Abstract
Introduction:
Identifying PLHIV in HIV care who are at particular risk of non-retention in care is an important element in improving their HIV care outcomes. The purpose of this study was to develop a risk prediction tool to identify PLHIV at risk of non-retention in care over the course of the next year.
Method:
We used stepwise logistic regression to assess sociodemographic, clinical and behavioral predictors of non-retention in HIV care. Retention in care was defined as having evidence of at least two encounters with an HIV care provider (or CD4 or viral load lab tests as a proxy measure for the encounter), at least 3 months apart within a year. We validated the risk prediction tool internally using the bootstrap method.
Results:
The risk prediction tool included a total of six factors: age group, race, poverty level, homelessness, problematic alcohol/drug use and viral suppression status. The total risk score ranged from 0 to 17. Compared to those in the lowest quartile (0 risk score), those who were in the middle two quartiles (score 1–4) and those in the upper quartile (>4 risk score) were more likely not to be retained in care (odds ratio [OR] 1.63 [CI; 1.39–1.92] and OR 4.82 [CI; 4.04–5.78] respectively). The discrimination ability for the prediction model was 0.651.
Conclusion:
We found that increased risk for non-retention in care can be predicted with routinely available variables. Since the discrimination of the tool was low, future studies may need to include more prognostic factors in the risk prediction tool.
Keywords: non-retention, HIV, AIDS, risk prediction tool, risk score
Introduction
The navigation of people living with HIV (PLHIV) across the HIV care continuum includes being diagnosed with HIV, linked to care, engaged in care, retained in care, adhering to antiretroviral therapy (ART), and having a suppressed HIV viral load.1 A goal of the United States (US) National HIV/AIDS Strategy is to increase the percentage of persons with diagnosed HIV infection who are retained in HIV medical care to at least 90 percent by 2020.2 The Centers for Diseases Prevention and Control (CDC) monitors retention in care using laboratory data from jurisdictions with complete reporting of CD4 and viral load test results. In 2015, only 57.2% of PLHIV were retained in care.3 Among 38 states with complete lab reporting for 2015 and 2016, none met the National HIV/AIDS Strategy 2020 target of 90%, 21 made progress toward the 2020 target, and 17 made no progress.3
Factors related to demographics, behavior, psychosocial and physical health affect retention in HIV care.4 Those factors include substance use,5–7 belonging to a racial ethnic minority group,5,8,9 mental health problems,6,7 young age,5,10–12 female gender,9,11 injection drug use (IDU) as the vector for infection,8–10 having public health insurance,13 health literacy,14 intimate partner violence,15 low socioeconomic status,7,9 past-year missed treatment visits16 and greater unmet socioeconomic needs such as housing, food, or transportation.7,9 Some studies have synthesized these factors and devised a risk prediction tool to identify people who might be poorly retained in HIV care. A study attempted to develop a clinical decision tool to estimate the probability of being lost to follow-up among adults initiating antiretroviral therapy in resource-limited settings.17 The study found that young age and advanced WHO disease stage were significant predictors of being lost to follow-up, but the model had weak ability to discriminate those who will remain in care from those who will be lost to follow-up. Another study developed a risk score to identify HIV-infected women who are most likely to be lost to follow-up in the postpartum period.18 Parity, education, employment status, WHO clinical stage, duration of combination ART during pregnancy, and number of antenatal care visits were found to predict being lost to follow-up. Woodward and his colleagues developed a risk prediction tool for medical appointment attendance among HIV-infected persons with unsuppressed viremia.19 They found that active substance abuse, poor adherence to daily medications, history of missing HIV care appointments, prior treatment failure, prior exposure to ART (defined as any prior exposure to nucleot(s)ide reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, and protease inhibitor classes OR a current regimen containing enfuvirtide), most recent CD4 + lymphocyte count < 100 copies/mm3, and most recent viral load > 200 copies/mL predicted poor medical appointment attendance.19
Poor retention in care can lead to undesirable HIV outcomes at the individual and population levels.9 Poor retention in care has been found to be associated with higher viral loads, lower CD4 cell counts,12 higher rates of ART failure, decreased likelihood of receiving antiretroviral therapy, increased HIV transmission risk behavior, increased hospitalization rates, and worse survival.9 Therefore, retention in HIV care is a key step to improve HIV outcomes and overall health of PLHIV. The aim of this study was to identify people in HIV care who are likely to be poorly retained in care over the course of the following year using sociodemographic, clinical, and laboratory information.
