Abstract
Background and Objectives
Although complete revascularization is known superior to incomplete revascularization in ST elevation myocardial infarction (STEMI) patients with multi-vessel coronary artery disease (MVCD), there are no definite instructions on the optimal timing of non-culprit lesions percutaneous coronary intervention (PCI). We compared 1-year clinical outcomes between 2 different complete multi-vessel revascularization strategies.
Methods
From the Korea Acute Myocardial Infarction Registry-National Institute of Health, 606 patients with STEMI and MVCD who underwent complete revascularization were enrolled from November 2011 to December 2015. The patients were assigned to multi-vessel single-staged PCI (SS PCI) group (n=254) or multi-vessel multi-staged PCI (MS PCI) group (n=352). Propensity score matched 1-year clinical outcomes were compared between the groups.
Results
At one year, MS PCI showed a significantly lower rate of all-cause mortality (hazard ratio [HR], 0.42; 95% confidential interval [CI], 0.19–0.92; p=0.030) compared with SS PCI. In subgroup analysis, all-cause mortality increased in SS PCI with cardiogenic shock (HR, 4.60; 95% CI, 1.54–13.77; p=0.006), age ≥65 years (HR, 4.00; 95% CI, 1.67–9.58, p=0.002), Killip class III/IV (HR, 7.32; 95% CI, 1.68–31.87; p=0.008), and creatinine clearance ≤60 mL/min (HR, 2.81; 95% CI, 1.10–7.18; p=0.031). After propensity score-matching, MS PCI showed a significantly lower risk of major adverse cardiovascular event than SS PCI.
Conclusions
SS PCI was associated with worse clinical outcomes compared with MS PCI. MS PCI for non-infarct-related artery could be a better option for patients with STEMI and MVCD, especially high-risk patients.
Keywords: Myocardial infarction, Percutaneous coronary intervention, Myocardial revascularization, Coronary artery disease
INTRODUCTION
Up to 50% of patients presenting with ST elevation myocardial infarction (STEMI) have multi-vessel coronary artery disease (MVCD), and these patients had significant stenosis in at least one of the non-infarct-related arteries (IRAs).1),2),3) There is no argument about performing primary percutaneous coronary intervention (P-PCI) of culprit lesion, however, it is still controversial about whether and when to do non-IRA lesions percutaneous coronary intervention (PCI).4),5),6),7) There are 2 PCI options to archive complete multi-vessel revascularization. First, multi-vessel single-staged PCI (SS PCI) which defined as non-IRA lesions PCI at the same time of IRA lesion P-PCI; Second, multi-vessel multi-staged PCI (MS PCI) which defined as staged non-IRA lesions PCI after IRA lesion P-PCI during the initial hospitalization. The 2015 American Heart Association (AHA)/American College of Cardiology Foundation (ACCF) guidelines for the management of STEMI recommended that PCI of a non-IRA may be considered in patients with STEMI and MVCD who are hemodynamically stable, either at the time of P-PCI or as a planned staged procedure (Class IIb, B–R)8); the current 2017 European Society of Cardiology (ESC) guidelines for the management of STEMI recommended that PCI of a non-IRA should be considered before hospital discharge (Class IIa, A) and non-IRA PCI during the index procedure should be considered in patients with cardiogenic shock (Class IIa, C).9) Although several studies revealed that multi-vessel MS PCI is associated with better outcomes than multi-vessel SS PCI,10),11),12) large data on the difference in clinical outcomes are needed. Thus, we conducted our study to compare the clinical outcomes between 2 different complete multi-vessel revascularization strategies in Korean patients with STEMI and MVCD: multi-vessel SS PCI versus multi-vessel MS PCI during the index hospitalization.
METHODS
Study design and population
This study population was derived from the Korea Acute Myocardial Infarction Registry (KAMIR)-National Institutes of Health (NIH). The KAMIR-NIH is a prospective, multicenter, on-line based observational cohort study that is still ongoing. Patients with a diagnosis of acute myocardial infarction (AMI) from 20 centers (tertiary university PCI-capable hospitals) have been enrolled from November 2011 to December 2015 in Korea and followed up through 2018. The KAMIR-NIH contains all consecutive characteristics and clinical outcomes of Korean patients with AMI and it reflects prognostic and surveillance index. Data were collected by the attending physician and trained clinical research coordinators through a web-based case report form in the clinical data management system.13)
We consecutively selected patients with STEMI and MVCD from the database. The STEMI was diagnosed by a new ST elevation in ≥ 2 contiguous leads, measuring >0.2 mV in leads V1–3 or 0.1 mV in all other leads on 12-lead electrocardiography (ECG) with a concomitant increase in troponin-I or -T. The MVCD was confirmed by coronary angiography that significant stenosis (presence of diameter stenosis ≥50%, visually estimated) is seen in at least 1 of the non-IRA. Among them, patients with culprit only P-PCI, multi-vessel partial revascularization, facilitated PCI or delayed PCI, defined as a PCI was not performed within 12 hours of ischemic symptom presentation, fibrinolytic therapy, conservative treatment or missing data were excluded. These patients were divided into 2 groups according to the timing of non-IRA lesions PCI. Per clinician decision, non-IRA lesions PCI was performed at the same time of IRA lesion P-PCI (multi-vessel SS PCI, namely) or the separated staged procedure (multi-vessel MS PCI, namely). This study was approved by the Institutional Review Board of our medical institution and all patients provided written informed consent for participation in the registry.
Study outcomes
The primary outcome was all-cause mortality and the secondary outcomes were major adverse cardiovascular event (MACE) and cardiac death during the one year of follow-up.
