Abstract
Background:
Abnormal pulmonary arterial pressure (PAP) responses to exercise have been described in select individuals, however clinical and prognostic implications of exercise pulmonary hypertension (exPH) among broader samples remains unclear.
Objectives:
To investigate the association of exPH with clinical determinants and outcomes.
Methods:
We studied individuals with chronic exertional dyspnea and preserved ejection fraction who underwent cardiopulmonary exercise testing with invasive hemodynamic monitoring. ExPH was ascertained using minute-by-minute PAP and cardiac output (CO) measurements to calculate a PAP/CO slope, and exPH defined as a PAP/CO slope >3 mmHg/L/min. The primary outcome was cardiovascular (CV) hospitalization or all-cause mortality.
Results:
Among 714 individuals (age 57 years, 59% women), 296 (41%) had abnormal PAP/CO slopes. Over a mean follow-up of 3.7 ± 2.9 years, there were 208 CV or death events. Individuals with abnormal PAP/CO slope had a 2-fold increased hazard of future CV or death event (multivariable-adjusted HR 2.03, 95%CI 1.48–2.78, P<0.001). The association of abnormal PAP/CO slope with outcomes remained significant after excluding rest PH (n = 146, HR 1.75, 95%CI 1.21–2.54, P=0.003). Both pre- and post-capillary contributions to exPH independently predicted adverse events (P <0.001 for both).
Conclusions:
ExPH is independently associated with CV event-free survival among individuals undergoing evaluation of chronic dyspnea. Our findings suggest incremental value of exercise hemodynamic assessment to resting measurements alone in characterizing the burden of PH in individuals with dyspnea. Whether PH and PH subtypes unmasked by exercise can be used to guide targeted therapeutic interventions requires further investigation.
Keywords: pulmonary hypertension, exercise, cardiovascular disease
Condensed Abstract:
Exercise pulmonary hypertension (PH) has been described in select individuals, however clinical and prognostic implications among broader samples remains unclear. We examined patients with chronic exertional dyspnea and EF ≥50% referred for cardiopulmonary exercise testing with invasive hemodynamic monitoring. We found that exercise PH was associated with a 1.75-fold increased hazard of future cardiovascular or death event even after excluding rest PH. Both pre- and post-capillary contributions to exercise PH independently predicted adverse events. Our findings suggest incremental value of exercise hemodynamic assessment to resting measurements alone in characterizing the burden of PH in individuals with dyspnea.
Introduction
Pulmonary hypertension (PH) may complicate a wide spectrum of cardiopulmonary diseases and is associated with increased morbidity and mortality regardless of etiology. Moreover, both incidence and prevalence of PH appear to be increasing among adults (1). Traditionally, rest PH was defined as a resting mean pulmonary artery pressure (PAP) ≥25 mmHg (2), however recent revised recommendations use a mean PAP >20 mmHg in light of adverse clinical outcomes even among individuals with mean PAP 21–24 mmHg (3,4). Further, PH during exercise testing, has previously been defined as an absolute exercise PAP ≥30 mmHg (5). However, the latter exercise criterion for diagnosing PH were appropriately abandoned after participants at the WHO expert consensus conference in Dana Point in 2008 concluded that this cut-off value for exercise PH (exPH) was not sufficiently supported by the literature and that the “dose dependent” increment in PAP with increasing exercise intensity limits the physiologic significance of a single PAP cut-off to define exercise PH (exPH) (6).
More recently, there is increasing recognition that elevation in pulmonary pressures should be assessed in relation to the corresponding increase in cardiac output during exercise (the PAP/CO relationship) (3,7–9). There are also growing data suggesting the clinical importance of abnormal pulmonary vascular responses to exercise (7–12). Exercise PH has been described in the context of specific disease entities, including systemic sclerosis and carriers of BMPR2 mutations without clinically overt PH (13,14). In these settings, exercise PH is thought to predate the future development of bona fide PH and has been associated with worse clinical outcomes in select patient samples (15–17). However, the natural history of exPH and its pre- and post-capillary components among a broader range of patients with dyspnea on exertion remains unknown and was identified as a key knowledge gap in a recent consensus statement on ExPH (9). Here, we sought to investigate the clinical significance and correlates of exercise pulmonary vascular responses across a broad sample of individuals with chronic exertional dyspnea. Our primary hypothesis was that exPH would be associated with adverse clinical outcomes.
METHODS
Study Sample
We included consecutive patients with chronic dyspnea on exertion who underwent cardiopulmonary exercise testing (CPET) to characterize hemodynamic response to exercise at Massachusetts General Hospital between 2006 and 2017. Of 761 patients with preserved left ventricular ejection fraction (defined as LVEF ≥50%), we excluded participants with the following: history of heart or lung transplantation (n = 16), undergoing evaluation for lung transplantation (n = 6), complex adult congenital heart disease (n = 17), mitochondrial myopathy (n = 14), leaving 714 participants for this analysis. All participants provided informed consent, and the study was approved by the Massachusetts General Hospital institutional review board.
Ascertainment of Clinical Variables and Outcomes
At the time of CPET, participants underwent history and physical examination including assessment of body mass index and fasting blood draw. Blood specimens were immediately processed and stored at −80°C. Medical records were reviewed in detail to confirm medical history, including the presence of cardiovascular and lung disease (defined as at least moderate chronic obstructive pulmonary disease or interstitial lung disease). Pulmonary function testing and measurement of the diffusion capacity of carbon monoxide (DLCO) was performed. Clinical outcomes were adjudicated through 10/29/2018 after review of all available medical records and included: first cardiovascular (CV) hospitalization (defined as any hospitalization for principal cardiovascular cause, including heart failure, pulmonary hypertension, arrhythmia, coronary revascularization) and all-cause mortality, which was ascertained using the social security death index and electronic hospital records. The primary analysis examined CV event-free survival as the combination of CV hospitalization or death. Plasma N-terminal pro-B type natriuretic peptide was assayed using an electrochemiluminescence immunoassay (Roche, NT-proBNP, intra-assay coefficient of variation 2.4–3.8%) and high-sensitivity C-reactive protein was ascertained using an immunoturbidimetric assay (Roche, hsCRP, intra-assay coefficient of variation 0.4–8.4%).