Methods
We used retrospective data from the Miami-Dade County (Florida) Ryan White Program (RWP) Part A/ Minority AIDS Initiative (MAI) for the calendar years 2016–2017 to assess the relationship between sociodemographic, clinical and behavioral variables and risk of non-retention in HIV care, with a primary focus on routinely available variables. The RWP Part A provides core medical, medical case management, pharmaceutical, and related support services to low-income people with HIV in metropolitan areas heavily impacted by HIV/AIDS (“Eligible Metropolitan Areas,” or EMAs), to improve their access to HIV care and their health outcomes; the MAI program provides additional support for a subset of these services, targeted toward ethnic and racial minorities in these EMAs.
Study population
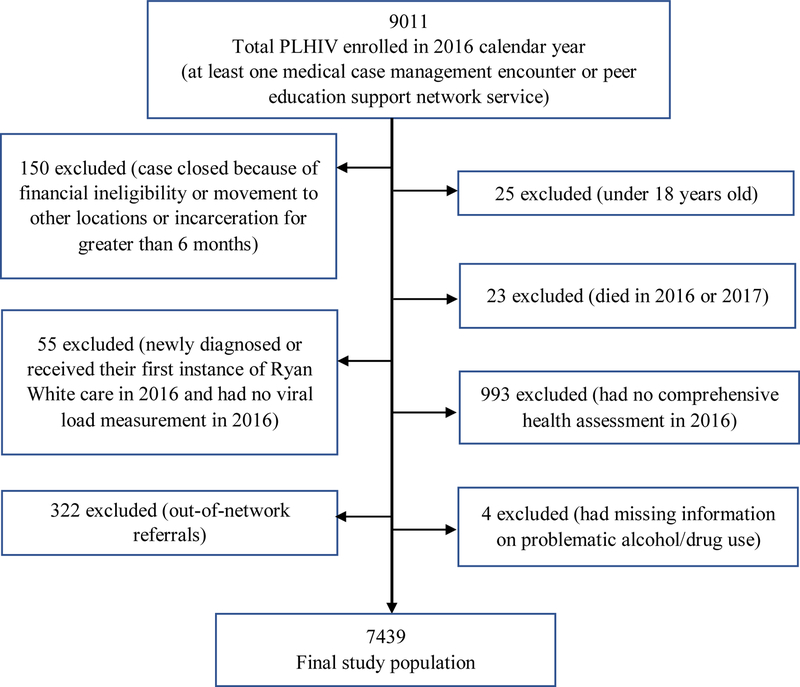
The population was PLHIV who were enrolled in (and receiving services from) the in RWP Part A/MAI program in the Miami-Dade EMA in 2016. Enrollment was defined as having received at least one medical case management encounter or peer education support network service in 2016. We measured risk factors in 2016, and the outcome (non-retention in care) was measured in 2017. Risk factors were obtained from the RWP’s comprehensive health assessment, patient intake assessment and laboratory results entered into the patient’s electronic medical records. The comprehensive health assessment is a health and social needs assessment of RWP patients that is completed every 6 months to determine the plan of care and needs for referrals to other services. Patient intake assessment includes demographic data collected at time of entry into the RWP. We excluded people who had no comprehensive health assessment in 2016, or were <18 years old in January 2016, who died in 2016 or 2017, or were out-of-network referrals in 2016 or 2017. Out-of-network referrals are people who were referred to the RWP from a non-RWP provider, receiving a single service but not receiving regular medical case management, and for whom assessments of retention would not be available. We also excluded clients if their case was closed because of movement to another state/county, financial ineligibility, or incarceration for greater than 6 months in 2016 and 2017. Moreover, clients diagnosed with HIV infection in 2016, and those who received their first RWP care in 2016 but who had no viral load measurement in 2016 were excluded from the analysis. We deleted four people who had missing information about problematic alcohol/drug use in 2016.
