Abstract
Inflammasomes are a specialized group of intracellular sensors that are key components of the host innate immune system. Autoinflammatory diseases are disorders of the innate immune system that are characterized by recurrent inflammation and serious complications. Dysregulation of the inflammasome is associated with the onset and progression of several autoinflammatory and autoimmune diseases, including cryopyrin-associated periodic fever syndrome, familial Mediterranean fever, rheumatoid arthritis, and systemic lupus erythematosus. In this review, we discuss the involvement of various inflammasome components in the regulation of autoinflammatory disorders and describe the manifestations of these autoinflammatory diseases caused by inflammasome activation.
Keywords: Inflammasome, inflammation, autoinflammatory syndrome, intracellular sensors
Summary Sentence
Review of how genetic alterations in inflammasome components instigate autoinflammatory disorders and the implications of inflammasome activation to the development and manifestations of autoinflammatory diseases.
1. INTRODUCTION
Inflammation is a protective response by the host to ensure the removal of detrimental stimuli and the development of a healing process to repair damaged tissue.[1] The innate immune system is the first line of defense against microbial and cellular insults. Germline-encoded pattern recognition receptors (PRRs) sense microbe-specific molecular signatures known as pathogen-associated molecular patterns (PAMPs) [2] and self-derived molecules from damaged cells known as damage-associated molecular patterns (DAMPs).[3, 4] These PRRs include members of the toll-like receptor,[5–7] nucleotide-binding oligomerization domain (NOD)-like receptor (NLR),[8, 9] retinoic acid-inducible gene-I–like receptor,[10] C-type lectin receptor,[11] and absent in melanoma 2 (AIM2)-like receptor families.[12, 13]
NLRs constitute a large family of intracellular PRRs, several of which are well characterized, such as NOD1, NOD2, and NLRP3 (Fig. 1A).[9, 14, 15] These NLRs regulate inflammation by inducing cytokines, chemokines, and antimicrobial genes. Upon activation, some NLRs form multiprotein complexes termed inflammasomes, whereas others mediate caspase-independent nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase signaling.[16] Inflammasome activation results in the oligomerization and activation of inflammatory caspases. Caspase-1 is the quintessential inflammatory caspase and is the primary enzyme responsible for the cleavage and activation of interleukin (IL)-1β and IL-18.[17] Upon activation, the inflammasome also promotes an inflammatory form of cell death termed pyroptosis, a process regulated by the formation of pores in the plasma membrane by the N-terminal domain of gasdermin D (GSDMD) (Fig. 1B).[18, 19] The current literature indicates that 5 PRRs assemble the inflammasome complex after sensing their respective stimuli: NLRP1, NLRP3, NLRC4, AIM2, and pyrin.[20, 21] Emerging evidence indicates that several other members of the NLR and PYHIN families, including NLRP2, NLRP6, NLRP7, NLRP12, and interferon (IFN)-γ–inducible protein 16 (IFI16), can also form inflammasomes, but there is little information available about their composition.[21]
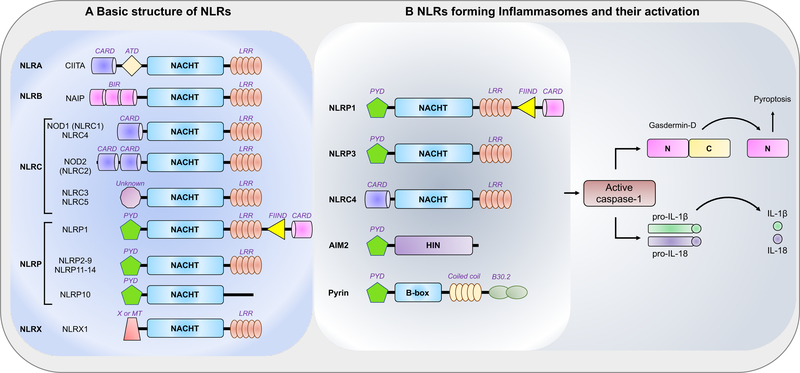
Figure 1. Schematic representation of the basic structure of individual NLRs and their activation.
(A) Schematic representation of the domains of individual human NLRs based on the human NLRs. Human NLRs are sub-classified into 5 categories: NLRA, NLRB, NLRC, NLRP, and NLRX. All 22 human NLRs contain a central NACHT domain, and all except NLRP10 contain a C-terminal ligand-sensing LRR domain. The N-terminal domains ascribe functional properties to the NLRs; however, the functions of the N-terminal domain of NLRC3 and NLRC5 and the C-terminal FIIND domain in NLRP1 are still unclear. The MT domain targets NLRX1 to the mitochondria, but no sequence homology with the traditional mitochondrial targeting sequence has been reported.