Cardiopulmonary Exercise Testing
All participants underwent insertion of a pulmonary artery catheter via the internal jugular vein and systemic arterial catheter via the radial artery if technically feasible after confirming ulnar arterial collateral circulation by an Allen’s test, followed by maximal upright cycle ergometry using a continuous ramp at 5 to 15 W/min after an initial 3-minute period of unloaded exercise (18). Serial gas exchange (MedGraphics, St. Paul, MN) and hemodynamic measures were assessed at rest and during exercise, including mean PAP and cardiac output (CO) calculated using the direct Fick method. PAP and pulmonary capillary wedge pressure (PCWP) measurements were ascertained at expiration over at least 3 respiratory cycles based on manual review of digitally recorded tracings. Rest PH was defined as a mean PAP >20 mmHg (3). Exercise pulmonary vascular responses were ascertained using serial PAP and CO measurements to calculate a PAP/CO slope, and an abnormal response or exercise pulmonary hypertension (exPH) or high PAP/CO slope was defined as a PAP/CO slope >3 mmHg/L/min as previously described (8, 19). As outlined in recent consensus documents, indexing of PAP to CO is preferable to using a single absolute cut-point value for exercise PAP to account for variable increases in flow with exercise (9). Further, the use of multipoint assessments of PAP, PCWP, and CO throughout exercise limit undue influence of peak exercise respirophasic pressure swings, with an average number of 10.0 ± 2.2 repeated hemodynamic measures within a given individual used to calculate PAP/CO slopes. The inter-observer coefficients of variance for a randomly selected subset of 100 PCWP and PAP measurements during exercise from this study were 4.0% and 2.6%, respectively. The previously reported coefficient of variation for repeated exercise PCWP measures over a period of 12 weeks was is 10% (19). Radionuclide first-pass ventriculography was also performed at rest and at peak exercise, with inter- and intra-observer coefficients of variation for assessment of RVEF <5%.
Statistical Analysis
Baseline characteristics and exercise parameters were summarized according to high vs normal PAP/CO slope groups using means and standard deviations, medians and inter-quartile ranges, or proportions as appropriate. Cross-sectional clinical determinants of PAP/CO slope were examined using age- and sex-adjusted analyses. A stepwise selection regression model was used to examine multivariable-adjusted determinants of PAP/CO slope with eligibility for entry and retention at P <0.05, forcing age and sex into the model. We first examined clinical comorbidities, and constructed a second model allowing both clinical covariates and pulmonary function tests for entry. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), the FEV1/FVC ratio, total lung capacity (TLC), and DLCO were expressed as % of predicted values, which were calculated using sex-specific regression models (21).
We examined Kaplan-Meier survival estimates for the primary outcome of cardiovascular event-free survival among patients with normal vs abnormal pulmonary vascular responses to exercise. We used Cox models to estimate age- and sex-adjusted risk of clinical outcomes by PAP/CO slope. Multivariable Cox models additionally adjusted for the presence of hypertension, myocardial infarction, heart failure, COPD, ILD, and smoking status. These covariates were chosen a priori given clinical relevance. We confirmed that the proportional-hazards assumption was met using Schoenfeld residuals. In secondary analyses we examined the association of each of the components of continuous PAP/CO slope with the primary outcome, including trans-pulmonary gradient (defined as PAP – PCWP) and pulmonary capillary wedge pressure exercise responses (TPG/CO and PCWP/CO slopes, respectively). Pressure/flow slopes were log-transformed due to right-skewed distributions. In sensitivity analyses, we examined the association of PAP/CO slope and the primary outcome after exclusion of (1) rest PH; (2) prevalent cardiovascular disease (defined as history of myocardial infarction or heart failure). In secondary analyses, we examined correlates and prognostic significance of PAP/CO slope among those without rest PH. To further evaluate the prognostic performance of PAP/CO measurements relative to that of alternative definitions of exPH, we examined the use of the single absolute cut-point of exercise PAP >30 mmHg, both with and without addition of total pulmonary resistance (=exPAP/CO) >3 mmHg/L/min in setting of rest PAP ≤20mmHg as previously described (20). Analyses were conducted using STATA v11.2 (College Station, Texas).
Results
A total of 714 participants with chronic dyspnea on exertion underwent CPET with invasive hemodynamic monitoring. The mean age was 57 ± 16 years, and 59% were women. A total of 296 (41%) had exercise PH, defined as a PAP/CO slope > 3 mmHg/L/min based on an average of 10±2 paired PAP and CO measurements per subject. Participants with abnormal PAP/CO slope were older and had a greater burden of clinical comorbidities including cardiovascular and pulmonary disease as displayed in Table 1. Specifically, those with high PAP/CO slope were more likely to have diabetes mellitus (22% vs 12%), prior heart failure (20% vs 7%), COPD (18% vs 5%), and ILD (13% vs 6%, P<0.05 for all). Of note, only 2% of all participants carried a prior diagnosis of pulmonary arterial hypertension. The median NT-proBNP level was 154 pg/mL among those with abnormal PAP/CO slope, and 52 pg/mL among those with normal PAP/CO slope (P<0.001). When examining rest vs exPH, we found 146 (20%) met criteria for rest PH, and an 184 (26%) participants had exPH with normal rest PAP.
