Abstract
Background and Objectives
Nationwide social inequalities of oral anticoagulation (OAC) usage after the introduction of non-vitamin K antagonist oral anticoagulants (NOACs) have not been well identified in patients with atrial fibrillation (AF). This study assessed overall rate and social inequalities of OAC usage after the introduction of NOAC in Korea.
Methods
Between January 2002 and December 2016, we identified 888,540 patients with AF in the Korea National Health Insurance system database. The change of OAC rate in different medical systems after the introduction of NOAC were evaluated.
Results
In all population, overall OAC use increased from 13.2% to 23.4% (p for trend <0.001), and NOAC use increased from 0% to 14.6% (p for trend <0.001). Compared with pre-reimbursement (0.48%), the annual increase of OAC use was significantly higher after partial (1.16%, p<0.001), and full reimbursement of OAC (3.72%, p<0.001). Full reimbursement of NOAC (adjusted odds ratio, 2.10; 95% confidence interval, 2.04–2.15) was independently associated with higher OAC use. However, the difference of overall OAC usage between tertiary referral hospitals and nursing or public health centers increased from 17.9% in 2010 to 36.8% in 2016. Moreover, usage rate of NOAC was significantly different among different medical systems from 37.2% at the tertiary referral hospital and 5.5% at nursing or public health centers.
Conclusions
Introduction of NOACs in routine practice for stroke prevention in AF was associated with improved rates of overall OAC use. However, significant practice-level variations in OAC and NOAC use remain producing social inequalities of OAC despite full reimbursement.
Keywords: Atrial fibrillation, Insurance, Anticoagulation, NOAC
INTRODUCTION
Stroke prevention is the principal management priority in patients with atrial fibrillation (AF) given its association with a 5-fold increase in stroke risk, and that 1 in 5 cases of stroke can be attributed to this arrhythmia.1),2) The non-vitamin K antagonist oral anticoagulants (NOACs) have been shown at least as effective and safe as warfarin with a lower incidence of intracranial hemorrhage.3) The preferred use of NOACs is recommended in guidelines,4),5),6) albeit with gradual uptake of their prescription in routine practice.7) Recent studies have suggested that the availability of NOACs may improve rates of use of overall oral anticoagulation (OAC) rate in patients with AF.8),9) Previously Lee et al.10) reported the differences in the utilization of antithrombotic therapy based on geographical regions and income levels among Korean population. However, social inequalities of OAC in different medical systems after introduction of NOAC have not been evaluated in large-scale nationwide studies. Accordingly, we analyzed data from the Korean National Health Insurance Service (NHIS) data to assess how the availability of NOACs has affected overall rate and social inequalities of OAC usage. We describe the temporal trends in OAC use, including both warfarin and NOACs, and patient factors associated with prescription of warfarin, NOACs. Second, we assessed the extent of practice-level variation in OAC and NOAC usage according to different medical systems.
METHODS
This nationwide study is based on the national health claims database established by the NHIS of Korea.11),12) The NHIS is the single insurer managed by the Korean government, and the vast majority (97.1%) of the Korean population are mandatory subscribers, with the remaining 3% of the population being medical aid subjects. Since 2006, information of Medical Aid beneficiaries has been incorporated into a single NHIS database. Therefore, the data extracted from the NHIS claims database are indeed based on the entire Korean population, in a nationwide cohort. The NHIS claims database includes diagnoses, procedures, biochemical test results, prescription records, and demographic information. The database is open to researchers, whose study protocols are approved by the official review committee. We confirmed diagnoses by using the International Classification of Disease, Tenth Revision (ICD-10) codes. This study was approved by the Institutional Review Board of Yonsei University Health System (4-2016-0179). The International Review Board waived the requirement to obtain informed consent, and this study was conducted in accordance with the tenets of the Declaration of Helsinki.
