Abstract
Objective
The causal association of human papillomavirus (HPV) in uterine cervical cancer was well established and this oncogenic virus was reported to be a biomarker for overall recurrence and central pelvic recurrence. The objective of the present systematic review and meta-analysis was to assess the role of HPV DNA testing in early detection of recurrence among cervical cancer survivors after radiotherapy.
Methods
We performed a systematic review and meta-analysis by means of searching electronic databases for published articles between January 1984 and June 2018, on the basis of standard systematic review guidelines prescribed by major agencies namely Cochrane Collaboration (https://www.cochrane.org) and Campbell Collaboration (https://www.campbellcollaboration.org). The meta-analysis component was further modified appropriately for the synthesis of sensitivity and specificity results.
Results
A total of 1,055 cervical cancer cases who had received pelvic radiation with or without chemotherapy from ten cohort studies were evaluated. The overall pooled sensitivity and specificity of HPV DNA testing was 0.84 (95% confidence interval [CI]= 0.66–0.94) and 0.35 (95% CI=0.20–0.54) respectively. The positive likelihood ratio was 1.3 (95% CI=1.0–1.7) and the negative likelihood ratio was 0.45 (95% CI=0.18–1.10) with an estimated diagnostic odds ratio of 3 (95% CI=1–9).
Conclusion
The screening for HPV DNA testing during follow-up facilitates early detection of recurrence after radiotherapy.
Keywords: Human Papillomavirus; Recurrence; Radiotherapy, Uterine Cervical Cancer
INTRODUCTION
The etiologic role of human papillomavirus (HPV) in cervical cancer was well recognized. Even though more than 200 types of HPV have been identified in humans [1], only thirteen have been classified as carcinogenic by the International Agency for Research on Cancer (IARC) [2]. HPV belongs to the Papillomaviridae family with 16 genera. Most of the carcinogenic types belong to either alpha 7 genus (HPV18, 39, 45, and 59) or alpha 9 genus (HPV16, 31, 33, 35, 52, and 58). The remaining oncogenic types were grouped under alpha 5 (HPV51) or 6 (HPV56 and HPV66). IARC has classified HPV 68 as probably carcinogenic with HPV26, 53, 67, 70, 73, and 82 were grouped as possibly carcinogenic. The causal association of HPV in uterine cervical carcinogenesis was well established and this oncogenic virus was stated to be a biomarker for overall recurrence and central pelvic recurrence which is more frequent in the initial 2 years after chemoradiation. The detection of high-risk HPV DNA after the definite therapy may point towards the risk of recurrence and variable survival outcome. The possibility of relapse of cervical cancer is high during the initial 2 years of completed treatment. As per the International Federation of Gynecology and Obstetrics staging, surgery is mainly reserved for early-stage diseases Ia, Ib1, and stage IIa. Meanwhile, stage IIb, IIIa, IIIb, IVa, and IVb are managed by chemoradiation. Clinical trials have revealed that concurrent Cisplatin-based chemoradiation is the standard regimen for treatment of stages Ib2, IIb–IVa [3,4]. However, almost one-fourth of the treated cases experience central pelvic recurrence after chemoradiation [5].
During follow-up Pap smear test also known as Pap test is often performed, after radical hysterectomy as well as chemoradiation to perceive the early dysplastic changes in the vaginal vault or cervix. However, the Pap test is not recommended during the first year after radiotherapy as well as hysterectomy owing to the alteration of cells and tissues [6]. Previous studies have reported a greater likelihood of relapse in cervical cancer cases with persistent high-risk HPV infection [7,8]. The integration of the viral genome into the host genome is an important event in the progression of a normal cell to the intraepithelial lesion and invasive cancer. The 2 molecular methods of HPV DNA testing are hybrid capture assay and polymerase chain reaction (PCR). The hybrid capture is a signal amplification assay that uses a combination of antibody capture and chemiluminescent signal detection. The system uses RNA probes that reacts with 13 high-risk HPV DNAs and 5 low risk HPV DNAs. The RNA: DNA hybrids are detected with multiple antibodies conjugated to alkaline phosphatase. The results are interpreted by reading the signal from chemiluminescent reaction. In PCR assays, DNA will be extracted from the clinical samples which will be amplified by 1-step PCR or nested PCR using general or type-specific primers targeting the L1 capsid region. PCR has been used for HPV detection, genotyping, and viral load determination. A broad spectrum of HPV types can be screened by means of primer-mediated PCR assays. Following amplification using primers, individual HPV genotypes are identified using a variety of methods. By means of real-time quantitative PCR, viral load (concentration) data can be generated from reaction curves generated. The Detection of HPV DNA by PCR testing may play an important role in the early recognition of recurrence, especially after pelvic irradiation [9,10].
