustained CNS infection [2]. Perivascular macrophages and microglial cells allow local replication within the CNS [2,4]. Studies have shown a greater rise in CSF HIV viral load than plasma during treatment interruption [4]. Compartmentalisation also causes a change from largely T-lymphocyte tropic blood viral strain to a macrophage-tropic CNS one over time [5], which is important for the shift from relatively mild T-lymphocyte driven meningeal infection to a more aggressive encephalitis and CNS injury [8].
Low-level viremia with CNS viral escape was considered the likely cause of the patient's neurological presentation with chorea. Circulating virus and subsequent neurological inflammation with widespread white matter changes on MRI, clinically manifested as chorea and confusion. We cannot exclude that this was the reason for her first presentation with encephalitis in 2012, although appropriate testing was not performed at the time.
Early initiation of ART in HIV-1 infection is known to be the most effective form of treatment and prevention, particularly in severe neurocognitive impairment [7]. While all forms of ART are found to protect the CNS to a certain extent, the question remains whether certain drugs that achieve higher levels in the CSF are more effective. Though controversial, the use of drugs with a high CNS penetration effectiveness (CPE) score is advised in patients with neurocognitive impairment [1,9,10]. The CPE score of the three regimens used in our case was comparable (3TC/ABC+booster darunavir [DRV/r]=8, elvitegravir [EVG]/cobicistat [c]/tenofovir [TDF]/emtricitabine [FTC]=7, DTG/ABC/3TC=8), which highlights the fact that medication potency and barrier to resistance is a strong determinant of HIV-1 control in the CNS. Ultimately, it was a DTG-based regimen which achieved sustained virological response and resolution of choreiform movements [11]. We have further considered whether timing of dosing in relation to oral intake would have altered the outcome as DRV and EVG/c/TDF/FTC should ideally be administered with food. However, despite a prolonged inpatient stay in early 2016 during which medications would have been appropriately administered, VL remained detectable prior to the final change in ART. It is likely that a repeat CSF viral load test would have confirmed viral suppression in CNS after the introduction of DTG. However, this was not pursued as the patient had improved clinically.
Whilst dolutegravir itself has impressive activity against HIV-1, we speculate that the combination of DTG/ABC/3TC and sodium valproate, a histone deacetylase (HDAC) inhibitor and latency-reversing agent (LRA), may have contributed to the eventual reduction in serum VL in our case. Persistence of latent HIV-1 with the ability to replicate in resting memory CD4 T cells is a major impediment to HIV1 eradication [8]. HDAC is an enzyme critical to HIV latency that represses viral transcription and thereby allows the virus to evade ART [8]. Sodium valproate thereby acts as an LRA and reactivates gene expression in latently-infected cells [12]. There have been conflicting study results with regard to the efficacy of sodium valproate to date, but other HDAC inhibitors which are concurrently under investigation include vorinostat, romidepsin and panobinostat [12].
In conclusion, with the introduction of potent combination ART and control of viral replication, severe new HIV-1-related neurological presentations are increasingly rare. However, when patients present with CNS manifestations such as chorea and confusion within the context of previous non-adherence to medication and potential resistance, CNS HIV-1 escape needs to be considered and change of treatment implemented, particularly in the presence of persistent low-level viremia.
Acknowledgements
Ethics approval and consent to participate not applicable.
Consent for publication
Written informed consent was obtained from the patient and her sister (next of kin), for publication of this case report and accompanying images. A copy of the signed consent is available for review if required.
Conflicts of interests
The authors have declared no conflicts of interest.
References
- 1. Nightingale S, Winston A, Letendre S et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 2014; 13: 1139– 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manji H, Jäger HR, Winston A. HIV dementia and antiretroviral drugs: 30 years of an epidemic. J Neurol Neurosurg Psychiatry 2013; 84: 1126– 1137. [DOI] [PubMed] [Google Scholar]
- 3. Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 2015; 12: 16– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad 2015; 1: 67– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cardoso F. HIV-related movement disorders; epidemiology, pathogenesis and management. CNS Drugs 2002; 16: 663– 668. [DOI] [PubMed] [Google Scholar]
- 6. Sankhla CS, Soman RN, Gupta NN et al. Movement disorder: a manifestation of HIV and its response to therapy. Neurol India 2009; 57: 789– 791. [DOI] [PubMed] [Google Scholar]
- 7. Yilmaz A, Gisslén M. Treatment of HIV in the central nervous system. Semin Neurol 2014; 34: 14– 20. [DOI] [PubMed] [Google Scholar]
- 8. Marban C, Forouzanfar F, Ait-Ammar A et al. Targeting the brain reservoirs: toward an HIV cure. Front Immunol 2016; 7: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brew BJ, Chan P. Update on HIV dementia and HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep 2014; 14: 468. [DOI] [PubMed] [Google Scholar]
- 10. Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers and effects of treatment. Curr HIV/AIDS Rep 2014; 11: 317– 324. [DOI] [PubMed] [Google Scholar]
- 11. Walmsley SL, Antela A, Clumeck N et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369: 1807– 1818. [DOI] [PubMed] [Google Scholar]
- 12. Manson McManamy ME, Hakre S, Verdin EM, Margolis DM. Therapy for latent HIV-1 infection: the role of histone deacetylase inhibitors. Antivir Chem Chemother 2014; 23: 145– 149. [DOI] [PMC free article] [PubMed] [Google Scholar]