MACE was defined as a composite of all-cause death, recurrent myocardial infarction (MI), repeat revascularization components (target lesion or target vessel revascularization), stent thrombosis and stroke. Each of the components was assessed individually. The cardiac death was defined as death from arrhythmia, pump failure or mechanical complications including free wall rupture and ventricular septal rupture. Recurrent MI was defined as an elevation in troponin-I or T level above the 99th percentile of the upper normal limit with concomitant ischemic symptom or change of ECG. Target lesion revascularization (TLR) was defined as any repeat PCI to treat luminal stenosis occurred within 5-mm edge of the stent in the target lesion. Target vessel revascularization (TVR) was defined as repeat PCI outside the 5mm edge of the stent or any segment of the target vessel. Stent thrombosis was defined as definite development of a stent-related thrombotic event, according to the Academic Research Consortium (ARC) classification.14)
Statistical analysis
The analysis was performed to examine the differences in adverse outcomes between the 2 groups. Continuous variables were presented as mean±standard deviation (SD) or median with interquartile range (IQR), and they were compared using the independent t-test or Mann-Whitney U test between the 2 groups. Categorical variables were expressed as raw numbers and percentages. Categorical variables were compared with Pearson's χ2 or Fisher's exact tests between the 2 groups. To minimalize the effect of selection bias in the comparison between SS PCI and MS PCI group, the propensity score was estimated using a multivariable logistic regression model that contained 12 covariates which were significantly different between 2 groups. Model discrimination was measured by the c-statistic and calibration was assessed by the Hosmer-Lemeshow goodness-of-fit test (c-statistic: 0.559, Hosmer-Lemeshow: p=0.126). Matching was performed using a greedy matching protocol (1:1 matching without replacement) with a caliper width of 0.03. We matched 162 patients of SS PCI to 162 patients of MS PCI. We estimated standardized differences for covariates shown significant differences in Tables 1 and 2 before and after propensity score matching to assess the balance of the covariates between the matched SS PCI group and MS PCI group. After matching, none of the covariates showed a standardized differences exceeding 5%, suggesting that all of the measured covariates were well balanced between the matched groups (Supplementary Figure 1).
Table 1. Baseline characteristics of the patients.
| Variable | All patients (n=606) | SS PCI (n=254) | MS PCI (n=352) | p value | |
|---|---|---|---|---|---|
| Age (years) | 62±12 | 62±12 | 62±12 | 0.906 | |
| Sex (male) | 486 (80.2) | 205 (80.7) | 281 (79.8) | 0.837 | |
| Body weight (kg) | 66.7±11.2 | 66.1±11.8 | 66.9±11.5 | 0.406 | |
| Body mass index* (kg/m2) | 24.3±3.0 | 24.0±3.4 | 24.3±2.8 | 0.253 | |
| Comorbidities | |||||
| Hypertension | 280 (46.2) | 124 (48.8) | 156 (44.3) | 0.284 | |
| Diabetes | 163 (26.9) | 78 (30.7) | 85 (24.1) | 0.078 | |
| Dyslipidemia | 64 (10.6) | 21 (8.3) | 43 (12.2) | 0.141 | |
| Current smoker | 279 (46.0) | 122 (48.0) | 157 (44.6) | 0.410 | |
| Previous myocardial infarction | 34 (5.6) | 15 (5.9) | 19 (5.4) | 0.859 | |
| Previous congestive heart failure | 7 (1.2) | 4 (1.6) | 3 (0.9) | 0.460 | |
| Previous stroke | 35 (5.8) | 18 (7.1) | 17 (4.8) | 0.290 | |
| Laboratory findings | |||||
| Hemoglobin (g/dL) | 14.4±1.8 | 14.3±1.9 | 14.4±1.8 | 0.577 | |
| Creatinine (mg/dL) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.9 (0.7–1.0) | 0.024 | |
| Creatinine clearance | 82.9±32.5 | 80.1±42.9 | 83.4±32.6 | 0.261 | |
| Total cholesterol (mg/dL) | 184±47 | 181±44 | 186±49 | 0.155 | |
| High-density lipoprotein (mg/dL) | 40 (34–47) | 41 (34–48) | 39 (33–47) | 0.141 | |
| Low-density lipoprotein (mg/dL) | 114 (91–139) | 109 (85–130) | 117 (94–144) | 0.012 | |
| Triglyceride (mg/dL) | 109 (69–172) | 110 (66–173) | 18 (71–176) | 0.991 | |
| CK-MB (IU/L) | 124 (44–276) | 118 (42–276) | 132 (50–277) | 0.285 | |
| NT- proBNP (pg/mL) | 114 (41–593) | 162 (69–858) | 99 (41–496) | 0.184 | |
| Baseline LVEF (%) | 51±10 | 48±11 | 52±9 | <0.001 | |
| LVEF ≤35% | 46 (7.8) | 27 (11.1) | 19 (5.4) | 0.013 | |
| In-hospital medication | |||||
| Aspirin | 603 (99.5) | 251 (98.8) | 352 (100.0) | 0.073 | |
| Clopidogrel/prasugrel/ticagrelor | 601 (99.2) | 250 (98.4) | 351 (99.7) | 0.167 | |
| ACE inhibitor/ARB | 470 (77.6) | 195 (76.8) | 275 (78.1) | 0.694 | |
| Beta-blocker | 513 (84.7) | 205 (80.7) | 308 (87.5) | 0.030 | |
| Calcium-channel blocker | 21 (3.5) | 9 (3.5) | 12 (3.4) | 1.000 | |
| Statin | 555 (91.6) | 224 (88.2) | 331 (94.0) | 0.012 | |
| Glycoprotein IIb/IIIa inhibitor | 140 (23.1) | 44 (17.3) | 96 (27.3) | 0.005 | |
| Hemodynamic status | |||||
| Systolic blood pressure (mmHg) | 124±31 | 126±32 | 123±31 | 0.317 | |
| Heart rate (bpm) | 76±19 | 79±19 | 74±19 | 0.001 | |
| Initial Killip class III/IV | 87 (14.4) | 49 (19.3) | 38 (10.8) | 0.005 | |
| Cardiogenic shock | 84 (42.9) | 43 (48.9) | 41 (38.0) | 0.147 | |
| Symptom to balloon time (min) | 186 (125–432) | 189 (130–283) | 184 (123–302) | 1.000 | |
| Door to balloon time (min) | 59 (46–71) | 59 (46–74) | 59 (47–70) | 0.774 | |
| In-hospital duration (days) | 5 (4–7) | 4 (3–6) | 6 (4–8) | <0.001 | |
Values are number (%) for categorical values; median and interquartile rage (25–75%) or means±standard deviation for continuous variables.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; CK-MB = creatine kinase-myocardial band; LVEF = left ventricular ejection fraction, MS PCI = multi-staged percutaneous coronary intervention; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; SS PCI = single-staged percutaneous coronary intervention.
