Abstract
Objective
To evaluate survival and recurrence in stage II endometrial cancer in relation to uterine risk stratification. Outcome for stage II was compared before and after the introduction of lymph node (LN) resection and omission of all postoperative radiotherapy.
Methods
The cohort consisted of 4,380 endometrial carcinoma patients radically operated (no visual tumor, all distant metastasis removed) (2005–2012) including 461 stage II. Adjusted Cox regression was used to compare survival and actuarial recurrence rates.
Results
Uterine risk factors (low-, intermediate-, and high-) were the strongest predictors of survival and recurrence in stage II. Stage II low-risk having a prognosis comparable to low-risk stage I (grade 1–2, <50% myometrial invasion), whereas cervical invasion significantly increased the risk of recurrence and decreased cancer-specific survival in intermediate- and high-risk compared to the corresponding stage I risk groups. In 355 cases of 708 with cervical stromal invasion, LN-resection showed 27.9% with LN metastasis and upstaged 18.1% from stage II to IIIC resulting in longer survival and lower recurrence in LN-resected compared to non-LN resected stage II. Radical as compared to simple hysterectomy did not alter survival. Treatment with external beam radiotherapy decreased local recurrence without affecting survival.
Conclusion
Uterine risk groups are the strongest predictors for survival and recurrence in stage II patients and should be considered when advising adjuvant therapy. LN-resected stage II had increased survival and decreased recurrence. Omitting radiotherapy increase vaginal recurrence without affecting survival.
Keywords: Endometrial Cancer, Stage 2, Lymphadenectomy, Recurrences, Survival
INTRODUCTION
Stage II endometrial cancer patients are a heterogeneous group with the common feature of cervical stromal invasion but otherwise different characteristics (grade, superficial or deep myometrial invasion), and both endometrioid and non-endometrioid tumors are included.
Management of stage II disease is therefore controversial with respect to both surgery and postoperative treatment. Lymph node (LN) staging of pelvic and paraaortic LNs is recommended [1]. Cervical stromal invasion may be identified by magnetic resonance imaging (MRI) preoperatively with a sensitivity of 80%, but is otherwise difficult to recognize before or during surgery [2,3,4,5]. It is therefore important to determine whether reoperation is necessary in case of undiscovered cervical stromal invasion, especially in the presence of low-risk uterine characteristics (stage II with grade 1 and 2 with <50% myometrial invasion). Radical hysterectomy is used to ensure radical borders in cases with parametrial invasion, but cervical stromal invasion does not seem to predict parametrial invasion [6], and radical hysterectomy does not seem to increase survival [7,8] and is therefore no longer recommended [1]. Omentectomy is only recommended in case of serous histology.
The European guidelines recommend brachytherapy for LN-resected stage II patients with grade 1 and 2 tumors, brachytherapy or external beam radiotherapy (EBRT) for grade 3, EBRT for non-staged, and adjuvant chemotherapy for serous histology [1]. In a previous Danish national study, 29% of International Federation of Gynaecology and Obstetrics (FIGO) 88 stage II cases recurred within the first 14 years after surgery and 16.4% recurring outside the field where radiation traditionally would have been delivered, indicating that some type of systemic adjuvant therapy may be needed [9].
The Danish national guidelines introduced pelvic LN resection for stage II cases in 2005 and omitted EBRT in 2010, and according to the present and past guidelines, no patients are recommended brachytherapy. Patients are instead offered postoperative adjuvant chemotherapy in the ongoing ENGOT-EN2-DGCG/EORTC-55102 trial [10]. The identification of more specific prognostic factors in this heterogeneous group of patients with only cervical stromal invasion in common may help tailor both surgical and adjuvant therapy and thereby reduce the very high recurrence rates demonstrated in Danish stage II endometrial cancer patients [9].
Applying the same risk stratification in stage II cases as were applied in stage I cases could be valuable to determine whether survival, risk and type of recurrence including risk of LN metastasis are dependent on cervical stromal invasion.
Our aim was to evaluate survival and recurrence in stage II endometrial cancer in relation to uterine risk stratification after LN resection was introduced and all radiotherapy omitted. We further aim to identify subgroups of Danish stage II endometrial cancers with favorable prognosis by evaluating survival and risk and type of recurrence to help tailor surgery and adjuvant therapy for this heterogeneous group of patients.
MATERIALS AND METHODS
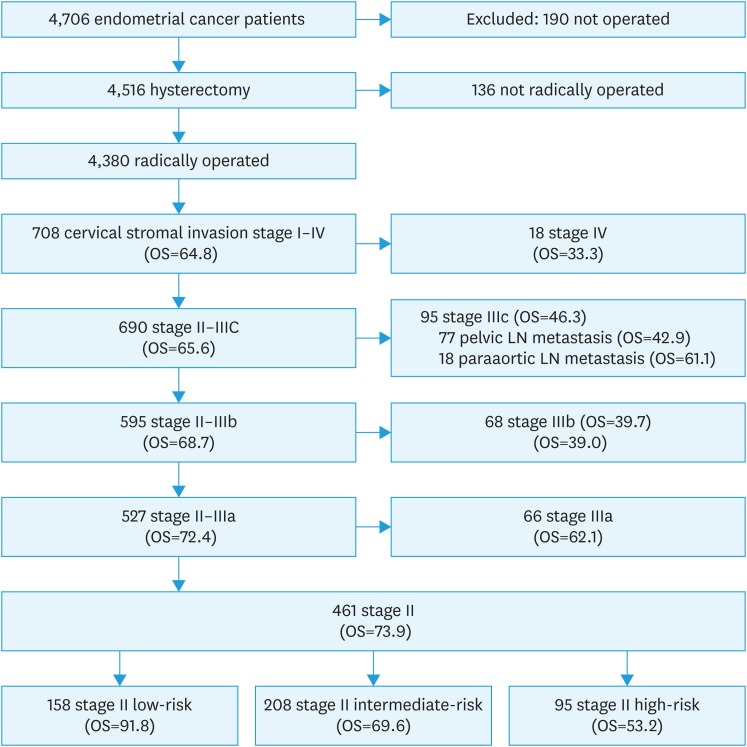
The validated Danish Gynecological Cancer Database (DGCD) years 2005–2012 includes 4,380 radically operated Danish patients with endometrial cancer (excluding sarcomas and carcinosarcomas) [11,12]. Details of the database have been published previously [13] In the present study, 708 cases with cervical stromal invasion were included, and of these 461 were stage II cases. Inclusion of patients is demonstrated in Fig. 1. As FIGO stage (88 to 2009) changed during the study period, all cases were reclassified to FIGO 2009 using the pathology reports [13]. All treatment of endometrial cancer was centralized to 6 centers in 2010 and all together 528 of the 708 patients with cervical stromal invasion were operated in one of the 6 centers (74.6%)
Fig. 1. Number of patients included in the study and the OS of sub-groups.