Table 1.
Baseline characteristics by high and normal PAP/CO slope
| High PAP/CO N=296 | Normal PAP/CO N=418 | P-value | |
|---|---|---|---|
| Age, years | 63 (13) | 52 (16) | <0.001 |
| Women, n (%) | 170 (57) | 252 (60) | 0.58 |
| Body-mass index, kg/m2 | 29.1 (6.1) | 29.2 (6.7) | 0.79 |
| Hypertension, n (%) | 184 (62) | 189 (45) | <0.001 |
| Diabetes mellitus, n (%) | 65 (22) | 50 (12) | <0.001 |
| Current smoker, n (%) | 12 (4) | 10 (2) | 0.21 |
| Past/present smoker, n (%) | 149 (53) | 131 (33) | <0.001 |
| Pulmonary arterial hypertension, n (%) | 8 (3) | 6 (1) | 0.24 |
| Prior HFpEF, n (%) | 58 (20) | 29 (7) | <0.001 |
| Myocardial infarction, n (%) | 16 (5) | 13 (3) | 0.13 |
| Interstitial lung disease, n (%) | 38 (13) | 23 (6) | 0.001 |
| COPD, n (%) | 54 (18) | 22 (5) | <0.001 |
| Coronary artery disease , n (%) | 62 (21) | 53 (13) | 0.003 |
| Valve disease, n (%) | 39 (13) | 17 (4) | <0.001 |
| Connective tissue disease, n (%) | 24 (8) | 37 (9) | 0.70 |
| Biomarkers (n=678) | |||
| NT-proBNP, pg/mL median (IQR) | 154 (64, 535) | 52 (26, 127) | <0.001 |
| hsCRP, pg/mL median | 2.5 (1.2, 5.3) | 1.5 (0.6, 3.8) | <0.001 |
| Pulmonary function test (n=671) | |||
| % predicted FEV1, % | 78 (22) | 92 (18) | <0.001 |
| % predicted FVC, % | 84 (20) | 96 (18) | <0.001 |
| % predicted FEV1/FVC, % | 93 (14) | 96 (10) | <0.001 |
| % predicted DLCO, % | 62 (21) | 80 (20) | <0.001 |
| COPD GOLD stage 3*, n (%) | 25 (9) | 6 (2) | <0.001 |
| Severe ILD*, n (%) | 6 (2) | 5 (1) | 0.36 |
GOLD stage 3 or greater defined as FEV1/FVC < 70% predicted and FEV1< 50% predicted. ILD defined as FVC <50% pred and DLCO <=35% predicted (33)
Exercise Parameters Associated with Exercise PH
Patients with abnormal PAP/CO slopes had lower absolute and % predicted peak VO2 compared to those with normal PAP/CO slopes despite similar respiratory exchange ratios. Both rest and exercise PAP were higher among those with abnormal PAP/CO slopes (Table 2). In age- and sex-adjusted analyses, elevated PAP/CO slope was associated with a 2.7 ml/kg/min lower peak VO2 compared with normal PAP/CO slope (β −2.70, s.e. 0.37, P<0.001). Invasive hemodynamic assessment demonstrated higher systemic blood pressures and intracardiac pressures and lower cardiac outputs at rest and with exercise among those with abnormal PAP/CO slope. Further, those with high PAP/CO slope had a 1.1 wu steeper TPG/CO slope (β 1.09, s.e. 0.10, P<0.001) and a 2.4 mmHg/L/min steeper PCWP/CO slope (β 2.39, s.e. 0.18, P<0.001) in age- and sex-adjusted analyses, suggesting both pre- and post-capillary components to the higher PAP/CO slope.
Table 2.
Exercise Parameters by PAP/CO slope
| High PAP/CO slope | Normal PAP/CO slope | |||
|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | |
| Peak VO2, ml/kg/min | 14.0 (4.1)* | 18.8 (6.0) | ||
| % predicted peak VO2, % | 46 (12)* | 58 (16) | ||
| Work, watts | 82 (35)* | 116 (47) | ||
| VE/VCO2 slope | 39 (10)* | 36 (9) | ||
| Peak respiratory exchange ratio | 1.16 (0.14) | 1.17 (0.12) | ||
| A-aO2 | 31 (22)* | 22 (21) | ||
| VD/VT | 33 (10)* | 28 (11)* | 28 (11) | 20 (10) |
| PaO2 | 88 (14)* | 87 (21)* | 97 (14) | 99 (18) |
| PaCO2 | 37 (5) | 35 (6)* | 36 (5) | 34 (5) |
| PETCO2 | 32 (5)* | 34 (5)* | 32 (5) | 35 (5) |
| C(a-v)O2 | 6.0 (1.2)* | 11.4 (2.2)* | 5.5 (1.2) | 11.1 (2.0) |
| Hemodynamic parameters | ||||
| Systolic blood pressure, mmHg | 152 (26)* | 189 (32)* | 142 (21) | 182 (27) |
| Heart rate, bpm | 75 (14)* | 126 (26)* | 77 (16) | 144 (24) |
| Pulmonary artery pressure, mmHg | 20 (6)* | 43 (11)* | 15 (4) | 32 (7) |
| Pulmonary capillary wedge pressure, mmHg | 8 (4)* | 25 (8)* | 6 (2) | 18 (6) |
| Transpulmonary gradient, mmHg | 12 (5)* | 18 (9)* | 9 (3) | 13 (5) |
| Fick cardiac output, L/min | 4.7 (1.5)* | 9.3 (2.7)* | 5.5 (1.4) | 13.0 (3.0) |
| O2 pulse | 9.2 (3.1)* | 10.9 (3.4) | ||
| PAP/CO slope | 5.8 (4.6)* | 1.9 (0.6) | ||
| TPG/CO slope | 1.7 (1.9)* | 0.6 (0.5) | ||
| PCWP/CO slope | 4.0 (3.1)* | 1.4 (1.1) | ||
| Ventriculography | ||||
| Right ventricular ejection fraction, % | 50 (8)* | 50 (9)* | 52 (8) | 55 (8) |
| Left ventricular ejection fraction, % | 62 (9)* | 65 (8)* | 64 (8) | 69 (7) |
denotes P<0.05 comparing high vs normal PAP/CO slope groups
Lastly, both resting right and left ventricular systolic function were lower among those with elevated PAP/CO slopes (Table 2). Further, 38% of those with high PAP/CO slope had abnormal right ventricular reserve (defined as the inability to augment right ventricular EF with exercise), compared with only 26% among those with normal PAP/CO slope (P=0.001). After adjusting for age and sex, high PAP/CO slope conferred a 1.9-fold increased odds of abnormal right ventricular reserve (OR 1.86, 95% CI 1.28, 2.72, P=0.001). By contrast, there were no differences in left ventricular reserve by PAP/CO slope groups (P=0.18 in age- and sex-adjusted analysis).