Measurements
The following variables were considered in the development of the risk prediction model; age (18–24, 25–39 and ≥40 years), sex assigned at birth (male/female), race (Black/other), transgender status (yes/no), Hispanic ethnicity (yes/no), homelessness (includes homeless patients and patients in transient or transitional housing) (yes/no), CDC-defined AIDS status as of 2016 (yes/no), viral suppression in 2016 (yes/no), getting the food he/she needs (yes/no), access to transportation for healthcare/dental/social service appointments (yes/no), alcohol/drug use resulting in any problem in patients daily activity or legal issue or hazardous situation (yes/no), history of injection drug use, including injection drug use as the self-reported vector for the original HIV infection (yes/no), self-reported feelings of depression or anxiety (yes/no), and income <100% of the federal poverty level (FPL) (yes/no). Federal poverty level <100% in 2016 was defined as having a household income less than $11,880 for a single person.20 Problematic alcohol/drug use was derived from three questions namely; (a) Has alcohol/drug use resulted in hazardous situation, (b) Has alcohol/drug use resulted in legal problems, and (c) Is your alcohol/drug use preventing you from carrying out your daily activities? History of injection drug use (IDU) includes injection drug users, and men who have sex with men who are also injection drug users.
Outcome
The outcome of the study was non-retention in HIV care in 2017. We defined retention in care as having evidence of at least two occurrences of any combination of (a) face-to-face encounter(s) with a Ryan White Program medical care professional, or (b) laboratory tests (CD4 or viral load), at least three months apart during 2017.
Analysis
First, we selected risk factors to be included in the bivariate analysis based on evidence from literature and completeness of information in the dataset, and we estimated unadjusted odds ratios. Variables associated with non-retention in HIV care at p-value<0.1 in bivariate analysis were included in the initial multivariate logistic regression model. We used stepwise backward elimination, retaining variables which maintained significance at P < 0.05 in the final model. We used Akaike information criterion (AIC) to check the model fit.21 We checked for any confounding effect of the excluded variables in the final model. Discrimination was assessed using concordance statistic or C-statistic (which is equal to the area under the receiver operating characteristic [ROC] curve), and calibration was assessed using calibration plots by dividing subjects into deciles of risk.22
We validated the risk score tool internally using the bootstrap method with the original derivation data set. A total of 1000 samples were created by sampling with replacement, and each bootstrap sample was the same size as the original derivation sample. For each sample, the model was refitted following the same method adopted in the derivation process. We computed model performance (C-statistic) on each bootstrap sample and compared with the model performance in the original data to calculate optimism (magnitude of bias). The optimism adjusted C-statistic was computed by subtracting the optimism from the original C-statistic.23
Finally, we generated a simple integer-based risk score for each predictor variable by multiplying the beta coefficients by 10 and rounding to the nearest integer.18,23 The total risk score was calculated by adding each component together. We divided the population into strata based on quartiles of the total risk score by placing cut points at the 25th, 50th and 75th percentiles.24,25 We also calculated the sensitivity and specificity at each risk score cutoff point. The predictive performance of the risk score was evaluated by means of discrimination and calibration. All analyses were conducted using SAS software V. 9.4 (SAS Institute Cary, NC). This study was approved by the Florida International University Institutional Review Board.
Results
Of the total 9011 PLHIV enrolled in RWP in 2016, 7439 people were included in our analysis. A total of 1572 PLHIV were excluded for various reasons (Figure 1). About 24% (1759) of the 7439 were not retained in HIV care during 2017. The mean age and standard deviation of the study population was 44.4±11.9. About 64% of the population were older than 40 years, 59.7% were Black, 76.2% were male, and 55.7 were Hispanic (Table 1).
Figure 1.
Diagram for exclusion of participants from the present study
Table 1.
