SUMMARY
Niche cells often wrap membrane extensions around stem cell surfaces. Niche wrapping has been proposed to retain stem cells in defined positions and affect signaling [eg. 1, 2]. To test these hypotheses and uncover additional functions of wrapping, we investigated niche wrapping of primordial germ cells (PGCs) in the C. elegans embryonic gonad primordium. The gonad primordium contains two PGCs that are wrapped individually by two somatic gonad precursor cells (SGPs). SGPs are known to promote PGC survival during embryogenesis and exit from quiescence after hatching, although how they do so is unknown [3]. Here, we identify two distinct functions of SGP wrapping that are critical for PGC quiescence and survival. First, niche cell wrapping templates a laminin-based basement membrane around the gonad primordium. Laminin and the basement membrane receptor dystroglycan function to maintain niche cell wrapping, which is critical for normal gonad development. We find that laminin also preserves PGC quiescence during embryogenesis. Exit from quiescence following laminin depletion requires glp-1/Notch and is accompanied by inappropriate activation of the GLP-1 target sygl-1 in PGCs. Independent of basement membrane, SGP wrapping performs a second, crucial function to ensure PGC survival. Endodermal cells normally engulf and degrade large lobes extended by the PGCs [4]. When SGPs are absent, we show that endodermal cells can inappropriately engulf and cannibalize the PGC cell body. Our findings demonstrate how niche cell wrapping protects germ cells by manipulating their signaling environment and by shielding germ cells from unwanted cellular interactions that can compromise their survival.
Keywords: Primordial germ cell, stem cell, niche, Notch, wrapping, basement membrane, quiescence, cell death, dystroglycan, laminin
RESULTS
SGP Wrapping Templates the Gonadal Basement Membrane
SGPs migrate to and wrap around PGCs during the first half of embryogenesis (Figure 1A). A basement membrane (BM) surrounds the gonad primordium as well as other organs, and depletion of several BM components, including laminin, disrupts gonad organization in larvae [5–7]. BM assembles when laminin trimers containing α, β and γ subunits concentrate on cellular surfaces and recruit additional BM components [8]. lam-1 encodes the sole C. elegans laminin ß. Functional LAM-1GFP [9] concentrated on outward-facing SGP surfaces (SGPMem) soon after SGPs wrapped the PGCs but was not present on SGP surfaces facing the PGCs (Figures 1B and S1A). C. elegans expresses two laminin a subunits, EPI-1 and LAM-3, which can function redundantly [5]. Consistent with observations in larvae [5], we detected only EPI-1 in the gonadal BM, whereas both proteins were present in many other BMs (Figures 1C and 1D). Laser-ablating the SGP precursors to block SGP-PGC interactions prevented LAM-1GFP from enriching around the naked PGCs (Figures 1E and S1B), indicating that SGP wrapping of PGCs templates the gonadal BM.
Figure 1. SGP wrapping templates the gonadal basement membrane.
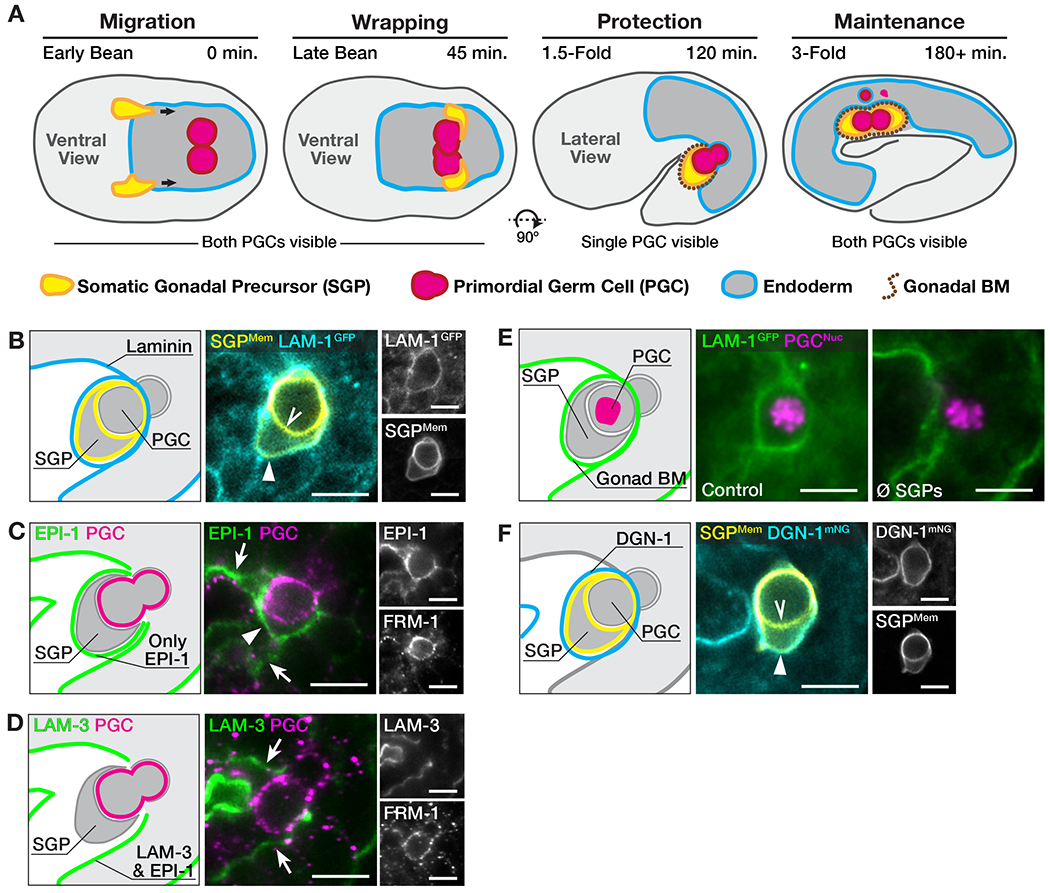
(A) Stages of gonad primordium formation. The embryo rotates onto its side between late-bean and 1.5-fold stages. (B) Localization of LAM-1GFP to outward-facing (arrowhead) but not inward-facing (chevron) SGP surfaces. (C-D) Localization of EPI-1 and LAM-3. Arrowhead, gonadal BM; arrow, non-gonadal BM. FRM-1 marks membranes. (E) LAM-1GFP in the gonad primordium of control and SGP-ablated (∅ SGPs) embryos. (F) DGN-1mNG localization to the outward-facing (arrowhead) but not inward-facing (chevron) surfaces of the SGPs. Scale bars, 5μm. See Figure S1.