*Body mass index is defined as the weight divided by the square of the body height.
Table 2. Procedural characteristics.
| Variable | All patients (n=606) | SS PCI (n=254) | MS PCI (n=352) | p value | |
|---|---|---|---|---|---|
| Infarct-related vessel | <0.001 | ||||
| Left anterior descending artery | 263 (43.4) | 131 (51.6) | 132 (37.5) | ||
| Left circumflex artery | 78 (12.9) | 42 (16.5) | 36 (10.2) | ||
| Right coronary artery | 248 (40.9) | 66 (26.0) | 182 (51.7) | ||
| Left main coronary artery | 17 (2.8) | 15 (5.9) | 2 (0.6) | ||
| Number of diseased vessels | < 0.001 | ||||
| 2 vessel disease | 466 (76.9) | 203 (79.9) | 263 (74.7) | ||
| 3 vessel disease | 96 (15.8) | 21 (8.3) | 75 (21.3) | ||
| Left main disease | 44 (7.3) | 30 (11.8) | 14 (4.0) | ||
| Pre TIMI flow 0/1 | 445 (73.4) | 172 (67.7) | 273 (77.6) | 0.009 | |
| ACC/AHA lesion classification | 0.400 | ||||
| Type A/B1 | 57 (9.4) | 27 (10.6) | 30 (8.5) | ||
| Type B2/C | 549 (90.6) | 227 (89.4) | 322 (91.5) | ||
| Puncture route | 0.839 | ||||
| Radial access | 125 (20.9) | 54 (21.3) | 71 (20.6) | ||
| Femoral access | 472 (79.1) | 199 (78.7) | 237 (79.4) | ||
| Treatment of IRA lesion | 0.018 | ||||
| Drug-eluting stent | 574 (95.0) | 233 (92.1) | 341 (97.2) | ||
| Bare-metal stent | 10 (1.7) | 7 (2.8) | 3 (0.9) | ||
| Balloon only angioplasty | 20 (3.3) | 13 (5.1) | 7 (2.0) | ||
| Number of stents at IRA lesion | 1.0±0.3 | 1.0±0.2 | 1.0 ± 0.3 | 0.746 | |
Values are number (%) for categorical values; means±standard deviation for continuous variables.
ACC = American College of Cardiology; AHA = American Heart Association; IRA = infarct-related artery; MS PCI = multi-staged percutaneous coronary intervention; SS PCI = single-staged percutaneous coronary intervention; TIMI = thrombolysis in myocardial infarction.
The cumulative events analyses were performed using time-to-event data by the Kaplan-Meier method, survival curves were compared using the log-rank test. Patients were censored at the time of event occurred or at last follow-up. Kaplan-Meier curves were plotted for the time to the occurrence of the clinical outcomes. A multivariate Cox proportional hazards regression was used to estimate hazard ratios (HRs), with 95% confidence interval (CI) in both all patients and patients of matched groups. Only variables with a p value <0.05 in the univariate analysis were used to evaluate HRs as estimates for each endpoint.
All statistical tests were considered 2-tailed and statistical significant was defined as a p value of <0.05. The Statistical Package for Social Sciences software, version 24.0 (SPSS, Inc., Chicago, IL, USA) was used for analyses.
RESULTS
Patients and characteristics
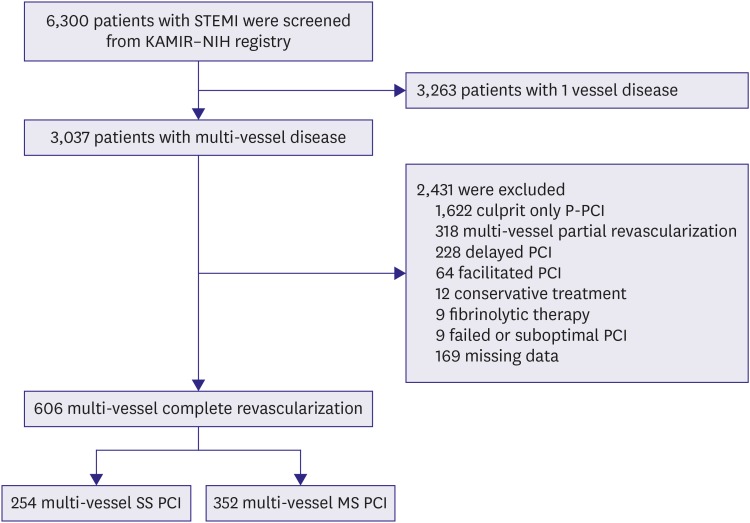
Between November 2011 and December 2015, total of 6,300 patients with a diagnosis of STEMI were enrolled and screened. Of 3,037 STEMI patients with MVCD, 2,418 patients were excluded for various reasons. Finally, 606 patients who were performed complete multi-vessel revascularization during the initial hospitalization were enrolled in our study. Among them, 254 patients (42%) underwent SS PCI and 352 patients (58%) underwent MS PCI (Figure 1).
Figure 1. Study population flow chart. The patients with STEMI and multi-vessel coronary artery disease were enrolled. Among them, 254 patients underwent multi-vessel SS PCI and 352 patients underwent multi-vessel MS PCI.
KAMlR–NIH = Korea Acute Myocardial Infarction Registry-National Institute of Health; MS PCI = multi-staged percutaneous coronary intervention; P-PCI = primary percutaneous coronary intervention; PCI = percutaneous coronary intervention; STEMI = ST elevation myocardial infarction; SS PCI = single-staged percutaneous coronary intervention.