Study population
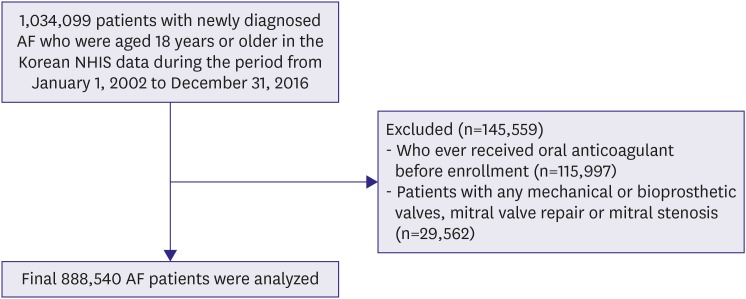
In the Korean NHIS data, 1,034,099 patients with prevalent AF who were aged ≥18 years were identified during the period from January 1, 2002 to December 31, 2016. The following were exclusion criteria: 1) those who ever received treatment with OAC before AF diagnosis (n=115,997), to exclude patients who had taken OAC for reasons other than AF such as venous thrombosis and 2) those with any of following conditions such as mitral valve stenosis and prosthetic valve replacement (ICD-10 codes I050, I052, I342; n=29,562). Finally 888,540 patients were included in the analysis (Figure 1). The annual prevalence of AF was calculated by dividing the number of AF patients of each year with exception for AF patients who died in previous year by the number of total Korean residents of that year. The annual incidence of AF was the number of incident cases of AF divided by the number of person-years at risk among all Korean residents of that year who had never been diagnosed as AF. AF was identified with ICD-10 codes; I48 (AF and atrial flutter), I48.0 (AF), and I48.1 (atrial flutter). To ensure accuracy, diagnosis was established based on one inpatient or 2 outpatient records of ICD-10 codes in the database.13),14) To evaluate the accuracy of our definition of AF, we conducted a validation study in 2 hospitals with 628 randomly chosen patients with the ICD-10 code I48. The patients were ascertained to have AF if it was documented by electrocardiogram examinations. The positive predictive value was found to be 94.1%.
Figure 1. Flowchart of study cohort enrollment.
AF = atrial fibrillation; NHIS = National Health Insurance Service.
Baseline comorbidities and endpoints
Baseline comorbidities were identified from medical claims according to ICD-10 codes and prescription codes and all comorbidities were established based on one inpatient or 2 outpatient records of ICD-10 codes in the database, similar to previous studies based with NHIS cohort. Hypertension, diabetes mellitus, heart failure, peripheral arterial disease, a history of myocardial infarction, a history of stroke and/or transient ischemic attack (TIA), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and end-stage renal disease (ESRD) were all assessed. The CHA2DS2-VASc score (2 points each for age 75 years or older and previous TIA or stroke; and 1 point each for heart failure, hypertension, age 65 years or older, diabetes mellitus, vascular disease, and female sex) for each subject was estimated at the end of the screening period, and every year during the follow-up period. Definitions of comorbidities are presented in Supplementary Table 1. Economic status was categorized into 3 groups based on the total amount of national health insurance premiums paid by the insured individual in each year, which is proportional to the individual's income: low, intermediate, and high economic status.15)
The primary outcome was prescription of any OAC (warfarin, dabigatran, rivaroxaban, or apixaban). The rates of OAC use calculated by the continued use of OAC in each period with considering prescription date and amount. The difference in the rates of OAC use according to the different criteria was presented in Supplementary Figure 1. Secondary outcomes included prescription of any NOAC (dabigatran, rivaroxaban, or apixaban) and of the individual NOACs. Prescription data were obtained from NHIS outpatient encounter documentation. For patients with multiple encounters, the last encounter was used in the analysis.
Statistical analysis
In Korea, NOACs were partially and fully reimbursed by the national insurance system in January 2013 and July 2015, respectively. We then examined temporal trends in the rates of use of any OAC, warfarin, and NOACs. For each month during the study period, we determined the proportion of patients who received therapeutic OAC with warfarin, dabigatran, rivaroxaban, or apixaban. If patients had >1 visit per month, the last study visit from each patient in each month was used for the analysis. A Cochrane-Armitage test for trend analysis was then performed to evaluate for changes in rates of use of any OAC over time.
We then examined the association between patient-level variables and the use of OAC. We used separate hierarchical, multivariable logistic regression models to better understand the individual contributions of patient factors to OAC use. Patient-level variables for this model included demographics (age and sex), comorbid conditions and risk factors (hypertension, diabetes mellitus, dyslipidemia, heart failure, previous stroke or TIA, vascular diseases, CKD or ESRD, COPD, and the use of antiplatelet agents). We also calculated the total CHA2DS2-VASc score.