LN, lymph node; OS, overall survival.
Stage II cases were further divided into original uterine risk group without consideration of cervical stromal invasion. Uterine risk groups were defined as follows: 1) low-risk stage II: grades 1/2, <50% myometrial invasion, 2) intermediate-risk stage II: grades 1/2, >50% myometrial invasion, or grade 3, <50% myometrial invasion), and 3) high-risk stage II: grade 3, >50% myometrial invasion or non-endometrioid (clear cell, serous, undifferentiated carcinoma if >10% of the tumor). Stage II cases were further divided into LN or not LN staged and into given and not given EBRT.
The database is validated yearly [11]. Using uniform guidelines, the operating gynecologist performed the primary registration of surgery cases with regard to cervical stromal invasion. Stage II case were offered radical hysterectomy type C and also bilateral salpingo-oophorectomy when recognized preoperatively. Cervical invasion was recognized preoperatively from biopsy of visual tumor on cervix and in few cases by MRI (approximately 30 patients) during 2 Danish studies on the ability of MRI to determent stage of endometrial cancer [3,4]. Peritoneal washings, intraabdominal assessment and at that time omentectomy were offered to women with serous, clear and undifferentiated tumors. Routine pelvic lymphadenectomy was recommended if age and comorbidities permitted. No further criteria for omitting lymphadenectomy were specified but were left to the surgeon and patient to decide. Some institutions also undertook para-aortic lymphadenectomy in high-risk cases. All enlarged nodes were removed.
The pathologists reported according to uniform guidelines described previously [13].
EBRT was recommended for stage II until 2010 (mainly 50 Gy in 27 fractions). Only 40% of stage II received radiotherapy before 2010 and 2.8% after 2010. After 2011 stage II patients were instead offered participation in the ongoing ENGOT-EN2-DGCG/EORTC-55102 protocol (a randomized phase 3 trial where effect of postoperative chemotherapy (6 series of paclitaxel-carboplatin) is compared with postoperative observation alone (standard strategy) [10]. Patients not participating in this study was instead offered 5 years follow-up with no further treatment (every 3–6 months for 3–5 years). No cases received brachytherapy.
Missing data in the DGCD and causes of death were retrieved from patients’ medical records, the Danish Central Person Register, the death register of the Danish National Board of Health, and from pathology reports using the Danish pathology database, as described previously [13]. All histologically verified recurrences (568) were obtained from the pathology database, and another 90 non-histologically verified recurrences identified by checking the medical record of patients that had subsequently died.
Medical records were reviewed for patients with known recurrences to retrieve site, time and treatment of recurrences, as described previously [9,13]. If a patient had a recurrence in more than one location, we also registered the most “severe” first recurrence, defined as the recurrence with the lowest cancer-specific survival in the order of distant>abdominal>pelvic>vaginal, to enable the determination of isolated vaginal and pelvic recurrences.
Data were analyzed using STATA 11 (StataCorp, College Station, TX, USA) [14]. Kaplan–Meier estimates and actuarial recurrence rates were used to compute actuarial survival and recurrence rates. In all, 5 of 461 stage II patients had left Denmark and were lost to follow-up. Recurrence-free survival was estimated using time from surgery to first recurrence, censoring patients dying from causes other than endometrial cancer. Student's t-test was used to calculate differences between means and Person χ2 for differences between categorical parameters. Differences between actuarial survival and recurrence rate in different groups were calculated using adjusted Cox regression analysis after adjustment for age (20–59, 60–69, 70–79, and over 80) American Society of Anesthesiologists comorbidity index (1, 2, 3–4, and unknown [1/461]), grade (grades 1, 2, 3, or serous, clear, undifferentiated), type of hysterectomy (simple, radical, or unknown [25/461]), LN resection (yes/no), and adjuvant therapy with EBRT (yes/no) or chemotherapy (yes/no) as categorical parameters.
No approval from The Danish Ethics Committees was needed. The study was approved by the Danish Data Protection Board (Journal No. 2010-41-4627) and by the Danish health authorities (Journal No. 3-3013-297/1/).
RESULTS
The mean observation time for survival was 9.0±2.3 years (range, 5.1–13.1) and for recurrences at least 60 months. Details of patients included are shown in Table 1 and Fig. 1.
Table 1. Comparison of epidemiological, surgical, and histological characteristics of patients with II stage endometrial cancer in relation to uterine risk group, postoperative external beam radiation and LN resection.