Baseline characteristics are shown in Tables 1 and 2. Two treatment groups were similar in age, sex, the prevalence of diabetes, hypertension and dyslipidemia as cardiovascular risk factors (Table 1). The patients in the SS PCI group had lower ejection fractions and showed more incidence of severe left ventricular systolic dysfunction than those in MS PCI group. Regarding the in-hospital medication, beta-blocker, statin, and glycoprotein IIb/IIIa inhibitor were prescribed more frequently in the MS PCI group. Procedure-related characteristics are shown in Table 2. The number of diseased vessel and locations of culprit lesion on coronary angiography were significantly different between the 2 groups. A total number of the implanted stent at culprit lesion was similar between the two groups. After propensity-score matching, there were no significant differences in baseline clinical, procedural characteristics between the 2 groups, except in-hospital duration. (Supplementary Tables 1 and 2)
Clinical outcomes
We summarized the clinical outcomes during the 1-year of follow-up (Table 3). The mean follow-up duration was 347 days. In multivariate Cox regression analysis, the patients treated with MS PCI strategy had a significantly lower rate of all-cause mortality (adjusted HR, 0.42; 95% CI, 0.19–0.92; p=0.030) compared with those treated with SS PCI strategy at 1-year. While the overall clinical adverse event rates such as MACE, cardiac death, and its components were lower in the MS PCI group, there were no statistical differences between the two groups after adjustment. In the propensity-matched patients, MS PCI group showed a significantly lower rate of MACE than SS PCI (adjusted HR, 0.48; 95% CI, 0.24–0.96; p=0.038; Table 4).
Table 3. One-year clinical outcomes in all patients.
| Outcomes | SS PCI (n=254) | MS PCI (n=352) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI)* | p value | HR (95% CI)† | p value | ||||
| 30 days outcomes | |||||||
| MACE§ | 22/254 (8.7) | 15/352 (4.3) | 0.48 (0.25–0.93) | 0.025 | 0.94 (0.44–1.99) | 0.864 | |
| All-cause mortality | 17/254 (6.7) | 6/352 (1.7) | 0.25 (0.10–0.63) | 0.001 | 0.62 (0.20–1.95) | 0.409 | |
| Cardiac death | 13/254 (5.1) | 5/352 (1.4) | 0.27 (0.10–0.76) | 0.008 | 0.53 (0.14–1.97) | 0.344 | |
| 30 days to 1-year outcomes‡ | |||||||
| MACE§ | 21/232 (9.1) | 21/337 (6.2) | 0.65 (0.35–1.20) | 0.164 | 0.65 (0.34–1.25) | 0.196 | |
| All-cause mortality | 10/237 (4.2) | 6/346 (1.7) | 0.40 (0.15–1.11) | 0.068 | 0.35 (0.11–1.08) | 0.068 | |
| Cardiac death | 6/241 (2.5) | 3/347 (0.9) | 0.34 (0.08–1.34) | 0.105 | 0.23 (0.04–1.18) | 0.078 | |
| 1-year outcomes | |||||||
| MACE§ | 43/254 (16.9) | 36/352 (10.2) | 0.56 (0.36–0.88) | 0.012 | 0.74 (0.46–1.21) | 0.230 | |
| All-cause mortality | 27/254 (10.6) | 12/352 (3.4) | 0.31 (0.16–0.60) | 0.001 | 0.42 (0.19–0.92) | 0.030 | |
| Cardiac death | 19/254 (7.5) | 8/352 (2.3) | 0.29 (0.13–0.67) | 0.003 | 0.42 (0.16–1.06) | 0.066 | |
| Non-cardiac death | 8/254 (3.1) | 4/352 (1.1) | 0.34 (0.10–1.13) | 0.079 | 0.87 (0.19–4.11) | 0.873 | |
| Myocardial infarction | 7/254 (2.8) | 13/352 (3.7) | 1.28 (0.51–3.21) | 0.598 | 1.47 (0.52–4.41) | 0.464 | |
| TVR/TLR | 8/254 (3.1) | 11/352 (3.1) | 0.92 (0.37–2.29) | 0.859 | 0.91 (0.34–2.43) | 0.851 | |
| Definite stent thrombosis | 4/254 (1.6) | 7/352 (2.0) | 1.29 (0.38–4.41) | 0.685 | 1.07 (0.28–4.12) | 0.919 | |
| Stroke | 5/254 (2.0) | 3/352 (0.9) | 0.42 (0.10–1.74) | 0.228 | 0.20 (0.04–1.13) | 0.069 | |
Values are number (%) for categorical values.
MACE = major adverse cardiovascular event; MS PCI = multi-staged percutaneous coronary intervention; SS PCI = single-staged percutaneous coronary intervention; TLR = target lesion revascularization; TVR = target vessel revascularization.
*HR are for the multi-vessel MS PCI groups compared with multi-vessel SS PCI group; †Adjusted Cox hazard regression analysis included age, sex, medications (statin, glycoprotein IIb/IIIa inhibitor), initial heart rate, left ventricular ejection fraction, pre TIMI flow (0–1 vs. 2–3), Initial Killip class (I–II vs. III–IV), infarct related vessel (IRA), treatment of IRA lesion, number of vessel disease; ‡Subjects who had an adverse events in initial 30 days were excluded in 30 days to 1-year outcomes data. Cox hazard regression analysis also included same variables used in 30 days outcomes and 1-year outcomes; §MACE defined as a composite of all-cause death, myocardial infarction, stroke, TVR, TLR, stent thrombosis.
Table 4. One-year clinical outcomes in propensity score matched patients.