(B) Among the NLRs, NLRP1, NLRP3, and NLRC4 participate in the formation of the inflammasome platform. AIM2 and pyrin, which do not belong to the NLR family, have also been shown to form part of a specific inflammasome. Inflammasome activation leads to caspase-1 activation that in turn cleaves its downstream effectors, the newly identified pyroptosis executioner gasdermin D and the pro-forms of the cytokines IL-1β and IL-18.
Because of its role in inflammation, inflammasome activity needs to be tightly regulated at multiple levels. Priming or “signal 1”, typically delivered via NF-κB activation by toll-like receptor ligands, stimulates NLRP3 and IL-1β transcription and prepares the cell for a vigorous response upon activation.[9] Regulation of the NLRP3 inflammasome by non-transcriptional priming and deubiquitination has also been reported to be an important intermediate step for priming.[22] Subcellular compartmentalization contributes to inflammasome regulation, and anti-inflammatory signals may block inflammasome assembly by preventing the co-localization of inflammasome components.[23] Other adaptor and signaling molecules, such as caspase-8, also participate in inflammasome regulation.[21] The ultimate assembly and activation of the inflammasome requires “signal 2” in the form of ligands specific for different inflammasome scaffolds or cellular metabolic changes. These can include signals as diverse as crystalline materials, oxidative stress, potassium efflux, and nucleic acids.[24–27]
The innate immune response is programmed for immediate action, often resulting in overwhelming and self-perpetuating inflammation that can be detrimental to the host. The term “autoinflammatory disease” was coined to distinguish a set of inflammatory autosomal dominant diseases from self‐directed autoimmune diseases.[28] Autoinflammatory syndromes are characterized by recurrent attacks of systemic inflammation with fevers and are distinguished from autoimmune diseases by the absence of autoantibody and autoantigen-specific T and B cells.[29, 30] Chronic inflammation presents a major threat for the development and progression of autoinflammatory diseases, and inflammasome activation is pivotal to instigate inflammatory responses in macrophages. The inflammasomes are key for resisting microbial infections; yet, mutations in inflammasome sensor-encoding genes are involved in several hereditary autoinflammatory syndromes.[30] In this review, we discuss the contribution of inflammasomes in inflammatory/autoimmune disorders. Insight into the mechanisms and functions of inflammasome activation may lead to the development of novel therapeutics for the treatment of autoinflammatory disorders.
2. INFLAMMASOME-MEDIATED AUTOINFLAMMATORY DISEASES
2.1. CRYOPYRIN-ASSOCIATED PERIODIC SYNDROMES (CAPS)
Since the initial association of NLRP3 with a rare autosomal dominant fever disorder known as familial cold autoinflammatory syndrome (FCAS) [31], 2 phenotypically similar diseases, Muckle-Wells syndrome and neonatal-onset multisystem inflammatory disease (NOMID), also known as chronic infantile neurological cutaneous and articular syndrome (CINCA) [32, 33] containing NLRP3 mutations have been identified. These 3 phenotypically distinct disorders caused by mutations in the NLRP3 gene are referred to as cryopyrinopathies or CAPS.[34] Patients with CAPS experience recurrent episodes of fever with localized neutrophilic inflammation in multiple tissues, a hive-like rash, joint pain and swelling, red eyes, and headaches. The spectrum of CAPS ranges from the relatively mild FCAS to the intermediate Muckle-Wells syndrome and to the severe NOMID (Table 1). [35] CAPS-associated NLRP3 mutations are inherited in an autosomal dominant manner or are secondary to de novo mutations.[36] These mutations result in a gain of function with constitutive activation of the NLRP3 inflammasome and a resultant excess in IL-1β production.[37] Monocytes from patients with CAPS show enhanced inflammasome activation compared with the cells from normal human controls, both in the presence or absence of a low-dose stimulation or mild hypothermia.[38–40] Results of several studies in mouse models suggest that inflammasome function increases in CAPS, with increase in caspase-1 activity, release of IL-1β, and various forms of cell death observed.[41–43] Remarkably, all NOMID-associated inflammatory symptoms are prevented upon ablation of GSDMD [44], suggesting that GSDMD-dependent actions are required for the pathogenesis of NOMID in mice.
Table 1.