| Variables | All stage II | Stage II divided according to uterine risk group | LN resection | EBRT | ||||
|---|---|---|---|---|---|---|---|---|
| Low-risk | Intermediate-risk | High-risk | −LN | +LN | −RT | +RT | ||
| Number | 461 | 158 | 208 | 95 | 248 | 213 | 344 | 117 |
| Age (yr) | 68.0±10.9 | 64.6±11.6 | 69.2*±10.3 | 70.9*±9.5 | 68.8±11.3 | 67.0±10.3 | 69.2±11.3 | 64.4*±8.82 |
| ASA | 1.77±0.68 | 1.60±0.7 | 1.81†±0.66 | 1.95*±0.66 | 1.79±0.72 | 1.74±0.62 | 1.85±0.69 | 1.52*±0.58 |
| BMI | 27.8±6.29 | 27.6±6.9 | 28.4±5.9 | 27.0±6.0 | 27.4±6.28 | 28.3±6.29 | 27.9±6.26 | 27.6±6.4 |
| Grade 1 | 213 (46.2) | 107 (67.7) | 106§ (51.0) | - | 127 (51.2) | 86§ (40.4) | 167 (48.6) | 46‡ (39.3) |
| Grade 2 | 138 (29.9) | 51 (32.3) | 87 (41.8) | - | 82 (33.1) | 56 (26.3) | 90 (26.2) | 48 (41.0) |
| Grade 3 | 50 (10.8) | - | 15 (7.2) | 35§ (36.8) | 15 (6.1) | 35 (16.4) | 43 (12.5) | 7 (6.0) |
| Serous, clear, undiff. | 60 (13.0) | - | - | 60 (63.2) | 24 (9.7) | 36 (16.9) | 44 (12.8) | 16 (13.7) |
| Deep myometrial invasion | 255 (55.3) | 0 | 193* (92.8) | 62* (65.3) | 130 (52.4) | 125 (58.7) | 200 (58.1) | 55* (47.0) |
| Cervical glandular involvement | 175 (38.0) | 41 (26.0) | 74† (35.6) | 60* (63.2) | 90 (36.3) | 85 (39.9) | 133 (38.7) | 42 (35.9) |
| Endometrioid | 401 (87.0) | 158 (100) | 208 (100) | 35* (36.8) | 224 (90.3) | 177† (83.1) | 300 (87.2) | 101 (86.3) |
| Non-endometrioid | 60 (13.0) | - | - | 60 (63.2) | 24 (9.7) | 36 (16.9) | 44 (12.8) | 16 (13.7) |
| LVSI of know status | 94/299 (31.4) | 9/87 (10.3) | 62/153§ (40.5) | 23/59§ (39.0) | 49/152 (32.2) | 45/147 (30.6) | 69/229 (30.1) | 25/70 (35.7) |
| Unknown LVSI | 162 (35.1) | 71 (44.9) | 55 (26.4) | 36 (37.9) | 96 (38.7) | 66 (31.0) | 115 (33.4) | 47 (40.2) |
| Simple hysterectomy | 363 (78.7) | 127 (80.4) | 172 (86.7) | 64† (67.4) | 236 (95.2) | 127§ (59.6) | 256 (74.4) | 107§ (91.5) |
| Radical hysterectomy | 73 (15.8) | 20 (12.7) | 25 (12.0) | 28 (29.5) | 4 (1.6) | 69 (32.4) | 65 (18.9) | 8 (6.8) |
| Unknown | 25 (5.4) | 11 (7.0) | 11 (5.3) | 3 (3.2) | 8 (3.2) | 17 (8.0) | 23 (6.7) | 2 (1.7) |
| Nodal staging | 213 (46.2) | 56 (35.4) | 96* (46.1) | 61* (64.2) | 0 | 213* (100) | 192 (55.8) | 21* (18.0) |
| Pelvic LN resection | 213 (46.2) | 56 (35.4) | 96 (46.1) | 61 (64.2) | 0 | 213* (100) | 192 (55.8) | 21* (18.0) |
| Paraaortic LN resection | 11 (2.4) | 1 (0.6) | 4 (1.9) | 6 (6.3) | 0 | 11 (5.2) | 11 (3.2) | 0 |
| No adjuvant therapy | 296 (64.2) | 100 (63.3) | 138 (66.4) | 58§ (61.1) | 125 (50.4) | 171§ (80.3) | 296 (86.1) | 0§ |
| RT | 115 (25.0) | 47 (29.8) | 49 (23.6) | 19 (20.0) | 94 (37.9) | 21* (9.9) | - | 115 (98.3) |
| Chemo | 48 (10.4) | 10 (6.3) | 20 (9.6) | 18 (19.0) | 27 (10.9) | 21 (9.9) | 48 (14.0) | 0* |
| RT+Chemo | 2 (0.43) | 1 (0.6) | 1 (0.5) | - | 2 (0.8) | - | - | 2 (1.7) |
| Death <5 years | 120 (26.0) | 13 (8.2) | 63* (30.3) | 44* (46.3) | 75 (30.2) | 45† (21.1) | 97 (28.2) | 23 (19.7) |
| From cancer | 69 (15.0) | 6 (3.8) | 34* (16.4) | 29* (30.5) | 41 (16.5) | 28 (13.2) | 50 (14.5) | 19 (16.2) |
| From others | 51 (11.0) | 7 (4.4) | 29† (13.9) | 15† (15.8) | 34 (13.7) | 17 (8.0) | 47 (13.7) | 4† (3.4) |
Data are shown as mean±standard deviation or number (%).
ASA, American Society of Anesthesiologists; BMI, body mass index; EBRT, external beam radiotherapy; LN, lymph node; LVSI, lymphovascular space invasion; RT, radiation therapy; Undiff., undifferentiated.
*p<0.001, †p<0.05 using Student's t-test or χ2 test when intermediate-risk and high-risk were compared to low-risk or RT against no RT or LN resection against no LN resection; ‡p<0.001, §p<0.05 using χ2 test distribution when intermediate-risk and high-risk were compared to low-risk or between RT and no RT or LN resection and no LN resection.
Cervical stromal invasion was present in 17.6% (795/4,516) of all cases that had undergone hysterectomy (including 136 cases not radically operated). Of cases with stromal invasion, 58.0% (461 cases) were stage II.
1. Uterine risk groups
We evaluated whether the uterine risk groups were an independent predictor for overall cancer-specific and progression-free survival in final stage II cases by multivariate Cox analysis (Table 2) and found uterine risk group to be an independent risk factor for overall survival (OS), cancer specific and progression free survival. Uterine risk groups are stronger predictor of survivals and recurrences then lymphovascular space invasion (LVSI) and LN status. (Table 2)
Table 2. Predictors of 5-year survival or recurrence of stage II endometrial cancer were calculated for prognostic factors (uterine risk group, LVSI status, and LN status) and for treatments factors (type of hysterectomy, LN resection, postoperative EBRT, and chemotherapy) by univariate and multivariate Cox analysis.
