Abstract
Background
Type 2 diabetes mellitus (T2DM) is associated with an increased risk for dementia. The effects of hypoglycemia on dementia are controversial. Thus, we evaluated whether hypoglycemia increases the risk for dementia in senior patients with T2DM.
Methods
We used the Korean National Health Insurance Service Senior cohort, which includes >10% of the entire senior population of South Korea. In total, 5,966 patients who had ever experienced at least one episode of hypoglycemia were matched with those who had not, using propensity score matching. The risk of dementia was assessed through a survival analysis of matched pairs.
Results
Patients with underlying hypoglycemic events had an increased risk for all-cause dementia, Alzheimer's dementia (AD), and vascular dementia (VaD) compared with those who had not experienced a hypoglycemic event (hazard ratio [HR], 1.254; 95% confidence interval [CI], 1.166 to 1.349; P<0.001 for all-cause dementia; HR, 1.264; 95% CI, 1.162 to 1.375; P<0.001 for AD; HR, 1.286; 95% CI, 1.110 to 1.490; P<0.001 for VaD). According to number of hypoglycemic episodes, the HRs of dementia were 1.170, 1.201, and 1.358 in patients with one hypoglycemic episode, two or three episodes, and more than three episodes, respectively. In the subgroup analysis, hypoglycemia was associated with an increased risk for dementia in both sexes with or without T2DM microvascular or macrovascular complications.
Conclusion
Our findings suggest that patients with a history of hypoglycemia have a higher risk for dementia. This trend was similar for AD and VaD, the two most important subtypes of dementia.
Keywords: Dementia; Diabetes mellitus, type 2; Hypoglycemia
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with a 1.5 to 2.5-fold increased risk for dementia, and this trend is similar for both Alzheimer's disease (AD) and vascular dementia (VaD), which are the most common subtypes of dementia [1,2]. With the increasing numbers of elderly people with T2DM in the general population, dementia is especially important due to its impact on self-management and quality of life. Therefore, identifying modifiable risk factors for dementia is crucial to public health. The mechanisms underlying the association between T2DM and dementia are not fully understood, but they are multifactorial, including poor glucose control, hypoglycemia, inflammatory mediators, and rheological factors [3].
Hypoglycemia is a potentially serious complication during diabetes treatment and it may be an obstacle to optimal glycemic control. As glucose is the primary source of energy for the brain, hypoglycemia may cause mild or severe neurological deficits, including coma [4,5]. Although the short-term effects of hypoglycemia on cognitive function have been well documented, it is unclear whether patients with hypoglycemia will develop dementia. A few epidemiological studies have shown that severe hypoglycemia is associated with a greater risk for dementia [6,7,8,9], but others have reported no significant association between hypoglycemia and subsequent dementia [10,11]. In addition, because the pathogeneses of AD and VaD are very different, the same hypoglycemic assault may have different effects on the progression of dementia according to subtype. However, previous studies have not estimated dementia risk according to dementia subtype.
Therefore, the purpose of the current study was to investigate the association between hypoglycemia and subsequent dementia in older adults with T2DM by conducting a propensity score matching analysis to minimize selection bias. We also identified the effect of hypoglycemia on dementia according to each subtype. We used the Korean National Health Insurance Service Senior cohort, which contains 14 years of follow-up data for >10% of all older adults in South Korea.
METHODS
Study design and data source
This study was a population-based retrospective observational study and was approved by the Institutional Review Board of the Korea National Institute for Bioethics Policy (http://www.irb.or.kr/, P01-201811-21-008), which waived the requirement for informed consent because all patient data were de-identified.
Data source
We used the Korean National Health Insurance Service Senior cohort version 3.0 (January 1, 2002 to December 31, 2015), which contains around 550,000 patients (>10% of the entire South Korea senior population in 2002). This dataset was extracted from the Korean National Health Insurance Service, which covers over 99% of the South Korean population, using a stratified random sampling method with 1,476 strata; thus, it is representative of the entire Korean senior population. This data set included information on the socioeconomic status of Korean National Health Insurance Service recipients; status was based on income and assets, such as property and automobile ownership.