Clinical Correlates of Exercise Pulmonary Vascular Response
In a multivariable stepwise model, independent predictors of abnormal PAP/CO slope included age, smoking history, and prior HF and chronic lung disease. Specifically, a history of HF, ILD, or COPD each conferred a more than 2-fold increased odds of abnormal PAP/CO slope (P≤0.003 for all, Supplemental Table 1). In models including pulmonary function testing, ILD, COPD, and smoking were no longer significant, but lower % predicted FEV1/FVC ratio, and DLCO each independently predicted abnormal PAP/CO slope (P <0.01 for all).
Exercise Pulmonary Vascular Responses Predict Clinical Outcomes
Over a mean follow-up of 3.7 ± 2.9 years after exercise testing, 167 participants experienced a non-elective CV hospitalization and 80 died. There were 39 individuals experiencing CV hospitalization followed by death, hence 208 experienced the combined endpoint of CV hospitalization and/or death. A total of 32% of CV hospitalizations were attributed to heart failure (primary signs and symptoms of congestion requiring IV diuretics) in isolation, with less frequent hospitalizations related to coronary artery disease, valvular heart disease, or arrhythmias. As shown in Figure 1, patients with high PAP/CO slope had worse CV event-free survival compared with the normal PAP/CO slope group (P log-rank <0.0001). When subdivided into patients with rest PH (rest PAP > 20mmHg) versus exPH (rest PAP ≤25 mmHg and PAP/CO slope >3 mmHg/L/min), both groups had worse CV event-free survival compared with patients without evidence of either rest or exercise PH (Central Illustration, P log-rank <0.0001 for all pairwise comparisons).
Figure 1. Pulmonary hemodynamic responses to exercise are associated with future clinical outcomes.
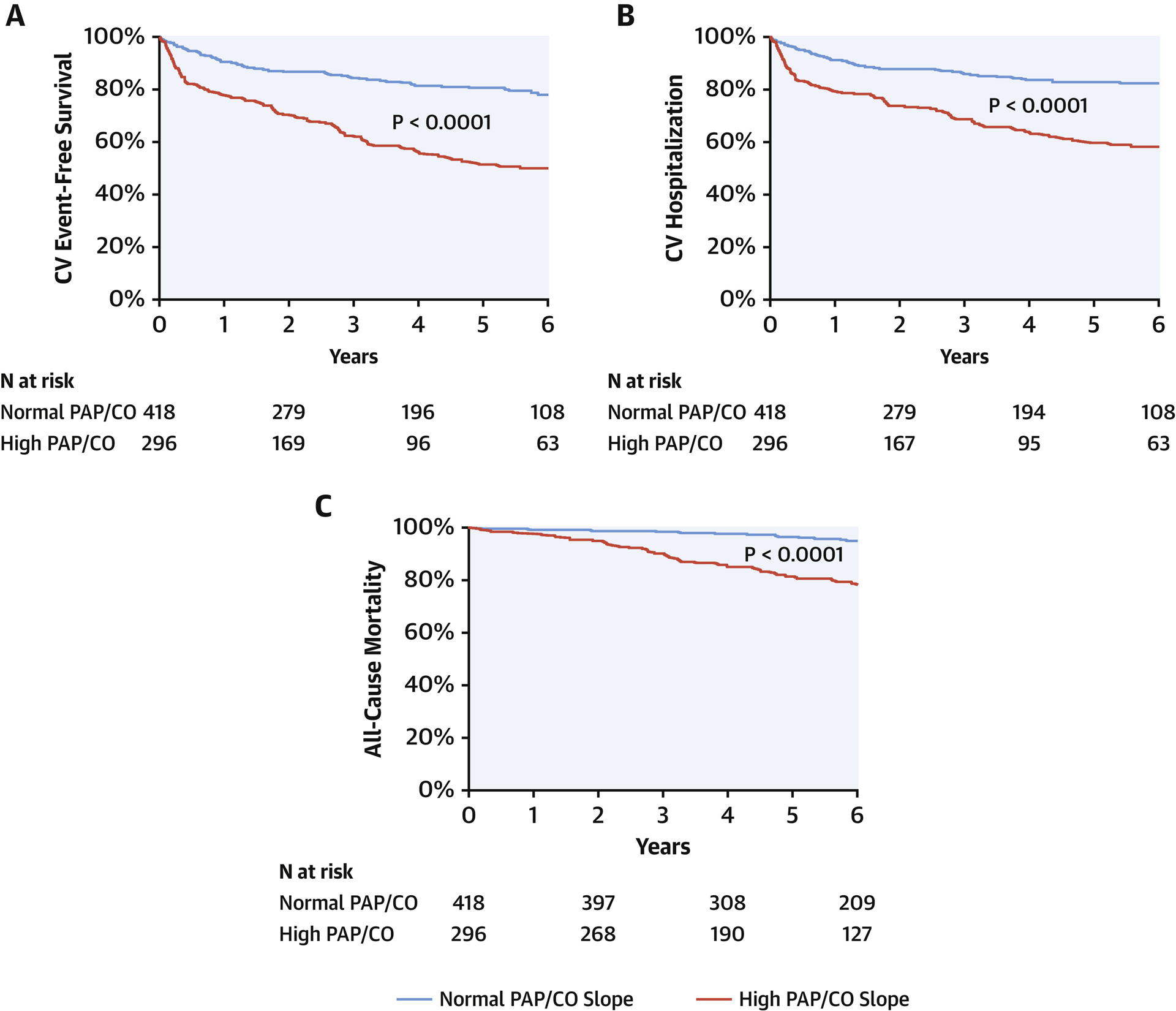
Kaplan-Meier curves for clinical outcomes in patients with high PAP/CO slope (defined as PAP/CO slope >3 mmHg/L/min, shown in solid line) vs normal PAP/CO slope (dotted line). Panel A shows combined primary endpoint of CV hospitalization or all-cause death, panel B shows CV hospitalization, and panel C shows all-cause death.