Basement Membrane has Distinct Roles in Initiating and Maintaining SGP Wrapping
To determine the role of the gonadal BM, we examined SGPs and PGCs in laminin-depleted late-stage embryos. Depleting lam-1 or lam-2 (the sole laminin γ) blocks laminin trimer assembly, disrupts BM formation (Figure S2A), and causes late embryonic arrest [5, 7, 10]. In most lam-1(RNAi) and lam-2(RNAi) embryos, one or both SGPs were not wrapped around a PGC (Figures 2A and 2B). epi-1(RNAi) embryos showed nearly identical phenotypes, whereas lam-3 mutant embryos had no defects in SGP wrapping (Figures 2A, 2B, S2B and S2C). The distinct phenotypes of epi-1(RNAi) and lam-3 mutant embryos suggest that loss of gonadal BM, which contains EPI-1 but not LAM-3, underlies the SGP wrapping defects of laminin-depleted embryos.
Figure 2. Basement membrane maintains SGP wrapping and GLP-1-regulated PGC quiescence.
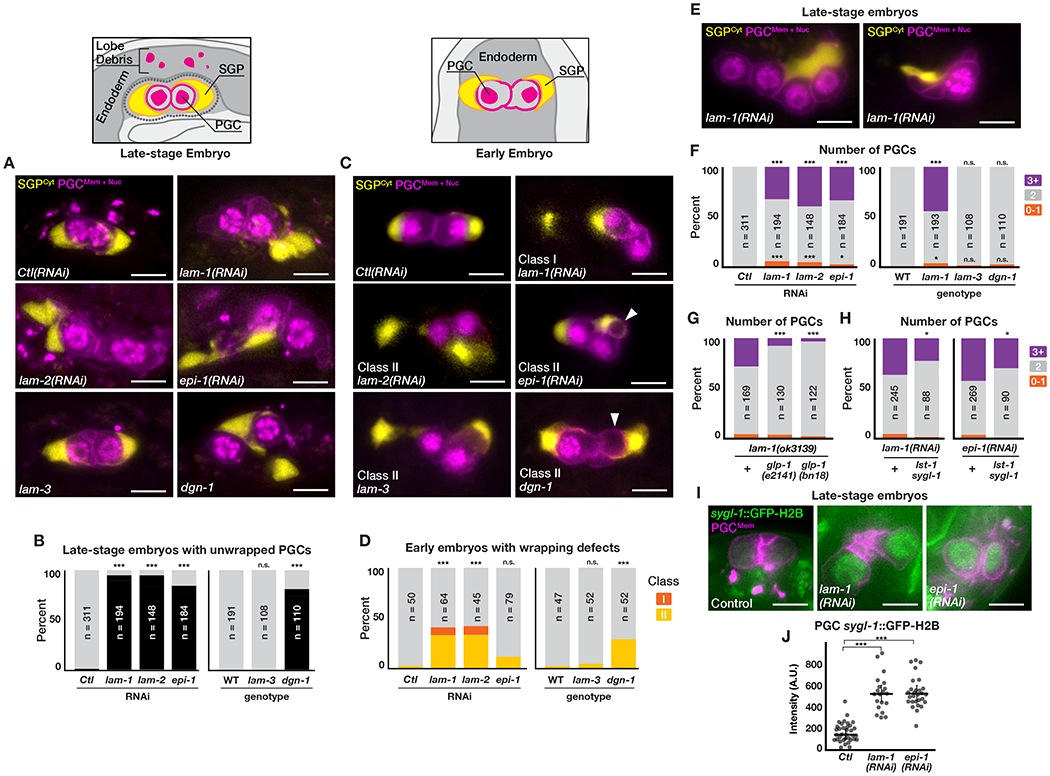
(A) SGP wrapping of PGCs in late-stage embryos. (B) Quantification of SGP wrapping defects in late-stage embryos. (C) SGP wrapping of PGCs in early embryos; arrowheads, SGPs wrapping PGC lobes. (D) Quantification of SGP wrapping defects in early embryos; see Results for phenotype Classes. (E) Extra (left) or reduced (right) PGC number in lam-1(RNAi) embryos. (F) Quantification of PGC number in late-stage embryos. (G) Suppression of extra PGCs in lam-1 mutants by glp-1. (H) Partial suppression of extra PGCs in lam-1(RNAi) and epi-1(RNAi) embryos by lst-1 sygl-1. (I) sygl-1::GFP-H2B reporter in PGCs of the indicated genotype. (J) Quantification of sygl-l::GFP-H2B expression in PGCs. Bar is median and error bars are 95% C.I. (B,D,F–H) Fisher’s exact test; (J) two-tailed t-test; ***p≤0.001, *p≤0.05, n.s. not significant. Scale bars, 5 μm. See Figures S2 and S3.
