Abstract
Background and Objectives
To reveal the detail mechanism of miR-484 on myocardial ischemia-reperfusion (MI/R) injury.
Methods
Rats model of MI/R injury was established based on control (Con; sham operate) group, ischemia-reperfusion (I/R) group, miR-484 treatment (miR) group, and I/R-negative control (IR-C) group, followed by pathological and interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β expression evaluation. Then the myocardial apoptosis, as well as the expression of miR-484, caspase-3, and caspase-9 in myocardium were examined. Finally, the regulatory relation between miR-484 and SMAD family member 7 (SMAD7) was predicated, followed by verification analysis.
Results
Compared with Con group, the expression of miR-484 in I/R and IR-C group was decreased. Compared with I/R and IR-C group, the expression of miR-484 was increased in miR group. Compared with Con group, the expression levels of IL-6, TNF-α, and IL-1β in cardiac myocytes of I/R group and IR-C group were increased. Compared with Con group, the apoptotic index, membrane potential of I/R, and the expression of caspase-3/9 were increased in IR-C group. Compared with the I/R and IR-C groups, the apoptotic index of myocardial cells in the ischemic region was decreased, the membrane potential was increased, and the expression of caspase-3/9 was decreased significantly in the miR group. SMAD7 was the target gene of miR-484.
Conclusions
MiR-484 protected myocardial cells from I/R injury by suppressing caspase-3 and caspase-9 expression during cardiomyocyte apoptosis. MiR-484 reduced the expression of IL-6, TNF-α, and IL-1β in MI/R. MiR-484 might alleviate the decreasing of mitochondrial membrane potential in MI/R cells.
Keywords: Apoptosis, Mitochondrial membrane potential, Caspase-3, Caspase-9
INTRODUCTION
Ischemia-reperfusion (I/R) injury is the tissue damage caused when blood supply returns to the tissue after a period of ischemia. It is a complex process involving several cell types (such as cardiomyocytes), soluble proinflammatory mediators, as well as cellular and molecular signals. For patients with acute myocardial infarction, timely and effective treatment of choice for reducing acute myocardial ischemic injury is necessary as myocardial reperfusion strategy.1) However, the process of reperfusion can itself induce cardiomyocyte death, known as myocardial I/R (MI/R) injury, for which there is still no effective therapy. Anyway, reperfusion of ischemic myocardium is necessary to salvage tissue from eventual death. Thus, revealing the molecular and cellular mechanism during the MI/R injury is urgently needed to decrease the occurrence of MI/R injury.2)
MicroRNAs (miRNAs) are small non-coding single-stranded RNA molecules that regulate gene expression, post-transcriptionally. Recently, several studies have suggested that miRNAs contribute to I/R injury by altering key signaling elements, thus making them potential therapeutic targets.3),4) Wang et al.3) show that miR-494 protects against MI/R injury by targeting both proapoptotic and antiapoptotic proteins. Based on rats' model, previous study indicates that miR-30b has anti-apoptotic effect in early phase of MI/R injury. As a member of miRNAs, miR-484 is down-regulated in heart disease progression. Bioinformatics analysis and luciferase reporter assay indicate that long non-coding RNA (lncRNA; such as LINC00339) directly binds to miR-484, and miR-484 inhibitor abrogates the collagen synthesis inhibition induced by lncRNA.4) Actually, the function of miRNAs in disease is visualized by taking part in the inflammation process and regulating apoptosis-related factors such as caspase-3 and caspase-9.5),6) Targeted regulation of caspase-3 or caspase-9 gene expression can directly affect the outcome of MI/R injury.7),8) The inhibition of caspase-3 affects the myocyte apoptosis and left ventricular remodeling in the I/R of rat heart.9) However, the detail function of miR-484 on influence factors including caspase-3 and caspase-9 during MI/R injury has not been fully investigated yet.
In this study, male sprague-dawley (SD) rats were used to construct MI/R injury model. All rats were randomly divided into control (Con; sham operate) group, I/R group, miR-484 treatment (miR) group, and I/R negative control (IR-C) group. Based on the MI/R injury model, the hemodynamics, hematoxylin-eosin (HE), inflammation factors analysis, mitochondrial membrane potential, cardiomyocyte apoptosis, as well as expression of miR-484 in cardiomyocytes were investigated. We hope to reveal the detail function of miR-484 on MI/R injury.
METHODS
Establishment of rat ischemia-reperfusion injury model
A total of 40 male SD rats (180–220 g, 6 weeks, SPF grade) were purchased from the Laboratory Animal Center of Peking University Medical Department. All rats were randomly divided into Con group (n=10), I/R group (n=10), miR group (n=10), and IR-C group (n=10). Rats were generally anesthetized by sodium pentobarbital (50 mg/kg), and made every effort to reduce pain during operation. Based on a rapid thoracotomy to expose the heart, the left coronary artery was ligated and compressed with a water-filled balloon (Medtronic, Inc, Santa Rosa, CA, USA) for 20 minutes to cause anterior myocardial ischemia. Then, the water in the balloon was sucked out to relieve the pressure on the blood vessel, and the elevated ST-segment (by electrocardiogram) returned to normal or decreased more than 50% was considered as the successful establishment of I/R injury model. Finally, 2 hours reperfusion was performed on rats. Rats in Con group were placed suture without ligation. Rats in miR group were injected with miR-484 agomir (1×103mol/L; GenePharma, Shanghai, China) in myocardium at 24 hours before the establishment of MI/R injury model (5 points were randomly selected on the surface of the myocardium, and 10 μL miR-484 agomir were given at each point). Meanwhile, the negative control (NC) sequence was injected into the myocardium at 24 hours before the establishment of I/R model in the IR-C group (5 points were randomly selected on the surface of the myocardium, and 10 μL miR-484 agomir were given at each point). All the experimental schemes were approved by the Ethics Committee of China Medical University (Shenyang, China; permit No. SCXK 2019-0023), and all experiments were in accordance with the guide for the care and use of laboratory animals established by United States National Institutes of Health (Bethesda, MD, USA).