Central Illustration. Cardiovascular Event-Free Survival Among Individuals with Dyspnea by Pulmonary Hypertension Status.
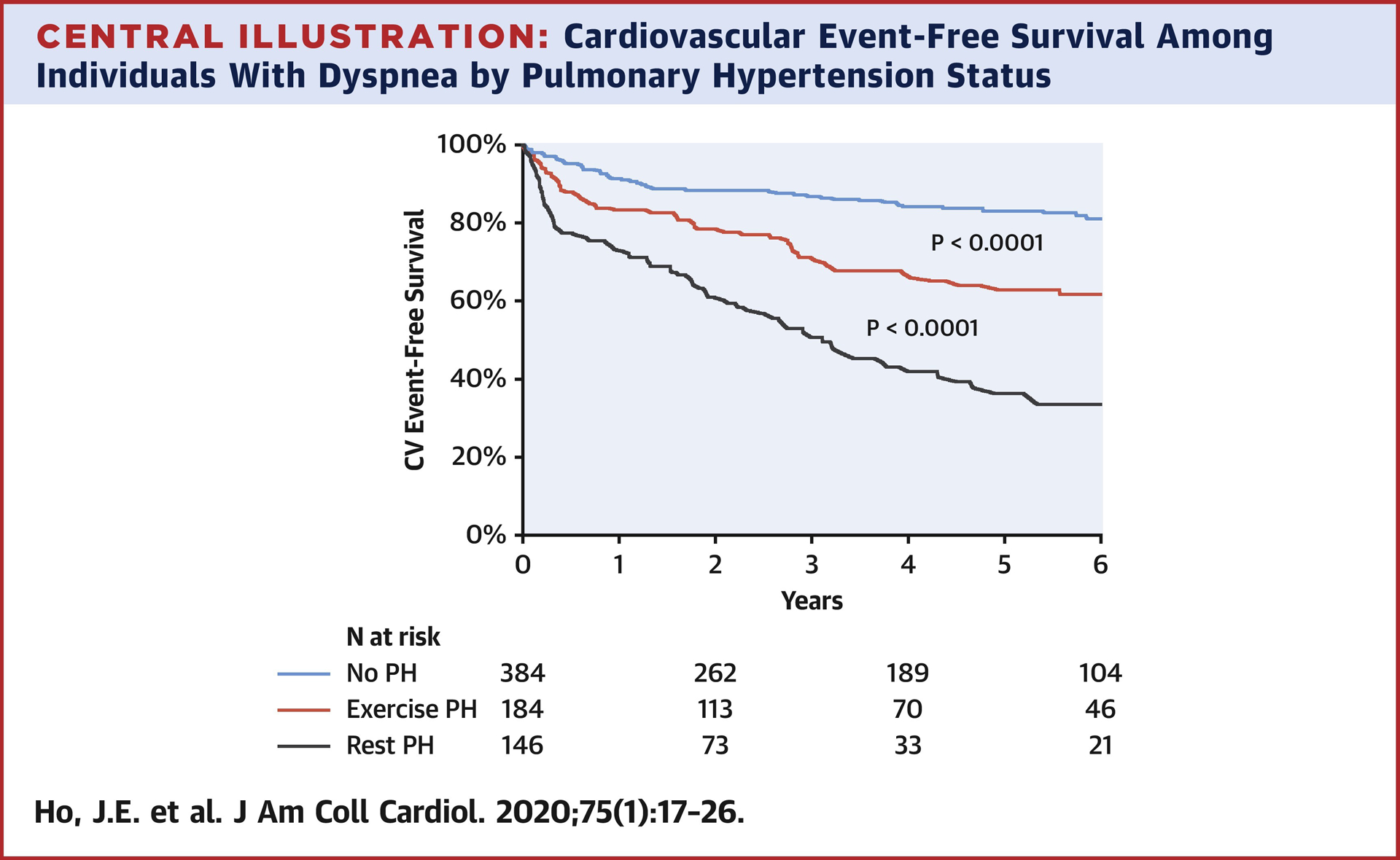
Colors designate those without PH (blue), rest PH (red, defined as rest PAP >20 mmHg), and exercise PH (yellow, defined as rest PAP ≤20 mmHg and PAP/CO slope >3 mmHg/L/min).