| Variables | Overall survival | Cancer-specific survival | Progression-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | % (95% CI) | HR/aHR (95% CI) | % (95% CI) | HR/aHR (95% CI) | % (95% CI) | HR/aHR (95% CI) | |||
| Prognostic factors* | 461 | 73.9 (69.6–77.7) | 84.1 (80.3–87.2) | 76.3 (72.1–80.0) | |||||
| Uterine risk group | |||||||||
| Low-risk | 158 | 91.8 (86.3–95.1) | 96.2 (91.6–98.3) | 90.5 (84.7–94.1) | |||||
| Intermediate-risk | 208 | 69.6 (62.9–75.4) | 4.17‡/3.60‡ (1.95–6.65) | 82.2 (75.9–86.9) | 4.87‡/4.09§ (1.68–9.98) | 73.0 (66.1–78.6) | 3.02‡/2.63§ (1.44–4.79) | ||
| High-risk | 95 | 53.2 (42.6–62.7) | 7.89‡/6.82‡ (3.56–13.07) | 66.1 (54.9–75.1) | 11.26‡/10.41‡ (4.15–26.11) | 58.3 (47.2–67.8) | 5.51‡/5.08‡ (2.68–9.65) | ||
| No LVSI | 205 | 77.6 (71.2–82.7) | 87.2 (81.6–91.1) | 82.0 (75.9–86.7) | |||||
| LVSI | 94 | 62.8 (52.2–71.7) | 1.94§/1.47 (0.94–2.30) | 72.2 (61.4–80.5) | 2.46§/1.84‡ (1.04–3.26) | 62.9 (52.0–72.1) | 2.41‡/1.93§ (1.19–3.14) | ||
| Unknown LVSI | 162 | 75.7 (68.3–81.6) | 1.12/1.08 (0.70–1.67) | 86.8 (80.2–91.3) | 1.05/1.02 (0.56–1.86) | 76.6 (69.1–82.6) | 1.37/1.38 (0.86–2.21) | ||
| No LN resection | 248 | 69.6 (63.4–74.9) | 81.9 (76.2–86.3) | 75.9 (69.9–80.9) | |||||
| Negative LNs | 213 | 78.9 (72.8–83.8) | 0.67§/0.60§ (0.40–0.89) | 86.4 (80.9–90.4) | 0.76/0.56§ (0.34–0.94) | 76.8 (70.4–82.0) | 0.95/0.80 (0.53–1.20) | ||
| Treatment factors† | |||||||||
| Simple hysterectomy | 363 (78.7) | 73.7 (68.9–77.9) | 84.4 (80.0–87.8) | 76.7 (71.9–80.8) | |||||
| Radical hysterectomy | 73 (15.8) | 69.9 (57.9–79.0) | 1.13/1.70 (0.98–2.96) | 79.9 (68.4–87.6) | 1.29/1.65 (0.82–3.29) | 70.5 (58.4–79.7) | 1.27/1.39 (0.81–2.39) | ||
| Unknown type of hysterectomy | 25 (5.4) | 88.0 (67.3–96.0) | 0.42/0.68 (0.21–2.20) | 91.7 (70.6–97.9) | 0.51/0.82 (0.19–3.45) | 87.5 (66.1–95.8) | 0.49/0.57 (0.17–1.83) | ||
| No LN resection | 248 (53.8) | 69.6 (63.4–74.9) | 81.9 (76.2–86.3) | 75.9 (70.0–80.9) | |||||
| LN resection | 213 (46.2) | 78.9 (72.8–83.8) | 0.67§/0.50§ (0.31–0.81) | 86.4 (80.9–90.4) | 0.76/0.53‡ (0.31–0.99) | 76.8 (70.4–82.0) | 0.95/0.59§ (0.36–0.97) | ||
| No postoperative EBRT | 344 (74.6) | 71.7 (66.6–76.1) | 84.3 (79.8–87.8) | 74.4 (69.3–78.8) | |||||
| Postoperative EBRT | 117 (25.4) | 80.3 (71.9–86.5) | 0.66/0.66 (0.39–1.11) | 83.4 (75.3–89.1) | 1.06/0.89 (0.47–1.68) | 81.7 (73.4–87.7) | 0.67/0.47§ (0.26–0.82) | ||
| No postoperative chemotherapy | 411 (89.2) | 72.9 (68.4–77.0) | 83.5 (79.4–86.8) | 75.2 (70.6–79.2) | |||||
| Postoperative chemotherapy | 50 (10.9) | 81.7 (67.8–90.0) | 0.63/0.59 (0.28–1.23) | 88.9 (75.3–95.2) | 0.60/0.48 (0.18–1.27) | 85.4 (71.8–92.8) | 0.55/0.37§ (0.17–0.84) | ||
ASA, American Society of Anesthesiologists; aHR, adjusted hazard ratio; CI, confidence interval; EBRT, external beam radiotherapy; HR, hazard ratio; LN, lymph node; LVSI, lymphovascular space invasion.
*Adjusted for the other prognostic factors, age, and ASA; †Adjusted for the other treatment factors and age, ASA, grades 1, 2, 3, unfavorable tumor type; ‡p<0.001, §p<0.05.
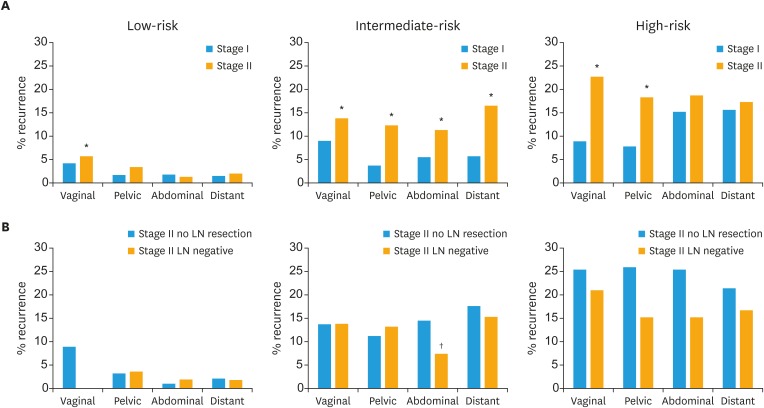
We further evaluated whether the subdivision of stage II into uterine risk groups would have the same predictive value as it does for stage I cases (Table 3 and Fig. 2). For stage II, survival decreased with increasing risk-group, resulting in a 4.0 times lower risk of survival for stage II intermediate-risk (adjusted hazard ratio [aHR]=3.99; 95% confidence interval [CI]=2.18–7.30; p<0.001) and 7.7 times lower risk of survival for high-risk as compared to stage II low-risk (aHR=7.72; 95% CI=4.01–14.9; p<0.001). The risk of both local and especially non-local recurrence was significantly higher for both intermediate-risk stage II and high-risk stage II as compared to low-risk stage II. We further compared the subdivision of stage II with the corresponding stage I risk groups. Survival and recurrence of low-risk stage II patients were almost comparable to low-risk stage I patients, except for significantly higher locoregional recurrences in stage II low-risk (8.9%) as compared to stage I low-risk (4.9%). However, intermediate- and high-risk stage II had decreased cancer-specific survival and increased recurrence compared to the corresponding intermediate- or high-risk stage I cases (Table 3 and Fig. 2).
