Abstract
Palbociclib, in conjunction with endocrine therapy, has been approved for the treatment of patients with advanced breast cancer. The common hematological toxicities associated with palbociclib are leukopenia and neutropenia. However, hematological malignancies have not been reported for palbociclib treatment. Here, for the first time, we present a case of acute lymphoblastic leukemia that was diagnosed in a patient undergoing treatment with letrozole and palbociclib for metastatic breast cancer. This case emphasizes the need for long term follow up of patients treated with palbociclib.
Keywords: Adverse effects, Breast neoplasms, Hematologic neoplasms, Palbociclib, Precursor cell lymphoblastic leukemia-lymphoma
INTRODUCTION
Breast cancer is the most common type of cancer among women worldwide. It is the leading cause of cancer related deaths in women (25%) and in the global population (14%) [1]. Hormone receptor (HR)-positive breast cancer is the largest therapeutic subtype, accounting for 55% to 77% of all cases of breast cancer globally [2].
Hormonal blockade is the most widely used treatment for patients with advanced HR-positive breast cancer [3]. However, acquired resistance to endocrine therapy can develop in a large subset of patients with metastatic breast cancer, necessitating the discovery of new therapeutic approaches [4].
Palbociclib (Ibrance®; Pfizer, New York, USA) is a small molecule inhibitor of cyclin-dependent kinase 4 and 6 (CDK 4/6). Based on the results of an international randomized, double-blind, placebo-controlled clinical trial comparing palbociclib plus letrozole versus letrozole monotherapy for the first-line treatment of postmenopausal women with estrogen-receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer (PALOMA-2), the United States Food and Drug Administration granted regular approval for the use of palbociclib in combination with an aromatase inhibitor as the initial endocrine based therapy for the treatment of HR-positive, HER2-negative, advanced or metastatic breast cancer in postmenopausal women [5]. The hematological adverse effects associated with palbociclib treatment include leukopenia, neutropenia, anemia, and thrombocytopenia. However, hematological malignancies have not been reported for palbociclib treatment.
For the first time, we present the case of a patient who was diagnosed with acute lymphoblastic leukemia (ALL) while undergoing treatment with letrozole and palbociclib for metastatic breast cancer.
CASE REPORT
A 62-year-old woman was referred to our institution following the detection of a nodule in the left breast by screening mammography in April 2018. She had no significant past medical history or family history of breast cancer and was not taking any medications. The patient was married and have given birth once. Physical examination revealed a round mass having a diameter of 1.5 cm in the upper region of the left breast.
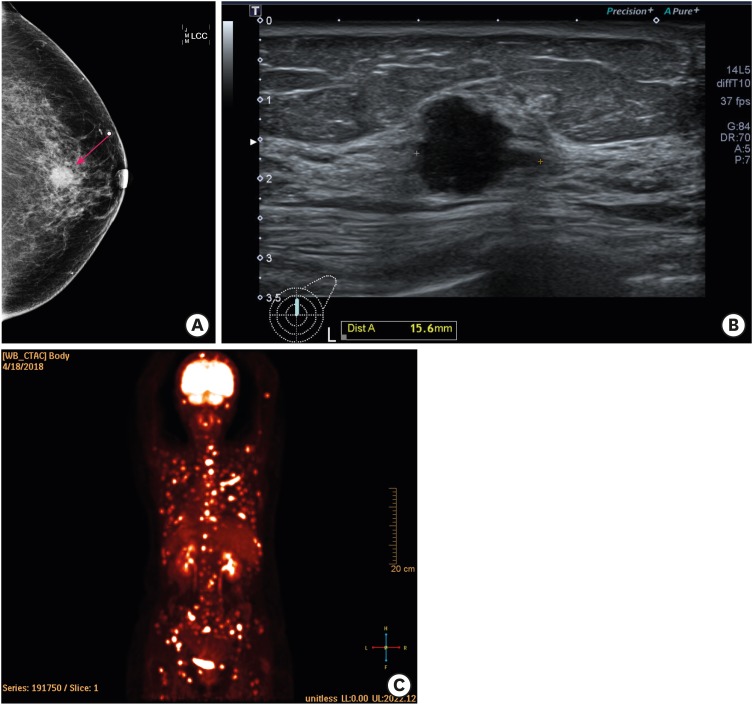
Mammography revealed an indistinct, irregular, high density mass of dimension 1.6 cm × 1.7 cm in the upper region of the left breast (Figure 1A). On ultrasonography, a microlobulating irregular hypoechoic mass, having a diameter of 1.6 cm, was found at the 12 o'clock position (Figure 1B). Ultrasonography did not reveal any enlarged lymph nodes in the left axilla. The irregular mass was classified as Breast Imaging-Reporting and Data System category 5. Breast magnetic resonance imaging revealed that the total extent of the mass was 3.5 × 2.5 × 2.0 cm. Contrast enhanced chest-abdomen-pelvis computed tomography (CT) only revealed a mass in the left breast with no evidence of systemic metastasis. However, positron emission tomography-CT (PET-CT) revealed hypermetabolic bone lesions throughout the skeletal system, including the skull, entire spine, both sides of the rib cage, clavicles, scapulae, humerus, pelvic bones, and femurs (Figure 1C). The patient did not have any bone pain.