In multivariable Cox models, high PAP/CO slope was independently associated with >2-fold increased risk of CV hospitalization or death (HR 2.03, 95%CI 1.48, 2.78, P<0.001) in models adjusted for potential clinical confounders including age, sex, hypertension, prior heart failure, COPD, ILD, and smoking status (Table 3). High PAP/CO slope remained independently associated with CV hospitalization or death after additional adjustment for baseline resting PAP (HR 1.64, 95%CI 1.15, 2.34, P=0.006). Among the subset of individuals with rest PAP 21–29 mmHg, PAP/CO slope >3 mmHg/L/min was similarly associated with CV event-free survival (multivariable-adjusted HR 1.91, 95%CI 1.15–3.19, P=0.01).
Table 3.
High PAP/CO slope is associated with clinical outcomes
| Sample | Outcome | Age- and sex-adjusted model | Multivariable-adjusted model* | ||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| All, n=714 | CV hospitalization or death, n=208 | 2.29 (1.70, 3.10) | <0.001 | 2.03 (1.48, 2.78) | <0.001 |
| CV hospitalization, n=167 | 2.39 (1.71, 3.34) | <0.001 | 2.25 (1.58, 3.20) | <0.001 | |
| All-cause mortality, n=80 | 2.76 (1.64, 4.66) | <0.001 | 2.10 (1.22, 3.60) | 0.007 | |
| Sensitivity analyses | |||||
| Excluding rest PH, n=146 | CV hospitalization or death, n=116 | 1.94 (1.33, 2.82) | <0.001 | 1.75 (1.21, 2.54) | 0.003 |
| Excluding prevalent CVD, n=184 | CV hospitalization or death, n=125 | 2.22 (1.49, 3.31) | <0.001 | 1.98 (1.31, 2.99) | 0.001 |
Multivariable model adjusted for age, sex, hypertension, prevalent heart failure, COPD, ILD, smoking status. HR represents high PAP/CO group with normal PAP/CO serving as referent.
In sensitivity analyses, high PAP/CO slope remained a significant predictor of CV event-free survival after excluding individuals with rest PH (n=146 excluded, Table 3; multivariable-adjusted HR 1.75, 95%CI 1.21–2.54, P <0.001) as well as prevalent cardiovascular disease (n=184; HR 1.98, 95%CI 1.31–2.99, P=0.001).
In secondary analyses, the use of an alternative definition of exPH (elevated peak exercise PAP PAP of >30 mmHg with total pulmonary resistance >3 among individuals with rest PAP ≤20 mmHg) was also associated with CV event free survival (n=195, HR 2.01, 95%CI 1.37–2.95, P<0.001). Further, indviduals with exPH defined by either this or PAP/CO slope appeared to have similar outcomes (P log rank 0.11). By contrast, in the absence of elevated PAP/CO slope, individuals with an absolute peak exercise PAP of >30 mmHg (n=173) had similar CV event-free survival as those without any evidence of PH (Supplemental Figure 1, P log rank 0.43).
Intrinsic and Post-Capillary Components of Exercise Pulmonary Vascular Responses
In secondary analyses, we separately examined the TPG and PCWP components of exercise PAP response using analogous multi-point TPG/CO and PCWP/CO slopes as predictors of clinical outcomes. As expected, correlations between PAP/CO slope and its components were significant (Spearman’s rho = 0.49, P<0.0001 for TPG/CO slope; rho = 0.78, P<0.0001 for PCWP/CO slope).
In multivariable-adjusted Cox models, both TPG/CO slope and PCWP/CO slope were independently associated with the primary outcome of CV event-free survival (Table 4). Specifically, a 1-standard-deviation increase in TPG/CO slope was associated with a 27% increased hazard (multivariable-adjusted HR 1.27, 95%CI 1.14–1.42, P<0.001), and a 1-standard-deviation increase in PCW/CO slope was associated with a 28% increased hazard of CV hospitalization or death (HR 1.28, 95%CI 1.17–1.41, P<0.001).
Table 4.
TPG and PCWP components of PAP/CO as predictors of clinical outcomes
| CV hospitalization or death | CV hospitalization | Death | ||||
|---|---|---|---|---|---|---|
| HR (95% CI)* | P-value | HR (95% CI)* | P-value | HR (95% CI)* | P-value | |
| PAP/CO slope | 1.31 (1.20, 1.43) | <0.001 | 1.21 (1.08, 1.36) | 0.001 | 1.22 (1.07, 1.39) | 0.002 |
| TPG/CO slope | 1.27 (1.14, 1.42) | <0.001 | 1.22 (1.07, 1.39) | 0.002 | 1.21 (1.08, 1.36) | 0.001 |
| PCW/CO slope | 1.28 (1.17, 1.41) | <0.001 | 1.21 (1.08, 1.36) | 0.001 | 1.22 (1.07, 1.39) | 0.002 |
Multivariable model adjusted for age, sex, hypertension, prevalent heart failure, COPD, ILD, smoking status. HR per 1-standard deviation increase in log-transformed pressure/flow slope.