Table 3. Survival and risk of recurrence for II as compared to stage I endometrial cancer after stratification into risk group according to risk factors (grade, myometrial invasion, unfavorable tumor types). Two adjusted Cox comparisons were performed 1. Intermediate- or high-risk were compared to low-risk (2 right columns) and 2. Stage II with uterine low-, intermediate- and high-risk features were compared to corresponding risk groups of stage I to evaluate whether cervical invasion in itself is a risk factor (Differences marked with ‡).
| Variables | All | Uterine risk group | HR (95% CI) Compared to low-risk | |||||
|---|---|---|---|---|---|---|---|---|
| Low-risk | Intermediate-risk | High-risk | Intermediate-risk | High-risk | ||||
| Stage I | 3,426 | 2,247 | 873 | 306 | ||||
| 5-year survival | ||||||||
| OS | 86 (85–87) | 91 (89–92) | 82 (80–85) | 65 (59–70) | 1.49* (1.19–1.86) | 3.37* (2.55–4.47) | ||
| Cancer-specific survival | 95 (94–95) | 97 (96–98) | 94 (92–95) | 79 (74–83) | 1.49 (0.84–2.67) | 6.13* (3.74–10.07) | ||
| Recurrence-free survival | 90 (89–91) | 94 (93–95) | 85 (82–87) | 73 (68–78) | 2.20* (1.69–2.86) | 4.41* (3.13–6.22) | ||
| Actuarial recurrences <5 years | ||||||||
| Locoregional recurrences | 7.1 (6.3–8.1) | 4.9 (4.1–5.9) | 10.8 (8.8–13.1) | 14.3 (10.6–19.0) | 1.98* (1.46–2.68) | 3.19* (2.07–4.91) | ||
| Non locoregional recurrences | 5.6 (4.9–6.5) | 2.5 (1.9–3.3) | 8.1 (6.4–10.2) | 22.9 (18.4–28.3) | 2.85* (1.94–4.20) | 8.83* (5.63–13.8) | ||
| Pelvic or paraaortic LN recurrences | 2.8 (2.3–3.5) | 1.2 (0.8–1.8) | 4.5 (3.3–6.2) | 11.1 (7.8–15.6) | 3.09* (1.77–5.37) | 7.71* (4.01–14.8) | ||
| Non-local LN recurrences | 1.3 (0.9–1.7) | 0.6 (0.3–1.0) | 2.0 (1.2–3.3) | 5.5 (3.2–9.3) | 3.20† (1.41–7.30) | 7.21* (2.66–19.6) | ||
| Stage II | 461 | 158 (34.3) | 208 (45.1) | 95 (20.6) | ||||
| 5-year survival | ||||||||
| OS | 74‡ (70–78) | 92 (86–95) | 70‡ (63–75) | 53 (43–63) | 3.99* (2.18–7.30) | 7.72* (4.01–14.9) | ||
| Cancer specific-survival | 84‡ (80–87) | 96 (92–98) | 82‡ (76–87) | 66§ (55–75) | 4.97* (2.07–12.0) | 12.5* (5.00–31.5) | ||
| Recurrence free-survival | 76‡ (72–80) | 90§ (85–94) | 73‡ (66–79) | 58§ (47–68) | 3.03* (1.69–5.45) | 6.39* (3.34–12.2) | ||
| Actuarial recurrences <5 years | ||||||||
| Locoregional recurrences | 17.9‡ (14.6–21.9) | 8.9§ (5.4–14.6) | 20.1‡ (15.0–26.6) | 30.7‡ (21.8–42.2) | 2.35† (1.24–4.43) | 4.77* (2.32–9.79) | ||
| Non locoregional recurrences | 15.4‡ (12.2–19.2) | 3.3 (1.4–7.8) | 19.7‡ (14.6–26.3) | 27.9 (19.4–39.1) | 6.54* (2.53–16.9) | 11.9* (4.32–33.0) | ||
| Pelvic or paraaortic LN recurrences | 9.9‡ (7.4–13.3) | 2.0 (0.6–6.2) | 14.9‡ (10.4–21.0) | 13.4 (7.4–23.7) | 7.96* (2.37–26.7) | 7.34† (1.88–28.6) | ||
| Non-local LN recurrences | 2.5 (1.4–4.7) | 0.7 (0.1–4.7) | 3.5 (1.6–7.7) | 3.8 (1.2–11.5) | 5.42 (0.63–46.8) | 9.21 (0.82–103.8) | ||
Survival is given as % (95% CI) and risk of recurrence is given as actuarial recurrences rate in % (95% CI).
aHR is represented adjusted for significant or potential confounders by multivariate analysis including, age, American Society of Anesthesiologists, grade, type of hysterectomy, lymph node resection, adjuvant EBRT or chemotherapy. Cox was not adjusted for grades 1, 2, 3, unfavorable tumor types when evaluating risk groups as this confounder is also represented in the risk groups.
aHR, adjusted hazard ratio; CI, confidence interval; EBRT, external beam radiotherapy; HR, hazard ratio; OS, overall survival.
*p<0.001, †p<0.05: aHR testing intermediate- or high-risk against low-risk; ‡p<0.001, §p<0.05: aHR testing risk group stage II against corresponding to risk group stage I.
Fig. 2. Five-year actuarial recurrence rates (vaginal, pelvic, abdominal, and distant) for (A) low-, intermediate-, and high-risk stage II compared to corresponding stage I risk groups and (B) stage II patients lymph-node staged as compared to non-LN staged.