Population characteristics and model of risk variables associated with non-retention in care among PLHIV (N=7439)
| Characteristics during 2016 | Total population (n) |
Not retained in care n (%) | Bivariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
p-value | Adjusted OR (95% CI) |
Coefficient (Beta) | Score | |||
| Total | 7439 | 1759 (23.7) | |||||
| Age (years) | <0.001 | ||||||
| 18–24 | 413 | 142 (34.4) | 1.85 (1.57–2.42) | 1.51 (1.21–1.90) | 0.05 | 1 | |
| 25–39 | 2256 | 608 (30.0) | 1.38 (1.22–1.54) | 1.31 (1.16–1.48) | 0.21 | 2 | |
| ≥40 | 4770 | 1009 (21.2) | Ref | Ref | |||
| Race | <0.001 | ||||||
| Other | 4443 | 889 (20.0) | Ref | Ref | |||
| Black | 2996 | 870 (29.0) | 1.64 (1.47–1.82) | 1.37 (1.22–1.53) | 0.16 | 2 | |
| Income below 100% of FPL | <0.001 | ||||||
| No | 4178 | 840 (20.1) | Ref | Ref | |||
| Yes | 3261 | 919 (28.2) | 1.56 (1.40–1.74) | 1.24 (1.11–1.40) | 0.11 | 1 | |
| Homeless | <0.001 | ||||||
| No | 6983 | 1567 (22.4) | Ref | Ref | |||
| Yes | 456 | 192 (42.1) | 2.51 (2.07–3.05) | 1.80 (1.46–2.23) | 0.27 | 3 | |
| Alcohol/drug use resulted in any problem in daily activity, legal issue or hazardous situation | <0.001 | ||||||
| Yes | 192 | 106 (55.2) | 4.17 (3.12–5.57) | 2.36 (1.72–3.23) | 0.43 | 4 | |
| No | 7247 | 1653 (22.8) | Ref | Ref | |||
| Virally suppressed | <0.001 | ||||||
| Yes | 6232 | 1224 (19.6) | Ref | Ref | |||
| No | 1207 | 535 (44.3) | 3.26 (2.86–3.71) | 2.69 (2.35–3.07) | 0.49 | 5 | |
| Sex assigned at birth | 0.56 | ||||||
| Male | 5667 | 1349 (23.8) | Ref | ||||
| Female | 1772 | 410 (23.1) | 1.04 (0.92–1.18) | ||||
| Hispanic ethnicity | <0.001 | ||||||
| Yes | 4143 | 813 (19.6) | Ref | ||||
| No | 3296 | 846 (25.7) | 1.41 (1.28–1.58) | ||||
| Are you feeling depressed or anxious? | <0.001 | ||||||
| Yes | 1146 | 305 (30.5) | 1.52 (1.33–1.75) | ||||
| No | 6293 | 1409 (22.4) | Ref | ||||
| Are you getting the food you need? | <0.001 | ||||||
| Yes | 7322 | 1717 (23.5) | Ref | ||||
| No | 117 | 42 (35.9) | 1.83 (1.25–2.68) | ||||
| CDC-defined AIDS | 0.13 | ||||||
| Yes | 3041 | 746 (24.5) | 1.09 (0.98–1.21) | ||||
| No | 4398 | 1013 (23.0) | Ref | ||||
| History of IDU | 0.030 | ||||||
| No | 7309 | 1714 (23.5) | Ref | ||||
| Yes | 130 | 45 (34.6) | 1.73 (1.20–2.49) | ||||
| Access to transportation to appointments | 0.01 | ||||||
| Yes | 6726 | 1563 (23.2) | Ref | ||||
| No | 713 | 196 (27.5) | 1.25 (1.05–1.49) | ||||
| Transgender | 0.44 | ||||||
| No | 7396 | 1751 (23.7) | Ref | ||||
| Yes | 43 | 8 (18.6) | 0.74 (0.34–1.59) | ||||
OR: Odds Ratio; FPL: Federal Poverty Level; IDU: Injection Drug Use; AIDS: Acquired Immunodeficiency Syndrome; CI: Confidence Interval; CDC: Centers for Diseases Control and Prevention
The multivariate logistic model included variables that were significant at p-value <0.1 in the bivariate analysis.
These include all the variables in the table except sex assigned at birth, AIDS status and transgender status.
Scores were assigned to each risk factor by multiplying each beta obtained from the stepwise logistic regression model by 10.
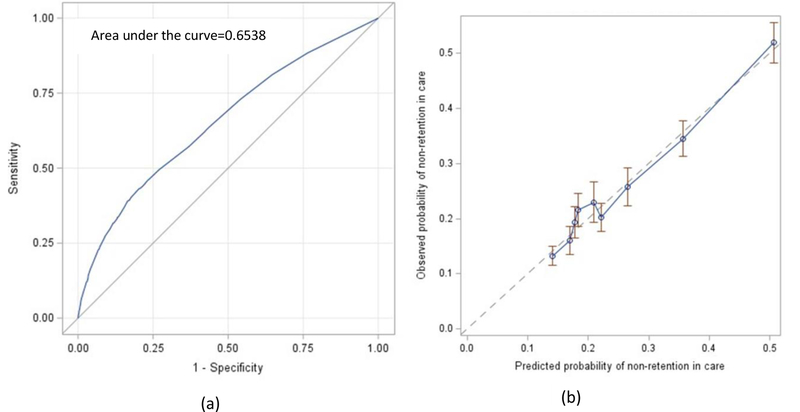
Of the 14 potential variables considered, 11 variables were associated with non-retention in HIV care at p-value <0.1. In the bivariate analysis, age in 2016, race, poverty level, homelessness, alcohol/drug use resulting in any problem in daily activity, legal issue or hazardous situation, viral suppression status, Hispanic ethnicity, feeling depressed or anxious and food need were significant at p-value <0.001; whereas history of IDU and access to transportation were significant at p-value <0.05 (Table 1). Sex assigned at birth, transgender status, and AIDS status as of 2016 were not associated with retention in care in 2017 (p-value>0.1). In the stepwise logistic regression analysis, six variables maintained significance level at p-value <0.05 level in the final model (Table 1). The six variables were age, race, poverty level, homelessness, alcohol/drug use resulting in any problem in daily activity, legal issue or hazardous situation, and viral suppression status. The discrimination of the overall model with the 6 variables was 0.654 (Figure 2(a)), and after adjusting for optimism, the discrimination was 0.651. Based on the calibration plot, the agreement between the observed and predicted proportion of events of non-retention in HIV care showed good apparent calibration (Figure 2(b)).