There is a sparsity of data regarding the prognostic role of HPV DNA testing in cervical cancer follow-up from low-income countries which bear 85% global cervical cancer burden [11] and available results are conflicting. In the present literature review, we tried to analyze, whether HPV testing in the post-radiotherapy setting will be helpful in detecting the early recurrence in cervical cancer cases.
In India, cervical cancer incidence rates are often underestimated and 5-year age-standardized relative survival rate of cervical cancer is much lower than that of many South East Asian countries [12]. This is mainly attributed to delayed diagnosis, inadequate treatment and absence of regular follow-up. Molecular detection of HPV may facilitate early diagnosis of residual and early recurrent cancers after radiotherapy [13]. During the initial years, molecular testing is more sensitive than cytology because of radiation induced distinct morphologic changes. We observed a limited number of quantitative analysis regarding this topic.
MATERIALS AND METHODS
The systematic review was carried out as per the standard systematic review guidelines prescribed by major agencies namely Cochrane Collaboration (https://www.cochrane.org) and Campbell Collaboration (https://www.campbellcollaboration.org). We further modified the meta-analysis component suited for the synthesis of sensitivity and specificity results.
1. Description of the condition
HPV DNA positivity: HPV DNA positivity is indicative of intermittent viral shedding from latent infection, reinfection, a newly acquired infection, autoinoculation from epithelial sites (oral cavity and anogenital areas), or transient detection of viral DNA from a recent sexual act [14].
HPV DNA negativity: HPV DNA negativity implies host specific virologic control or latency rather than viral clearance or eradication [14].
Recurrence (relapse) of cervical cancer: If the regrowth of the local tumour or distant metastasis occurs after 6 months of definitive treatment, the cancer is considered recurrent [15].
Residual disease is the one which develops within 6 months of primary treatment [16]. Majority of the recurrences happen within the first 2 years of treatment and 90% relapses occur within 5 years [17].
2. Study protocol
A comprehensive electronic literature search was performed to assess all the published literature in English between 1984 and 2018 regarding the persistence of HPV in cervical cancer cases after radiotherapy. The electronic databases included were PubMed/Medline, Scopus, and Google Scholar. Relevant articles in English were retrieved combining search terms of “human papillomavirus OR HPV AND cervical cancer AND radiotherapy NOT head and neck cancer.” A manual library search for articles published in the peer-reviewed journals was performed. The search builders were checked for their reproducibility before the final search. The references cited in the retrieved articles were also analyzed to increase the search sensitivity. The search was last updated on September 30th, 2018.
The accuracy of the test was assessed by sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and odds ratio (OR) including 95% confidence interval (CI).
3. Inclusion process and criteria:
Cohort studies in which histologically confirmed cervical cancer cases treated by radiotherapy, screened by HPV molecular assays and followed up for at least 1-year post-radiation were included. Studies with no molecular testing for HPV, no data on relapses/recurrences, no histological correlation with outcome, carried out in immunocompromised individuals and not followed up for 1 year were excluded.