Hemodynamic measurement
Transthoracic echocardiography was performed on rats in each group before operation and 2 hours after reperfusion. Left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS) were used to evaluate the cardiac function of rats.
Hematoxylin-eosin staining investigation
Myocardial tissues of rats in each group were fixed with 10% neutral formaldehyde solution for 24 hours, followed by routine dehydration, transparency, paraffin immersion, embedding, and coronal section (4 μm). The sections were dewaxed with xylene, rehydrated with ethanol (serially diluted), and then stained with hematoxylin. The pathological changes were observed under light microscope.
Ultrastructural observation
Myocardial tissue (10 mm3) was fixed with 2.5% glutaraldehyde and washed twice with phosphate buffer saline (PBS). After fixed with 1% starvation acid and gradient dehydrated by acetone, the embedded sections were stained with uranium acetate-lead oryzate (BD Company, Franklin Lakes, NJ, USA). Then, ultrastructural changes were observed under transmission electron microscopy (TEM; Hitachi High-Technologies, Tokyo, Japan).
Enzyme linked immunosorbent assay for inflammatory factors
The expression of inflammatory factors was evaluated using enzyme linked immunosorbent assay (ELISA). Ischemic myocardial tissues of each group were cut and added into pre-cooled PBS/ice-cold PBS and homogenated in ice bath, followed by absorption of homogenate. The supernatant was centrifuged for 5–10 minutes at 4°C (5,000g). The levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β in ischemic myocardium were detected by ELISA kit (BioSource International, Camarillo, CA, USA). All operations were carried out in strict accordance with ELISA kit instructions.
Detection of mitochondrial membrane potential changes
Mitochondrial membrane potential changes in myocardial cells were detected by JC-1 fluorescence labeling. Ischemic myocardium was harvested at the end of reperfusion to form a single cell suspension (106/mL). Then, the suspension was mixed with JC-1 solution (Invitrogen, Carlsbad, CA, USA) and cultured in incubator for 20 minutes. The relative fluorescence intensity of cells was measured by flow cytometry (Cell Lab Quanta; Beckman Coulter, Brea, CA, USA).
Cell apoptosis detection
Terminal deoxynucleotidyl transferase-mediated biotinylated nick end-labeling (TUNEL) assay was performed to detect myocardial cellular apoptosis in ischemic region. Ischemic myocardial cells (1cm×1cm×0.5cm) were harvested at the end of reperfusion, followed by fixing with 4% paraformaldehyde solution. The tissues were dewaxed by xylene, rehydrated by gradient alcohol, and then placed in PBS for 5–10 minutes. The TUNEL labeling procedure was carried out strictly according to the kit instructions (Ruisai Biotechnology Co., Ltd., Shanghai, China). Then, the tissues were stained (hematoxylin), dehydrated, transparent, sealed and then observed under microscope. Positive stained cells (brown) represented the apoptotic cells.
Real-time polymerase chain reaction analysis
Extraction of total RNA from cells was performed using the TRIzol reagent (Invitrogen), and reverse transcripted using SYBR Green PCR Master Mix kit (Promega, Madison, WI, USA) following the manufacturer's instructions. The real-time polymerase chain reaction (RT-PCR) was performed on ABI7500 (Thermo Fisher Scientific, Waltham, MA, USA), and U6 small nuclear B non-coding RNA (forward: 5′- TGCGGGTGCTCGCTTCGGCAGC -3′; reverse: 5′- CCAGTGCAGGGTCCGAGGT -3′) was used as the endogenous control to normalize the level of messenger RNA (mRNA). The oligo (dT) primer was used for the RT reaction for gene expression. β-actin (forward: 5′- CGTGACATTAAGGAGAAGCTG -3′; reverse: 5′- CTAGAAGCATTTGCGGTGGAC -3′) was used as the endogenous control to normalize the level of genes. In detection of miR-484 expression (forward: 5′- CGACGGATCCAAGCGCACCCTTCACTTC -3′; reverse: 5′- GCTCGAATTCCGCTTCAAGGTTCCTTTCG -3′), the PCR program included 95°C for 10 minutes, 40 cycles of 95°C for 10 seconds, 60°C for 60 seconds, and 72°C for 60 seconds. All analyses were performed in triplicate and calculated by 2−ΔΔCt.10)
Western blot
The expression of caspase-3 and caspase-9 in myocardial cells were detected by Western blot. Simply, the ischemic myocardium cell lysate was prepared in cold RIPA buffer (0.15 mmol/L NaCl, 0.05 mmol/L Tris (HCl) pH 7.3, 1% Triton X-100, and 1% sodium deoxycholate). After centrifugation, proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane (Millipore, Burlington, MA, USA). Then the membrane was blocked with 5% skim milk for 2 hours, and incubated with anti-rabbit caspase-3 and caspase-9 (1:1,000; Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C. After washed with tris-buffered saline with tween 20 for 3 times, the membrane was incubated with corresponding fluorescence labeled secondary antibodies (1:1,000; Cell Signaling Technology) for 1 hours at 25°C. Protein bands were visualized and analyzed using Gel imaging system (Thermo Fisher Scientific) and Image-Pro-Plus software, respectively. The β-actin was used as internal reference. Both primary and secondary antibodies were purchased from Cell Signaling Technology, Inc.
Cell culture and transfection
Myocardial tissue was evenly inoculated into Dulbecco's Modified Eagle Medium high-sugar medium containing 1 mL fetal bovine serum for culture (37°C, 5% CO2), and then added with 0.25% trypsin-EDTA (1 mL) until cell fusion.