Figure 2.
a) Discrimination of the final model b) Calibration of the final model
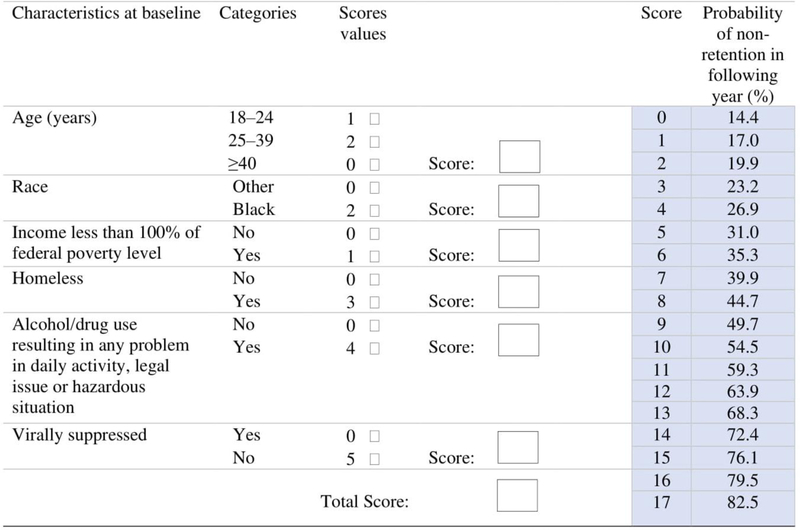
The final risk prediction tool included 6 risk factors present in 2016 that can be used to predict non-retention in HIV care over the course of 2017. Each risk factor contributed additively to an overall risk score, as follows: having unsuppressed viral load had a risk score of 5, being homeless had a risk score of 3, being Black had a risk score of 2, being in the age group 18–24 had a risk score of 1, being in the age group 25–39 had a risk score of 2, having income below 100% of the federal poverty level had a risk score of 1, and alcohol/drug use resulting in any problem in daily activity, legal issue or hazardous situation had a risk score of 4. The minimum total risk score was 0 for a person without any of the risk factors, and the maximum possible risk score was 17. A person with a total risk score of 0 had 14.4% probability of not being retained in HIV care in 2017, and a person with a total risk score of 17 had 82.5% probability of not being retained in HIV care. As the risk score increased, the probability of non-retention in care increased. Every one-point increase in the risk score scale was associated with OR 1.22 (95% CI; 1.20–1.24) increase in non-retention in care. The discrimination of the risk score was 0.650. We divided the risk scores into three categories based on quartiles placing cut points at the 25th and 75th percentiles. There were 1559 (21.0%) people in the first quartile (0 risk score), and 211 (13.5%) of these were not retained in HIV care. In the second and third quartiles (score 1–4), there were 4331 (58.2%) people, and in the upper quartile (>4 risk score) there were 1549 people (20.8%). About 20% (882) of those in the second and third quartiles and 43.0% (666) of those in the upper quartile were not retained in HIV care. Compared to those in the first quartile, those who were in the middle two quartiles and those in the upper quartile were more likely not to be retained in care (OR 1.63 [CI; 1.39–1.92] and OR 4.82 [CI; 4.04–5.78], respectively). The cutoff value of 4 had a sensitivity of 43% and specificity of 80% and a cutoff value of 5 had a sensitivity of 38% and specificity of 84%. Similarly, a cutoff value of 3 in the risk score had a sensitivity of 56% and specificity of 65%.