4. Data extraction
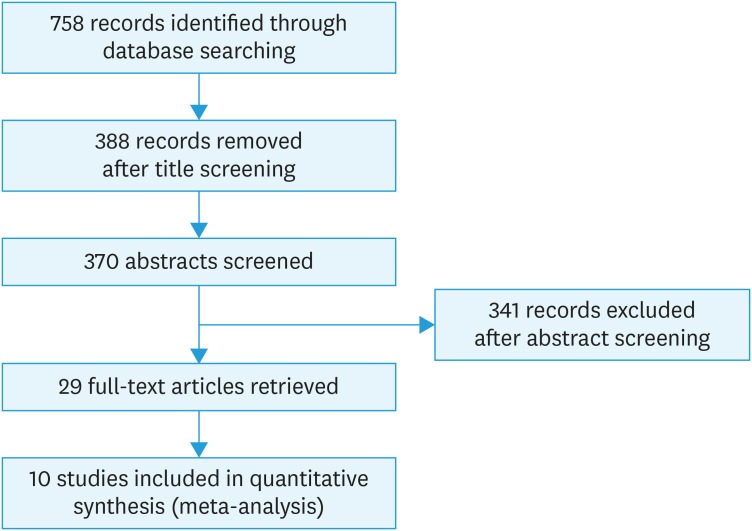
The pre-designed and pre-tested proforma was employed to extract the data. The proforma focused on the year of publication, study setting, country, pre/post radiation status, HPV DNA testing, follow-up and relapse rate. From the full-text articles, data were extracted by one reviewer which was reviewed by a second reviewer. The disagreements were discussed with a third reviewer, who was an expert and consensus was drawn. We carried out a 3-stage selection process for the final inclusion of the studies in the systematic review. In the first stage, one reviewer assessed each title from 758 titles (records) for the appropriateness for inclusion in the review. If found inappropriate, the articles were rejected (n=388) and all the other articles were moved to the second stage of selection. In the second stage, abstract of 370 titles were obtained and 2 reviewers independently scrutinized all such abstracts. All the non-relevant and duplicate studies were rejected (n=341) and the remaining 29 studies were moved to the third stage. In the third stage, full-text articles of 29 studies were obtained and were reviewed by 2 authors independently. The studies chosen by both the reviewers were included and in the case of disagreement between reviewers, a third reviewer adjudged the selection process. Studies with no molecular testing for HPV, no data on relapses/recurrences, no histological correlation with outcome and not followed up for a minimum period of 1 year were excluded. Ten prospective cohort studies were included in the quantitative synthesis (meta-analysis). The study selection process is shown in the PRISMA chart (Fig. 1).
Fig. 1. PRISMA chart detailing the study selection process. The flow diagram demonstrating the number of studies identified, records screened, full-text articles evaluated for the eligibility, and the studies included in the systematic review and meta-analysis.
5. Quality assessment
A checklist was applied to assess the quality of the studies selected after the abstract and content review. This checklist included 14 questions using the National Institutes of Health (National Heart, Lung and Blood Institute) checklist for observational, cohort and cross-sectional studies [18]. All the fourteen questions were applicable to our study and all the aspects of the methodology like study objectives, defined study population, the participation rate of eligible subjects, clearly defined exposure as well as outcome measures, measurement of exposure prior outcome, sufficient duration of follow-up and, the statistical adjustment of confounding variables were evaluated. Each question was graded as Yes, No, and Others—CD, can not determine/NA, not applicable/NR, not reported. The studies with 11–14 ‘Yes’ responses, were considered good and studies with 7–10 ‘Yes’ responses were considered fair. Studies with 6 or less than 6 ‘Yes’ responses were considered to be of poor quality.
6. Statistical analysis
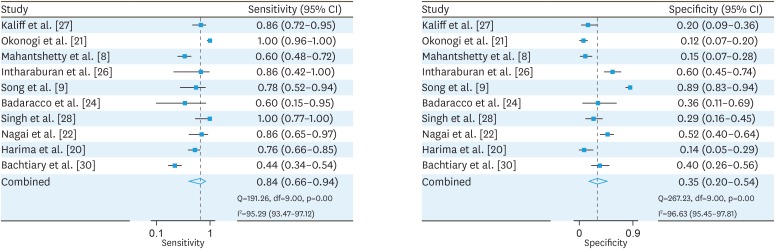
Meta-analysis was performed in STATA version 13.0 (College Station, TX, USA). The forest plot was constructed using midas package in STATA. The midas package uses “mixed-effects logistic regression framework” to provide the pooled estimate of sensitivity and specificity. Anticipating substantial methodological as well as statistical heterogeneity we adopted a random effects model for meta-analysis. An attempt was made to reduce the heterogeneity by considering cohort studies which carried out high-risk HPV DNA testing and followed up the cases for a minimum period of 1 year. The sensitivity and specificity estimates of individual studies with 95% CI, pooled sensitivity and specificity with 95% CI, chi-square statistic for heterogeneity and I2 Statistic are depicted in forest plot (Fig. 2).
Fig. 2. Forest plots of pooled sensitivity and specificity of human papillomavirus DNA testing after radiation of cervical cancer cases.
CI, confidence interval.
7. Assessment of publication bias
For assessment of publication bias Egger's test was used [19]. The Egger's test includes a weighted linear regression with standardized effect estimate as the dependent variable and precision as the independent variable. In the present study loge diagnostic OR was considered as the effect estimate and the precision was given as 1/standard error of loge diagnostic OR. Weights were assigned by the inverse variance approach (1/variance of the effect estimate). A statistically significant slope coefficient provides evidence for presence of publication bias.