Myocardial cells at logarithmic growth stage were transfected with SMAD7 siRNA (si-SMAD7), si-SMAD7 NC (si-NC) (Santa Cruz Biotechnology, Dallas, TX, USA), miR-484 agomir, miR-484 antagomir, and miR-484 antagomir NC (miR-NC) (GenePharma). Myocardial cells of IR group were divided into 4 groups, including si-NC+miR-NC, si-SMAD7+miR-NC, si-SMAD7+miR-484 antagomir, and si-NC+miR-484 antagomir group. Lifectamine 2000 transfection kit (11668030; Thermo Fisher Scientific) was used for transfection. All transfection operations were carried out in strict accordance with the transfection instructions of Lipfectamine 2000. The transfected cells were made into cell suspension and inoculated into 24-well plates with 1×105 cell per well. Cells were cultured in a constant temperature incubator (5% CO2, 37°C, and 95% humidity).
Luciferase reporter assay
The regulatory relation between SMAD7 and miR-484 was predicted based on Target Scan (http://www.targetscan.org/). Wild type (SMAD7-WT) or mutant (SMAD7-MT) fragment of the SMAD7 3'UTR containing miR-484 was synthesized. Then, the WT or MT sequences were cloned into the Promega vector. Cardiomyocytes were co-transfected with a MT sequence in combination with miR-484 agomir or NC, and were named miR-484 agomir group and MT+NC group, respectively. In addition, wild-type sequences were co-transfected with miR-484 agomir or NC, which named as WT+miR-484 agomir group and WT+NC group respectively. After 48 hours of transfection, the fluorescence activity intensity of each group of cells was measured using a luciferase kit (Beijing Yuanping Biotechnology Co., Ltd., Beijing, USA).
RNA immunoprecipitation assay
RNA immunoprecipitation (RIP) detection was performed using the Magna RIP Kit (17-700; Millipore). Cell lysates (SiHa and HeLa) were incubated in RIP buffer containing magnetic beads and human anti-ago2 antibody coupling. Based on the normal immunoglobulin G (IgG) as controls, the immunoprecipitated RNA was then isolated by proteinase K, and finally the purified RNA was detected by quantitative RT-PCR (qRT-PCR).
Statistical analyses
All experiments were performed with 3 replications, and all data were expressed as mean±standard deviation. Statistical analysis was performed by SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Comparison between different groups was determined by t-test (2 groups) or 1-way analysis of variance (more than 2 groups). The p value less than 0.05 was considered to be significantly different.
RESULTS
MiR-484 was down-regulated in myocardial ischemia-reperfusion tissues
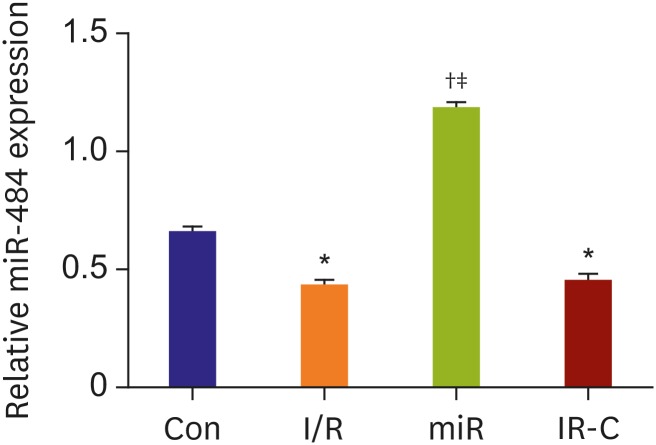
The expression of miR-484 in myocardial tissues after I/R treatment was detected by RT-PCR. The results showed that compared with Con group, the expression of miR-484 in myocardium of rats in both I/R group and IR-C group was decreased significantly (p<0.05). Moreover, the expression of miR-484 in miR group was increased significantly (p<0.05) than that in both I/R and IR-C group. However, there was no significant difference in miR-484 expression between I/R group and IR-C group (p>0.05) (Figure 1).
Figure 1. The expression of miR-484 in myocardial ischemia reperfusion of rats. The X-axis represented 4 groups including Con group, I/R group, miR group, and IR-C group.
Con = control (sham operate); I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; miR = miR-484 treatment.
*p<0.05 compared with Con group; †p<0.05 compared with I/R group; ‡p<0.05 compared with IR-C group.
Hemodynamic analysis
The echocardiographic measurement or echocardiographic analysis was performed in MI/R injury rats. The results showed that there were no significant differences in LVEDV, LVESV, LVEF, and LVFS among 4 groups before surgery. After 2 hours reperfusion, LVEDV and LVESV in both I/R group and IR-C group were increased significantly than those in Con group (p<0.05), while LVEF and LVFS were decreased significantly (p<0.05). Moreover, compared with I/R group and IR-C group, LVEDV and LVESV in miR group were decreased significantly (p<0.05), while LVEF and LVFS were increased significantly (p<0.05). However, there were no differences in LVEDV, LVESV, LVEF, and LVFS between I/R group and IR-C group (p>0.05) (Table 1).