To assess the effect of practice level on OAC use, we divided medical institutions by scale as follows: tertiary referral hospital, secondary care hospital, primary care hospital, and other healthcare facilities such as public health center, nursing hospital, etc. Then we compared the changes in the utilization rates of any OAC, warfarin, and NOACs within one year after the AF diagnosis of each practice level by year, among patients with more than 2 points of CHA2DS2-VASc score. Statistical analyses were performed using SPSS version 23.0 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics and temporal trends in overall OAC use
Patients were categorized by reimbursement, and their characteristics were compared (Table 1). In general, patients after full reimbursement of NOAC had higher rates of comorbidities, and higher CHA2DS2-VASc scores.
Table 1. Baseline characteristics.
| Reimbursement of NOAC | ||||
|---|---|---|---|---|
| Before (n=405,910) | Partial (n=524,843) | Full (n=685,801) | ||
| Age (years) | 64.2±14.6 | 64.3±14.7 | 65.1±14.3 | |
| Male | 211,235 (52.0) | 275,045 (52.4) | 360,731 (52.6) | |
| Heart failure | 101,477 (25.0) | 148,530 (28.3) | 227,001 (33.1) | |
| Hypertension | 278,048 (68.5) | 363,191 (69.2) | 502,006 (73.2) | |
| Diabetes mellitus | 86,459 (21.3) | 118,615 (22.6) | 167,335 (24.4) | |
| Stroke/TIA | 82,399 (20.3) | 114,941 (21.9) | 174,193 (25.4) | |
| Myocardlal infarction | 57,026 (14.1) | 71,855 (13.7) | 97,384 (14.2) | |
| Peripheral arterial disease | 26,194 (6.5) | 43,386 (8.3) | 72,009 (10.5) | |
| Dyslipidemia | 217,568 (53.6) | 304,409 (58.0) | 445,085 (64.9) | |
| CKD/ESRD | 21,919 (5.4) | 30,441 (5.8) | 47,320 (6.9) | |
| COPD | 110,813 (27.3) | 147,481 (28.1) | 211,227 (30.8) | |
| CHA2DS2-VASc score | ||||
| 0 (male) or 1 (female) | 66,163 (16.3) | 84,499 (16.1) | 100,237 (14.6) | |
| 1 (male) | 46,680 (11.5) | 59,832 (11.4) | 74,177 (10.8) | |
| 2 | 70,628 (17.4) | 89,748 (17.1) | 116,696 (17.0) | |
| 3 | 68,599 (16.9) | 88,174 (16.8) | 114,325 (16.8) | |
| 4 | 59,669 (14.7) | 77,152 (14.7) | 102,294 (14.9) | |
| 5 | 43,838 (10.8) | 57,733 (11.0) | 77,606 (11.3) | |
| 6 | 27,602 (6.8) | 36,739 (7.0) | 53,602 (7.8) | |
| 7 | 15,830 (3.9) | 21,519 (4.1) | 30,981 (4.5) | |
| ≥8 | 6,901 (1.7) | 9,447 (1.8) | 15,883 (2.3) | |
| Mean CHA2DS2-VASc score | 3.04±1.86 | 3.05±1.92 | 3.10±1.95 | |
Values are mean±standard deviation or number (%).
NOAC = non-vitamin K antagonist oral anticoagulant; TIA = transient ischemic attack; CKD = chronic kidney disease; ESRD = end-stage renal disease; COPD = chronic obstructive pulmonary disease; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female).
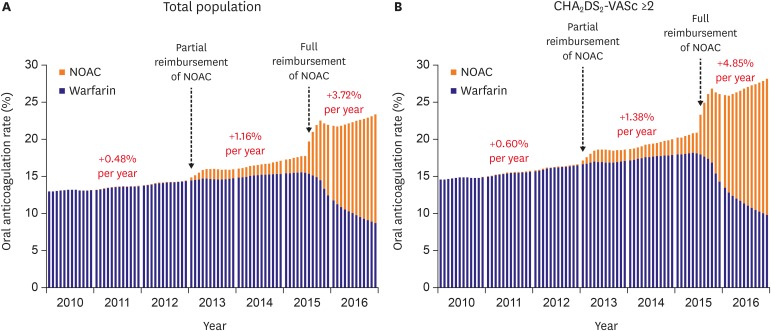
In all population, overall OAC use increased from 13.2% to 23.4% (p for trend <0.001), and NOAC use increased from 0% to 14.6% (p for trend <0.001). OAC use increased from 13.2% to 18.0% before the full reimbursement of NOAC (p for trend <0.001), and further increased to 23.4% after the full reimbursement of NOAC (p for trend <0.001). Compared with pre-reimbursement (0.48%), the annual increase of OAC was significantly higher after partial (1.16%, p<0.001) and full reimbursement of OAC (3.72%, p<0.001) (Figure 2A). In the subgroup of AF patients with CHA2DS2-VASc score ≥2, the rate of use of overall OAC increased from 14.6% in 2010 to 28.1% in 2016 (p for trend <0.001). The annual increase of OAC was 0.60%, 1.38% and 4.85% at before, after partial and full reimbursement of NOACs. The annual increase of OAC significantly increased after partial (p<0.001) and full reimbursement of OAC (p<0.001) (Figure 2B).