Discussion
In this study, we derived and internally validated a risk prediction tool for non-retention in HIV care in the next year using retrospective data from Miami-Dade County RWP Part A/MAI. This risk prediction tool can be used in clinical settings by HIV care providers to identify PLHIV who will not be retained in HIV care in the next year. We found that the risk score constitutes age group, race, poverty level, homelessness, problematic alcohol/drug use and viral suppression status. These variables can be extracted easily from medical records or by interviewing the patient and can be implemented in a variety of settings.
The individual factors included in our risk prediction tool have been previously found to predict retention in care. Consistent with findings in previous studies, unsuppressed viral load and age group predict retention in HIV care.5,10–12,17,19 Similarly, persons living with HIV who are homeless or have low economic status have been found to be poorly retained in care.7,9,26,27 This is likely due to unmet social service needs.27 People who use alcohol/drugs are at increased risk of poor adherence and non-retention in HIV care.28–31 This may be due to the behavioral factors associated with alcohol/drug use. Moreover, being Black/African American has been identified as a risk factor increasing non-retention in care. Historical and cultural factors as well as structural racism may affect the retention of African Americans in HIV care.32 Therefore, inclusion of Black race in the risk prediction tool is likely a proxy for underlying social, cultural, and economic factors. Inclusion of race in the risk prediction tool may lead to unconscious bias by health care providers about Blacks. Addressing racial bias needs comprehensive, multifaceted, and evidence-based interventions at the individual and organizational level including leadership commitment to a cultural inclusion, diversity training, self-reflection on personal biases, mentorship and sponsorship, and cultural competency.33
We stratified the population into quartiles, and patients with a total risk score >4 were classified in the fourth quartile. The risk of non-retention in care showed a graded increase across the quartiles. Those who were in the fourth quartile were about 5 times as likely not to be retained in care compared with those who were in the first quartile. A cutoff value of 5 in the risk score had a sensitivity of 38% and a specificity of 84%. This cutoff identified 20.8% of our study population with the highest likelihood of non-retention in care for intervention. Based on this risk score cutoff, non-viral suppression, independent of other factors in the risk score, contributes to one third of the total risk score. Thus, viral suppression is a good predictor to use for identifying patients that may benefit from a retention intervention. Alternatively, a lower cutoff point in the risk score would yield higher sensitivity and lead to targeting a larger proportion of the population for a retention intervention.
Previous risk prediction tools developed to predict patient adherence to appointments or retention in care were either restricted to specific populations or had different outcome definitions. The study by McNairy et al. measured lost to follow-up based on a single clinic or pharmacy visit during 365 days after ART initiation.17 Our definition of retention in care was based on two or more clinic visits or laboratory tests at least three months apart during a year. Bengtson et al. developed a risk prediction tool among HIV-infected women and they included different predictors specific to pregnant women such as parity and number of antenatal care visits.18 Woodward et al. used a tool previously developed for virologic failure to stratify patients based on medical appointment attendance (defined based on a single visit) among persons with unsuppressed viremia.19 The definition of the outcome and the target population are different from ours. Some factors such as substance use and viral suppression were common predictors in our risk prediction tool and theirs. However, Woodward et al included additional predictors such as prior treatment failure, adherence to daily medications, history of missing HIV care appointments, and prior exposure to ART which may be better predictors of retention in care but are not readily collected in our study.
The risk prediction tool is intended to be used in HIV care settings, where the characteristics of the target population are similar to ours. Upon arrival of a patient to the HIV care setting, an HIV care provider could assess the probability of a patient not being retained in HIV care in the next year using this checklist. Depending on the availability of resources, HIV care providers may arrange for an intervention to support retention based on severity of risk in order to improve HIV outcomes34,35 and reduce HIV transmission.36,37 Retention in HIV care can be improved by incorporating informational, motivational, and behavioral skill components.9 Peer navigators and clinic-wide marketing (e.g., posters, brochures) including targeted messages on staying in care which were delivered at minimal effort and cost, have been found to be effective in improving clinic attendance.7,38,39 Designating a staff person to helps with appointments, referrals, system navigation, service coordination, and transportation may improve retention in HIV care.7,40 Enhancing personal contact with patients and asking open-ended questions in regular conversations at every office visit may help to identify specific ART adherence and retention support services.7,41
Our study has several limitations. First, in our analysis, we included variables that are routinely collected and easily available to care providers. However, these variables were not strong predictors of non-retention in care. The discriminative ability of our study is low (0.651),42 although it is higher than that of the study by McNairy et al.17 Moreover, we were not able to find a risk score cutoff with higher sensitivity and specificity. This indicates that other predictive variables could have been included in the risk prediction tool to improve its discriminative ability. Factors such as adherence to daily medications, sexually transmitted infections, previous appointment attendance, prior treatment failure,19 and other unmet needs7,9 may increase the discriminative ability of the risk prediction tool. However, information about these factors may not be routinely accessible to the HIV care providers, or collecting these factors may require additional resources and increase the workload for HIV care providers or support staff. Although the discrimination level is relatively low, this tool can be used in situations where these additional variables are not available. Second, we used RWP Part A/MAI data to develop and internally validate our risk prediction tool. The Ryan White Program provides medical care, medical case management, anti-retroviral prescription drugs and other support for PLHIV without health insurance. Thus, Ryan White Program participants may not be representative of all PLHIV. Third, people newly diagnosed with HIV infection may behave differently due to experiencing additional challenges related to acceptance of their diagnosis and stigma. Therefore, they may require a different risk prediction tool. Finally, of those enrolled in 2016, we were not able to find laboratory results for 917 people during 2017. In a separate analysis, we excluded those people and the results were similar with the model that included those 917 people.