We established when SGP wrapping defects arose by capturing movies of lam-1(RNAi) and lam-2(RNAi) early embryos as the gonad primordium assembled. Two classes of defects were apparent (Figures 2C and 2D). In Class I embryos, one SGP failed to wrap its PGC (8%). In Class II embryos, an SGP that initially failed to wrap the PGC cell body recovered by a later stage (33%). Because gonadal BM formation requires SGP wrapping, we infer that these wrapping initiation defects result from loss of non-gonadal BM. Additionally, since a considerably larger fraction of late-stage laminin-depleted embryos showed wrapping defects (95%, Figure 2B), we conclude that laminin has roles in both initiating and maintaining SGP wrapping. We propose that non-gonadal BM is required for migratory SGPs to transition to a wrapped state [11], and that gonadal BM, after being assembled by SGPs, ensures that SGP wrapping is maintained. The latter conclusion is supported by the finding that epi-1(RNAi) embryos have only minor defects in wrapping initiation (Figure 2D) but defects as severe as those of lam-1(RNAi) and lam-2(RNAi) embryos in wrapping maintenance (Figure 2B).
The wrapping maintenance defects of laminin-depleted embryos suggest that SGPs utilize a BM receptor to prevent their wrapping membranes from retracting. In larvae, the laminin receptor DGN-1/dystroglycan is enriched in gonadal BM and is required for gonad organization [6], suggesting that DGN-1 could anchor SGP membranes to the gonadal BM. We detected endogenously tagged DGN-1 (DGN-1mNG) [12] at outward-facing SGP surfaces soon after SGP wrapping was complete (Figures 1F and S1C). Most SGPs in dgn-1 mutant late-stage embryos failed to maintain wrapping (Figures 2A and 2C), even though dgn-1 is not required to form the gonadal BM [6] and comparatively fewer dgn-1 mutant embryos showed wrapping initiation defects (Figure 2D). These findings suggest that DGN-1 is the major BM receptor responsible for maintaining SGP wrapping and also plays a role in wrapping initiation.
Basement Membrane Ensures PGC Quiescence by Inhibiting GLP-1/Notch Signaling
Wild-type embryos always contain two PGCs (Figure 2F), which remain quiescent until the embryo hatches in the presence of food [13]. Unexpectedly, we observed 3-4 PGCs in many lam-1(RNAi) and lam-2(RNAi) embryos, and confirmed these RNAi results using lam-1(ok3139) mutants (Figures 2E and 2F). In movies of lam-1(RNAi) embryos, extra PGCs always resulted from a single extra division of one or both PGCs (Figure S3A, 8/8 embryos). epi-1(RNAi) embryos but not lam-3 mutant embryos also contained extra PGCs (Figure 2F), suggesting that gonadal BM is required for PGCs to remain quiescent before embryos hatch and begin feeding. dgn-1 mutant embryos never contained extra PGCs (Figure 2F), despite the requirement for dgn-1 in maintaining SGP wrapping, indicating that gonadal BM maintains SGP wrapping and inhibits PGC proliferation through distinct mechanisms.
Loss of daf-18/pten causes PGCs in newly hatched larvae to divide even in the absence of food, and this phenotype is suppressed by mutations in its downstream effector akt-1 [14]. However, lam-1(RNAi) and lam-1(RNAi) akt-1 embryos had equivalent numbers of extra PGCs (Figure S3B), suggesting that the daf-18 pathway is not responsible for extra PGCs in laminin-depleted embryos. In larvae and adults, GLP-1/Notch is required to maintain germline stem cell identity and number [13], although GLP-1 is not known to regulate embryonic PGCs. We tested whether GLP-1 was required for extra PGCs in laminin-depleted embryos using two temperature-sensitive glp-1 mutations. Both alleles significantly suppressed the extra PGC phenotype of lam-1 mutant embryos (Figure 2G). In larvae, GLP-1 promotes germline stem cell maintenance by activating its redundant transcriptional targets sygl-1 and lst-1 [15]. A sygl-1 transcriptional reporter [15], which is not normally expressed in embryonic PGCs (Figure 2I), was significantly upregulated in lam-1(RNAi) and epi-1(RNAi) embryos (Figures 2I and 2J), and sygl-1 lst-1 double mutants partially suppressed the PGC proliferation phenotype of lam-1(RNAi) and epi-1(RNAi) embryos (Figure 2H). Together, these findings suggest that loss of gonadal BM inappropriately activates GLP-1/Notch signaling in PGCs, causing loss of PGC quiescence. Because we did not observe extra PGCs in wild-type or laminin-depleted embryos with ablated SGPs (Table 1), it is likely that activation of GLP-1 in laminin-depleted embryos requires signaling from the SGPs, which are normally in contact with the gonadal BM.
Table 1.