Figure 1. Images of breast cancer (A) Mammogram showing an irregular high density mass in the upper region of the left breast (indicated by arrows). (B) Ultrasonography image showing a microlobulating irregular hypoechoic mass at the 12 o'clock position. (C) 18F-fluorodeoxyglucose positron emission tomography/computed tomography image demonstrating hypermetabolism in the mass in the left breast and diffuse bone.
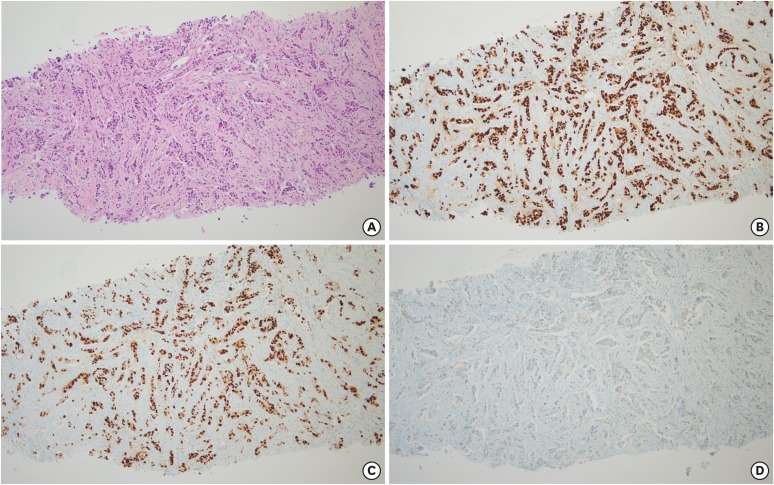
Needle biopsy of the mass in the left breast revealed an invasive ductal carcinoma (Figure 2A). The results of immunochemistry were as follows: estrogen receptor (95%), progesterone receptor (95%), HER2 (−), and Ki 67 index (15%) (Figure 2B). The nuclear and histological grades of the carcinoma were determined to be 2 and II, respectively. Based on these findings, the patient was diagnosed with HR-positive, HER2-negative metastatic breast cancer. Since she was postmenopausal and as the metastatic sites were confined to the bones, she was started on 2.5 mg letrozole and 150 mg palbociclib in April 2018.
Figure 2. Histopathological findings of breast cancer. (A) Hematoxylin-eosin staining showing invasive ductal carcinoma at a magnification of 100×. (B-D) The results of immunohistochemistry revealed that the tumor was estrogen receptor-positive, progesterone receptor-positive, and human epidermal growth factor receptor 2-negative (magnification 100×).
The patient experienced grade 2 neutropenia after the first cycle of letrozole and palbociclib, and dose adjustment for palbociclib was not required. The response to 3 cycles of letrozole and palbociclib was a stable disease. However, she developed grade 3 neutropenia and fever after seven cycles of therapy, and the dose of palbociclib was reduced to 100 mg in November 2018. During the time she was undergoing treatment with letrozole and palbociclib, her hemoglobin level was 11–12 g/dL. After ten cycles of therapy, she developed anemia and her hemoglobin level, as of March 2019, was 8.4 g/dL. After eleven cycles of treatment, the hemogram worsened in general. The total leukocyte count, hemoglobin, and platelet count were 1,340 per µL, 5.5 g/dL, and 27 × 103 per µL, respectively. The letrozole and palbociclib therapy was discontinued, and she received packed red blood cell and plateletpheresis transfusion. Three weeks later, the hemogram improved, and the total leukocyte count, hemoglobin, and platelet count were 3,690 per µL, 7.9 g/dL, and 147 × 103 per µL, respectively. She subsequently resumed treatment with the 12th cycle of letrozole and palbociclib. Two weeks later, she developed grade 4 thrombocytopenia, and her platelet count was 33 × 103 per µL. Although palbociclib was discontinued for one week, the thrombocytopenia continued to worsen, her platelet count dropped to 11 × 103 per µL, and she developed multiple bruises. Palbociclib was discontinued for one month, and she frequently received platelet transfusion. The tumor response continued to be stable disease after the completion of 11 cycles. However, as the thrombocytopenia did not improve, she was admitted to the hospital for bone marrow biopsy in June 2019. The hemogram revealed that her hemoglobin was 6.5 g/dL, total leukocyte count was 1,650 per µL (differential leukocyte count: neutrophils 47%, lymphocytes 48%, monocytes 3%, and immature cells 2%), and platelet count was 30 × 103 per µL.