Discussion
We prospectively studied pulmonary arterial pressure responses to exercise among patients with chronic dyspnea on exertion and related invasive hemodynamics to clinical outcomes. In this rigorously phenotyped sample of >700 patients with >7,000 matched PAP and CO measurements throughout exercise, we find the following: (1) clinical determinants of exercise PH, as defined by a PAP/CO slope >3mmHg/L/min, include older age and underlying cardiovascular and pulmonary disease; (2) exercise PH is associated with worse functional capacity and abnormal right ventricular contractile reserve; and (3) the presence of exercise PH predicts worse CV event-free survival. Importantly, adverse prognosis remains associated with exercise PH even in the absence of rest PH, or known CVD in our sample. Further, in dissecting pre- and post-capillary contributions to exercise PH, we find that both components are independently associated with clinical outcomes. We also find that exPH predicts outcomes among patients with mild resting PH (mPAP 21–29 mmHg). These findings suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings coupled with a growing body of work defining pulmonary vascular responses to exercise further suggest that re-introduction of an exercise-based definition of PH in PH guidelines merits consideration.
Exercise elevations in mean PAP ≥30mmHg with PVR >1 wu have been previously shown to be associated with intermediate functional capacity relative to patients with normal exercise hemodynamics and patients with resting PAH(10). Exercise pulmonary responses have also previously been described among smaller samples in the context of specific disease entities. In these settings, exercise PH portended the future development of PH in patients with systemic sclerosis or connective tissue disease and among family members of patients with pulmonary arterial hypertension (PAH) carrying BMPR2 mutations (13–16). Exercise PH also predicted worse clinical outcomes among patients with PAH or chronic thromboembolic PH, and improved with initiation of pulmonary-specific vasodilator therapy among PAH patients (17, 22, 23). Our study represents the largest sample of individuals with chronic dyspnea to date with comprehensive ascertainment of exercise PH. We build upon previous studies focused on distinct disease conditions with outcomes, and extend findings by underscoring the clinical significance of exercise pulmonary vascular responses across a broad sample of individuals with chronic exertional dyspnea and preserved LVEF.
While rest PH as previously defined (mean PAP ≥25 mmHg) clearly portends adverse outcomes, more recent studies demonstrate worse prognosis even among individuals with mean PAP in the 21–24 mmHg range (4,24,25). The new definition of PH, which represents PAH when present in conjunction with PVR ≥3 based on the definition advanced at the WHO Nice Consortium, in turn appears associated with exercise PH (25). We find that even in the absence of rest PH based on the new definition, exercise PH is associated with worse cardiovascular event-free survival in our sample, albeit with intermediate risk when compared with rest PH (Central Illustration). In addition, in patients with mild PH (mPAP 21–29 mmHg) those with a steep PAP/CO slope have a worse prognosis than those with a normal PAP/CO slope. This suggests that exercise testing can be used to unmask abnormal PAP in at-risk individuals and to refine risk in individuals with mild resting PH. Whether unmasking PH with exercise will inform initiation of efficacious preventive and targeted treatment strategies in the future needs to be evaluated. In addition, these findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate exPH (26), particularly in light of the consistent findings related to PAP/CO slopes in previous studies including both modalities (8).
It is important to note that PAP/CO slope may be abnormal in the setting of pulmonary vascular remodeling, vasoconstriction, or cardiac dysfunction with elevated left-sided filling pressures (7). Our study offers more granular insights into cardiac vs pulmonary vascular contributions to exercise PH. In a prior study of patients with systemic sclerosis, elevated PAP/CO slopes were observed without concomitant increases in PCW/CO slope, suggesting predominantly intrinsic pulmonary vascular dysfunction (27). By contrast, we find that in our inclusive sample with a range of cardiopulmonary comorbidities, intrinsic pre- and post-capillary components of exercise PH ascertained via TPG/CO and PCWP/CO slopes, respectively, are both independently associated with prognosis. Our study highlights the functional significance of exPH in that abnormal PAP/CO slope is associated with reduced exercise capacity and abnormal right ventricular reserve with exercise. These findings are consistent with prior studies relating exercise pulmonary vascular function and distensibility to functional capacity and outcomes in various smaller samples (11,27–29).
There are several limitations that deserve mention. We studied a consecutive sample of patients referred for cardiopulmonary exercise testing in the setting of chronic dyspnea. As such, we recognize that referral bias could have influenced results, and generalizability to other samples needs to be considered. Repeated invasive CPET has not been routinely performed at our institution after its use for initial diagnostic evaluation in order to limit patient burden. As such, our study focused on relationships of one-time assessments of exPH to clinical outcomes. Future investigation of serial rest and exercise hemodynamic measurements are warranted to further characterize the natural history of exPH, including its relationship to long-term outcomes and PAH progression. This will be informed by ongoing work by the international PEX-NET consortium (30). Further work is also needed to determine the relative prognostic value of exercise hemodynamic measurements performed in the supine vs. upright position and across a broad range of referral cohorts. These limitations notwithstanding, strengths of our study include rigorous ascertainment of hemodynamic responses throughout exercise, performance of upright exercise to reflect the usual physiologic state of exercise in humans, use of a PAP/CO slope to eliminate reliance on accurate zero-level measurements which are less well established in the upright vs. supine position (31), simultaneous assessments of peak exercise capacity and rest and exercise RVEF to ascertain the functional significance of exPH, and the comprehensive follow up that permitted ascertainment of outcomes in relation to exPH. As described recently, indexing PAP to CO is preferable to an absolute PAP cut point to define abnormal exPH (7,9,32), and indeed we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP of >30 mmHg does not portend worse outcomes compared with those with normal exercise PAP.
In sum, we demonstrate that exercise PH is associated with worse CV event-free survival even in the absence of rest PH across a broad sample of individuals with chronic dyspnea. Our findings highlight the potential clinical relevance of exercise PH within the overall spectrum of PH and the role of exercise testing to uncover early manifestations of PH. Future studies are needed to examine whether identification of this at-risk population may lend itself to targeted therapeutic interventions.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
Abnormal pulmonary arterial pressure responses to exercise are independently associated with adverse clinical outcomes among patients with chronic dyspnea, even when pulmonary arterial pressure is normal at rest.