Figure 2. Temporal trends in overall oral anticoagulation prescription in total atrial fibrillation population (A) and patients with CHA2DS2-VASc score ≥2 (B).
NOAC = non-vitamin K antagonist oral anticoagulant; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female).
Warfarin use also increased from 13.0% to 15.6% before the full reimbursement of NOAC (p for trend <0.001), but finally decreased to 8.8% during the study period. Any NOAC use increased from 0% to 14.6% (p for trend <0.001).
Reimbursement of NOAC and OAC use
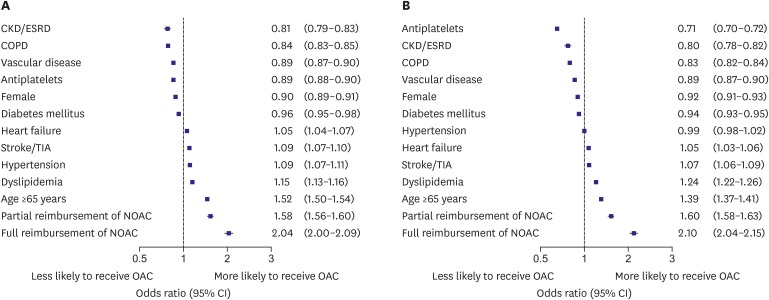
In all patients, patient characteristics associated with OAC use are shown in Figure 3A. In the multivariate models, patient factors associated with lower rates of use of OAC included CKD or ESRD, COPD, vascular disease, use of antiplatelet agents, female sex and diabetes mellitus. Patient factors associated with higher rates of use of OAC included heart failure, hypertension, previous stroke or TIA, dyslipidemia and age older than 65 years. Partial (adjusted odds ratio [OR], 1.58; 95% confidence interval [CI], 1.56–1.60) and full reimbursement of NOAC (adjusted OR, 2.04; 95% CI, 2.00–2.09) were independently associated with higher OAC use in total population.
Figure 3. Adjusted patient factors associated with overall OAC use within 1 year from AF diagnosis in total AF population (A) and patients with CHA2DS2-VASc score ≥2 (B).
CKD = chronic kidney disease; ESRD = end-stage renal disease; COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulant; CI = confidence interval; AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female).
In patients with CHA2DS2-VASc score ≥2, patient factors associated with lower rates of use of any OAC included use of antiplatelet agents, CKD or ESRD, COPD, vascular disease, female sex, diabetes mellitus and hypertension (Figure 3B). Higher rates of use of any OAC included heart failure, previous stroke or TIA, dyslipidemia and age older than 65 years. Partial (adjusted OR, 1.60; 95% CI, 1.58–1.63) and full reimbursement of NOACs (adjusted OR, 2.10; 95% CI, 2.04–2.15) was independently associated with higher OAC use.