| Outcomes | SS PCI (n=162) | MS PCI (n=162) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI)* | p value | HR (95% CI)† | p value | ||||
| 30 days outcomes | |||||||
| MACE | 8/162 (4.9) | 6/162 (3.7) | 0.75 (0.26–2.15) | 0.586 | 0.71 (0.23–2.16) | 0.546 | |
| All-cause mortality | 4/162 (2.5) | 2/162 (1.2) | 0.50 (0.09–2.72) | 0.420 | 0.67 (0.07–6.16) | 0.725 | |
| Cardiac death | 3/162 (1.9) | 2/162 (1.2) | 0.66 (0.11–3.97) | 0.653 | 1.22 (0.12–12.05) | 0.865 | |
| 30 days to 1-year outcomes‡ | |||||||
| MACE | 15/154 (9.7) | 9/156 (5.8) | 0.53 (0.24–1.25) | 0.146 | 0.46 (0.19–1.12) | 0.087 | |
| All-cause mortality | 6/158 (3.8) | 3/160 (1.9) | 0.49 (0.12–1.96) | 0.314 | 0.40 (0.09–1.78) | 0.230 | |
| Cardiac death | 5/159 (3.1) | 1/160 (0.6) | 0.19 (0.02–1.65) | 0.133 | 0.13 (0.01–1.31) | 0.083 | |
| 1-year outcomes | |||||||
| MACE | 23/162 (14.2) | 15/162 (9.3) | 0.60 (0.31–1.17) | 0.137 | 0.48 (0.24–0.96) | 0.038 | |
| All-cause mortality | 10/162 (6.2) | 5/162 (3.1) | 0.49 (0.17–1.44) | 0.197 | 0.37 (0.12–1.24) | 0.111 | |
| Cardiac death | 8/162 (4.9) | 3/162 (1.9) | 0.37 (0.10–1.38) | 0.138 | 0.26 (0.06–1.13) | 0.072 | |
| Non-cardiac death | 2/162 (1.2) | 2/162 (1.2) | 1.01 (0.14–7.19) | 0.990 | 1.80 (0.19–16.95) | 0.609 | |
| Myocardial infarction | 5/162 (3.1) | 5/162 (3.1) | 0.79 (0.21–2.94) | 0.726 | 0.72 (0.26–1.99) | 0.527 | |
| TVR/TLR | 6/162 (3.7) | 4/162 (2.5) | 0.67 (0.19–2.38) | 0.535 | 0.59 (0.16–2.17) | 0.429 | |
| Stroke | 4/162 (2.5) | 1/162 (0.6) | 0.24 (0.03–2.19) | 0.208 | 0.07 (0.01–1.66) | 0.098 | |
Values are number (%) for categorical values.
MACE = major adverse cardiovascular event; MS PCI = multi-staged percutaneous coronary intervention; SS PCI = single-staged percutaneous coronary intervention; TLR = target lesion revascularization; TVR = target vessel revascularization.
*HR are for the multi-vessel MS PCI groups compared with multi-vessel SS PCI group; †Adjusted Cox hazard regression analysis included various clinical variables including age, sex, use of statin, left ventricular ejection fraction, Initial Killip class (I-II vs. III-IV), and Target vessel; ‡Subjects who had an adverse events in initial 30 days were excluded in 30 days to 1-year outcomes data. Cox hazard regression analysis also included same variables used in 30 days outcomes and 1-year outcomes.
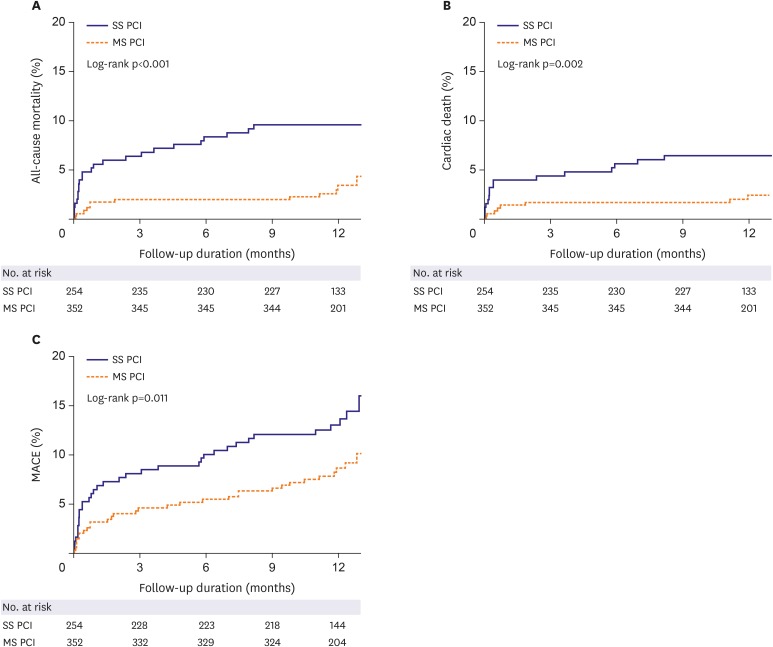
The Kaplan-Meier curves for cumulative event rates of clinical outcomes at 1-year of follow-up were shown according to the revascularization strategy (Figure 2 and Supplementary Figure 2). SS PCI group had a higher rate of all-cause mortality (27% vs. 12%), cardiac death (19% vs. 8%), and MACE (16.9% vs. 10.2%) at 1-year. When we compared the incidence of clinical outcomes divided into early (30 days events) and late stage (30 days to 1 year events) between SS PCI and MS PCI group, all-cause mortality, cardiac death, and MACE of SS PCI were significantly higher within 30 days, but not 30 days to 1 year in the Kaplan-Meier curve analyses. (Table 3 and Supplementary Figure 2).
Figure 2. Event rates of all-cause mortality, cardiac death and MACE for the entire patients at 1-year of follow-up. The Kaplan-Meier curves for cumulative event rates of all-cause mortality (A), cardiac death (B) and MACE (C) were shown according to the type of revascularization. Multi-vessel SS PCI group showed a higher rate of all-cause mortality (27% vs. 12%), cardiac death (19% vs. 8%), and MACE (16.9% vs. 10.2%) at 1-year of follow-up.
MACE = major adverse cardiovascular event; MS PCI = multi-staged percutaneous coronary intervention; SS PCI = single-staged percutaneous coronary intervention.
Subgroups analysis
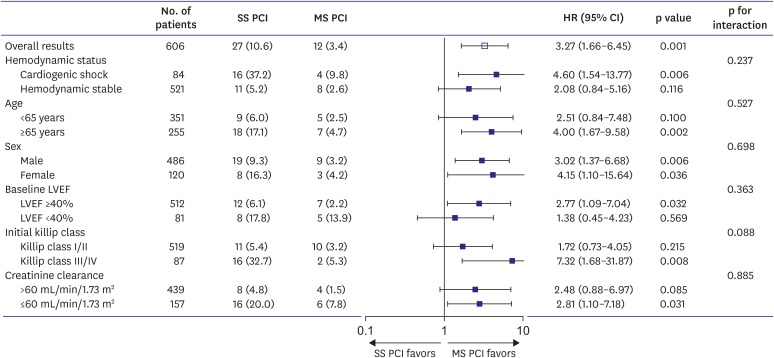
In the pre-specified subgroup analysis, SS PCI group had an increased risk of adverse outcomes compared to MS PCI group, and the similar trend was shown in all subgroups (Supplementary Tables 3 and 4). As shown in the Figure 3, there were significantly higher rates of all-cause mortality in the subgroup of cardiogenic shock (HR, 4.60; 95% CI, 1.54–13.77; p=0.006), age ≥65 year (HR, 4.00; 95% CI, 1.67–9.58; p=0.002), Killip class III/IV (HR, 7.32; 95% CI, 1.68–31.87; p=0.008) and creatinine clearance ≤60 mL/min/1.73 m2 (HR, 2.81; 95% CI, 1.10–7.18; p=0.031) in the SS PCI group. The Kaplan-Meier curves of the subgroup for all-cause mortality and cardiac death at 1-year were showed in Supplementary Figure 3.