Inclusion and exclusion criteria
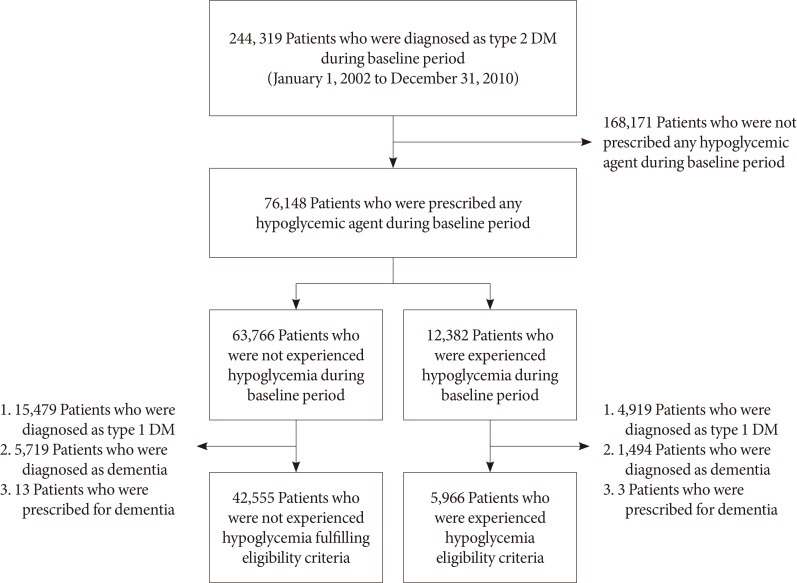
Patients diagnosed with T2DM and prescribed oral hypoglycemic agents or insulin were enrolled (Fig. 1). The index date was January 1, 2011, and patients were divided into two groups according to the presence of hypoglycemia (International Classification of Diseases, 10th revision [ICD-10] codes E11.63, E15, E16.0, E16.1, and E16.2) before the index date. Patients who experienced at least one hypoglycemic episode were placed in the hypoglycemia group, which was then divided into three groups according to the number of hypoglycemic episodes (one episode, two or three episodes, or more than three episodes). Patients with type 1 diabetes mellitus (T1DM; ICD-10 code E10) or dementia diagnoses (ICD-10 codes F00, F01, F02, F03, F04, F05, G30, or G31) or a medication prescribed to treat dementia (donepezil, memantin, rivastigmine, or galantamine) before the index date (January 1, 2002 to December 31, 2010) were excluded. The loss of samples is described in Fig. 1. Because the Korean National Health Insurance Service Senior cohort consists of patients ≥60 years old in 2002, all patients were >68 years of age on the index date.
Fig. 1. Flow chart of the sample selection. DM, diabetes mellitus.
Study outcome and subgroup analysis
The primary outcome was the first diagnosis of all-cause dementia (ICD-10 codes F00, F01, F02, F03, F04, F05, G30, or G31). The secondary outcome was the first diagnosis of AD (F00, G30) or VaD (F01). Additionally, we assessed differences in the risk of dementia according to the number of hypoglycemic episodes by dividing the sample into three groups. Subgroup analyses were performed according to sex, age (<75 and ≥75 years), and the presence of T2DM microvascular (any diagnosis of diabetic nephropathy, neuropathy, or retinopathy) or macrovascular complications (any diagnosis of stroke, transient ischemic attack, acute myocardial infarction, other ischemic heart disease, and peripheral artery occlusive disease).