PGC loss after SGP ablation
| Genotype | Perturbation or class | Number of PGCs |
n | p valueb | |||
|---|---|---|---|---|---|---|---|
| Two PGCs | One PGC | No PGCs | % Death | ||||
| wild type | None | 132 | 0 | 0 | 0% | 132 | |
| wild type | Control ablation | 15 | 0 | 0 | 0% | 15 | 1 |
| wild type | Double SGP ablation | 16 | 16 | 8 | 40% | 40 | <0.0001 |
| wild type | Single SGP ablation | 10 | 5 | 0 | 17 % | 15 | <0.0001 |
| ced-3 | Double SGP ablation | 7 | 6 | 2 | 33 % | 15 | 0.762 |
| ced-10 | Double SGP ablation | 12 | 1 | 0 | 4% | 13 | 0.0011 |
| lst-4 | Double SGP ablation | 17 | 1 | 1 | 8% | 19 | 0.0005 |
| ehn-3 + Ctl(RNAi) | Two SGPs present | 76 | 0 | 0 | 0% | 76 | |
| ehn-3 + hnd-1(RNAi) | Two SGPs present | 57 | 2 | 0 | 2% | 59 | 0.189 |
| ehn-3 + hnd-l(RNAi) | One SGP present | 62 | 29 | 1 | 17% | 92 | <0.0001 |
| ehn-3 + hnd-l(RNAi) | No SGPs present | 23 | 6 | 0 | 10% | 29 | 0.0003 |
| end-1 end-3 rescueda | Double SGP ablation | 5 | 7 | 6 | 53 % | 18 | |
| end-1 end-3 | Double SGP ablation | 17 | 1 | 0 | 3 % | 18 | 0.0001 |
| Ctl(RNAi) | Double SGP ablation | 5 | 6 | 4 | 47% | 15 | |
| lam-1(RNAi) | Double SGP ablation | 6 | 4 | 2 | 34% | 12 | 0.452 |
| lam-2(RNAi) | Double SGP ablation | 3 | 8 | 1 | 42% | 12 | 0.696 |
end-1(ok558) end-3(ok1448) rescued with irEx568 [end-1(+), end-3(+), sur-5::dsRed]
p values were calculated using Fisher’s Exact Test, comparing between samples the fraction of embryos with fewer than two PGCs. Ablations performed in a wild-type background were compared with unperturbed WT embryos. Ablations in ced-3, ced-10 and lst-4 embryos were compared with ablated wild-type embryos, ehn-3 + hnd-1(RNAi) embryos were compared with ehn-3 + Ctl(RNAi) embryos. Ablated end-1 end-3 embryos were compared with rescued ablated end-1 end-3 embryos. Ablated lam-1(RNAi) and lam-2(RNAi) embryos were compared with ablated Ctl(RNAi) embryos.
SGPs Promote PGC Survival by Preventing Endodermal Cell Cannibalization
Surprisingly, a small number of laminin-depleted embryos showed the opposite phenotype - too few PGCs (Figures 2E and 2F). Movies of lam-1(RNAi) embryos showed that loss of PGCs resulted from PGC death (9/9 embryos, Figure 3A). Dying PGCs transitioned through two distinct stages. Initially, both membrane and nuclear mCherry markers concentrated within the cytoplasm (Figure 3A, 4:00). Subsequently, the dying PGC condensed into compact debris (Figure 3A, 4:50). In contrast to apoptotic corpses, which are refractile when viewed by DIC, dying PGCs did not appear distinct (Figure S4A). PGC death in lam-1(RNAi) embryos always correlated with a complete failure of SGP wrapping (Class I phenotype, Figure 2D) when the gonad first forms (9/9 embryos), suggesting that PGC death is normally prevented by SGP wrapping. We conclude that laminin promotes PGC survival, independently of its later role in maintaining gonad integrity, by enabling SGP wrapping when the gonad primordium assembles.
Figure 3. Unwrapped PGCs are cannibalized by endodermal cells.

(A) Time-lapse sequence showing PGC death in a lam-1(RNAi) embryo (PGC before death, chevron; degrading PGC, arrowheads). Times are hours:min relative to gonad primordium formation. (B) Relative positions of SGPs, PGCs and endodermal cells in control and SGP-ablated (∅ SGPs) late-stage embryos. Arrowheads, engulfed (middle panel) or degrading (right panel) PGC cell body. (C) PGC position at the time of gonad assembly in control and SGP-ablated embryos. Dashed line, midline. (D) Analysis of variance in PGC position in control and SGP-ablated embryos, compared using an F-test for equality of variance (***p≤0.001; control, n=26; SGP-ablated, n=35). Mean(bar) and S.D. are indicated. (E) end-1 end-3 SGP-ablated embryo showing surviving PGC cell bodies (arrowheads) and persistent lobes. (F-G) PGC cell body (arrowhead, identified by DIC) inside of endodermal cells in ced-10 and lst-4 mutant SGP-ablated embryos. Arrow, unengulfed lobes. Scale bars, 5 μm. See Figure S4.
To directly test whether SGPs are required for PGC survival, we laser-ablated the SGP precursors. Using DIC microscopy, Kimble and White [3] reported that PGCs were missing in SGP-ablated embryos but did not determine when or how they disappeared. We found that one or both PGCs were frequently absent when SGP-ablated embryos hatched (40% lost), in contrast to control-ablated embryos (Table 1 and Figure S4A). Approximately half as many PGCs (17%) were lost in embryos with one ablated SGP precursor, and the surviving PGC invariably contacted the un-ablated SGP (Table 1 and Figure S4A). We confirmed these findings using a genetic approach by examining ehn-3 hnd-1(RNAi) L1 larvae, which specifically lack SGPs [16] (Table 1). In contrast to apoptotic cell death, which requires ced-3/caspase [17], ced-3 was not required for PGC death in embryos lacking SGPs (Table 1). These findings strongly suggest that contact with an SGP prevents PGC death through a non-apoptotic mechanism. Depleting laminin did not enhance the PGC death seen when SGP precursors were ablated (Table 1), further supporting the conclusion that laminin prevents PGC death by ensuring that SGPs are able to find and wrap PGCs.
As the gonad primordium forms, PGCs form large lobes that are cannibalized and digested in late-stage embryos by endodermal cells [4]. We observed that compact debris from dead PGCs in SGP-ablated embryos was always present inside of endodermal cells (8/8 embryos), and in some embryos we observed a PGC cell body within an endodermal cell (4/16 embryos, Figure 3B), suggesting that SGP wrapping might prevent endodermal cells from cannibalizing the PGC cell body. Supporting this idea, in SGP-ablated embryos PGCs were often positioned abnormally (Figures 3C and 3D), and their cell body frequently contacted endodermal cells (Figures S4B and S4C). These defects were present at early stages when the gonad primordium normally forms.