Figure 3.
Subgroups analysis for all-cause mortality. The Cox regression analyses revealed that the multi-vessel SS PCI group had a higher rate of all-cause mortality compared to multi-vessel MS PCI group in all subgroups. Especially, high-risk patients such as cardiogenic shock, Killip class III/IV, creatinine clearance ≤60 mL/min/1.73 m2 in the multi-vessel SS PCI group had lower mortality rates in the multi-vessel MS PCI group.
CI = confidence interval; LVEF = left ventricular ejection fraction; HR = hazard ratio; MS PCI = multi-staged percutaneous coronary intervention; SS PCI = single-staged percutaneous coronary intervention.
DISCUSSION
We conducted our study to determine the optimal timing of non-IRA lesions PCI in Korean patients with MVCD and STEMI. The main findings of our study are that MS PCI strategy was superior to reduce 1-year all-cause mortality compared with SS PCI strategy in patients with STEMI and MVCD. Moreover, in patients with cardiogenic shock, age ≥65, Killip class III/IV or creatinine clearance ≤60 mL/min/1.73 m2, the risk of all-cause mortality was significantly decreased in MS PCI group than in SS PCI group. After propensity score matching, MS PCI showed a significantly lower rate of MACE than SS PCI.
It is well known that MVCD in the STEMI patients associated with a higher rate of adverse cardiac outcomes compared with single vessel disease.3),6) Non-IRA strategy of MVCD in the STEMI patients depends on whether or not there is a cardiogenic shock. According to the current ESC practice guidelines, non-IRA lesions PCI at the time of P-PCI was considered in patients with cardiogenic shock.9) Because SS PCI might improve hemodynamic stability, it should be considered during the primary procedure in patients with cardiogenic shock. However, this recommendation is based just on a consensus opinion (the level of evidence C). Also, no randomized clinical trials compared the 2 complete revascularization strategies. Moreover, some data which are not favor of SS PCI in cardiogenic shock patients were not included in current ESC STEMI practice guideline.15),16),17) Recently, several meta-analyses documented that SS PCI did not improve clinical outcomes over culprit lesion only PCI in patients with cardiogenic shock.15),16) Thiele et al.17) also reported random clinical trial that 1-year mortality did not differ significantly between culprit-lesion only PCI group and immediate multi-vessel PCI group in patients with AMI, cardiogenic shock, and MVCD. Their result archived improved clinical outcomes in all-cause mortality or renal replacement therapy in culprit-lesion-only PCI group over immediate multi-vessel PCI group (relative risk, 0.87; 95% CI, 0.76–0.99; p=0.048). Lee et al.18) reported with KAMIR-NIH registry that multi-vessel PCI (complete revascularization) showed more improved all-cause death and reduced non-IRA repeat revascularization than IRA only PCI, but the multi-vessel PCI group included about 40% of patients underwent MS PCI. However, these studies, unlike our study, are not also a comparison of 2 complete revascularization strategies.
In hemodynamically stable patients, recent guidelines recommended that non-IRA lesions routine revascularization should be considered before hospital discharge, but not proposed the optimal timing of revascularization (immediate vs. staged).8),9) This guideline's recommendation was derived from several meta-analyses and random clinical trials.19),20),21),22),23),24) The Complete Versus Culprit-Lesion Only Primary PCI (CvLPRIT) trial showed that in-hospital complete revascularization of significant non-culprit lesions resulted in improved clinical outcomes compared with the treatment of culprit lesion only.23) The Preventive Angioplasty in Acute Myocardial Infarction (PRAMI) trial also demonstrated that complete multi-vessel revascularization during the index procedure showed superior composite primary outcome compared with the culprit artery only P-PCI.24) The Third Danish Study of Optimal Acute Treatment of Patients with ST-Segment Elevation Myocardial Infarction - Primary PCI in Multivessel Disease (DANAMI-3 PRIMULTI) was compared fractional flow reserve (FFR)-guided complete revascularization with infarct-artery-only revascularization. The observed relative risk reduction in favor of complete revascularization was 44% (p=0.004).22) Smits et al.21) compared immediate FFR-guided complete revascularization and IRA only revascularization with ischemia-guided staged PCI. FFR-guided complete revascularization reduced the risk of a composite cardiovascular outcome as compared with IRA only revascularization. Most of these data compared complete revascularization strategies including SS PCI and MS PCI with IRA only revascularization.
Differ from these all reports, our study compared 2 complete revascularization strategies (immediate vs. staged). MS PCI showed improved 1-year clinical outcomes compared with SS PCI and showed clinical benefit in high-risk patients with cardiogenic shock, the elder (≥65 years), high Killip class (III/IV), low creatinine clearance (≤60 mL/min/1.73 m2). These better clinical outcomes in MS PCI group are likely to be explained by the following reasons. First, the enhanced thrombotic and inflammatory environment of STEMI contributes to a higher risk of procedural complications as compared with elective procedures.25),26),27) Factors that increase risk are related to the complexity and duration of the procedure in the case with multi-vessel PCI for STEMI. Second, when performing multi-vessel PCI of the significant non-IRA lesion, the PCI will be performed without objective evidence for the presence of myocardial ischemia. If SS PCI is performed on the lesions that are not accurately assessed the significance of coronary artery stenosis, it would not give a benefit in hemodynamically unstable conditions and high-risk patients.28) FFR-guided PCI could provide a clue of myocardial ischemia.21),22) Third, generally, contrast use increases in SS PCI, which may be less well tolerated in the patient with STEMI, especially radiocontrast nephropathy develops; it could aggravate clinical outcomes.29) Finally, unexpected peri-procedural complications in the non-IRA may be poorly tolerated due to the simultaneous impairment of the culprit and non-IRA lesions.