Statistical analysis
All statistical analyses were performed using R software version 3.3.3 (R Development Core Team, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC, USA). All values are presented as mean±standard deviation. The differences between patients with and without an underlying hypoglycemic event were adjusted with propensity score matching using the nearest-neighbor technique with a caliper of 0.1 on the probability scale. We set age, sex, socioeconomic status (index date), diagnoses (1 year before the index date), and prescribed drugs (180 days before the index date) as confounding variables (all variables presented in Table 1) and those were used to calculate propensity scores. The balance achieved by matching propensity scores was assessed using standardized differences, t-tests (age), chi-square tests (all other variables except age and socioeconomic status), and Mantel-Haenszel chi-square tests (socioeconomic status); an absolute standardized difference between groups <0.1 was considered negligible. After the propensity score matching, a Kaplan-Meier curve and Cox proportional hazards model were used to evaluate the dementia risk of hypoglycemia. Because all confounding variables were adjusted in propensity score matching, 1 minus Kaplan-Meier estimate, and a univariate Cox regression analysis were performed.
Table 1. Baseline characteristics of propensity score matched patients.
| Characteristic | Patients without underlying hypoglycemia | Patients with underlying hypoglycemia | SMD | P value |
|---|---|---|---|---|
| Number | 5,966 | 5,966 | ||
| Age, yr | 75.82±5.34 | 75.82±5.41 | <0.001 | 0.947 |
| Male sex, % | 37.81 | 38.50 | 0.014 | 0.778 |
| Socio-economic status | 0.042 | 0.427 | ||
| 1st to 4th of 11 quantiles | 1,628 (27.29) | 1,627 (27.27) | ||
| 5th to 8th of 11 quantiles | 1,822 (30.54) | 1,860 (31.18) | ||
| 9th to 11th of 11 quantiles | 2,516 (42.17) | 2,479 (41.55) | ||
| Hypertension | 86.24 | 85.82 | 0.012 | 0.401 |
| Dyslipidemia | 58.85 | 59.65 | 0.016 | 0.394 |
| Chronic kidney disease | 7.58 | 7.71 | 0.005 | 0.945 |
| End-stage renal disease | 3.62 | 4.22 | 0.031 | 0.473 |
| Any malignancy | 11.33 | 11.26 | 0.002 | 0.439 |
| Migraine | 4.53 | 4.51 | 0.001 | 0.654 |
| Asthma | 19.18 | 19.01 | 0.004 | 0.971 |
| Chronic obstructive pulmonary disease | 14.28 | 13.88 | 0.012 | 0.685 |
| Connective tissue disease | 5.88 | 6.22 | 0.014 | 0.828 |
| Atrial fibrillation | 4.51 | 4.64 | 0.006 | 0.172 |
| Heart failure | 8.3 | 8.41 | 0.004 | 0.945 |
| Osteoporosis | 25.96 | 26.22 | 0.006 | 0.699 |
| Cerebrovascular disease | ||||
| Ischemic stroke | 15.37 | 15.39 | <0.001 | 0.533 |
| Hemorrhagic stroke | 0.62 | 0.85 | 0.027 | 0.914 |
| Transient ischemic attack | 4.24 | 3.97 | 0.014 | 0.608 |
| Acute myocardial infarction | 4.61 | 4.27 | 0.016 | 0.587 |
| Other ischemic heart disease | 28.43 | 28.03 | 0.009 | 0.768 |