In summary, we developed a relatively simple prediction tool that can be used to identify PLHIV who are at risk of non-retention in HIV care. This tool includes characteristics that are routinely collected in healthcare settings. These factors include age group, race, poverty level, homelessness, problematic alcohol/drug use and viral suppression status. The risk prediction tool has low discrimination power but could be a good alternative tool in situations where additional data is not available. Further research should include better predictive variables to enhance the accuracy of this risk prediction tool.
Figure 3.
Point scores for all risk factors in the logistic regression model. The predicted probabilities of non-retention in HIV care for the total score ranged from 14.4% for a patient with 0 total score to 82.5% for a patient with 17 total score. To get the total score for individual person, we should add the scores of the six variables. For example, for patient who is 20 years old (Score=1), White (Score=0), income equal to or higher than 100% of FPL (Score=0), homeless (Score=3), with no problematic alcohol/drugs use (Score=0), and has unsuppressed viral load (Score=5), the total score will be 9 (1+0+0+3+0+5). A person with total score of 9 had 49.7% probability of not being retained in care in the next year.
Acknowledgments
This research was supported in part by National Institute on Minority Health and Health Disparities (NIMHD) under Award Numbers R01MD012421, R01MD013563, 5S21MD010683, K01MD013770, U54MD012393, and Florida International University graduate school dissertation year fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: Updates, goals, and recommendations for the future. AIDS Res Ther 2016;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National HIV/AIDS Strategy: Updated to 2020. Available at: https://files.hiv.gov/s3fs-public/nhas-update.pdf (2015, accessed 22 May 2019).
- 3.Centers for Disease Control and Prevention. HIV Prevention Progress Report, 2019. Available at: https://www.cdc.gov/hiv/pdf/policies/progressreports/cdc-hiv-preventionprogressreport.pdf (2019, accessed 22 May 2019).
- 4.Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of Adult Retention in HIV Care: A Systematic Review. AIDS Behav 2018;22(3):752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano TP, Hartman C, Gifford AL, et al. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials 2009;10(5):299–305. [DOI] [PubMed] [Google Scholar]
- 6.Dombrowski JC, Simoni JM, Katz DA, et al. Barriers to HIV care and treatment among participants in a public health HIV care relinkage program. AIDS Patient Care STDs 2015;29:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Diseases Control and Prevention. HIV Treatment and Care: Information for Health Care Providers. Available at: https://www.cdc.gov/hiv/clinicians/treatment/treatment-clinicians.html (2018, accessed 25 September 2019).