RESULTS
Through electronic database search, 758 articles were identified and screened for eligibility and 388 titles were excluded. Abstracts of 370 titles were reviewed and 29 abstracts were found to be eligible for the full-text review. Full-text articles were retrieved for 29 studies. For the final meta-analysis, ten studies were qualified.
Most of the available data were from Japan, India, Austria, Italy, Korea, and Sweden. The number of enrolled cancer cases varied between 18 and 204. Only pre-radiation HPV DNA status was checked in 2 studies included for meta-analysis [20,21]. Five studies subjected the patients to molecular tests pre- as well as post-radiotherapy [9,22,23,24,25]. Study participants treated by concurrent chemoradiotherapy were also included for molecular testing in 3 studies considered for meta-analysis [8,24,26]. High-risk HPV persistence post-radiotherapy was reported to be associated with recurrence in most of the studies. The HPV detection rate after radiation and/or chemoradiation ranged between 18.6% and 93% [8,9,27]. As shown in the Table 1, studies screened patients starting from third month of pelvic irradiation reported higher persistence of HPV infection. Even though the study which reported the highest rate of detection screened the cases immediately after radiation, did not specify the exact time interval [28]. Since 8–12 weeks duration is essential to experience the maximum therapeutic effect of radiation, most of the studies performed HPV DNA testing starting from 12 weeks after completion of radiotherapy. The occurrence of high-risk HPV DNA was observed to be a significant predictor of local relapse and overall survival rate.
Table 1. Cohort studies regarding HPV DNA detection in cervical cancer cases after radiotherapy.
| Reference | Year | Region | No. of cases | Concurrent chemotherapy received | HPV DNA positive (pre-RT) | HPV DNA positive (post-RT) | Time of HPV testing after treatment | HPV types detected | Follow-up (mo) | Recurrences (HPV+ and HPV−ve cases) | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bachtiary et al. [30] | 2002 | Austria | 106 | No | - | 42 cases (43.7) | Every 3 months | 16, 18, 31, 33, 45, 69, 73 | Mean 50 (45.8–54.2) | 27 in HPV+ve | Good |
| 18 in HPV−ve | |||||||||||
| Harima et al. [20] | 2002 | Japan | 84 | No | 64 (50) | - | Not done | 16, 18, 31, 33, 58, 52 | Mean 31.3 (1–78.5) | 37 in HPV+ | Good |
| 5 in HPV−ve | |||||||||||
| Nagai et al. [22] | 2004 | Japan | 97 | No | 97 | 55 (56.7) | Every 3 months | 16, 18, 45, 56 | Mean 52.4 (6–102) | 19 in HPV+ | Good |
| 3 in HPV−ve | |||||||||||
| Singh et al. [28] | 2006 | Kolkata, India | 56 | No | - | 44 (78.5) | Once soon after treatment | HPV-16, 18 other high-risk HPV | 60 | 14 in HPV+ | Good |
| 0 in HPV−ve | |||||||||||
| Badaracco et al. [24] | 2010 | Italy | 18 | 3/18 received | 16 | 10 (62.5) | Once soon after treatment | 16, 18, 58, 45, 31 | 18 | 3 in HPV+ | Good |
| 2 in HPV−ve | |||||||||||
| Song et al. [9] | 2010 | Korea | 156 | No | 123 | 29 (18.6) | 1, 3, 6, 12 months | High-risk HPV not typed | 24 | 14 in HPV+ | Good |
| 4 in HPV−ve | |||||||||||
| Intharaburan et al. [26] | 2012 | Thailand | 55 | Yes | - | 25 (45.5) | 2 months | 16, 18, 52, 58 | Mean 13 (3–22) | 6 in HPV+ | Good |
| 1 in HPV−ve | |||||||||||
| Mahantshetty et al. [8] | 2017 | Mumbai, India | 135 | 129/135 received | 126 | 89 (70.6) | Every 3 months till 24 months | 16, 18 | 24 | 44 in HPV+ | Good |
| 29 in HPV−ve | |||||||||||
| Okonogi et al. [21] | 2017 | Japan | 83 | No | 69 (83.1) | - | - | 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, 68 | 72 | 37 in HPV+ | Good |
| 6 in HPV−ve | |||||||||||
| Kaliff et al. [27] | 2018 | Sweden | 204 | No | - | 189 (93) | Not available | 16, 18, 31, 33, 35, 39, 45, 56 | 60 | 83 in HPV+ | Good |
| 0 in HPV−ve |
Values are presented as number (%).
HPV, human papillomavirus; RT, radiotherapy; ve, negative.