Selection of the complete revascularization strategy as SS PCI versus MS PCI was based on a physician decision and the reasons why a physician chose that treatment among 2 options were not prospectively collected. During the early period of patient recruitment in this study from 2011 to 2015, there were no strong recommendations for multi-vessel revascularization for STEMI patients with multi-vessel disease. So, decision of the treatment strategy between SS PCI and MS PCI is usually made by medical status of the individual patients and decision of cardiologists with different policies. As revascularization strategies depend on the operator's decision, we could not exclude the possibility that MS PCI group might have a less severe ischemic driven non-IRA lesion. In this study, matching of the 2 strategy groups was balanced with statistical power but cannot overcome the independency of randomization study. That is, we could not collect the reasons for non-IRA revascularization such as spontaneous ischemia or intermediate- or high-risk findings on the predischarge noninvasive test. Further study about FFR-guided revascularization should overcome this problem.
Although our data showed all subgroup shown in Figure 3 favors MS PCI strategy, we suggest that SS PCI strategy could be considered carefully in low-risk patients such as stable hemodynamics, the young (<65 years), low Killip class I/II, and relatively normal creatinine clearance (≥60 mL/min/1.73 m2) shown in Supplementary Table 3.
Our study has several limitations. First, our study is based on a prospective, observational registry, but a small-scale, non-randomized study. Although statistical adjustment including propensity score matching was performed, propensity score-matched patients were too small to evaluate the differences of the outcomes between two groups. This issue could lower the statistic power. A propensity score matching could not replace randomization when exploring clinical data with important clinical implication. Second, although the median hospitalization period of MS PCI group was 6 days, we do not know the exact time between the index procedure and staged PCI. Fourth, KAMIR registry has no angiographic core lab to describe the procedure. We could not find additional procedure time, difficulty of procedure, amount of used contrast, endurance of the patients, and significance of lesion. These limited data of the detailed angiographic findings and exact reason why involved physicians decided to perform SS vs. MS PCI hard to suggest clear-cut criteria to decide the strategy of PCI.
In conclusion, our study revealed that SS PCI was associated with worse clinical outcomes compared with MS PCI. MS PCI for non-IRA could be a better option for patients with STEMI and MVCD, especially high-risk patients. Although MS PCI is better option for all patients with STEMI and MVCD, SS PCI strategy could be considered carefully in low-risk patients such as stable hemodynamics, the young, low Killip class I/II, relatively normal creatinine clearance.
Footnotes
Funding: This work was supported by a research fund of Chungnam National University (2017), a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A1029617), and a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention, Republic of Korea.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Ahn KT, Oh JK, Gwon HC, Jeong JO.
- Data curation: Ahn KT, Oh JK, Jin SA, Jeong MH, Chae SC, Kim YJ, Kim HS, Cho MC, Gwon HC, Jeong JO, Seong IW.
- Formal analysis: Ahn KT, Oh JK, Seong SW, Jin SA, Jeong MH, Kim CJ, Jeong JO.
- Funding acquisition: Jeong MH, Jeong JO.
- Investigation: Seong SW, Jin SA, Lee JH, Choi SW, Kim CJ, Kim HS, Cho MC, Gwon HC, Jeong JO, Seong IW.
- Methodology: Ahn KT, Oh JK, Jin SA, Lee JH, Choi SW, Gwon HC, Jeong JO.
- Project administration: Lee JH, Choi SW, Chae SC, Kim YJ, Kim CJ, Cho MC, Gwon HC, Jeong JO.
- Resources: Lee JH, Choi SW, Jeong MH, Chae SC, Kim YJ, Kim CJ, Kim HS, Cho MC, Gwon HC, Jeong JO, Seong IW.
- Software: Ahn KT, Oh JK, Lee JH, Choi SW, Kim YJ, Jeong JO.
- Supervision: Oh JK, Lee JH, Choi SW, Jeong JO, Seong IW.
- Validation: Oh JK, Choi SW, Jeong JO.
- Visualization: Ahn KT, Oh JK, Seong SW, Jeong JO.
- Writing - original draft: Ahn KT, Oh JK, Jeong JO.
- Writing - review & editing: Ahn KT, Jeong JO.
SUPPLEMENTARY MATERIALS
Baseline characteristics in propensity score-matched patients
Procedural characteristics in propensity score-matched patients
Clinical outcomes at 1-year in pre-specified subgroups
Clinical outcomes at 1-year in subgroups of propensity score-matched patients
Absolute standardized differences in baseline covariates between SS PCI and MS PCI group before and after propensity score matching. We estimated standardized differences for covariates shown significant differences in Table 1, 2 before and after propensity score matching to assess the balance of the covariates between the matched SS PCI group and MS PCI group. After matching, none of the covariates showed a standardized difference exceeding 5%, suggesting that all of the measured covariates were well balanced between the matched groups.
Early (within 30 days) and late stage (30 days to 1year) event rates of the all-cause mortality, cardiac death, and MACE for the entire patients between SS PCI and MS PCI group. The Kaplan-Meier curves for cumulative event rates of all-cause mortality (A, B), cardiac death (C, D) and MACE (E, F) were shown divided into early (30 days events) and late stage (30 days to 1 year events), respectively. These Kaplan-Meier curves are shown the incidence of clinical outcomes divided into early (30 days events) and late stage (30 days to 1 year events) between SS PCI and MS PCI group, all-cause mortality, cardiac death, and MACE were significantly occurred within 30 days, but not 30 days to 1 year.
Event rates of the all-cause mortality and cardiac death in pre-specified subgroups at 1-year of follow-up. These Kaplan-Meier curves are shown for cumulative event rates of all-cause mortality and cardiac death in cardiogenic shock patients (A), normal hemodynamic patients (B), age ≥65 years patients (C), age <65 years patients (D), Killip class III/IV (E), and Killip class I/II (F). The multi-vessel SS PCI group had a significantly higher rate of all-cause mortality and cardiac death in the subgroup of cardiogenic shock, age ≥65 and Killip class III/IV.