Practice level variation and social inequalities in OAC use
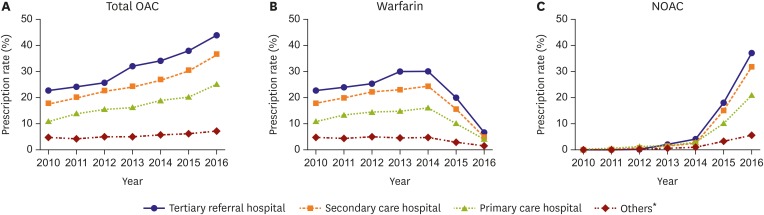
Significant practice-level variation was evident in the use of OAC. Figure 4 illustrates the temporal trends in OAC initiation within 1 year after AF diagnosis according to the practice level. The use of any OAC in eligible patients ranged from 4.9% to 22.8% in 2010, and from 7.2% to 44.0% in 2016 according to the practice level. The difference of OAC usage between tertiary referral hospital and nursing or public health centers increased from 17.9% in 2010 to 36.8% in 2016 (Figure 4A). The rate of OAC use significantly increased from 22.8% to 44.0% in tertiary referral hospitals (p<0.001), from 17.9% to 36.7% in secondary care hospitals (p<0.001), and from 11.0% to 25.2% at primary care hospitals (p<0.001). OAC use remained without improvement in other healthcare facilities such as nursing hospital or public health center.
Figure 4. Practice level variation in total OAC (A), warfarin (B), and NOAC (C) use for AF patients with CHA2DS2-VASc score ≥2.
OAC = oral anticoagulant; NOAC = non-vitamin K antagonist oral anticoagulant; AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female).
*Others include nursing hospital, public health centers, etc.
Warfarin use increased from 2010 to 2014, before the full reimbursement of NOAC, but finally decreased in 2016 compared to 2010 at all practice-levels (Figure 4B). In 2016, the rate of NOAC use ranged from 5.5% to 37.2% according to the practice level (Figure 4C). NOAC use increased dramatically from 0% to 37.2% at the tertiary referral hospital, 31.8% at secondary care hospitals, 20.9% at primary care hospitals, and 5.5% at other healthcare facilities (for all, p for trend <0.001). Practice level variation in the prescription rate of individual NOAC among AF patients along time elapse is presented in Supplementary Figure 2.
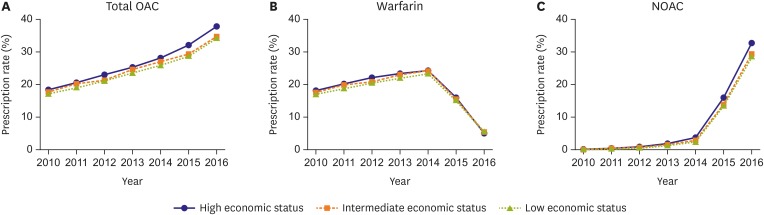
In addition, we further analyzed OAC usage according to the patients' economic status (Figure 5). The increase in NOAC use was slightly higher in patients with high economic status compared to the others, but the use of NOAC showed an even trend across the 3 economic status groups, without significant difference.
Figure 5. OAC usage according to the patients' economic status with total OAC (A), warfarin (B), and NOAC (C).
OAC = oral anticoagulant; NOAC = non-vitamin K antagonist oral anticoagulant.
DISCUSSION
In Korean AF patients, we found that the rate of overall OAC use increased from 15.6% to 26.9% following the introduction and reimbursement of NOACs. Second, the full reimbursement of NOACs was strongly associated with higher OAC use, which suggests that the change in insurance policy for NOACs might have contributed to improved overall OAC prescription rates among eligible AF patients. Finally, significant practice variation was present in OAC and NOAC usage among different medical systems. This finding shows increased social inequalities of OAC usage after the introduction of NOAC despite full reimbursement of NOAC.
The study illustrates how incentivisation of NOAC use by offering reimbursement improves OAC uptake. Underuse of OAC in patients with AF is not uncommon,16),17) and is known to be associated with increased risk of stroke and systemic thromboembolism.18),19) Before 2014, <20% of the incident and prevalent AF patients in Korea were prescribed OAC.16),17) Similar to other recent reports,20),21) we found that overall rates of OAC for AF increased following the introduction of NOACs. Given that randomized clinical trials have demonstrated relative safety and efficacy of the NOACs compared with warfarin, especially evident amongst Asian patients,22) and these alternatives to warfarin have likely contributed to increased rate of OAC utilization. Our results suggested the early trends in adoption of NOACs persist, although they appear to be used more as de novo therapy for those who were not previously anticoagulated, rather than as a transition from warfarin.
In Korea, NOACs were partially reimbursed by the national insurance system in January 2013. In period of partial reimbursement, insurance benefits were applied only to patients whose international normalized ratio level did not achieve therapeutic level even with considerable dose adjustment. Overall OAC prescription rates increased during this period, but only to a limited extent. The increase in OAC prescription at this time might be mainly due to expansion of consensus on the prescription of anticoagulants rather than the application of insurance benefit itself.