1. Viral factors augmenting the recurrence rate
Studies have reported that cases associated with HPV DNA clearance after radiation demonstrate better prognosis and viral persistence may imply apparent treatment failure [24,29]. In comparison to a single genotype, infection with multiple genotypes was associated with poor prognosis and early recurrences [30]. The study from Chennai reported that the occurrence of HPV58, HPV18 and multiple infections were associated with increased probability of recurrence following radiotherapy [31]. Cervical cancers with infections by many HPV genotypes including at least 1 high-risk genotype were observed to be having 5 times greater risk of radiotherapy failure when compared to cancer cases positive for a single high-risk HPV DNA [31].
The persistence of HPV18 after radiotherapy would result in a 4-fold increased risk of recurrence [32]. HPV 18 was observed to be more resistant to surgery as well as to chemoradiation, suggesting more aggressive nature of this genotype [33]. The occurrence of high-risk HPV DNA at 24 months post-radiotherapy implies a greater risk of local recurrence [9]. A recent study from Sweden observed that detection of certain high-risk genotypes including HPV16 and 18 during follow-up was associated with greater frequency of distant metastases [27]. There is vast variation in HPV DNA detection tests employed in studies and the detection rates varied between 18.6% and 93.0% [9,33,34]. In comparison with cervical cancer cases associated with HPV-16, the relative risk of death in patients with HPV negative cancers was 1.64 [34]. The systematic review and meta-analysis by Li et al reported that the pre-treatment HPV DNA status is a clinically useful prognostic biomarker in cervical cancer cases [35]. There exists a higher risk of local recurrence if HPV DNA persists after radiation [22]. HPV detection after radiation is a significant tool to assess the effectiveness of treatment [24].
A low HPV load prior radiotherapy or surgery was observed to be having poor prognosis and short disease free survival as a result of aggressive nature of the malignancy [36]. Post-radiation, high viral load observed in late-stage cervical cancers was associated with higher recurrence rate in certain studies [9,24,25,28]. Poorly differentiated tumours were observed to be having low viral load in contrast to well or moderately differentiated tumours. The overall pooled sensitivity of HPV DNA testing was 0.84 (95% CI=0.66–0.94). The overall pooled specificity of HPV DNA testing was 0.35 (95% CI=0.20–0.54). The positive likelihood ratio was 1.3 (95% CI=1.0–1.7) implying that a positive result is more likely to occur in people with relapse than people without the relapse. The negative likelihood ratio was 0.45 (95% CI=0.18–1.10). The estimated OR was 3 (95% CI=1–9) denoting that a cervical cancer case with positive HPV DNA test after radiation has 3 times the odds of developing the recurrence in comparison to cervical cancer tested negative for HPV DNA after radiation. The p-value for the slope coefficient is 0.12, which is statistically non-significant (Table 2). There is no evidence for presence of publication bias.
Table 2. The table depicting the results of Egger's test.
| Coefficient | Estimate (95% CI) | p-value |
|---|---|---|
| Intercept | 3.67 (−0.33, 7.68) | 0.07 |
| Slope | −1.90 (−4.39, 0.58) | 0.12 |
CI, confidence interval.
DISCUSSION
The meta-analysis presented here included ten prospective cohort studies published in the last two decades, assessing the role of HPV DNA testing to detect early relapse among 1,055 cervical cancer cases who had received radiation. Studies from Austria, Japan, India, Italy, Korea, Sweden, and Thailand were qualified for the meta-analysis [8,9,20,21,22,24,26,27,28,30]. The present study observed a high pooled sensitivity of HPV DNA based screening for detection of relapses after pelvic irradiation of cervical cancer. The cervical cancer cases screened positive for HPV DNA has 3 times the odds of recurrence or relapse. Meanwhile pooled specificity was low and thus HPV testing is not confirmatory of recurrence. This is in accordance with the previous studies reporting a low specificity of high-risk HPV testing during follow-up [37]. The present literature review observed vast variation in the HPV detection rate which was attributed to the disparities in the duration of follow-up, staging of enrolled cancer cases, the timing of procurement of samples in relation to pelvic irradiation, age group of enrolled cases and methods of radiotherapy. Nevertheless, a positive high-risk HPV test is the most significant independent predictor of relapse [9]. Study participants were treated by concurrent chemoradiotherapy in only 3 studies considered for meta-analysis and there was vast disparity in the number of cases treated by concurrent chemoradiation. As the low number of studies limit the power of regression to detect significant effects, meta-regression was not carried out.