To test if endodermal cells are necessary for PGC death, we examined endoderm-less end-1 end-3 mutants [18]. Most end-1 end-3 embryos contained two SGPs that contacted the PGCs, even though PGC position was often aberrant [19]. Remarkably, in end-1 end-3 mutants lacking SGPs, only 3% of PGCs died (Table 1 and Figure 3E), in contrast to control SGP-ablated embryos (rescued end-1 end-3 mutants), in which 53% of PGCs died (Table 1). These findings indicate that in the absence of SGPs, PGCs die when they are cannibalized by endodermal cells.
Endodermal cells assemble a scission complex of F-actin and dynamin to cut off PGC lobes, and scission complex formation requires CED-10/Rac and LST-4/SNX9 [4]. To determine whether a scission step is needed for endodermal cells to cannibalize the PGC cell body, we ablated SGP precursors in ced-10 and lst-4 mutant embryos, in which most PGC lobes persist [4]. PGCs rarely died in ced-10 and lst-4 mutants lacking SGPs (Table 1), and we detected PGC cell bodies surrounded by endodermal cells connected to external, unengulfed lobes (Figures 3F and 3G; ced-10, 5/15 embryos). We conclude that SGPs normally prevent endodermal cells from engulfing, severing, and digesting the PGC cell body.
DISCUSSION
Our findings show that formation of the C. elegans PGC niche is a multi-step developmental process involving initial wrapping by niche (SGP) cells, local assembly of basement membrane on niche cell membranes, and dystroglycan-mediated adhesion to gonadal BM that anchors niche cell wrapping membranes in place. PGCs may play an active role in promoting wrapping, as their descendants have been described to do in larvae [20]. Although the function of wrapping by niche cells is poorly understood, previous studies have suggested that wrapping regulates stem cell behavior by modulating the local signaling environment [2, 21, 22]. We showed that SGP wrapping influences PGC signaling by establishing a gonadal BM, which maintains PGC quiescence by inhibiting GLP-1/Notch activity. While it is not yet apparent how gonadal BM inhibits Notch signaling, BM could influence the availability of Notch ligands or regulators, similar to the role of collagen in locally enriching the Notch regulator BMP to promote Drosophila intestinal stem cell self-renewal [23]. These findings raise the intriguing possibility that changes in gonadal BM structure or composition after hatching might be needed to promote GLP-1/Notch signaling in larval germ cells. As Notch is required for the maintenance of many types of stem cells [eg. 24, 25], its regulation by niche BM might provide an underappreciated yet critical signaling input.
We uncovered an additional, unexpected function for niche cell wrapping – providing physical protection to stem cells from potentially lethal interactions with neighboring cells – in this case cannibalism of PGCs by endodermal cells. We propose that the initial wrapping of the PGC cell body by SGPs ensures that endodermal cells only have access to the PGC lobes, which they subsequently consume [4]. This role of SGP wrapping is most likely independent of the gonadal BM since SGPs are required to prevent inappropriate contact between the PGC cell body and endodermal cells at the earliest stages of gonad primordium formation, before the gonadal BM is apparent; because PGCs in laminin-depleted embryos never die if they are initially wrapped successfully by an SGP; and since a far-greater percentage of PGCs die in SGP-ablated embryos than in lam-1 mutant embryos. Collectively, our findings reveal the critical importance of niche cell wrapping in creating a BM that regulates germ cell quiescence, and in protecting germ cells from cellular interactions that can compromise their survival.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeremy Nance, Jeremy.Nance@med.nyu.edu. All materials described in this manuscript are either commercially available or available from the authors upon request. Raw data are available upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Worm Strains
Worms were cultured under standard conditions at room temperature [26] except for glp-1 temperature sensitive mutants, which were maintained at 20°C. To block glp-1 function in the gonad primordium, glp-1 (ts) embryos were shifted to 25.5°C after the 128-cell stage in order to bypass required GLP-1 functions during the first few cleavage cycles of embryogenesis. Strain N2 (Bristol) was used as wild type. Strains utilized in this study are listed in detail in the Key Resources Table. The akt-1(ok525) allele is a 1251bp deletion that removes the kinase domain and is predicted to be a null.