Table 1. The results of hemodynamic measurement in myocardial ischemia reperfusion injury of rats.
| Groups | LVEDV (mL) | LVESV (mL) | LVEF (%) | LVFS (%) | |
|---|---|---|---|---|---|
| Preoperative | |||||
| Con | 0.20±0.02 | 0.04±0.01 | 83.34±5.15 | 44.61±5.34 | |
| I/R | 0.21±0.02 | 0.03±0.01 | 84.28±4.81 | 45.09±4.97 | |
| miR | 0.22±0.02 | 0.04±0.01 | 83.15±4.07 | 44.73±3.79 | |
| IR-C | 0.21±0.02 | 0.03±0.01 | 82.85±3.93 | 43.47±4.98 | |
| Postoperative | |||||
| Con | 0.22±0.02 | 0.05±0.01 | 82.38±4.52 | 44.35±4.03 | |
| I/R | 0.25±0.02* | 0.10±0.02* | 61.84±3.56* | 28.08±3.79* | |
| miR | 0.22±0.01†,‡ | 0.04±0.01†,‡ | 78.33±5.65†,‡ | 39.72±3.74†,‡ | |
| I/R-C | 0.24±0.01* | 0.11±0.02* | 62.49±4.15* | 28.44±4.19* | |
Con = control (sham operate); I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; LVEDV = left ventricular end diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end systolic volume; LVFS = left ventricular fractional shortening; miR = miR-484 treatment.
*Compared with Con group, p<0.05; †Compared with I/R group, p<0.05; ‡Compared with IR-C group, p<0.05.
Ultrastructural observation of myocardial tissue in rats
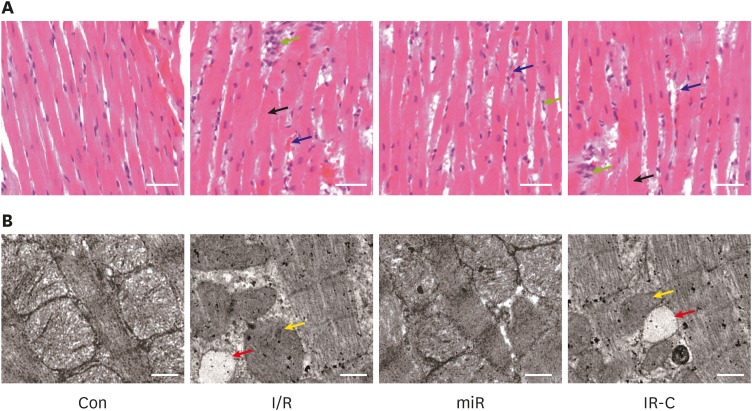
HE staining showed that the histomorphology of myocardial tissues in Con group was basically normal. Cardiomyocyte edema, erythrocyte exudation, inflammatory cell infiltration, and myocardial fibers arrangement disorder were observed in both I/R and IR-C group. In miR group, the above pathological characteristics were relieved in some degrees (Figure 2A).
Figure 2. Histopathological changes of ischemic myocardium in rats. (A) Hematoxylin-eosin staining of myocardial tissues (×200, bar=100 μm); green, black, and blue arrows represented inflammatory cell infiltration, cardiomyocyte edema, and erythrocyte exudation, respectively. (B) Transmission electron microscopy of mitochondrial structure in myocardial cells (×10,000, bar=1 μm); yellow, and red arrows represented vacuolation, and mitochondrial swelling and unclear cristae, respectively.
Con = control (sham operate); I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; miR = miR-484 treatment.
TEM showed that the mitochondrial structure in myocardial cells of Con group was normal. Mitochondrial swelling, vacuolation and unclear cristae were observed in cells of both I/R group and IR-C group. In miR group, the mitochondrial damage was relieved in some degrees (Figure 2B).
MiR-484 reduced the expression of inflammatory factors in myocardium of ischemia-reperfusion
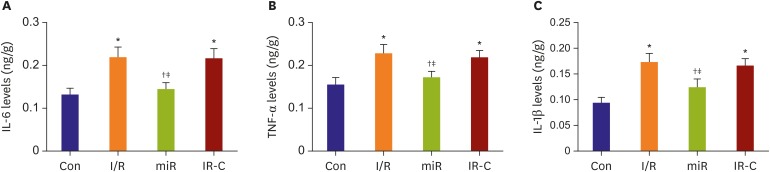
ELISA showed that the concentrations of IL-6, TNF-α, and IL-1β in myocardium of I/R group and IR-C group were significantly higher than those of Con group (p<0.05). Meanwhile, compared with I/R group and IR-C group, the concentrations of IL-6, TNF-α, and IL-1β in miR group were significantly decreased (p<0.05) (Figure 3).
Figure 3. The concentrations of inflammatory factors in myocardial tissue of I/R rats. (A) The concentrations of IL-6 in each group. (B) The concentrations of TNF-α in each group. (C) The concentrations of IL-1β in each group.
Con = control (sham operate); IL = interleukin; I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; miR = miR-484 treatment; TNF = tumor necrosis factor.
*p<0.05 compared with Con group; †p<0.05 compared with I/R group; ‡p<0.05 compared with IR-C group.
Mitochondrial membrane potential in ischemic myocardium
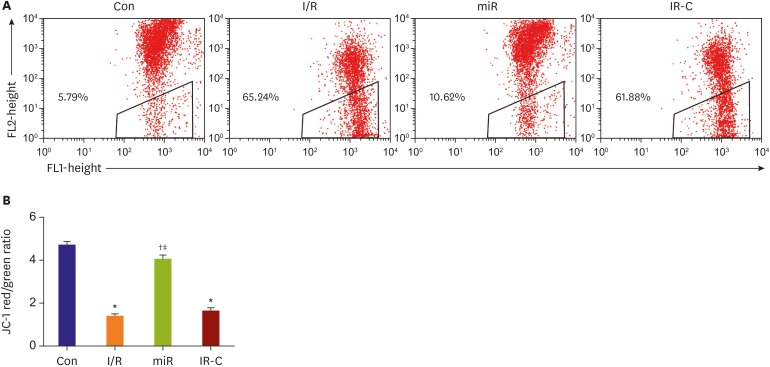
Flow cytometry results showed that the mitochondrial membrane potential was decreased significantly (p<0.05) in both I/R group (65.24%) and IR-C group (61.88%) than that in Con group (5.79%). Compared with I/R group and IR-C group, the occurrence of mitochondrial membrane potential was increased significantly in miR group (10.62%) (p<0.05) (Figure 4).