| Other heart disease | 22.18 | 21.77 | 0.01 | 0.826 |
| Peripheral artery disease | 2.77 | 2.63 | 0.008 | 0.476 |
| Microvascular complications of diabetes | ||||
| Neuropathy | 22.95 | 23.21 | 0.006 | 0.533 |
| Nephropathy | 8.95 | 9.34 | 0.013 | 0.271 |
| Retinopathy | 20.62 | 20.62 | <0.001 | 0.254 |
| Alcohol usea | 2.83 | 2.65 | 0.011 | 0.736 |
| Obesitya | 0.02 | 0.03 | 0.011 | 0.465 |
| Medication use | ||||
| Anti-diabetic medicine | ||||
| Metformin | 67.83 | 67.77 | 0.001 | 0.583 |
| Sulfonylurea | 73.57 | 72.93 | 0.014 | 0.254 |
| Thiazolidinedione | 9.1 | 9.52 | 0.014 | 0.105 |
| DPP-4i | 9.29 | 8.73 | 0.019 | 0.722 |
| α-Glucosidase inhibitor | 23.33 | 22.9 | 0.01 | 0.662 |
| Meglitinide | 4.76 | 5.43 | 0.03 | 0.777 |
| Insulin | 15.35 | 16.46 | 0.03 | 0.878 |
| Anti-hypertensive agent | ||||
| Calcium channel blocker | 35.89 | 35.97 | 0.002 | 0.446 |
| ACEI | 10.46 | 10.81 | 0.011 | 0.463 |
| ARB | 52.46 | 52.03 | 0.009 | 0.728 |
| β-Blocker | 18.67 | 18.54 | 0.003 | 0.573 |
| α-Blocker | 3.1 | 3.07 | 0.002 | 0.532 |
| Diuretics | 33.44 | 33.02 | 0.009 | 0.473 |
| Aspirin | 45.31 | 45.31 | <0.001 | 0.956 |
| P2Y12 inhibitor | 14.08 | 13.68 | 0.012 | 0.438 |
| Warfarin | 1.91 | 2.15 | 0.017 | 0.949 |
| Other antiplatelet | 9.45 | 9.35 | 0.003 | 0.925 |
| NOAC | 0.08 | 0.05 | 0.013 | 0.705 |
| Lipid-lowering agent | ||||
| Statin | 35.77 | 35.97 | 0.004 | 0.789 |
| Fibrate | 3.4 | 3.3 | 0.006 | 0.757 |
| Ezetimibe | 1.42 | 1.51 | 0.007 | 0.819 |
Values are presented as mean±standard deviation, number (%), or percentage. The mean±standard deviation standardized difference of all covariates was 1.06%±0.09%.
SMD, standardized mean difference; DPP-4i, dipeptidyl-peptidase IV inhibitor; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor antagonists; NOAC, novel oral anticoagulant.
aConfirmed using the International Classification of Diseases (10th revision) diagnosis codes.
RESULTS
In total, 48,521 patients were included in the cohort and 220,385 person-years were considered. After propensity score matching, 5,966 patients comprised both groups. The baseline characteristics of the matched group are presented in Table 1; all absolute values of standardized differences were <0.1; thus, all confounding variables were considered properly adjusted by propensity score matching. The mean follow-up period of the matched pairs was 1,591.1 days.
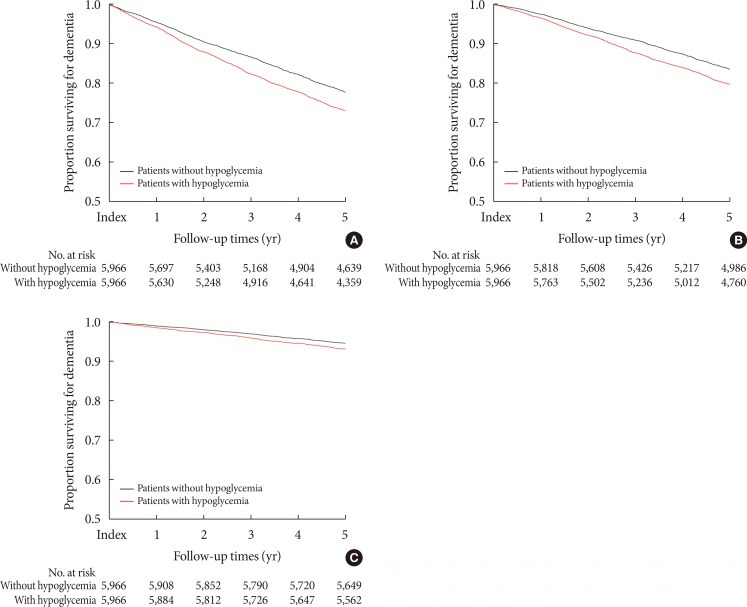
During the study period, 2,934 patients were newly diagnosed with dementia. The patients with at least one hypoglycemia episode showed an increased risk of all-cause dementia compared with those without hypoglycemia (hazard ratio [HR], 1.254; 95% confidence interval [CI], 1.166 to 1.349; P<0.001) (Table 2, Fig. 2). Patients with underlying hypoglycemia were prone to AD and VaD (HR, 1.264; 95% CI, 1.162 to 1.375, P<0.001 for AD; and HR, 1.286; 95% CI, 1.110 to 1.490; P<0.001 for VaD) (Table 2, Fig. 2).