Clinical features and pathophysiology of monogenic autoinflammatory diseases.
| Disease | Responsible gene, protein |
Inheritance pattern |
Clinical features | Treatment |
|---|---|---|---|---|
| CAPS[38, 53] | NLRP3, NLRP3 (cryopyrin) | Autosomal dominant | Fever, rigors, urticaria-like rash, arthralgia/arthritis, headache, conjunctivitis FCAS: episodes lasting < 24 h; cold-induced Muckle-Wells syndrome: episodes lasting 1–2 d; progressive neurosensory hearing loss NOMID: chronic daily symptoms; bony overgrowth of knees, chronic aseptic meningitis, papilledema, uveitis, developmental delay, seizures |
IL-1 blockade FCAS: anakinra, canakinumab, rilonacept Muckle-Wells syndrome: anakinra, canakinumab, rilonacept NOMID: anakinra, canakinumab |
| FMF[64, 72] | MEFV, PYRIN | Autosomal recessive | Episodes lasting 1–3 d, abdominal pain, chest pain, arthralgia/arthritis, myalgia | Colchicine, anakinra, canakinumab |
| NLRC4-associated disorders[66, 109] | NLRC4, NLRC4 | Autosomal dominant | Variable; recurrent fever, diarrhea, vomiting, urticaria-like rash, arthritis; might have MAS-like symptoms | Anakinra, anti–IFN-γ mAb |
| PAPA[154, 155] | PSTPIP1, PSTPIP1 | Autosomal dominant | Episodes induced by mild trauma or infection; fever, arthralgia/arthritis, severe cystic acne, recurrent ulcers, sterile skin abscesses | Corticosteroids, etanercept, anakinra; acne might require oral tetracycline or isotretinoin |
| HIDS or MKD[156] | MVK, mevalonate kinase | Autosomal recessive | Episodes lasting 7–10 d; fever, arthralgia, cervical lymphadenopathy, abdominal pain, maculopapular rash | NSAIDs, etanercept, anakinra |
| TRAPS[157] | TNFSFR1A, TNFSFR1A | Autosomal dominant | Episodes lasting days to weeks; fever, periorbital edema, arthritis/myalgia, abdominal pain, centrifugal rash | Corticosteroids, anakinra, canakinumab, etanercept, NSAIDs |
| DIRA[158, 159] | IL1RN, IL-1RA | Autosomal recessive | Chronic symptoms noted in neonatal period; failure to thrive, irritability, pustular rash, hepatosplenomegaly, oral ulcers, joint swelling, arthritis | Anakinra |
Many mutations described in the NLRP3 gene are located in the nucleotide-binding domain (NBD) (Fig. 2A); yet, few mutations have been characterized in animal models, and several discordant effects in human and mouse models were found.[45–47] Some of the variants represent somatic gain-of-function mutants of NLRP3 restricted to myeloid cells.[48, 49] The development of knock-in mice with CAPS-associated mutations in the mouse homolog of NLRP3 have helped to investigate the pathophysiological mechanisms of these mutations. These models reproduce the autoinflammatory phenotypes found in human patients. However, the tissue-specific models showed increased mortality dependent on cellular location, especially in myeloid cells, which does not occur in humans.[38, 50] Knock-in mice globally expressing the D301N NLRP3 mutation, an orthologue of the D303N NLRP3 mutation found in human patients with NOMID, present with neutrophilia in the blood, inflammatory cell recruitment in knee joints, and high levels of inflammatory mediators in the serum with specific bone-remodeling abnormalities and osteolysis.[51] These features were also observed in myeloid cell-specific D301N expression, demonstrating that this is a gain-of-function mutation within osteoclasts and that it is associated with the degradation of poly (ADP-ribose) polymerase 1, an inhibitor of osteoclastogenesis.[51, 52]
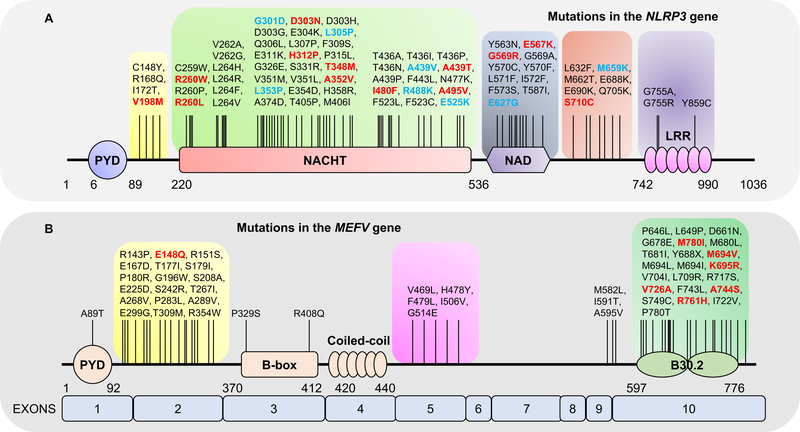
Figure 2. Schematic outline of mutations in the domains of NLRP3 and PYRIN.