Singh et al. [28] observed that the detection of high HPV DNA titres in plasma and tissue samples is a very sensitive biomarker of recurrence. HPV is an epitheliotropic virus requiring differentiating squamous epithelium for the life cycle. A recent study, reported HPV positivity in serum samples of patients with HPV-associated cervical, and oropharyngeal cancers [38]. A statistically significant association with viral load and prognosis was not reported. Meanwhile, HPV DNA positivity is the single most important predictor of recurrence at any time during follow-up [37].
Better response to radiotherapy and overall survival rate were observed in HPV positive cancers of head and neck [39], vagina [40], and cervix [37] when compared to cancers with HPV negativity. Elevated levels of mutated p53 may be attributed to the low survival rate of HPV negative cancer patients. The apoptosis of damaged DNA as a result of radiation therapy depends on tumour suppressor gene p53. Despite viral E6 protein-mediated degradation of p53, a fraction of p53 is still functional in HPV positive tumours conferring improved response to radiotherapy unlike HPV negative cancers [27]. Infection with multiple genotypes of HPV would result in a further decline in p53 owing to increased viral oncoprotein levels.
Research on targeted treatment at HPV viral molecular pathways such as proteasome inhibitors and histone deacetylase inhibitors as well as customized viral immunotherapy is in progress leading to more effective control of this cancer.
In the final analysis, cohort studies with minimum 1-year follow-up published in high quality, indexed journals were included. To the best of our knowledge, this is the first meta-analysis reporting the pooled sensitivity, pooled specificity, positive likelihood ratio, negative likelihood ratio and an OR of HPV based screening of cervical cancers treated by radiotherapy. Most of the previous reports focused on HPV DNA status prior to treatment. The present review will be helpful for Oncologists in modifying the current follow-up approaches.
The present systematic review and meta-analysis included published studies in English language and we could not review relevant articles published in other languages. Due to a limited number of follow-up studies, we had to include studies with even small sample size. Another limitation is that the present review did not consider various modes of radiotherapy received.
Annual HPV DNA testing during the initial 2 years of follow-up may facilitate early recognition of recurrence in cervical cancer survivors. Large cohort studies involving cervical cancer cases tested HPV positive before and after radiation are essential for deciding the optimal timing of molecular tests.
Further research is mandatory to understand the mechanism behind the variation in radio-sensitivity of different HPV genera. Hospital-based cohort studies enrolling HPV associated cervical cancer cases receiving radiotherapy from African, Eastern European, and Latin American countries having the highest disease burden are essential. Pretreatment HPV genotyping facilitating personalized treatment may improve recurrence-free survival rate. If further studies identify that persistent HPV virus is a strong predictor or recurrence in the post-irradiated setting, a simple hysterectomy may have a role in preventing central recurrence in a small subset of patients. De-intensification of radiation treatment in selected HPV-positive oropharyngeal squamous cell cancers, as well as cervical cancers, have to be considered after large clinical trials. Long-term follow-up studies using various clinical samples for better understanding of the natural history of HPV and blood stream spread resulting in distant metastases are essential.
Footnotes
Funding: Financial support from ICMR project File No.5/8/7/15/2010/ECD-I.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.S.
- Data curation: S.S., K.S., D.B., R.N.
- Formal analysis: S.S., K.S., D.B.
- Funding acquisition: A.G.
- Investigation: S.S.
- Methodology: S.S., R.N.
- Project administration:
- Resources: S.S., A.G.
- Software: R.N.
- Supervision: S.S.
- Validation: S.S., D.B., R.N.
- Writing - original draft: S.S.
- Writing - review & editing: S.S., K.S., D.B., A.G.