- 8.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. JAIDS 2013;62:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giordano TP. Retention in HIV care: what the clinician needs to know. Top Antivir Med 2011;19(1):12–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Nosyk B, Lourenço L, Min JE, et al. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn.’ AIDS 2015;29(13):1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lourenço L, Colley G, Nosyk B, et al. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS ONE 2014;9:e115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi A, Youmans E, Gibson JJ, et al. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses 2011;27(7):751–8. [DOI] [PubMed] [Google Scholar]
- 13.Tedaldi EM, Richardson JT, Debes R, et al. Retention in care within 1 year of initial HIV care visit in a multisite US cohort: who’s in and who’s out? J Int Assoc Provid AIDS Care 2014;13:232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldrop-Valverde D, Guo Y, Ownby RL, et al. Risk and protective factors for retention in HIV care. AIDS Behav 2014;18(8):1483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer KR, Brant J, Gupta S, et al. Intimate partner violence: a predictor of worse HIV outcomes and engagement in care. AIDS Patient Care STDs 2012;26(6):356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pence BW, Bengtson AM, Boswell S, et al. Who Will Show? Predicting Missed Visits Among Patients in Routine HIV Primary Care in the United States. AIDS Behav 2019;23(2):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNairy ML, Jannat-Khah D, Pape JW, et al. Predicting death and lost to follow-up among adults initiating antiretroviral therapy in resource-limited settings: Derivation and external validation of a risk score in Haiti. PLoS One 2018;13(8):e0201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengtson AM, Chibwesha CJ, Westreich D, et al. A risk score to identify HIV-infected women most likely to become lost to follow-up in the postpartum period. AIDS Care 2016;28(8):1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodward B, Person A, Rebeiro P, et al. Risk Prediction Tool for Medical Appointment Attendance Among HIV-Infected Persons with Unsuppressed Viremia. AIDS Patient Care STDS 2015;29(5):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Department of Health and Human Services. Computations for the 2016 poverty guidelines. 2016. Available at: https://aspe.hhs.gov/computations-2016-poverty-guidelines (2016, accessed 14 August 2019).
- 21,Lee YH, Bang H, Kim DJ. How to Establish Clinical Prediction Models. Endocrinol Metab (Seoul) 2016;31(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York: Springer, 2009. [Google Scholar]
- 24.Traeger A, Henschke N, Hübscher M, et al. Development and validation of a screening tool to predict the risk of chronic low back pain in patients presenting with acute low back pain: a study protocol. BMJ Open 2015;5(7):e007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan TC, Shea YF, Luk KH, et al. Development and validation of a prognostic index for 2-year mortality in Chinese older residents living in nursing homes. Geriatr Gerontol Int 2012;12(3):555–62. [DOI] [PubMed] [Google Scholar]
- 26.Rajabiun S, Tryon J, Feaster M, et al. The Influence of Housing Status on the HIV Continuum of Care: Results From a Multisite Study of Patient Navigation Models to Build a Medical Home for People Living With HIV Experiencing Homelessness. American Journal of Public Health 2018;108(S7):S539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolitski RJ, Kidder DP, Fenton KA. HIV, homelessness, and public health: critical issues and a call for increased action. AIDS Behav 2007;11(6 Suppl):167–71. [DOI] [PubMed] [Google Scholar]
- 28.Vagenas P, Azar MM, Copenhaver MM, et al. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review: Alcohol and the HIV Continuum of Care. Curr HIV/AIDS Rep 2015;12(4):421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edison L, Hughes D, Drenzek C, et al. Prevalence and indicators of viral suppression among persons with diagnosed HIV infection retained in care - Georgia, 2010. MMWR Morb Mortal Wkly Rep 2014;63(3):55–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Williams EC, Hahn JA, Saitz R, et al. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res 2016;40(10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwadz M, de Guzman R, Freeman R, et al. Exploring How Substance Use Impedes Engagement along the HIV Care Continuum: A Qualitative Study. Front Public Health 2016;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman R, Gwadz MV, Silverman E, et al. Critical race theory as a tool for understanding poor engagement along the HIV care continuum among African American/Black and Hispanic persons living with HIV in the United States: a qualitative exploration. Int J Equity Health 2017;16(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcelin JR, Siraj DS, Victor R, et al. The Impact of Unconscious Bias in Healthcare: How to Recognize and Mitigate It. J Infect Dis 2019;220(Supplement_2):S62–S73. [DOI] [PubMed] [Google Scholar]
- 34.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaner JS. Treatment as prevention--a double hat-trick. Lancet 2011;378:208–209. [DOI] [PubMed] [Google Scholar]
- 36.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah M, Risher K, Berry SA, et al. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis 2016;62:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner LI, Marks G, Craw JA, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis 2012;55(8):1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner LI, Marks G, Wilson TE, et al. Clinic-wide intervention lowers financial risk and improves revenue to HIV clinics through fewer missed primary care visits. J Acquir Immune Defic Syndr 2015;68(4):472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okeke NL, Ostermann J, Thielman NM. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr HIV/AIDS Rep 2014;11(4):376–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis 2014;59(5):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation 2010;121(15):1768–77. [DOI] [PubMed] [Google Scholar]