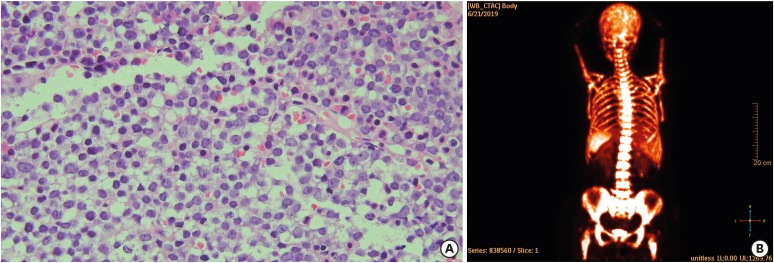
Bone marrow examination revealed medium sized cells with moderate amounts of basophilic cytoplasm, cytoplasmic vacuoles, loose nuclear chromatin, and multiple prominent nucleoli, which accounted for 41.2% of the total nucleated cells (Figure 3A). Flow cytometry analysis of the bone marrow aspirate revealed leukocytes with low cluster of differentiation (CD) 45 expression and leukocytes with CD10, CD19, CD22, CD56, cytoplasmic CD79a, and lambda chain expression. Immunohistochemical staining of the biopsy section revealed positivity for CD10, B-cell lymphoma 2, MYC, Ki-67, terminal deoxynucleotidyl transferase, Pax5, CD56, and CD43. Conventional cytogenetic and/or fluorescent in situ hybridization studies revealed a normal karyotype devoid of cytogenetic abnormalities. Based on these findings, a confirmatory diagnosis of precursor B cell ALL (B-ALL) with aberrant CD56 expression was made. PET-CT demonstrated diffuse hypermetabolism along the axial and appendicular bones as well as in the spleen (standardized uptake value–7.4) (Figure 3B). There was no residual hypermetabolism in the tumor in the left breast. The patient achieved complete remission after receiving induction chemotherapy as per the conventional protocol for B-ALL. Concurrently, she received letrozole for breast cancer, and she is presently continuing treatment with letrozole.
Figure 3. Histology of bone marrow biopsy and 18F-FDG PET/CT scan of acute lymphoblastic leukemia (A) Bone marrow examination showing abnormal lymphocytes with hematoxylin-eosin staining, at a magnification of 400×, (B) 18F-FDG PET/CT image demonstrating diffuse hypermetabolism along the whole axial and appendicular bones and spleen with no residual hypermetabolism in the mass in the left breast.
FDG = fluorodeoxyglucose; PET/CT = positron emission tomography/computed tomography.
This case report was approved by the Institutional Review Boards of Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB No. 30-2019-77) and written in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
DISCUSSION
The G1-S phase is an important restriction point in the normal cell cycle that is deregulated in cancer cells, resulting in uncontrolled cell proliferation. CDKs including CDK4 and CDK6 play a critical role in the G1-S phase transition [6]. The inhibition of CDKs blocks the phosphorylation of the downstream retinoblastoma protein, leading to cell cycle arrest in the G1 phase in vitro and tumor regression in vivo [7,8]. The oral administration of the CDK4/6 inhibitor palbociclib alone is associated with significant tumor reduction in human tumor xenografts [6]. Several CDK 4/6 inhibitors including palbociclib, abemaciclib, and ribociclib have shown promising anticancer effects and manageable toxicity in clinical trials.
Palbociclib is the first CDK 4/6 inhibitor to be approved to be used in conjunction with aromatase inhibitors for the treatment of patients with advanced breast cancer [5]. Palbociclib is also used in combination with fulvestrant to treat advanced-stage or metastatic, HR-positive, HER2-negative breast cancer that has progressed on hormonal therapy in postmenopausal women [9]. The most common adverse events (AEs) reported in patients receiving palbociclib plus letrozole in randomized clinical trials were neutropenia (79.5%), leukopenia (39.0%), fatigue (37.4%), nausea (35.1%), arthralgia (33.3%), alopecia (32.9%), and diarrhea (26.1%). Grade 3–4 neutropenia and leukopenia were observed in 66% and 25% of the patients, respectively. Anemia and thrombocytopenia were reported in 24.1% and 15.5% of the cases, respectively. Grade 3–4 thrombocytopenia was rarely observed i.e., in 1.6% of the patients [5]. The long-term pooled safety analysis of palbociclib was recently published that reports the long-term effects after up to 50 months of treatment [10]. Based on these results, there are no specific cumulative or delayed toxicities associated with palbociclib.