Figure 4. Mitochondrial membrane potential in myocardial cells. (A) Red-green fluorescence in myocardial cells detected by flow cytometry. (B) Red/green ratio of full-color fluorescence.
Con = control (sham operate); I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; miR = miR-484 treatment.
*p<0.05 compared with Con on group; †p<0.05 compared with I/R group; ‡p<0.05 compared with IR-C group.
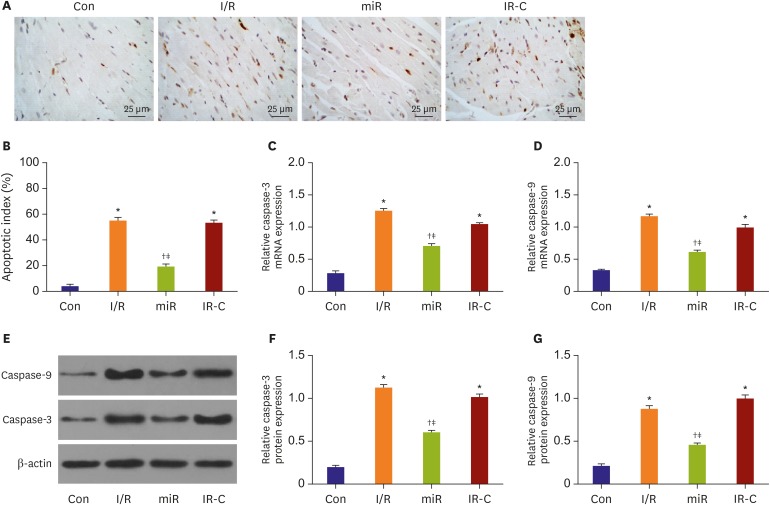
MiR-484 reduced apoptosis of ischemic cardiomyocytes
TUNEL assay showed that the positive apoptotic cells were brown (Figure 5A). Compared with Con group, the apoptotic rate of ischemic cells in I/R and IR-C groups was increased significantly (p<0.05). Meanwhile, compared with I/R group and IR-C group, the apoptotic cells in ischemic area of miR group were significantly reduced (p<0.05). However, the difference in the number of apoptotic cells between IR-C group and I/R group was not significant (p>0.05) (Figure 5B).
Figure 5. MiR-484 reduced apoptosis in myocardium cell of ischemia reperfusion rats. (A) The result of TUNEL assay (×400, bar=25 μm). (B) Myocardial apoptotic index. (C) The expression level of caspase-3. (D) The caspase-9 mRNA expression detected by RT-PCR. (E) The expression level of caspase-3 and caspase-9 protein detected by Western blot. (F) The expression of caspase-3 protein in different groups. and (G) The expression of caspase-9 protein in different groups.
Con = control (sham operate); I/R = ischemia-reperfusion; IR-C = ischemia-reperfusion negative control; miR = miR-484 treatment; mRNA = messenger RNA; RT-PCR = real-time polymerase chain reaction; TUNEL = terminal deoxynucleotidyl transferase-mediated biotinylated nick end-labeling.
*p<0.05 compared with Con on group; †p<0.05 compared with I/R group; ‡p<0.05 compared with IR-C group.
RT-PCR and Western blot results showed that caspase-3 and caspase-9 expression in I/R and IR-C groups were significantly higher than those in Con group (p<0.05). Meanwhile, caspase-3 and caspase-9 expression in miR group were significantly lower than those in I/R and IR-C group (p<0.05). However, the differences of expression levels of caspase-3/caspase-9 were not significant between I/R group and IR-C group (p<0.05) (Figure 5C-G).
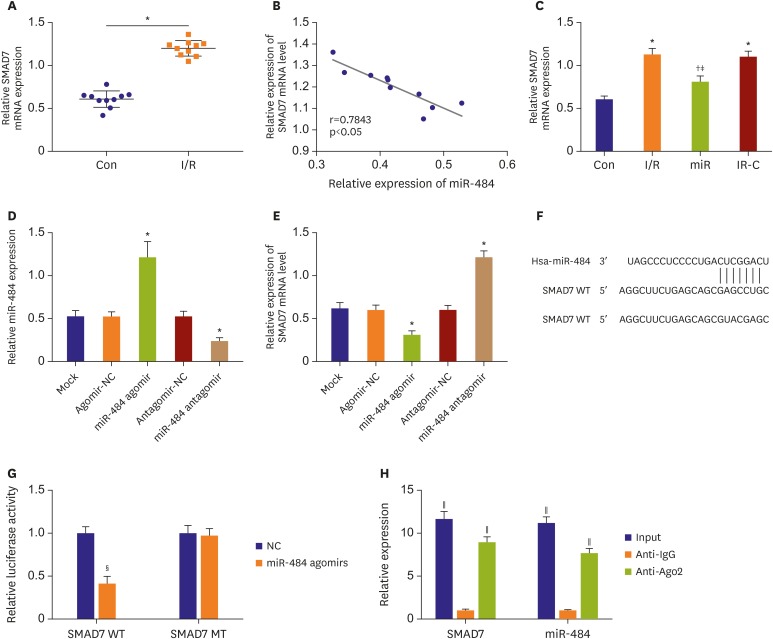
SMAD family member 7 was the target gene of miR-484
The expression of SMAD7 in myocardial tissue was detected by qRT-PCR. The results showed that the expression of SMAD7 was increased in ischemic myocardium compared with normal myocardium (Figure 6A). In addition, there was a significant negative correlation between SMAD7 and miR-484 expression (p<0.05, Figure 6B). After transfection of miR-484, qRT-PCR was used to detect the expression of SMAD in myocardial tissues. The result showed that compared with the Con group, the expression of SMAD7 mRNA in the myocardial tissues of the IR group and the IR-C group was significantly up-regulated (all p<0.05, Figure 6C). Compared with the IR and IR-C groups, SMAD7 mRNA expression was significantly down-regulated (p<0.05) in miR group. In addition, miR-484 was overexpressed and silenced in myocardial cells by the transfection of miR-484 agomir and antagomir, respectively (p<0.05) (Figure 6D). qRT-PCR showed that the transfection of miR-484 agomir and antagomir significantly downregulated and upregulated SMAD7 in myocardial cells (p<0.05) (Figure 6E). Target Scan showed that the binding site of SMAD7 and miR-484 was the 3'-UTR region (Figure 6F). The luciferase reporter gene assay showed that the intensity of luciferase activity was significantly decreased in cells co-transfected with SMAD7-Wt and miR-484 agomir group (p<0.05, Figure 6G). Rip analysis showed that the expression of SMAD7 and miR-484 was significantly increased in anti-ago2 group compared with anti-IgG (all p<0.05, Figure 6H).