References
- 1.Karolinska Institutet. International HPV reference center [Internet] Stockholm: Karolinska Institutet; 2018. [cited 2018 Oct 17]. Available from: https://ki.se/en/labmed/international-hpv-reference-center. [Google Scholar]
- 2.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: International Agency for Research on Cancer; 2012. [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, et al. ACR appropriateness Criteria® on advanced cervical cancer expert panel on radiation oncology-gynecology. Int J Radiat Oncol Biol Phys. 2011;81:609–614. doi: 10.1016/j.ijrobp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Cancer Society. Follow-up after treatment for cervical cancer [Internet] Toronto: Canadian Cancer Society; 2018. [cited 2018 Oct 10]. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/cervical/treatment/follow-up/?region=on. [Google Scholar]
- 7.Holloway RW, Farrell MP, Castellano C, Barnes WA, Lewandowski G, Jenson B, et al. Identification of human papillomavirus type 16 in primary and recurrent cervical cancer following radiation therapy. Gynecol Oncol. 1991;41:123–128. doi: 10.1016/0090-8258(91)90270-f. [DOI] [PubMed] [Google Scholar]
- 8.Mahantshetty U, Teni T, Naga P, Hotwani C, Umesh S, Kannan S, et al. Impact of HPV 16/18 infection on clinical outcomes in locally advanced cervical cancers treated with radical radio (chemo) therapy - a prospective observational study. Gynecol Oncol. 2018;148:299–304. doi: 10.1016/j.ygyno.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Song YJ, Kim JY, Lee SK, Lim HS, Lim MC, Seo SS, et al. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int J Cancer. 2011;129:896–902. doi: 10.1002/ijc.25741. [DOI] [PubMed] [Google Scholar]
- 10.Yu MC, Austin RM, Lin J, Beck T, Beriwal S, Comerci JT, et al. The role of high-risk human papilloma virus testing in the surveillance of cervical cancer after treatment. Arch Pathol Lab Med. 2015;139:1437–1440. doi: 10.5858/arpa.2014-0534-OA. [DOI] [PubMed] [Google Scholar]
- 11.LaVigne AW, Triedman SA, Randall TC, Trimble EL, Viswanathan AN. Cervical cancer in low and middle income countries: addressing barriers to radiotherapy delivery. Gynecol Oncol Rep. 2017;22:16–20. doi: 10.1016/j.gore.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobdey S, Sathwara J, Jain A, Balasubramaniam G. Burden of cervical cancer and role of screening in India. Indian J Med Paediatr Oncol. 2016;37:278–285. doi: 10.4103/0971-5851.195751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Azevedo AE, Carneiro FP, Neto FF, Bocca AL, Teixeira LS, de Queiroz Maurício Filho MA, et al. Association between human papillomavirus infection and cytological abnormalities during early follow-up of invasive cervical cancer. J Med Virol. 2012;84:1115–1119. doi: 10.1002/jmv.23303. [DOI] [PubMed] [Google Scholar]
- 14.Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 2017;9:E267. doi: 10.3390/v9100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotto LSJ, Graham JB, Pickren JW. Postmortem findings in cancer of the cervix: an analysis of 108 autopsies in the past 5 years. Am J Obstet Gynecol. 1960;80:791–794. [Google Scholar]
- 16.Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030–1038. doi: 10.1056/NEJM199604183341606. [DOI] [PubMed] [Google Scholar]
- 17.Halpin TF, Frick HC, 2nd, Munnell EW. Critical points of failure in the therapy of cancer of the cervix: a reappraisal. Am J Obstet Gynecol. 1972;114:755–764. doi: 10.1016/0002-9378(72)90897-6. [DOI] [PubMed] [Google Scholar]
- 18.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126:2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies [Internet] Boston, MA: Boston College Department of Economics; 2009. [cited 2019 Apr 18]. Available from: https://ideas.repec.org/c/boc/bocode/s456880.html. [Google Scholar]
- 20.Harima Y, Sawada S, Nagata K, Sougawa M, Ohnishi T. Human papilloma virus (HPV) DNA associated with prognosis of cervical cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:1345–1351. doi: 10.1016/s0360-3016(01)02796-1. [DOI] [PubMed] [Google Scholar]
- 21.Okonogi N, Kobayashi D, Suga T, Imai T, Wakatsuki M, Ohno T, et al. Human papillomavirus genotype affects metastatic rate following radiotherapy in patients with uterine cervical cancer. Oncol Lett. 2018;15:459–466. doi: 10.3892/ol.2017.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai Y, Toma T, Moromizato H, Maehama T, Asato T, Kariya K, et al. Persistence of human papillomavirus infection as a predictor for recurrence in carcinoma of the cervix after radiotherapy. Am J Obstet Gynecol. 2004;191:1907–1913. doi: 10.1016/j.ajog.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee R, Mandal B, Bandopadhyay S. Detection of HPV DNA in cervical carcinomas after treatment in India. Int J Hum Genet. 2005;5:27–31. [Google Scholar]