Despite the considerable increase in overall OAC use observed in the present study, several challenges remain regarding the effective and sufficient use of OAC for eligible AF patients. Although overall OAC use increased during our study period, a large number of patients with AF without a documented contraindication to OAC were still not receiving OAC.
Significant practice-level variation existed for overall OAC and NOAC use. Although NOAC was dramatically increased in tertiary referral hospitals, its increase was minimal in primary care, nursing and public health centers. This result is in consistent with a data from the ORBIT-AF (Outcomes Registry for Better Quality of Care in the Treatment of AF) registry, which demonstrated higher rates of OAC utilization among patients with AF seen by electrophysiology specialists compared with general cardiologists and primary care providers.23) Electrophysiology specialists in referral medical institutions are seemingly more aggressive in terms of stroke prevention, and they adhere better to treatment guidelines compared with general practitioner providers. This might represent a higher likelihood of patients without contraindication to OAC being referred to electrophysiology practices and an enhanced attention to the need for OAC by electrophysiology providers.21) One additional possibility for the difference in OAC utilization is likely to be due to differences in age distribution between the practice levels. The mean age of patients in nursing hospital or public health center was significantly higher than those in tertiary referral hospital (78.9±11.3 vs. 64.0±15.4 years, p<0.001). Previous studies demonstrated that extreme old age is associated with underutilization of OAC among AF patients.24),25) The present study also shows that which practice level is associated with NOAC use over time. Further research is needed to reduce variation and improve use of OAC for patients at risk of stroke associated with AF, by streamlining primary and secondary patient management pathways.26) This is of particular importance, given that a significant portion of patients at high risk of stroke related to AF receive no OAC therapy.27)
Our study results should be interpreted in the context of the following limitations, given the natures of the nationwide registry database we used. First, baseline AF diagnosis and other comorbidities were dependent on the ICD-10 codes; therefore, the diagnosis of AF could be inaccurate although the method for the diagnosis has been validated in previous studies, and our internal validation found a high correlation with actual AF diagnosis. Second, although our analysis provided some insights regarding the effect of national insurance system on the change of treatment pattern we observed, we could not account for all variables that might have influenced the use of OAC. Third, the present nationwide study only enrolled the entire Korean population, whether the results can be extrapolated to other populations remains uncertain. Fourth, the present study investigated only up to 1.5 years after the expansion of NOAC insurance coverage in Korea. Future studies of data after 2017 will further expand the issues discussed in this study. Despite these limitations, this study had a large sample size and included a long follow-up period of the entire Korean adult population.
The introduction of NOACs in routine practice for stroke prevention in AF was associated with improved rates of overall OAC use for AF. However, significant practice-level variations in OAC and NOAC use remained producing social inequalities of OAC despite full reimbursement.
ACKNOWLEDGEMENTS
The National Health Information Database was provided by the National Health Insurance Service of Korea (NHIS) (NHIS-2018-4-029). The authors thank the National Health Insurance Service for its cooperation.
Footnotes
Funding: This study was supported by a research grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2017R1A2B3003303, 2017R1C1B1008292), and grants from the Korean Healthcare technology R&D project funded by the Ministry of Health & Welfare (HI16C0058, HI15C1200).
Conflict of Interest: Gregory Y.H. Lip: Consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo. No fees are received personally. None declared for other authors.
- Conceptualization: Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Lip GYH, Joung B.
- Data curation: Yu HT, Yang PS.
- Formal analysis: Yu HT, Yang PS, Hwang J, Ryu S, Jang E.
- Funding acquisition: Joung B.
- Investigation: Yu HT, Lip GYH, Joung B.
- Methodology: Yu HT.
- Supervision: Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Lip GYH, Joung B.
- Validation: Yu HT, Yang PS, Joung B.
- Writing - original draft: Yu HT.
- Writing - review & editing: Yu HT, Lip GYH, Joung B.
SUPPLEMENTARY MATERIALS
ICD-10 codes used for defining the comorbidities
The rates of OAC use calculated by different criteria (CHA2DS2-VASc score ≥2). (A) by the continued use of OAC in each period with considering prescription date and amount, (B) by whether OAC was prescribed for 30 days or more before the index date, (C) by whether OAC was prescribed at least once before the index date.