References
- 1.Muller DW, Topol EJ, Ellis SG, et al. Multivessel coronary artery disease: a key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Am Heart J. 1991;121:1042–1049. doi: 10.1016/0002-8703(91)90661-z. [DOI] [PubMed] [Google Scholar]
- 2.Toma M, Buller CE, Westerhout CM, et al. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. Eur Heart J. 2010;31:1701–1707. doi: 10.1093/eurheartj/ehq129. [DOI] [PubMed] [Google Scholar]
- 3.Corpus RA, House JA, Marso SP, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493–500. doi: 10.1016/j.ahj.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Vlaar PJ, Mahmoud KD, Holmes DR, Jr, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol. 2011;58:692–703. doi: 10.1016/j.jacc.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry) Am J Cardiol. 2009;104:507–513. doi: 10.1016/j.amjcard.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Varani E, Balducelli M, Aquilina M, et al. Single or multivessel percutaneous coronary intervention in ST-elevation myocardial infarction patients. Catheter Cardiovasc Interv. 2008;72:927–933. doi: 10.1002/ccd.21722. [DOI] [PubMed] [Google Scholar]
- 7.Kalarus Z, Lenarczyk R, Kowalczyk J, et al. Importance of complete revascularization in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am Heart J. 2007;153:304–312. doi: 10.1016/j.ahj.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 10.Kornowski R, Mehran R, Dangas G, et al. Prognostic impact of staged versus “one-time” multivessel percutaneous intervention in acute myocardial infarction: analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2011;58:704–711. doi: 10.1016/j.jacc.2011.02.071. [DOI] [PubMed] [Google Scholar]
- 11.Politi L, Sgura F, Rossi R, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. 2010;96:662–667. doi: 10.1136/hrt.2009.177162. [DOI] [PubMed] [Google Scholar]
- 12.Di Mario C, Mara S, Flavio A, et al. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent. 2004;6:128–133. doi: 10.1080/14628840310030441. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Chae SC, Oh DJ, et al. Multicenter cohort study of acute myocardial infarction in Korea: interim analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health registry. Circ J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 14.Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081–1092. doi: 10.1016/j.jcin.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Kolte D, Sardar P, Khera S, et al. Culprit vessel-only versus multivessel percutaneous coronary intervention in patients with cardiogenic shock complicating ST-segment-elevation myocardial infarction: a collaborative meta-analysis. Circ Cardiovasc Interv. 2017;10:e005582. doi: 10.1161/CIRCINTERVENTIONS.117.005582. [DOI] [PubMed] [Google Scholar]
- 16.de Waha S, Jobs A, Eitel I, et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2018;7:28–37. doi: 10.1177/2048872617719640. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H, Akin I, Sandri M, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710. doi: 10.1056/NEJMoa1808788. [DOI] [PubMed] [Google Scholar]
- 18.Lee JM, Rhee TM, Hahn JY, et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2018;71:844–856. doi: 10.1016/j.jacc.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Galvão Braga C, Cid-Álvarez AB, Redondo Diéguez A, et al. Multivessel versus culprit-only percutaneous coronary intervention in ST-segment elevation acute myocardial infarction: analysis of an 8-year registry. Rev Esp Cardiol (Engl Ed) 2017;70:425–432. doi: 10.1016/j.rec.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Bangalore S, Toklu B, Wetterslev J. Complete versus culprit-only revascularization for ST-segment-elevation myocardial infarction and multivessel disease: a meta-analysis and trial sequential analysis of randomized trials. Circ Cardiovasc Interv. 2015;8:e002142. doi: 10.1161/CIRCINTERVENTIONS.114.002142. [DOI] [PubMed] [Google Scholar]
- 21.Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- 22.Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 23.Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 25.Heusch G, Kleinbongard P, Böse D, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 26.Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573–583. doi: 10.1016/j.jacc.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 27.Peterson ED, Dai D, DeLong ER, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanratty CG, Koyama Y, Rasmussen HH, Nelson GI, Hansen PS, Ward MR. Exaggeration of nonculprit stenosis severity during acute myocardial infarction: implications for immediate multivessel revascularization. J Am Coll Cardiol. 2002;40:911–916. doi: 10.1016/s0735-1097(02)02049-1. [DOI] [PubMed] [Google Scholar]
- 29.Assali AR, Brosh D, Ben-Dor I, et al. The impact of renal insufficiency on patients’ outcomes in emergent angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2007;69:395–400. doi: 10.1002/ccd.20939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics in propensity score-matched patients
Procedural characteristics in propensity score-matched patients
Clinical outcomes at 1-year in pre-specified subgroups
Clinical outcomes at 1-year in subgroups of propensity score-matched patients
Absolute standardized differences in baseline covariates between SS PCI and MS PCI group before and after propensity score matching. We estimated standardized differences for covariates shown significant differences in Table 1, 2 before and after propensity score matching to assess the balance of the covariates between the matched SS PCI group and MS PCI group. After matching, none of the covariates showed a standardized difference exceeding 5%, suggesting that all of the measured covariates were well balanced between the matched groups.
Early (within 30 days) and late stage (30 days to 1year) event rates of the all-cause mortality, cardiac death, and MACE for the entire patients between SS PCI and MS PCI group. The Kaplan-Meier curves for cumulative event rates of all-cause mortality (A, B), cardiac death (C, D) and MACE (E, F) were shown divided into early (30 days events) and late stage (30 days to 1 year events), respectively. These Kaplan-Meier curves are shown the incidence of clinical outcomes divided into early (30 days events) and late stage (30 days to 1 year events) between SS PCI and MS PCI group, all-cause mortality, cardiac death, and MACE were significantly occurred within 30 days, but not 30 days to 1 year.
Event rates of the all-cause mortality and cardiac death in pre-specified subgroups at 1-year of follow-up. These Kaplan-Meier curves are shown for cumulative event rates of all-cause mortality and cardiac death in cardiogenic shock patients (A), normal hemodynamic patients (B), age ≥65 years patients (C), age <65 years patients (D), Killip class III/IV (E), and Killip class I/II (F). The multi-vessel SS PCI group had a significantly higher rate of all-cause mortality and cardiac death in the subgroup of cardiogenic shock, age ≥65 and Killip class III/IV.