Table 2. The risk of dementia in patients with hypoglycemia compared to patients without hypoglycemia.
| Variable | Number | Events | HR | 95% CI | P value |
|---|---|---|---|---|---|
| All-cause dementia | 11,932 | 2,934 | 1.254 | 1.166–1.349 | <0.001 |
| Alzheimer's disease | 11,932 | 2,186 | 1.264 | 1.162–1.375 | <0.001 |
| Vascular dementia | 11,932 | 721 | 1.286 | 1.110–1.490 | <0.001 |
HR, hazard ratio; CI, confidence interval.
Fig. 2. Kaplan-Meier plots for (A) all-cause dementia, (B) Alzheimer disease, and (C) vascular dementia in patients with or without underlying hypoglycemia.
According to number of hypoglycemic episodes, the HRs of dementia were 1.170, 1.201, and 1.358 in patients with one hypoglycemic episode, two or three episodes, and more than three episodes, respectively (Table 3).
Table 3. The risk of dementia according to number of hypoglycemic episodes in patients with and without previous hypoglycemic episode.
| No. of previous hypoglycemic episodes | Number | Events | HR | 95% CI | P value |
|---|---|---|---|---|---|
| 1 | 4,622 | 1,159 | 1.170 | 1.043–1.313 | 0.008 |
| 2–3 | 2,946 | 550 | 1.201 | 1.016–1.421 | 0.032 |
| >3 | 4,354 | 256 | 1.358 | 1.060–1.740 | 0.016 |
HR, hazard ratio; CI, confidence interval.
In the subgroup analysis, patients were divided according to sex, age, or the presence of T2DM microvascular or macrovascular complications. Patients who experienced hypoglycemia showed an increased risk for dementia in all subgroups (Table 4).
Table 4. Subgroup analyses according to sex, age, and the presence of diabetic microvascular or macrovascular complications.
| Variable | Number | Events | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Male sex | 4,553 | 921 | 1.180 | 1.037–1.344 | <0.05 |
| Female sex | 7,379 | 2,013 | 1.297 | 1.188–1.416 | <0.001 |
| Patients aged ≥75 years | 6,210 | 1,893 | 1.197 | 1.093–1.310 | <0.001 |
| Patients aged <75 years | 5,722 | 1,041 | 1.376 | 1.217–1.556 | <0.001 |
| Patients with DM microvascular complications | 5,039 | 1,330 | 1.312 | 1.177–1.462 | <0.001 |
| Patients without DM microvascular complications | 6,893 | 1,604 | 1.208 | 1.095–1.332 | <0.001 |
| Patients with DM macrovascular complications | 5,089 | 1,354 | 1.226 | 1.102–1.365 | <0.001 |
| Patients without DM macrovascular complications | 6,843 | 1,580 | 1.276 | 1.155–1.409 | <0.001 |
HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
DISCUSSION
This population-based retrospective cohort study showed that patients with a history of hypoglycemia had a higher risk of dementia using the propensity score matching method with large cohort data. This association was persistent in both the AD and VaD subtypes. Patients who experienced repeated episodes of hypoglycemia had a higher risk of dementia. In the subgroup analysis, hypoglycemia increased the risk for dementia in both sexes with or without T2DM microvascular and macrovascular complications.
This is the first cohort study using the propensity score matching method to minimize selection bias. In addition, this is the first study to estimate dementia risk of hypoglycemia according to the specific dementia subtype. Our study included the largest number of patients who have ever experienced hypoglycemia compared with previous studies.