Translational Outlook:
More research is needed to clarify the therapeutic implications of an abnormal pulmonary hemodynamic response and how specific interventions influence the precapillary and post-capillary components of exercise-induced pulmonary hypertension.
Funding:
This work was supported by grants from the NIH/NHLBI R01-HL134893 (JEH), R01-HL140224 (JEH), R01-HL142809 (RM), R01-HL131029 (GDL), a Gilead Sciences Research Scholar Award (JEH), and the American Heart Association 15GPSGC24800006 (GDL) and the MGH Heart Failure Research Innovation Fund. Sponsors had no role in the design, conduct, or decision to publish the work.
ABBREVIATIONS
- CO
cardiac output
- CPET
cardiopulmonary exercise testing
- DLCO
diffusion capacity of carbon monoxide
- ExPH
exercise pulmonary hypertension
- FEV1
Forced expiratory volume in one second
- FVC
forced vital capacity
- PAP
mean pulmonary artery pressure
- LVEF
left ventricular ejection fraction
- PCWP
pulmonary capillary wedge pressure
- TLC
total lung capacity
- TPG
trans-pulmonary gradient
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Wijeratne DT, Lajkosz K, Brogly SB et al. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11:e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Galiè N, Rubin LJ et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BA, Hess E, Maddox TM et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation. 2016;133:1240–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman AP. Pulmonary circulation. Handbook of physiology The Respiratory system Circulation and nonrespiratory functions. 1985;1:93–166. [Google Scholar]
- 6.Simonneau G, Robbins IM, Beghetti M et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GD, Bossone E, Naeije R et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–79. [DOI] [PubMed] [Google Scholar]
- 8.Naeije R, Vanderpool R, Dhakal BP et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs G, Hervé P, Barbera JA et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50pii:1700578. [DOI] [PubMed] [Google Scholar]
- 10.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degani-Costa LH, Levarge B, Digumarthy SR, Eisman AS, Harris RS, Lewis GD. Pulmonary vascular response patterns during exercise in interstitial lung disease. Eur Respir J. 2015;46:738–49. [DOI] [PubMed] [Google Scholar]
- 12.Grünig E, Tiede H, Enyimayew EO et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–15. [DOI] [PubMed] [Google Scholar]
- 13.Kusunose K, Yamada H, Hotchi J et al. Prediction of Future Overt Pulmonary Hypertension by 6-Min Walk Stress Echocardiography in Patients With Connective Tissue Disease. J Am Coll Cardiol. 2015;66:376–84. [DOI] [PubMed] [Google Scholar]
- 14.Hinderhofer K, Fischer C, Pfarr N et al. Identification of a new intronic BMPR2-mutation and early diagnosis of heritable pulmonary arterial hypertension in a large family with mean clinical follow-up of 12 years. PLoS ONE. 2014;9:e91374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriels C, Lancellotti P, Van De Bruaene A et al. Clinical significance of dynamic pulmonary vascular resistance in two populations at risk of pulmonary arterial hypertension. Eur Heart J Cardiovasc Imag. 2015;16:564–70. [DOI] [PubMed] [Google Scholar]
- 16.Codullo V, Caporali R, Cuomo G et al. Stress Doppler echocardiography in systemic sclerosis: evidence for a role in the prediction of pulmonary hypertension. Arthritis Rheum. 2013;65:2403–11. [DOI] [PubMed] [Google Scholar]
- 17.Stamm A, Saxer S, Lichtblau M et al. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur Respir J. 2016;48:1658–67. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GD, Shah R, Shahzad K et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. [DOI] [PubMed] [Google Scholar]
- 19.Lewis GD, Murphy RM, Shah RV et al. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hervé P, Lau EM, Sitbon O et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46:728–37. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino R, Viegi G, Brusasco V et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 22.Hasler ED, Müller-Mottet S, Furian M et al. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest. 2016;150:57–67. [DOI] [PubMed] [Google Scholar]
- 23.Chaouat A, Sitbon O, Mercy M et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;44:704–13. [DOI] [PubMed] [Google Scholar]
- 24.Douschan P, Kovacs G, Avian A et al. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am J Respir Crit Care Med. 2018;197:509–16. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs G, Avian A, Tscherner M et al. Characterization of patients with borderline pulmonary arterial pressure. Chest. 2014;146:1486–93. [DOI] [PubMed] [Google Scholar]
- 26.Argiento P, Chesler N, Mulè M et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J. 2010;35:1273–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs G, Avian A, Wutte N et al. Changes in pulmonary exercise haemodynamics in scleroderma: a 4-year prospective study. Eur Respir J. 2017;50:1601708. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra R, Dhakal BP, Eisman AS et al. Pulmonary Vascular Distensibility Predicts Pulmonary Hypertension Severity, Exercise Capacity, and Survival in Heart Failure. Circ Heart Fail. 2016;9; pii:e0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau EMT, Godinas L, Sitbon O et al. Resting pulmonary artery pressure of 21–24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J. 2016;47:1436–44. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs G, Herve P, Olschewski H. The pulmonary haemodynamics during exercise–research network (PEX-NET) ERS Clinical Research Collaboration: investigating the prognostic relevance of exercise haemodynamics. Eur Respir J. 2019;53:1900458; [DOI] [PubMed] [Google Scholar]
- 31.Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190:252–57. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs G, Avian A, Olschewski H. Proposed new definition of exercise pulmonary hypertension decreases false-positive cases. Eur Respir J. 2016;47:1270–73. [DOI] [PubMed] [Google Scholar]
- 33.Ley B, Ryerson CJ, Vittinghoff E et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