Key Resource Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti-EPI-1 | Wadsworth Lab [5] | N/A |
| Chicken anti-LAM-3 | Wadsworth Lab [5] | N/A |
| Mouse anti-FRM-1 | Cho Lab [36] | N/A |
| Alexa 647 donkey anti-chicken IgY | Jackson ImmunoResearch | Cat# 703-606-155 |
| Alexa 488 goat anti-mouse IgG | Thermo Fisher Scientific | Cat# A28175 |
| Bacterial and Virus Strains | ||
| E. coli RNAi feeding strain | Caenorhabditis Genetics Center | HT115 |
| E.coli OP50 | Caenorhabditis Genetics Center | OP50 |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Trioxsalen | Sigma | Cat# T6137 |
| 7-Amino-4-Methylcoumarin | Sigma | Cat# 257370 |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| ced-10(n1993); xnls360 [mex-5p::mCherry-PHPLC1∂1::nos-2 3’UTR, unc-119(+)]; zuls70 [end-1p::GFP-CAAX, unc-119(+)] | [4] | FT1214 |
| Ist-4(xn45); xnls360; zuls70; lin-2(e1309) | [4] | FT1468 |
| end1(ok558) end3(ok1448) xnls360; irEx568 [end-1(+), end-3(+), sur-5::RFP]; xnls525 [ehp-3p::YFP, unc-119(+)]; xnEx295 [end-1p::CFP-CAAX, unc-119(+)] | This study | FT1701 |
| xnls360; naSi2 [mex-5p::mCherry-H2B::nos-2 3’UTR]; xnls525; xnEx295 | This study | FT1703 |
| ehn-3(q689); xnls360; naSi2; xnls525 | This study | FT1718 |
| naSi2; qyls10 [lam-1p::lam-1-GFP, unc-119(+)] | This study | FT1936 |
| ced-3(n717); naSi2; glh-1(xn82 [glh-1p::mCardinal-PH::glh-1 3’utr]) | This study | FT1939 |
| dgn-1(qy18 [dgn-1 ::mNeonGreen]); naSi2 | This study | FT1975 |
| dgn-1(cg121); cgEx308 [dgn-1(+), dgn-1p::dgn-1-GFP, rol-6(su1006)]; xnls360; naSi2; xnls525; xnEx295 | This study | FT1983 |
| xnls510 [ehn3p::mCherry-PH, unc-119(+)]; qyls10 | This study | FT2014 |
| dgn-1(qy18 [dgn-1::mNeonGreen]); xnls510 | This study | FT2046 |
| lam-3(ok2030)/tmC18 [dpy-5(tmls1200)]; xnls360; naSi2; xnls525 | This study | FT2052 |
| akt-1(ok525); naSi2 | This study | FT2075 |
| lst-1(ok814) sygl-1(tm5040)/tmC27 [unc-75(tmls1239)];naSi2 | This study | FT2160 |
| lam-1(ok3139); glp-1(e2141ts); naSi2; zuEx288 [lam-1(+), SUR-5::GFP] | This study | FT2163 |
| lam-1(ok3139); naSi2; zuEx288 | This study | FT2169 |
| lam-1(ok3139); glp-1(bn18ts); naSi2; zuEx288 | This study | FT2176 |
| Oligonucleotides | ||
| lam-1(RNAi) For: 5’–GTGCCGACATTACTCATTACG–3’ | This study | N/A |
| lam-1(RNAi) Rev: 5’–CTCCGAGTCTTGGATCTC–3’ | This study | N/A |
| lam-2(RNAi) For: 5’–CCCAAGAATCAATGAACTCGAA–3’ | This study | N/A |
| lam-2(RNAi) Rev: 5’–CATCCATTGGCACTGAATCC–3’ | This study | N/A |
| hnd-1(RNAi) For: 5’–CTGGAAACAATGCGGTTTCT–3’ | This study | N/A |
| hnd-1(RNAi) Rev: 5’–CCGGAAACGGACTTTACAAT–3’ | This study | N/A |
| Recombinant DNA | ||
| epi-1 feeding RNAi clone | Ahringer RNAi library [30] | clone K08C7.3 |
| lam-1 feeding RNAi clone | This study | PDCM101 |
| lam-2 feeding RNAi clone | This study | PDCM102 |
| hnd-1 feeding RNAi clone | This study | pDCM103 |
| Expression vector with ehn-3p::mCherry-PHPLC1∂1 | This study | pYA12 |
| Expression vector with ehn-3p::YFP | This study | pDCM03 |
| Expression vector with end-1p::CFP-CAAX | This study | pJN585 |
| Software and Algorithms | ||
| FIJI v2.0 | FIJI | https://fiji.sc/ |
| Zeiss Zen software v2.0 | Carl Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Adobe Photoshop CC v19.0 | Adobe Systems | https://www.adobe.com/products/photoshop.html |
| Other | ||
METHOD DETAILS
Transgene construction
ehp-3p::mCherry-PHPLC1∂1 (pYA12)
PCR was used to construct Gateway (Invitrogen) entry vectors for the ehn-3 promoter up to the second exon (5’ entry clone; base pairs −2751 to +323 of ehn-3) and mCherry-PHPLC1∂1 (middle entry clone, amplified from end-1p::mCherry-PHPLC1∂1 [19]). Together with the tbb-2 3’ UTR 3’ entry clone [27], entry clones were recombined with destination vector pCFJ150 [28] using MultiSite Gateway (Invitrogen). The pCFJ150 destination vector contains C. briggsae unc-119(+) for use as a transformation marker.
ehp-3p::YFP (pDCM03)
The mCherry-PHPLC1∂1 in pYA12 was replaced by YFP amplified from pPD134.99 (Fire Lab C. elegans Vector Kit, Addgene kit #1000000001) using Gibson Assembly [29].
end-1p::CFP-CAAX (pJN585).
GFP in plasmid end-1p::GFP-CAAX [30] was replaced with CFP amplified from plasmid pPD134.96 (Fire Lab C. elegans Vector Kit, Addgene kit #1000000001) using Gibson Assembly[29].
Worm transformation
Plasmids were injected into unc-119(ed3) worms (end-1p::CFP-CAAX was injected together with C. elegans unc-119(+) plasmid pJN254 [31]) to obtain extrachromosomal arrays [32]. ehn-3p::mCherry-PHPLC1∂1 and ehn-3p::YFP extrachromosomal arrays were integrated using Trioxsalen (Sigma, T6137) and UV irradiation, as described [33].