Figure 6. SMAD7 was the target gene of miR-484. (A) The expression of SMAD7 in ischemic myocardial tissue. (B) The correlation analysis of SMAD7 and miR-484. (C) The expression of SMAD7 mRNA in myocardial tissue. (D) The expression of miR-484 in myocardial cells transfected with miR-484 agomir and antagomir. (E) The expression of SMAD7 in myocardial cells transfected with miR-484 agomir and antagomir. (F) Target Scan predicted the target site for binding of SMAD7 to miR-484. (G) The dual luciferase reporter gene activity assay. (H) RNA immunoprecipitation analysis.
IgG = immunoglobulin G; mRNA = messenger RNA; MT = mutant; NC = negative control; SMAD7 = SMAD family member 7; WT = wild type.
*p<0.05 compared with Con group; †p<0.05 compared with IR group; ‡p<0.05 compared with IR-C group; §p<0.05 compared to the NC-mimics group; ∥p<0.05 compared with the anti-IgG group.
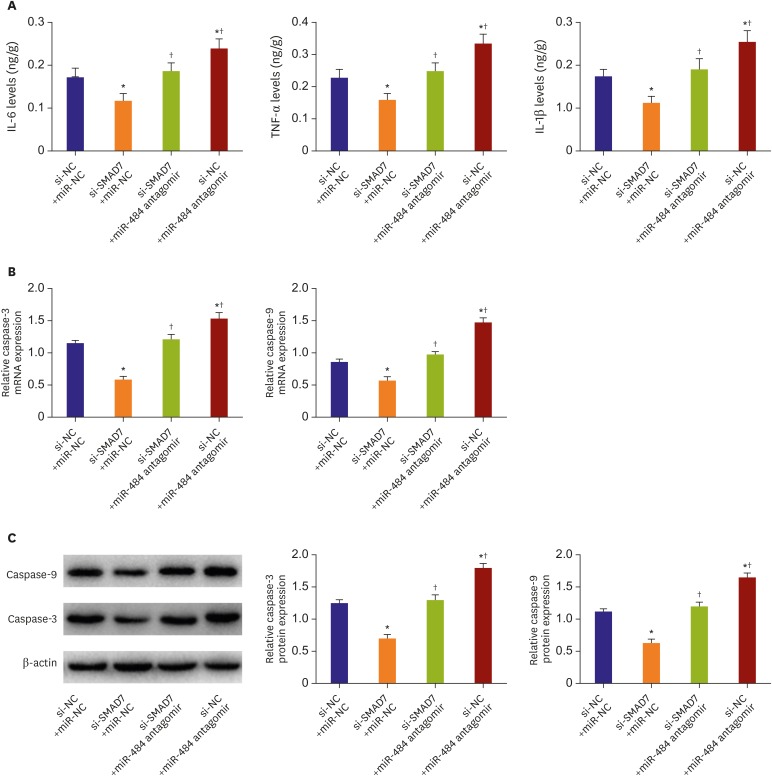
SMAD family member 7 eliminated the protective effect of miR-484 on ischemic myocardium
After transfection of SMAD7 siRNA with miR-484 antagomir, ELISA was used to detect the inflammation (Figure 7A). The result showed that IL-6, TNF-α, and IL-1β in the si-SMAD7+miR-NC group were significantly up-regulated compared to that in si-NC+miR-NC group (all p<0.05). Moreover, compared with the si-NC+miR-NC group, the level of inflammation in the si-NC+miR-484 antagomir group was significantly down-regulated (p<0.05). Furthermore, compared with the si-SMAD7+miR-NC group, the inflammatory levels of the si-SMAD7+miR-484 antagomir group and the si-NC+miR-484 antagomir group were significantly up-regulated (p<0.05). The qRT-PCR (Figure 7B) and Western blot (Figure 7C) were used to detect the expression of caspase-3 and caspase-9. The results showed that compared with the si-NC+miR-NC group, the caspase-3/9 mRNA and protein levels in the si-SMAD7+miR-NC group were significantly down-regulated (all p<0.05). Moreover, compared with the si-NC+miR-NC group, the caspase-3/9 mRNA and protein levels were significantly up-regulated in the si-NC+miR-484 antagomir group (all p<0.05). Furthermore, compared with the si-SMAD7+miR-NC group, the caspase-3/9 mRNA and protein levels were significantly up-regulated in both si-SMAD7+miR-484 antagomir group and si-NC+miR-484 antagomir group (all p<0.05).