Our study showed that hypoglycemia increased the risk of dementia by 25.4%, which conflicts with some previous studies. The Fremantle Diabetes Study found no evidence that hypoglycemia contributes to cognitive impairment in older adults with T2DM who had normal cognition at baseline [10]. However, the small sample size and relatively short follow-up duration may have limited the power to detect differences. In the Diabetes Control and Complication Trial on younger adults with T1DM, 40% of the patients experienced at least one hypoglycemic coma or seizure but did not decline cognitively [11]. Although this cohort was followed for 18 years, the mean age of the enrolled patients was 27 years, which was too young to show cognitive impairment. The same assault of hypoglycemia may have different impacts according to the age of the subject population, as levels of hippocampal neurogenesis steadily decrease with age [12]. The conflicting results may be due to differences in the demographics of the study populations.
Consistent with our results, three retrospective studies showed that at least one hypoglycemic episode increases the risk of dementia [6,7,9]. Additionally, repeated episodes of hypoglycemia were correlated with a higher risk of dementia [6,9]. Lin and Sheu [7] showed an increased risk of dementia in subjects with a history of hypoglycemia. However, they adjusted only for age, sex, insulin use, and five comorbidities, and included only 289 patients who had had at least one hypoglycemic episode, which is a relatively small sample size [7]. Another retrospective cohort study using the Kaiser Permanente Northern California Diabetes Registry of around 17,000 patients, with a mean age of 65 years, who were followed for 27 years, showed that one, two, and three or more episodes of hypoglycemia increased the risk of dementia, with HRs of 1.26, 1.80, and 1.94, respectively [6]. However, this cohort involved only approximately 1,500 patients with a hypoglycemic experience and did not control for either economic status, which is among the important risk factors for dementia, or medication use, including class of oral hypoglycemic agent or statins, which can affect dementia [13,14,15,16,17,18]. The third study, using the Clinical Practice Research Datalink (CPRD), which contains the medical records from 674 general practices in the United Kingdom, showed that hypoglycemia is associated with a higher risk of dementia [9]. Furthermore, the risk of dementia increased with the number of hypoglycemia episodes. However, CPRD is collected from general practice and may be biased relative to our data, which was collected from a primary hospital to a tertiary hospital, and this study also did not compensate for socioeconomic status, or medication use, such as each oral hypoglycemic agent class or statins. Compared to all of these previous studies using Cox proportional hazards models that compensated for insufficient confounding factors and included small numbers of patients with hypoglycemia, we used the propensity score matching method and more than 40 confounding variables, including socioeconomic status, oral hypoglycemic agent class, statin use, and many underlying diseases, and included the largest number of patients with hypoglycemia.
Several possible mechanisms have been suggested from animal studies for how hypoglycemia can cause dementia. Multiple animal and human magnetic resonance imaging studies have reported that severe hypoglycemia with coma can lead to selective neuronal cell death in vulnerable brain regions, such as the cortex and hippocampus [19]. This can lead to a decline in memory. In a rat model, significant learning and memory deficits were identified with behavioral testing 6 weeks after inducing 30 minutes of EEG isoelectricity with severe hypoglycemia, which could be attributed to neuronal death observed in the hippocampus of these animals [20]. In another animal study, prolonged moderate hypoglycemia without coma also caused neuronal damage in the prefrontal medial cortex, piriform cortex, and orbital cortex in rats [21].
Other explanations related to vascular and AD pathology are plausible. Increased adrenaline levels during hypoglycemic events enhance platelet and leucocyte activities, and increase the formation of fibrinogen [22,23]. Endothelial function may also be compromised during a hypoglycemic attack [24]. All of these factors contribute to micro- or macro-infarcts in the brain, causing vascular cognitive decline. Several studies have shown a possible link between hypoglycemia and AD pathology. In a study with rat primary cortical astroglial cells, hypoglycemia enhanced the expression of mRNA encoding the β-amyloid precursor protein. In a study with neuroblastoma cells, hypoglycemia induced tau hyperphosphorylation, which can lead to the formation of neurofibrillary tangles [25]. In the present study, a history of hypoglycemia increased both the AD and vascular subtypes of dementia. Although it is common to have a single patient with both AD and VD pathology, these subtypes can be difficult to distinguish. This study suggests that hypoglycemia can be related to both pathomechanisms and may make the brain more vulnerable to both pathologies.