ALL is the most prevalent type of cancer as well as the most common form of leukemia in children. Leukemia involves loss of normal proliferation and interruption in the complete differentiation into mature cells [11]. More than 80% of ALL cells in children stop proliferating in the G1 phase, and only a few cells remain in G0 [12]. CDK 4/6 inhibitors are known to induce cell cycle arrest at G1 [6]. Cell cycle arrest and subsequent apoptosis are considered to be the most relevant mechanisms of action of CDK 4/6 inhibitors. Numerous human cancer cells have genomic or transcriptional alterations that activate CDK 4/6 [13], and these cells are more sensitive to CDK 4/6 inhibitors than normal cells. However, it was not possible to predict whether normal cells have a particular dependence on CDK 4/6. Although this study did not establish the casual relationship between palbociclib and ALL, the findings suggested that palbociclib was related to ALL in this case. We can hypothesize that the inhibition of CDK 4/6 promoted cell cycle arrest, which contributed to the proliferation of leukemic cells in this patient.
Therapy-related leukemia has been frequently described following treatment with cytotoxic therapies, including chemotherapy and/or radiotherapy for solid or hematologic cancers [14,15]. The majority of such cases are therapy-related acute myeloid leukemia/myelodysplastic syndrome with well characterized cytogenetic and molecular features [14]. In contrast, therapy-related ALL (t-ALL) has been infrequently described and remains poorly defined. The frequency of t-ALL in therapy-related leukemia is 10% to 15% [14]. The latency from prior diagnosis of cancer to t-ALL varies among cases, with a median latency of 6.8 years (range, 0.8–50.7) [14]. Clinically, the incidence of t-ALL in older patients has a poorer prognosis than de novo ALL, and is more prevalent in women and individuals from a white ethnic background [14]. A cohort study of t-ALL reported that the Philadelphia chromosome was the most common cytogenetic abnormality, followed by the normal karyotype, and the mixed lineage leukemia gene arrangement [15]. In the present case, the time interval from the diagnosis of cancer to development of t-ALL was 1.16 years in a female patient with a normal karyotype. As the mechanisms underlying the incidence of t-ALL are poorly understood, it is difficult to prove the causal relationship between palbociclib and t-ALL. According to definition, t-ALL is described as ALL that develops in patients who have received cytotoxic cancer therapy, and therefore this case should be considered as t-ALL with caution.
To the best of our knowledge, this report is the first case of lymphoid malignancy diagnosed following palbociclib treatment. Palbociclib was recently made commercially available, from November 2016, and no hematological malignancies have been reported yet in association with palbociclib therapy in the literature. It is possible that ALL is associated with palbociclib treatment. This case emphasizes the need for further research for understanding hematological malignancy as an AE following palbociclib treatment. It also emphasizes the need for long term follow up of patients who receive palbociclib treatment.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Park JH.
- Data curation: Park JH, Park H, Roh EY, Kim JE, Chang MS.
- Resources: Roh EY, Kim JE, Chang MS.
- Writing - original draft: Park JH.
- Writing - review & editing: Park JH, Park H, Kim KH, Kim JS, Choi IS, Roh EY, Kim JE, Chang MS.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 3.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT. Changing concepts of hormone receptor-positive advanced breast cancer therapy. Clin Breast Cancer. 2013;13:159–166. doi: 10.1016/j.clbc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro . Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 10.Diéras V, Rugo HS, Schnell P, Gelmon K, Cristofanilli M, Loi S, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. J Natl Cancer Inst. 2019;111:419–430. doi: 10.1093/jnci/djy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matushansky I, Radparvar F, Skoultchi AI. Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin-dependent kinases in G1 . Proc Natl Acad Sci U S A. 2000;97:14317–14322. doi: 10.1073/pnas.250488697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller PR, Meier R, Hirt A, Bodmer JJ, Janic D, Leibundgut K, et al. Nuclear lamin expression reveals a surprisingly high growth fraction in childhood acute lymphoblastic leukemia cells. Leukemia. 1994;8:940–945. [PubMed] [Google Scholar]
- 13.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6:353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribera JM. Therapy-related acute lymphoblastic leukemia. Haematologica. 2018;103:1581–1583. doi: 10.3324/haematol.2018.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldoss I, Stiller T, Tsai NC, Song JY, Cao T, Bandara NA, et al. Therapy-related acute lymphoblastic leukemia has distinct clinical and cytogenetic features compared to de novo acute lymphoblastic leukemia, but outcomes are comparable in transplanted patients. Haematologica. 2018;103:1662–1668. doi: 10.3324/haematol.2018.193599. [DOI] [PMC free article] [PubMed] [Google Scholar]