Figure 7. SMAD7 eliminated the protective effect of miR-484 on ischemic myocardium. (A) The concentrations of inflammatory factors in myocardial tissue of each group. (B) The detection of caspase-3/9 mRNA expression by RT-PCR. (C) Western blot analysis of caspase-3 and caspase-9 protein levels in each group.
IL = interleukin; miR-NC = miR-484 antagomir negative control; mRNA = messenger RNA; RT-PCR = real-time polymerase chain reaction; si-SMAD7 = SMAD7 siRNA; si-NC = SMAD7 siRNA negative control; SMAD7 = SMAD family member 7; TNF = tumor necrosis factor.
*p<0.05 compared with the si-NC+miR-NC group; †p<0.05 compared with the si-SMAD7+miR-NC group.
DISCUSSION
Early reperfusion of ischemic cardiac tissues remains the most effective intervention for improving clinical outcome following myocardial infarction. However, myocardial reperfusion can lead to cardiomyocyte death and consequent loss of cardiac function.11) A previous study indicates that miRNAs play important roles in the process of MI/R injury.12) The up-regulation of miR-1 can decrease mitochondrial membrane potential, increase cytochrome-c release, and increase apoptosis.13) Via Akt- and p53-dependent pathway, the up-regulated miR-210 exerts cytoprotective effect in hypoxic cardiomyocytes.14) In mice model, the genetic deletion of miR-214 causes loss of cardiac contractility, increased apoptosis, and excessive fibrosis in response to I/R injury.15) Overexpression of miR-25 increases the survival rate of rats and inhibits sepsis-induced cardiomyocyte apoptosis.16) As an important member of miRNAs, miR-484 has been shown to take part in the process of cell proliferation and apoptosis.17) Previous study demonstrates that miR-484 can modulate cytidine deaminase axis and play various roles in cell apoptosis.18) Actually, sequential activation of caspases like caspase-3 and caspase-9 play central roles in the cell apoptosis.19) Down-regulation of caspase-3 can lead to the cardiomyocytes apoptosis in the I/R rat.9) A previous study indicates that caspase-3 is essential for pro-caspase-9 processing and cisplatin-induced apoptosis.20) Targeted regulation of caspase-3 or caspase-9 gene expression can directly affect the outcome of MI/R injury.7)8) Importantly, miR-484 triggers the migration and proliferation, as well as simultaneously reduces caspase-3 expression in tumor cells.21) In the current study, RT-PCR analysis showed that miR-484 was down-regulated in myocardial tissues of I/R rats. After miR-484 agomir treatment, the TUNEL assay showed that apoptotic cells in ischemic area were decreased significantly than non-treatment groups based on MI/R model. Meanwhile, Western-blot showed that the expression of caspase-3 and caspase-9 in cardiomyocytes were up-regulated with MI/R injury, while down-regulated after miR-484 agomir treatment. Thus, we speculate that miR-484 may protect myocardial cells from I/R injury by suppressing expression of caspase-3 and caspase-9 during cardiomyocyte apoptosis.
Inflammation is considered to be the most important cause of tissue injury in organs subjected to ischemia. In the process of I/R injury, the inflammation factors such as IL-6, TNF-α, and IL-1β are up-regulated in vivo.22) The incidence rate of acute MI/R injury is significantly lower in IL-6 deficient mice. A previous study shows that the protective effect of agonist in I/R injury in rats is realized by the down-regulation of IL-6, TNF-α, and IL-1β.23) In the current study, the ELISA assay showed that the contents of IL-6, TNF-α, and IL-1β were all increased in MI/R tissues, while decreased after miR-484 treatment. This result indicates that miR-484 may reduce the contents of inflammatory factors in myocardium of I/R rats. Actually, the level of inflammation in cells is negatively correlated with the level of mitochondrial membrane potential.24) A previous study indicates that mitochondrial membrane potential plays a vital role in the process of I/R.25) Sun et al.26) proved that change in mitochondrial membrane potential was the key mechanism in early warm hepatic I/R injury. Solhjoo and O'Rourke27) indicated that mitochondrial instability during regional I/R underlied arrhythmias in monolayers of cardiomyocytes. In this study, flow cytometry analysis showed that mitochondrial membrane potential in myocardial cell was decreased when I/R injury occurred. The miR-484 treatment significantly elevated the mitochondrial membrane potential. Thus, we speculate that miR-484 may alleviate the decreasing of mitochondrial membrane potential in MI/R cells.
SMAD7 is a major inhibitory regular in transforming growth factor (TGF)-β signaling pathway that can affect the activity of TGF-β.28) Zhang et al.29) proved that the inhibition of miR-92a attenuated myocardiocyte apoptosis induced by hypoxia/reoxygenation by targeting SMAD7. Yang et al.30) proved that inhibition of miR-15a protected against hypoxia/reoxygenation-induced apoptosis of cardiomyocytes by targeting SMAD7. In this study, we found that SMAD7 was a target gene of miR-484. SMAD7 eliminated the protective effect of miR-484 in MI/R cells. Our findings indicate that the upregulation of miR-484 may protect against MI/R by targeting SMAD7. The alleviating effects of the above miRs on MI/R may depend on SMAD7.
In conclusion, miR-484 might protect myocardial cells from I/R injury by suppressing expression of caspase-3 and caspase-9 during cardiomyocyte apoptosis. Moreover, miR-484 might reduce the expression of inflammatory factors including IL-6, TNF-α, and IL-1β in MI/R. Furthermore, miR-484 might alleviate the decreasing of mitochondrial membrane potential in MI/R cells.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Formal analysis: Li S, Jiang W.
- Investigation: Liu H.
- Methodology: Li S.
- Project administration: Liu H.
- Validation: Li Y.
- Writing - original draft: Liu H, Li S, Jiang W, Li Y.
- Writing - review & editing: Li Y.