LN, lymph node.
*p<0.05 testing low-, intermediate- and high-risk stage II patients against the corresponding stage I risk group; †p<0.05 using adjusted Cox analysis between either stage I or II or between stage II LN resected and not resected.
LVSI was not included in the risk stratification because LVSI status was unknown in 35% of stage II cases. To exclude LVSI as a confounder, we repeated the adjusted Cox analysis including LVSI status and found very similar results, except for a non-significant, but higher cancer-specific survival in high-risk stage II compared to stage I (p=5.6%).
As the inclusion of both LN staged and non-staged also could be a bias, we further compared the recurrences of the 3 uterine risk groups in staged and non-staged cases. Nevertheless, we demonstrated no significant differences between staged and non-staged cases except for a significantly lower risk of abdominal recurrences in the intermediate-risk LN staged cases as compared to non-staged groups (Fig. 2).
2. LN resection
LNs were removed in 50.1% of all cases with stromal invasion and in 46.2% of stage II patients. Examining all radically operated cases with stromal invasion (stage II–IV), the removal of LNs in 355 cases diagnosed 27.9% (99 patients: 95 patients stage IIIC and 4 patients stage IV) with LN metastasis. The risk of LN metastasis at primary surgery increased in relation to uterine risk groups (low-risk + stromal invasion: 8.5%, intermediate-risk + stromal invasion: 29.6%, and high-risk + stromal invasion: 36.8%). In all, 18.1% were upstaged from stage II to IIIC due to LN metastasis (low-risk: 8.2%, intermediate-risk: 22.0%, and high-risk: 22.8%), indicating the need for LN staging.
For stage II, the mean±standard deviation [SD] of pelvic LNs was 19.8±10.1 (95% CI=1–52), and in the 11 cases with paraaortic LN resection, the mean number was 4.9±3.1 (SD) (range, 1–11). Groups were not comparable because LN resected stage II patients had significantly higher tumor grade, more non-endometrioid tumors, more often radical hysterectomy and less postoperative adjuvant therapy than non-staged. Thus, LN-resected stage II cases had significantly higher overall, cancer-specific and recurrence-free survival and fewer vaginal and paraaortic LN recurrences than non-staged (Tables 2 and 4). As these finding may be a result of stage migration, survival was also compared including cases upstaged due to LN metastasis only (47 cases) to create comparative groups. This demonstrating no significant differences in OS (staged including LN metastasis/non-staged 74.2%/69.6%, hazard ratio [HR]=0.79; 95% CI=0.52–1.19; p=0.262), cancer-specific (81.3%/81.9%, HR=1.00; 95% CI=0.59–1.68; p=0.229), or recurrence-free survival (71.9/75.9%, HR=0.90; 95% CI=0.59–1.40; p=0.665), suggesting that LN staging in stage II increased survival, mainly due to stage migration.
Table 4. Five-year actuarial recurrence rates for 461 stage II endometrial cancer patients.
| Variables | All stage II | LN resection | Postoperative radiotherapy | |||
|---|---|---|---|---|---|---|
| No LN resection | LN resection | No radiotherapy | EBRT | |||
| Number | 461 | 248 | 213 | 344 | 117 | |
| a) Total recurrence rate | 105 (23.6) | 57 (24.1) | 48 (23.2)† | 84 (25.6) | 21 (18.2)† | |
| All vaginal | 53 (12.4) | 30 (12.9) | 23 (11.8)† | 45 (14.2) | 8 (7.3)† | |
| All pelvic | 41 (10.1) | 20 (9.3) | 21 (11.0) | 38 (12.7) | 3 (2.8)† | |
| All abdominal | 37 (9.1) | 22 (10.1) | 15 (7.9) | 27 (9.2) | 10 (8.9) | |
| All distant | 45 (11.2) | 22 (10.7) | 23 (11.9) | 33 (11.4) | 12 (11.0) | |
| b) Site of most severe 1. recurrence | ||||||
| Vaginal | 26 (6.2) | 16 (6.9) | 10 (5.4)† | 23 (7.4) | 3 (2.8)† | |
| Pelvic | 14 (3.6) | 7 (3.5) | 7 (3.8) | 13 (4.5) | 1 (1.1) | |
| Abdominal | 19 (4.6) | 12 (5.4) | 7 (3.8) | 14 (4.7) | 5 (4.5) | |
| Distant | 45 (11.2) | 22 (10.7) | 23 (11.9) | 33 (11.4) | 12 (11.0) | |
| c) Locoregional LN recurrences | 40 (9.9) | 21 (9.9) | 19 (10.0) | 32 (10.9) | 8 (7.4) | |
| All PL | 29 (7.3) | 14 (6.5) | 15 (8.1) | 27 (9.4) | 2 (1.8) | |
| All PA | 22 (5.5) | 16 (7.7) | 6 (3.1)† | 14 (4.8) | 8 (7.4) | |
| d) Extra abdominal LN | 10 (2.5) | 6 (3.0) | 4 (2.1) | 8 (2.8) | 2 (1.8) | |
Values are presented as number (%).
Recurrences were divided into 1. Given or not given RT 2. LN resected or not. Recurrences divided into a) Total recurrence rate: number of recurrences at each location: vaginal, pelvic, abdominal, or distant metastasis. If a patient had recurrences at several sites, the patient can be represented more than once, b) First recurrence: most serious first recurrence in order of distant>abdominal>pelvic>vaginal, c) Locoregional LN recurrences (pelvic or aortic LN), and d) Extra abdominal LN (inguinal, mediastinal, neck, and axillar).
aHR, adjusted hazard ratio; EBRT, external beam radiotherapy; HR, hazard ratio; LN, lymph node; PA, paraaortic lymph nodes; PL, pelvic lymph nodes.
†p <0.05 using aHR: HR/aHR: adjusted for significant or potential confounders by multivariate analysis including age, American Society of Anesthesiologists, grades 1, 2, 3, unfavorable tumor types, type of hysterectomy, LN resection, adjuvant EBRT, or chemotherapy.