This study had several strengths. First, we investigated the largest senior cohort, which contains all of the claims data for >10% of the entire elderly population in South Korea for 14 years. Second, we tried to exclude bidirectional effects as much as possible. Dementia is one of the major risk factors for hypoglycemia; thus, dementia and hypoglycemia have a bi-directional effect. All subjects in our study were free of dementia at baseline, as we excluded patients who were diagnosed with dementia or prescribed dementia medications during the 9-year period before the index date, to assess temporality in the relationship. Third, as described above, we minimized selection bias by using propensity score matching with 48 variables, such as demographics, socioeconomic status, medication use, and comorbidities.
However, several limitations should be considered. This was a retrospective analysis, and the claims database lacked results of any measurements, such as glycated hemoglobin or Mini-Mental State Exam scores, which may have influenced the results. Specifically, patients with mild cognitive impairment are more difficult to identify using a claims database without cognitive testing results. Thus, prospective cohort studies that include cognitive function tests to exclude mild cognitive impairment before enrollment are needed to confirm our results. Additionally, we analyzed the incidence of dementia and hypoglycemia according to ICD-10 diagnostic codes; however, there may have been some discrepancies between the medical diagnoses and the diagnostic codes in the claims data. However, a previous study showed that the dementia diagnoses in claims data were quite correct when compared with clinically diagnosed dementia [26]. In addition, because >99% of South Koreans are registered with National Health Insurance, and the dementia diagnostic codes are mandatory to earn insurance benefits when being prescribed dementia medication, it is highly unlikely that dementia would be found without the relevant diagnostic codes in the claims data. In terms of hypoglycemia, clinicians tend not to record hypoglycemia in actual practice, especially when it is mild, when entering diagnostic codes. Thus, this study is limited by the possibility that we underestimated the incidence of hypoglycemic episodes and were unable to assess the effects of mild hypoglycemia on dementia. Last, because this study only included the South Korean population, the results may not be generalizable to all ethnic groups. The incidence and proportion of dementia subtypes vary among ethnic groups [27,28]. Previous studies have shown that the Asian population has a greater proportion of VaD and a lower proportion of AD [29].
In summary, our study indicates that a history of hypoglycemia increases the risk for dementia in older adults with T2DM. This association is consistent for both AD and VaD. Caution is advised when treating T2DM in older individuals with hypoglycemia, to prevent deterioration of their cognitive ability.
ACKNOWLEDGMENTS
This study utilizes the data of the National Health Insurance Service (REQ0000016362) and results are unrelated to the opinion of the National Health Insurance Service.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1C1B5044056). The funding entities had no role in the conduct of this study or interpretation of its results.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Conception or design: Y.G.K., S.J.H.
- Acquisition, analysis, or interpretation of data: Y.G.K., D.G.P., S.J.H.
- Drafting the work or revising: Y.G.K., D.G.P., S.Y.M., J.Y.J., H.J.K., D.J.K., K.W.L., S.J.H.
- Final approval of the manuscript: Y.G.K., D.G.P., S.Y.M., J.Y.J., H.J.K., D.J.K., K.W.L., S.J.H.