References
- 1.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuxian X, Jianfeng G, Ke L. Influence of electricity needle on nuclear transcription factor NF-κB and amino acid transmitters of aging rat's ischemia reperfusion brain tissue. Tradit Chin Med Orthop Med. 2010;25:462, 465. [Google Scholar]
- 3.Wang X, Zhang X, Ren XP, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Li L, Li X, Wu S. Long noncoding RNA LINC00339 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting miR-484. Biochem Biophys Res Commun. 2018;503:3038–3043. doi: 10.1016/j.bbrc.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 5.Loison F, Zhu H, Karatepe K, et al. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J Clin Invest. 2014;124:4445–4458. doi: 10.1172/JCI76246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Zhang J, Jia Q, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010;24:195–201. doi: 10.3892/or_00000846. [DOI] [PubMed] [Google Scholar]
- 7.Thukkani AK, Shoghi KI, Zhou D, et al. PET imaging of in vivo caspase-3/7 activity following myocardial ischemia-reperfusion injury with the radiolabeled isatin sulfonamide analogue [(18)F]WC-4-116. Am J Nucl Med Mol Imaging. 2016;6:110–119. [PMC free article] [PubMed] [Google Scholar]
- 8.Sodhi RK, Singh M, Singh N, Jaggi AS. Protective effects of caspase-9 and poly(ADP-ribose) polymerase inhibitors on ischemia-reperfusion-induced myocardial injury. Arch Pharm Res. 2009;32:1037–1043. doi: 10.1007/s12272-009-1709-9. [DOI] [PubMed] [Google Scholar]
- 9.Kimuta M, Miura T, Okamura T, Iwamoto H, Iwatate M, Matsuzaki M. Effect of caspase-3 inhibitor on myocyte apoptosis and left ventricular remodeling in the ischemia-reperfused rat heart. J Card Fail. 1999;5:48. [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Marrone AK, Beland FA, Pogribny IP. The role for microRNAs in drug toxicity and in safety assessment. Expert Opin Drug Metab Toxicol. 2015;11:601–611. doi: 10.1517/17425255.2015.1021687. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Chen Q, Lew KS, Richards AM, Wang P. Discovery of potential therapeutic miRNA targets in cardiac ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2016;21:296–309. doi: 10.1177/1074248415604463. [DOI] [PubMed] [Google Scholar]
- 13.Yu XY, Song YH, Geng YJ, et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–552. doi: 10.1016/j.bbrc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011;301:H1519–30. doi: 10.1152/ajpheart.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurora AB, Mahmoud AI, Luo X, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Y, Sun F, Lei M. MiR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep. 2018;38:BSR20171511. doi: 10.1042/BSR20171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed FE, Jeffries CD, Vos PW, et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics. 2009;6:281–295. [PubMed] [Google Scholar]
- 18.Hu X, Ye F, Cao Z, et al. Abstract P4-07-11: dual characteristics of microRNA-484 modulated cytidine deaminase (CDA) axis in breast cancer: chemo-resistance and regulating cell proliferation; Thirty-Sixth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2013 Dec 10–14; Tue, USA. San Antonio: American Association for Cancer Research; 2013. [Google Scholar]
- 19.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 20.Blanc C, Deveraux QL, Krajewski S, et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 2000;60:4386–4390. [PubMed] [Google Scholar]
- 21.Li T, Ding ZL, Zheng YL, Wang W. MiR-484 promotes non-small-cell lung cancer (NSCLC) progression through inhibiting Apaf-1 associated with the suppression of apoptosis. Biomed Pharmacother. 2017;96:153–164. doi: 10.1016/j.biopha.2017.09.102. [DOI] [PubMed] [Google Scholar]
- 22.Lunsford KE, Baird BJ, Sempowski GD, et al. Upregulation of IL-1β, IL-6, and CCL-2 by a novel mouse model of pancreatic ischemia-reperfusion injury. Transplantation. 2013;95:1000–1007. doi: 10.1097/TP.0b013e318286483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Murase N, Maeda T, et al. Protective effect of TNF-α and IL-1 β inhibitor FR167653 on ischemia-reperfusion injury in rat small intestinal transplantation. Transplant Proc. 1998;30:2638. doi: 10.1016/s0041-1345(98)00760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol Aging. 2013;34:602–609. doi: 10.1016/j.neurobiolaging.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Ylitalo KV, Ala-Rämi A, Liimatta EV, Peuhkurinen KJ, Hassinen IE. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J Mol Cell Cardiol. 2000;32:1223–1238. doi: 10.1006/jmcc.2000.1157. [DOI] [PubMed] [Google Scholar]
- 26.Sun CK, Zhang XY, Sheard PW, Mabuchi A, Wheatley AM. Change in mitochondrial membrane potential is the key mechanism in early warm hepatic ischemia-reperfusion injury. Microvasc Res. 2005;70:102–110. doi: 10.1016/j.mvr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Solhjoo S, O'Rourke B. Mitochondrial instability during regional ischemia-reperfusion underlies arrhythmias in monolayers of cardiomyocytes. J Mol Cell Cardiol. 2015;78:90–99. doi: 10.1016/j.yjmcc.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Li D. Smad7 and fibrotic disorders. Acta Universitatis Medicinalis Secondae Shanghai. 2005;2:131–133. [Google Scholar]
- 29.Zhang B, Zhou M, Li C, et al. MicroRNA-92a inhibition attenuates hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting Smad7. PLoS One. 2014;9:e100298. doi: 10.1371/journal.pone.0100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Ding S, Xu G, Chen F, Ding F. MicroRNA-15a inhibition protects against hypoxia/reoxygenation-induced apoptosis of cardiomyocytes by targeting mothers against decapentaplegic homolog 7. Mol Med Rep. 2017;15:3699–3705. doi: 10.3892/mmr.2017.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]