3. Adjuvant EBRT or chemotherapy
We further compared stage II given or not given EBRT. Less comorbidity, higher grade, deeper myometrial invasion, fewer had radical hysterectomy and LN resection and chemotherapy were observed in the group 1 compared to the group 2 (Table 1). The 117 stage II cases given EBRT had significantly higher recurrence-free survival due to better local control (vaginal and pelvic recurrences), with no effect on survival compared to no radiotherapy (Tables 2 and 4). Chemotherapy in stage II significantly increased recurrence-free survival compared to no chemotherapy (Table 2)
4. Radical hysterectomy
Radical hysterectomy was only performed in 15.8% of stage II patients (Table 1). No differences in overall, cancer-specific or recurrence-free survival were observed between radical and simple hysterectomy (Table 2).
5. Comparison to historical data
We finally compared the results of the present study to prior Danish historical results from the time when EBRT was still recommended and LN resection was not performed [9,15]. For this comparison we had to create comparative groups by using same FIGO stage (FIGO 88) and also include patients upstaged from stage I to IIIC due to LN metastasis only (FIGO 88 stage IIb was compared to FIGO 2009 stage II including patients upstaged from II to IIIC due to LN metastasis only). We found no indication of decreased survival (DEMCA 1998–1999 OS, 65%; present 2005–2012, 72.0%).
DISCUSSION
In the present study, we demonstrate that uterine risk factors (low-, intermediate-, and high-) were the strongest predictors of survival and recurrence in stage II, even stronger than LVSI and LN status. Cases with stage II low-risk (grades 1–2, <50% myometrial invasion) had a prognosis that was almost comparable to low-risk stage I, whereas for intermediate- and high-risk, cervical stromal invasion significantly increased the risk of recurrence and decreased cancer-specific survival compared to the corresponding stage I intermediate- or high-risk groups. Furthermore, in cases with stromal invasion, pelvic LN staging diagnosed 27.9% with LN metastasis and upstaged 18.1% from stage II to stage IIIC. LN-resected stage II cases had significantly higher overall, cancer-specific, progression-free survival, probably due to stage migration. Postoperative EBRT increased recurrence-free survival due to a lower number of vaginal and pelvic recurrences with no change in survival, in agreement with earlier studies [16,17,18].
The major strength of the present study is the inclusion of an entire population centralized to a few hospitals, high number of cases, and loss of only a few patients to follow-up (5 of 461) patients with cervical stromal invasion. Selection bias is a limitation when comparing subgroups. Known confounders were controlled for, but unknown confounders cannot be ruled out. The lack of histological verification at all locations in cases with multiple recurrences is a limitation. Another limitation is that LVSI was not included in the definition of risk groups as 35% of cases had unknown LVSI status. Instead we used adjusted Cox analysis to analyze the effect of the risk stratification in stage II endometrial cancer on survival and recurrence, demonstrating similar results when LVSI status was included as a confounder.
Changing a nationwide strategy by omitting all radiotherapy and introducing LN staging in the treatment of stage II endometrial cancer needs to be evaluated. We therefore compared the results of the present study to prior Danish historical results from the time when EBRT was still recommended and LN resection was not performed, using almost comparative groups (including upstaged from II to IIIC), but we found no indication of decreased survival (stage II: DEMCA 1998–1999 OS, 65%; present 2005–2012, 72.0%), supporting the Danish strategy [15].
As we in the present study demonstrated a very high-risk of recurrences in stage II patients (5-year recurrence rates: 23.6%), a new strategy for adjuvant treatment needs to be considered. The low-risk of non-locoregional recurrences (3.3%) found in low-risk stage II indicates that no adjuvant therapy may be needed in this group, while the high-risk of recurrence in intermediate- (27%) and high-risk stage II (42%) cases underlines the need for effective adjuvant therapy. EBRT or brachytherapy for stage II was demonstrated to reduce local recurrences without affecting survival [17,19]. No study has clearly demonstrated a benefit of chemotherapy for stage II patients [20], but risk stratification to uterine risk groups has not been performed. However, in all 19.7% of intermediate- and 27.9% of high-risk stage II cases experienced non-local recurrences demonstrating that postoperative adjuvant systemic therapy must be considered.
Therefore LN resection for apparent stage II will upstaged patients that may benefit from adjuvant chemotherapy, but will also introduce a high-risk of lymph oedema and so fare no randomized studies have demonstrated a survival benefit from a full LN resection [21]. Therefore, for future perspective sentinel node may be a new approach to ensure proper staging with less harm especially less lymph oedema [22]. The LN-resected stage II patients in the intermediate- and high-risk groups still have a very high-risk of developing non-local recurrences (distant metastasis, 15%–17%). Tewari et al demonstrated that chemotherapy to stage III and IV patients with stromal invasion increased OS and PFS as compared to whole abdominal irradiation [23], but whether adjuvant therapy is a benefit in stage II patients awaits futures studies. The result of the ENGOT-EN2-DGCG/EORTC-55102 trial is pending and may add knowledge to the effect of chemotherapy in stage II patients [10]. Future research should focus on finding new treatment targets with the potential of reducing the risk of non-local recurrences and on the use biomarkers to differentiate between the very different characteristics (uterine risk group, histological tumor types, and molecular differences) seen in patients with stage II endometrial cancer [24].
In conclusion, in stage II endometrial cancers patients' uterine risk groups are strong predictors for survival and recurrence and should be considered when advising adjuvant therapy. LN staging for stage II endometrial cancers increases overall and cancer-specific survival probably due to stage migration. Omitting radiotherapy increases local vaginal recurrences without affecting survival.
ACKNOWLEDGMENTS
We would like to acknowledge all the doctors, nurses, and secretaries in the Danish departments of gynecology, pathology, oncology, and radiology for the time and effort they spent constantly keeping the database up to date and answering all our requests for data. They represent the foundation for all Danish Gynecological Cancer Database (DGCD) studies. We furthermore acknowledge the hard work of the secretary of the DGCD, and we thank Edwin Stanton Spencer for linguistic corrections.
Footnotes
Funding: The study was financially supported by the Health research fund of Copenhagen University Hospital and Hans & Nora Buchard's Fond.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Ø.G., H.C., D.M.
- Data curation: Ø.G., H.C., H.E.S.
- Formal analysis: Ø.G., H.C., D.M.