Practice level variation in the prescription rate of individual NOAC among AF patients along time elapse.
References
- 1.Kim TH, Yang PS, Uhm JS, et al. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48:1524–1530. doi: 10.1161/STROKEAHA.117.016926. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Yang PS, Kim D, et al. CHA2DS2-VASc score for identifying truly low-risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48:2984–2990. doi: 10.1161/STROKEAHA.117.018551. [DOI] [PubMed] [Google Scholar]
- 3.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 6.Joung B, Lee JM, Lee KH, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. 2018;48:1033–1080. doi: 10.4070/kcj.2018.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Kim TH, Cha MJ, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017;47:877–887. doi: 10.4070/kcj.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JD, Shewale AR, Dherange P, Talbert JC. A comparison of oral anticoagulant use for atrial fibrillation in the pre- and post-DOAC eras. Drugs Aging. 2016;33:427–436. doi: 10.1007/s40266-016-0369-y. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Lee YS, Kim TH, et al. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: a COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Korean J Intern Med. 2019 doi: 10.3904/kjim.2017.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SR, Choi EK, Han K, Cha MJ, Oh S. Prevalence of non-valvular atrial fibrillation based on geographical distribution and socioeconomic status in the entire Korean population. Korean Circ J. 2018;48:622–634. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YH, Han K, Ko SH, Ko KS, Lee KU Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, Yang PS, Jang E, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. doi: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Yang PS, Kim TH, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–1245. doi: 10.1016/j.jacc.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Yang PS, Yu HT, et al. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40:809–819. doi: 10.1093/eurheartj/ehy877. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Yang PS, Jang E, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018;104:2010–2017. doi: 10.1136/heartjnl-2017-312930. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Kim TH, Baek YS, et al. The trends of atrial fibrillation-related hospital visit and cost, treatment pattern and mortality in Korea: 10-year nationwide sample cohort data. Korean Circ J. 2017;47:56–64. doi: 10.4070/kcj.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang PS, Ryu S, Kim D, et al. Variations of prevalence and incidence of atrial fibrillation and oral anticoagulation rate according to different analysis approaches. Sci Rep. 2018;8:6856. doi: 10.1038/s41598-018-25111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorin L, Fauchier L, Nonin E, Charbonnier B, Babuty D, Lip GY. Prognosis and guideline-adherent antithrombotic treatment in patients with atrial fibrillation and atrial flutter: implications of undertreatment and overtreatment in real-life clinical practice; the Loire Valley Atrial Fibrillation Project. Chest. 2011;140:911–917. doi: 10.1378/chest.10-2436. [DOI] [PubMed] [Google Scholar]
- 19.Li CH, Liu CJ, Chou AY, et al. European Society of Cardiology guideline-adherent antithrombotic treatment and risk of mortality in Asian patients with atrial fibrillation. Sci Rep. 2016;6:30734. doi: 10.1038/srep30734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decker C, Garavalia L, Garavalia B, et al. Exploring barriers to optimal anticoagulation for atrial fibrillation: interviews with clinicians. J Multidiscip Healthc. 2012;5:129–135. doi: 10.2147/JMDH.S33045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540. [DOI] [PubMed] [Google Scholar]
- 22.Wang KL, Lip GY, Lin SJ, Chiang CE. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fosbol EL, Holmes DN, Piccini JP, et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. 2013;2:e000110. doi: 10.1161/JAHA.113.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 25.Proietti M, Laroche C, Opolski G, et al. ‘Real-world’ atrial fibrillation management in Europe: observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2017;19:722–733. doi: 10.1093/europace/euw112. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Lane DA, Sarwar S. Streamlining primary and secondary care management pathways for stroke prevention in atrial fibrillation. Eur Heart J. 2017;38:2980–2982. doi: 10.1093/eurheartj/ehx554. [DOI] [PubMed] [Google Scholar]
- 27.Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1:55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-10 codes used for defining the comorbidities
The rates of OAC use calculated by different criteria (CHA2DS2-VASc score ≥2). (A) by the continued use of OAC in each period with considering prescription date and amount, (B) by whether OAC was prescribed for 30 days or more before the index date, (C) by whether OAC was prescribed at least once before the index date.
Practice level variation in the prescription rate of individual NOAC among AF patients along time elapse.