RNAi
L4 larvae were fed E. coli bacterial strain HT115 expressing dsRNA from plasmid pPD129.36 (control) or derivatives containing sequences targeting specific genes. Worms were fed for two days at 23°C before progeny were collected for analysis. For all RNAi experiments, negative control embryos were from worms fed empty pPD129.36 vector. All results presented include pooled data from at least three independent trials. RNAi feeding plasmid targeting epi-1 was obtained from the Ahringer RNAi library (clone K08C7.3) [34]. All other RNAi feeding plasmids were constructed from PCR-amplified cDNA ligated into pPD129.36 vector using Gibson Assembly and the following primers:
lam-1: 5’–GTGCCGACATTACTCATTACG–3’, 5’–CTCCGAGTCTTGGATCTC–3’
lam-2: 5’–CCCAAGAATCAATGAACTCGAA-3’, 5’–CATCCATTGGCACTGAATCC–3’
hnd-1: 5’–CTGGAAACAATGCGGTTTCT-3’, 5’–CCGGAAACGGACTTTACAAT–3’
The effectiveness of laminin subunit RNAi was confirmed by assaying LAM-1GFP expression, which was absent in lam-1 (RNAi) embryos, and absent from gonadal basement membranes in lam-2(RNAi) and epi-1(RNAi) embryos (Figure S2). hnd-1 RNAi effectiveness was confirmed by the loss of SGP marker ehn-3p::YFP in the ehn-3(q689) genetic background (Table 1).
SGP laser ablation
Cells were ablated using a MicroPoint tunable laser (Oxford Instruments) with a 440 nm Coumarin dye cell installed on a Zeiss AxioImager and observed using a 100X 1.3NA objective lens. Embryos were mounted on a 3% agarose pad under a #1.5 coverslip. Nuclei in targeted cells were ablated with 10-15 laser pulses, until refractile debris was evident in the nucleus. Ablation of the SGP precursors was verified using DIC imaging or, in most experiments, by the failure of embryos to express the SGP-specific ehn-3p::YFP transgene. Ablated cells ceased dividing and were not engulfed by other cells during the course of the experiments. To eliminate both SGPs, the MSapp and MSppp blastomeres were targeted. Control unablated embryos were mounted on the same slides as experimental embryos and allowed to develop simultaneously. In control ablated embryos, the sisters of MSapp and MSppp were targeted (MSapa and MSppa). These embryos always contained two SGPs and two PGCs (15/15). In some cases, laser ablation caused damage to nearby control unablated embryos, which were mounted on the same slide. These experiments were discarded and repeated.
Immunostaining
Embryos were collected from rinsed gravid hermaphrodites that were incubated in water for 4 hours to allow eggs to age. Fixation and immunostaining were performed as described [35] using the following primary and secondary antibodies: chicken anti-EPI-1 (1:8) [5], chicken anti-LAM-3 (1:8) [5], mouse anti-FRM-1 (1:1000) [36], Alexa Fluor 647 donkey anti-chicken IgY (1:500, Jackson ImmunoResearch), and Alexa Fluor 488 goat anti-mouse IgG (1:1000, Invitrogen).
Microscopy
Images of fixed embryos and time-lapse movies were acquired on a Zeiss AxioImager.A2 equipped with an AxioCam 503 mono CCD camera, Uniblitz shutter, and 63X 1.4NA or 40X 1.3NA objective. Images of SGPs and PGCs in SGP ablation experiments at bean stage and 1.5-fold stage were acquired on a Leica SP5 II confocal microscope with a 63X 1.4NA objective. For live imaging, embryos were mounted on 3% agarose pads in Egg Salts buffer. L1 larva were immobilized by adding 1.5ul of 5mM levamisole to the coverslip before it was placed over the agarose pad. Three-fold embryos were immobilized using a custom-built gas exchange slide pressurized with nitrogen (3-5 PSI). The top of the chamber contains a #1.5 coverslip; embryos were placed on the inside of the coverslip, sandwiched under a 3% agarose pad. After ~10 minutes in nitrogen, embryos ceased moving, but resumed movement and completed development once the chamber was vented with room air. Unless otherwise specified, Z-stacks were spaced at 500nm. For time-lapse imaging, Z-stacks were acquired every 25 to 30 minutes as indicated. Image stacks were deconvolved using Zeiss Zen software, and processed in ImageJ and Adobe Photoshop.
QUANTIFICATION AND STATISTICAL ANALYSIS
Laminin intensity measurements
To measure LAM-1GFP intensity after SGP ablation, equal exposure times were used to capture images in control and SGP-ablated embryos, background fluorescence was subtracted using ImageJ, and maximum pixel intensity (averaged over a line 10 pixels in width) was measured across the gonadal BM on the side of the PGCs facing the endoderm and compared as a ratio with the same measurement across an unaffected reference BM (along the ventral side of the endoderm).
PGC nucleus position
To measure the position of PGC nuclei within the embryo, the following two measurements were made: 1) using ImageJ, the center of each nucleus was marked and its distance to the midline of the embryo was calculated by drawing the shortest possible line between that point and the midline; 2) using ImageJ, the center of each nucleus was marked and its distance to the anterior end of the embryo was calculated by drawing the shortest possible line between that point and a tangential line orthogonal to the anterior end of the eggshell. This distance was then normalized to the total length of the egg.
sygl-1::H2B-GFP intensity measurements
To measure H2B-GFP intensity in PGCs, equal exposure settings were used to capture images from embryos treated with each type of RNAi, total pixel intensity was measured in a 4μm diameter circle containing the PGC nucleus, and background fluorescence was subtracted by measuring GFP intensity in a region of the same size outside of the PGC.
Statistics and reproducibility
Data sets presented include at least three independent experiments and the minimum sample size relied upon was ten unless indicated otherwise. Power calculations were not used to determine sample sizes, and experiments were not randomized nor blinded before analysis. During data collection, damaged embryos were excluded from analysis. Statistical tests used are indicated in figure legends and table footnotes. In cases where multiple comparisons were made, p-values were corrected using the Bonferroni method. For t-test comparisons between two or more samples, data normality was assessed using the Shapiro-Wilk normality test.