- Funding acquisition: Ø.G.
- Investigation: Ø.G.
- Methodology: Ø.G., H.C., H.E.S., D.M.
- Project administration: Ø.G.
- Resources: Ø.G.
- Supervision: D.M.
- Writing - original draft: Ø.G., H.C., H.E.S., D.M.
- Writing - review & editing: Ø.G., H.C., H.E.S., D.M.
References
- 1.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksson LS, Lindqvist PG, Flöter Rådestad A, Dueholm M, Fischerova D, Franchi D, et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol. 2015;45:476–482. doi: 10.1002/uog.14645. [DOI] [PubMed] [Google Scholar]
- 3.Ørtoft G, Dueholm M, Mathiesen O, Hansen ES, Lundorf E, Møller C, et al. Preoperative staging of endometrial cancer using TVS, MRI, and hysteroscopy. Acta Obstet Gynecol Scand. 2013;92:536–545. doi: 10.1111/aogs.12103. [DOI] [PubMed] [Google Scholar]
- 4.Antonsen SL, Jensen LN, Loft A, Berthelsen AK, Costa J, Tabor A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer - a multicenter prospective comparative study. Gynecol Oncol. 2013;128:300–308. doi: 10.1016/j.ygyno.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo S, Femia M, Buscarino V, Franchi D, Garbi A, Zanagnolo V, et al. Endometrial cancer: an overview of novelties in treatment and related imaging keypoints for local staging. Cancer Imaging. 2018;18:45. doi: 10.1186/s40644-018-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe Y, Satou T, Nakai H, Etoh T, Dote K, Fujinami N, et al. Evaluation of parametrial spread in endometrial carcinoma. Obstet Gynecol. 2010;116:1027–1034. doi: 10.1097/AOG.0b013e3181f80a49. [DOI] [PubMed] [Google Scholar]
- 7.Takano M, Ochi H, Takei Y, Miyamoto M, Hasumi Y, Kaneta Y, et al. Surgery for endometrial cancers with suspected cervical involvement: is radical hysterectomy needed (a GOTIC study)? Br J Cancer. 2013;109:1760–1765. doi: 10.1038/bjc.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JD, Fiorelli J, Kansler AL, Burke WM, Schiff PB, Cohen CJ, et al. Optimizing the management of stage II endometrial cancer: the role of radical hysterectomy and radiation. Am J Obstet Gynecol. 2009;200:419.e1–419.e7. doi: 10.1016/j.ajog.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Ørtoft G, Hansen ES, Bertelsen K. Omitting adjuvant radiotherapy in endometrial cancer increases the rate of locoregional recurrences but has no effect on long-term survival: the Danish Endometrial Cancer Study. Int J Gynecol Cancer. 2013;23:1429–1437. doi: 10.1097/IGC.0b013e3182a5e77d. [DOI] [PubMed] [Google Scholar]
- 10.Danish Gynecological Cancer Group. Chemotherapy or observation in stage I-II intermediate or high risk endometrial cancer. ClinicalTrials.gov Identifier: NCT01244789 [Internet] Copenhagen: Danish Gynecological Cancer Group; 2018. [cited 2019 Sep 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT01244789. [Google Scholar]
- 11.Sørensen SM, Bjørn SF, Jochumsen KM, Jensen PT, Thranov IR, Hare-Bruun H, et al. Danish Gynecological Cancer Database. Clin Epidemiol. 2016;8:485–490. doi: 10.2147/CLEP.S99479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juhl CS, Hansen ES, Høgdall CK, Ørtoft G. Valid and complete data on endometrial cancer in the Danish Gynaecological Cancer Database. Dan Med J. 2014;61:A4864. [PubMed] [Google Scholar]
- 13.Ørtoft G, Lausten-Thomsen L, Høgdall C, Hansen ES, Dueholm M. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: a Danish Gynecological Cancer Group Study. J Gynecol Oncol. 2019;30:e84. doi: 10.3802/jgo.2019.30.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.StataCorp. Stata: release 11. Statistical software. Collage Station, TX: StataCorp; 2009. [Google Scholar]
- 15.Bertelsen K, Ortoft G, Hansen ES. Survival of Danish patients with endometrial cancer in the intermediate-risk group not given postoperative radiotherapy: the Danish Endometrial Cancer Study (DEMCA) Int J Gynecol Cancer. 2011;21:1191–1199. doi: 10.1097/IGC.0b013e318229264e. [DOI] [PubMed] [Google Scholar]
- 16.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 17.Fu HC, Chen JR, Chen MY, Hsu KF, Cheng WF, Chiang AJ, et al. Treatment outcomes of patients with stage II pure endometrioid-type endometrial cancer: a Taiwanese Gynecologic Oncology Group (TGOG-2006) retrospective cohort study. J Gynecol Oncol. 2018;29:e76. doi: 10.3802/jgo.2018.29.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matei D, Filiaci VL, Randall M, Steinhoff M, DiSilvestro P, Moxley KM, et al. A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. J Clin Oncol. 2017;35:5505. [Google Scholar]
- 19.Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 20.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost JA, Webster KE, Bryant A, Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2017;10:CD007585. doi: 10.1002/14651858.CD007585.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.How JA, O'Farrell P, Amajoud Z, Lau S, Salvador S, How E, et al. Sentinel lymph node mapping in endometrial cancer: a systematic review and meta-analysis. Minerva Ginecol. 2018;70:194–214. doi: 10.23736/S0026-4784.17.04179-X. [DOI] [PubMed] [Google Scholar]
- 23.Tewari KS, Filiaci VL, Spirtos NM, Mannel RS, Thigpen JT, Cibull ML, et al. Association of number of positive nodes and cervical stroma invasion with outcome of advanced endometrial cancer treated with chemotherapy or whole abdominal irradiation: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:87–93. doi: 10.1016/j.ygyno.2011.12.414. [DOI] [PubMed] [Google Scholar]
- 24.De Boer SM, Nout RA, Bosse T, Creutzberg CL. Adjuvant therapy for high-risk endometrial cancer: recent evidence and future directions. Expert Rev Anticancer Ther. 2019;19:51–60. doi: 10.1080/14737140.2019.1531708. [DOI] [PubMed] [Google Scholar]