- 24.Badaracco G, Savarese A, Micheli A, Rizzo C, Paolini F, Carosi M, et al. Persistence of HPV after radio-chemotherapy in locally advanced cervical cancer. Oncol Rep. 2010;23:1093–1099. doi: 10.3892/or_00000737. [DOI] [PubMed] [Google Scholar]
- 25.Kahla S, Kochbati L, Sarraj S, Ben Daya I, Maalej M, Oueslati R. Molecular detection of human papillomavirus and viral DNA load after radiotherapy for cervical carcinomas. Tumori. 2016;102:521–526. doi: 10.5301/tj.5000401. [DOI] [PubMed] [Google Scholar]
- 26.Intharaburan S, Tanapat Y, Tatanan K, Sangkhavasi K, Komolpis S, Buranawit K, et al. Human papillomavirus infection following radiation therapy or concurrent chemoradiation for invasive cervical cancer. J Med Assoc Thai. 2012;95(Suppl 5):S38–S41. [PubMed] [Google Scholar]
- 27.Kaliff M, Sorbe B, Mordhorst LB, Helenius G, Karlsson MG, Lillsunde-Larsson G. Findings of multiple HPV genotypes in cervical carcinoma are associated with poor cancer-specific survival in a Swedish cohort of cervical cancer primarily treated with radiotherapy. Oncotarget. 2018;9:18786–18796. doi: 10.18632/oncotarget.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh RK, Maulik S, Mitra S, Mondal RK, Basu PS, Roychowdhury S, et al. Human papillomavirus prevalence in postradiotherapy uterine cervical carcinoma patients: correlation with recurrence of the disease. Int J Gynecol Cancer. 2006;16:1048–1054. doi: 10.1111/j.1525-1438.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 29.Unger ER, Vernon SD, Thoms WW, Nisenbaum R, Spann CO, Horowitz IR, et al. Human papillomavirus and disease-free survival in FIGO stage Ib cervical cancer. J Infect Dis. 1995;172:1184–1190. doi: 10.1093/infdis/172.5.1184. [DOI] [PubMed] [Google Scholar]
- 30.Bachtiary B, Obermair A, Dreier B, Birner P, Breitenecker G, Knocke TH, et al. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int J Cancer. 2002;102:237–243. doi: 10.1002/ijc.10708. [DOI] [PubMed] [Google Scholar]
- 31.Munagala R, Donà MG, Rai SN, Jenson AB, Bala N, Ghim SJ, et al. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol. 2009;34:263–271. [PubMed] [Google Scholar]
- 31.Kim JY, Nam BH, Lee JA. Is human papillomavirus genotype an influencing factor on radiotherapy outcome? Ambiguity caused by an association of HPV 18 genotype and adenocarcinoma histology. J Gynecol Oncol. 2011;22:32–38. doi: 10.3802/jgo.2011.22.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CH, Chang CJ, Huang HJ, Hsueh S, Chao A, Yang JE, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007;25:3628–3634. doi: 10.1200/JCO.2007.11.2995. [DOI] [PubMed] [Google Scholar]
- 34.Lombard I, Vincent-Salomon A, Validire P, Zafrani B, de la Rochefordière A, Clough K, et al. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J Clin Oncol. 1998;16:2613–2619. doi: 10.1200/JCO.1998.16.8.2613. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Tan Y, Zhu LX, Zhou LN, Zeng P, Liu Q, et al. Prognostic value of HPV DNA status in cervical cancer before treatment: a systematic review and meta-analysis. Oncotarget. 2017;8:66352–66359. doi: 10.18632/oncotarget.18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng T, Feng Y, Zheng J, Huang Q, Liu J. Low initial human papillomavirus viral load may indicate worse prognosis in patients with cervical carcinoma treated with surgery. J Gynecol Oncol. 2015;26:111–117. doi: 10.3802/jgo.2015.26.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa S, Venturoli S, Origoni M, Preti M, Mariani L, Cristoforoni P, et al. Performance of HPV DNA testing in the follow-up after treatment of high-grade cervical lesions, adenocarcinoma in situ (AIS) and microinvasive carcinoma. Ecancermedicalscience. 2015;9:528. doi: 10.3332/ecancer.2015.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeannot E, Becette V, Campitelli M, Calméjane M, Lappartient E, Ruff E, et al. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus‐associated invasive carcinoma. J Pathol Clin Res. 2016;2:201–209. doi: 10.1002/cjp2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson GL, Helenius G, Andersson S, Sorbe B, Karlsson MG. Prognostic impact of human papilloma virus (HPV) genotyping and HPV-16 subtyping in vaginal carcinoma. Gynecol Oncol. 2013;129:406–411. doi: 10.1016/j.ygyno.2013.02.004. [DOI] [PubMed] [Google Scholar]