References
- 1.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 2.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 4.Warren RE, Frier BM. Hypoglycaemia and cognitive function. Diabetes Obes Metab. 2005;7:493–503. doi: 10.1111/j.1463-1326.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 5.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ. Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab. 2009;297:E194–E201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CH, Sheu WH. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J Intern Med. 2013;273:102–110. doi: 10.1111/joim.12000. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, Strotmeyer ES, Shorr RI, Metti A, Schwartz AV Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta HB, Mehta V, Goodwin JS. Association of hypoglycemia with subsequent dementia in older patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. 2017;72:1110–1116. doi: 10.1093/gerona/glw217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce DG, Davis WA, Casey GP, Clarnette RM, Brown SG, Jacobs IG, Almeida OP, Davis TM. Severe hypoglycaemia and cognitive impairment in older patients with diabetes: the Fremantle Diabetes Study. Diabetologia. 2009;52:1808–1815. doi: 10.1007/s00125-009-1437-1. [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couillard-Despres S, Iglseder B, Aigner L. Neurogenesis, cellular plasticity and cognition: the impact of stem cells in the adult and aging brain: a mini-review. Gerontology. 2011;57:559–564. doi: 10.1159/000323481. [DOI] [PubMed] [Google Scholar]
- 13.Prince M, Acosta D, Ferri CP, Guerra M, Huang Y, Llibre Rodriguez JJ, Salas A, Sosa AL, Williams JD, Dewey ME, Acosta I, Jotheeswaran AT, Liu Z. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet. 2012;380:50–58. doi: 10.1016/S0140-6736(12)60399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scazufca M, Menezes PR, Vallada HP, Crepaldi AL, Pastor-Valero M, Coutinho LM, Di Rienzo VD, Almeida OP. High prevalence of dementia among older adults from poor socioeconomic backgrounds in Sao Paulo, Brazil. Int Psychogeriatr. 2008;20:394–405. doi: 10.1017/S1041610207005625. [DOI] [PubMed] [Google Scholar]
- 15.Wu CK, Yang YH, Lin TT, Tsai CT, Hwang JJ, Lin JL, Chen PC, Chiang FT, Lin LY. Statin use reduces the risk of dementia in elderly patients: a nationwide data survey and propensity analysis. J Intern Med. 2015;277:343–352. doi: 10.1111/joim.12262. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Nie H, Xu Y, Zhang L, Wu Y. Association of statin use with risk of dementia: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2013;13:817–824. doi: 10.1111/ggi.12044. [DOI] [PubMed] [Google Scholar]
- 17.Chen PY, Liu SK, Chen CL, Wu CS. Long-term statin use and dementia risk in Taiwan. J Geriatr Psychiatry Neurol. 2014;27:165–171. doi: 10.1177/0891988714522702. [DOI] [PubMed] [Google Scholar]
- 18.Bohlken J, Jacob L, Kostev K. Association between the use of antihyperglycemic drugs and dementia risk: a case-control study. J Alzheimers Dis. 2018;66:725–732. doi: 10.3233/JAD-180808. [DOI] [PubMed] [Google Scholar]
- 19.Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int. 2013;63:331–343. doi: 10.1016/j.neuint.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tkacs NC, Pan Y, Raghupathi R, Dunn-Meynell AA, Levin BE. Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. J Cereb Blood Flow Metab. 2005;25:1645–1655. doi: 10.1038/sj.jcbfm.9600152. [DOI] [PubMed] [Google Scholar]
- 22.Fisher BM, Hepburn DA, Smith JG, Frier BM. Responses of peripheral blood cells to acute insulin-induced hypoglycaemia in humans: effect of alpha-adrenergic blockade. Horm Metab Res Suppl. 1992;26:109–110. [PubMed] [Google Scholar]
- 23.Dalsgaard-Nielsen J, Madsbad S, Hilsted J. Changes in platelet function, blood coagulation and fibrinolysis during insulin-induced hypoglycaemia in juvenile diabetics and normal subjects. Thromb Haemost. 1982;47:254–258. [PubMed] [Google Scholar]
- 24.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33:1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CW, Shih YH, Wu SY, Yang T, Lin C, Kuo YM. Hypoglycemia induces tau hyperphosphorylation. Curr Alzheimer Res. 2013;10:298–308. doi: 10.2174/1567205011310030009. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care. 2014;37:1009–1015. doi: 10.2337/dc13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulatao RA, Anderson NB, Cohen B. Critical perspectives on racial and ethnic differences in health in late life. Washington, DC: The National Academic Press; 2004. [PubMed] [Google Scholar]