DATA CODE AND AVAILABILITY
This study did not generate any datasets or code.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Sherwood for reagents, and Erika Bach, Jane Hubbard, and Prash Rangan for comments on the manuscript. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Funds were provided to J.N. by NYSTEM (C029561) and the NIH (R21HD084809 and R35GM118081), and to D.C.M. from the American Cancer Society Eastern Division (New York Cancer Research Fund Postdoctoral Fellowship, PF-16-098-01-DDC).
Footnotes
DECLARATION OF INTERESTS
The authors have no competing interests to declare.
REFERENCES
- 1.Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA, and Zon LI (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd DT, Knobel K, Affeldt K, Crittenden SL, and Kimble J (2014). A DTC niche plexus surrounds the germline stem cell pool in Caenorhabditis elegans. PLoS One 9, e88372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimble JE, and White JG (1981). On the control of germ cell development in Caenorhabditis elegans. Dev Biol 81, 208–219. [DOI] [PubMed] [Google Scholar]
- 4.Abdu Y, Maniscalco C, Heddleston JM, Chew TL, and Nance J (2016). Developmentally programmed germ cell remodelling by endodermal cell cannibalism. Nat Cell Biol 18, 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, and Wadsworth WG (2003). Laminin alpha subunits and their role in C. elegans development. Development 130, 3343–3358. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RP, Kang SH, and Kramer JM (2006). C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development 133, 1911–1921. [DOI] [PubMed] [Google Scholar]
- 7.Kao G, Huang CC, Hedgecock EM, Hall DH, and Wadsworth WG (2006). The role of the laminin beta subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev Biol 290, 211–219. [DOI] [PubMed] [Google Scholar]
- 8.Yurchenco PD (2015). Integrating Activities of Laminins that Drive Basement Membrane Assembly and Function. Curr Top Membr 76, 1–30. [DOI] [PubMed] [Google Scholar]
- 9.Ziel JW, Hagedorn EJ, Audhya A, and Sherwood DR (2009). UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol 11, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen JP, Reddy SS, and Priess JR (2012). Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development 139, 2050–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrschneider MR, and Nance J (2013). The union of somatic gonad precursors and primordial germ cells during Caenorhabditis elegans embryogenesis. Dev Biol 379, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naegeli KM, Hastie E, Garde A, Wang Z, Keeley DP, Gordon KL, Pani AM, Kelley LC, Morrissey MA, Chi Q, et al. (2017). Cell Invasion In Vivo via Rapid Exocytosis of a Transient Lysosome-Derived Membrane Domain. Dev Cell 43, 403–417 e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimble J, and Crittenden SL (2005). Germline proliferation and its control. WormBook, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuyama M, Rougvie AE, and Rothman JH (2006). C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol 16, 773–779. [DOI] [PubMed] [Google Scholar]
- 15.Kershner AM, Shin H, Hansen TJ, and Kimble J (2014). Discovery of two GLP-1/Notch target genes that account for the role of GLP-1/Notch signaling in stem cell maintenance. Proc Natl Acad Sci U S A 111, 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathies LD, Henderson ST, and Kimble J (2003). The C. elegans Hand gene controls embryogenesis and early gonadogenesis. Development 130, 2881–2892. [DOI] [PubMed] [Google Scholar]
- 17.Ellis HM, and Horvitz HR (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829. [DOI] [PubMed] [Google Scholar]
- 18.Owraghi M, Broitman-Maduro G, Luu T, Roberson H, and Maduro MF (2010). Roles of the Wnt effector POP-1/TCF in the C. elegans endomesoderm specification gene network. Dev Biol 340, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chihara D, and Nance J (2012). An E-cadherin-mediated hitchhiking mechanism for C. elegans germ cell internalization during gastrulation. Development 139, 2547–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon KL, Payne SG, Linden-High LM, Pani AM, Goldstein B, Hubbard EJA, and Sherwood DR (2019). Ectopic Germ Cells Can Induce Niche-like Enwrapment by Neighboring Body Wall Muscle. Curr Biol 29, 823–833 e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buszczak M, Inaba M, and Yamashita YM (2016). Signaling by Cellular Protrusions: Keeping the Conversation Private. Trends Cell Biol 26, 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Lewallen M, and Xie T (2013). Adhesion in the stem cell niche: biological roles and regulation. Development 140, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian A, and Jiang J (2014). Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. Elife 3, e01857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conboy IM, and Rando TA (2002). The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3, 397–409. [DOI] [PubMed] [Google Scholar]
- 25.Blanpain C, Lowry WE, Pasolli HA, and Fuchs E (2006). Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev 20, 3022–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merritt C, Rasoloson D, Ko D, and Seydoux G (2008). 3’ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol 18, 1476–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, and Jorgensen EM (2008). Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, and Smith HO (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6, 343–345. [DOI] [PubMed] [Google Scholar]
- 30.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, and Nance J (2011). The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol 21, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nance J, Munro EM, and Priess JR (2003). C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 130, 5339–5350. [DOI] [PubMed] [Google Scholar]
- 32.Mello CC, Kramer JM, Stinchcomb D, and Ambros V (1991). Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armenti ST, Lohmer LL, Sherwood DR, and Nance J (2014). Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 141, 4640–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. (2003). Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237. [DOI] [PubMed] [Google Scholar]
- 35.Anderson DC, Gill JS, Cinalli RM, and Nance J (2008). Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320, 1771–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi B, Kang J, Park YS, Lee J, and Cho NJ (2011). A possible role for FRM-1, a C. elegans FERM family protein, in embryonic development. Mol Cells 31, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