(A) Schematic representation of residue substitutions in the NLRP3 protein and reported point mutations. Mutations reported to be involved in Muckle-Wells syndrome and familial cold autoinflammatory syndrome (FCAS) are highlighted in red and blue, respectively.
(B) The domain organization of human PYRIN, indicating the four major domains and residue substitutions. Mutations reported to be involved in familial Mediterranean fever (FMF) are highlighted in red.
Recent data suggest there is a role for IL-18 in CAPS pathogenesis. Similar to IL-1β, IL-18 is also cleaved by caspase-1 and is often secreted at higher levels in CAPS-affected human monocytes and mouse hematopoietic cells.[53] Deletion of IL-18R, IL-1R, or IL-1β on a CAPS mutation background in mice has demonstrated that IL-18 signaling is vital to the initial development of inflammation in murine CAPS, whereas IL-1β leads to later systemic inflammation [53]. Interestingly, when CAPS-mutant mice were bred onto an Il1r–/–Il18–/– background, the mice exhibited reduced inflammatory activity, although the inflammatory activity was still significant.[53] Based on the known role of NLRP3 mutations in these inflammatory activities, it can be speculated that murine CAPS is mediated by NLRP3 inflammasome-dependent factors but independent of the biological activity of IL-1β and IL-18.
Treatment of CAPS with non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs has not provided any significant benefits for patients.[54, 55] However, dramatic improvements have resulted from using IL-1–targeted treatments, including anakinra, a recombinant human IL-1 receptor antagonist (IL-1RA); rilonacept, a recombinant fusion protein consisting of portions of the IL-1 receptor and IgG1 Fc region; and canakinumab, a humanized anti–IL-1β monoclonal antibody.[54, 55] Unlike anakinra and rilonacept, canakinumab has a long half-life of 28–30 days, requires administration once every 8 weeks, and is associated with minimal injection-site reactions but some increased risk of infections. Use of canakinumab in CAPS resulted in prompt and persistent improvement in the clinical and laboratory parameters of inflammation.[54, 56, 57]
2.2. FAMILIAL MEDITERRANEAN FEVER (FMF)
FMF is the most common autosomal recessive, autoinflammatory disorder; it is highly prevalent in individuals from the eastern Mediterranean region.[58, 59] It is characterized by episodic fever and neutrophil-mediated inflammation of serosal tissues (Table 1). MEFV has been described as the gene responsible for FMF.[60, 61] MEFV encodes for pyrin, a protein of 781 amino acids with an N-terminal pyrin domain, a central boxed-box and coiled-coil domain, and a C-terminal PRY/SPRY (B30.2) domain.[62] Pyrin is primarily expressed in myeloid cells including neutrophils, eosinophils, monocytes, dendritic cells, and synovial fibroblasts and forms an inflammasome complex in response to disturbances in cytoplasmic homeostasis.[63, 64]
More than 300 MEFV gene variants have been described (Fig. 2B) [65], but only 14 occur frequently in FMF (E148Q, E167D, T267I, P369S, F479L, I591T, M680I, I692del, M694I, M694V, K695R, V726A, A744S, and R761H). Interestingly, in contrast to the gain-of-function mutations in NLRP3, NLRC4, or NLRP1 observed in CAPS and in NLRC4-and NLRP1-associated inflammasomopathies [31, 66–68], FMF-associated MEFV mutations do not lead to constitutive pyrin inflammasome activation and require a specific stimulus, such as low doses of Clostridium difficile toxin B.[69–71] Most pathogenic MEFV mutations in humans occur in exon 10, which encodes the B30.2 domain. Strikingly, this domain is absent in the murine ortholog.[64]
To study the effect of these mutations, a collection of FMF knock-in (FMF-KI) mouse strains were generated that express a chimeric pyrin protein harboring the human B30.2 domain with mutations observed in patients with FMF.[64] One such FMF-KI strain is MefvV726A/V726A, which expresses the murine pyrin spliced to the B30.2 domain with a valine-to-alanine substitution at amino acid position 726. These mice develop an autoinflammatory disorder characterized by systemic wasting and neutrophilia that is caused by GSDMD-mediated IL-1β release in response to aberrant pyrin inflammasome activation independent of IL-1α and caspase-8.[64, 72, 73] Recent study results highlight the central role of tumor necrosis factor (TNF)/TNF receptor (TNFR) signaling in regulating the pyrin inflammasome and the distinct features of pyrin inflammasomopathy.[74] Intriguingly, colchicine therapy is primarily effective as a prophylactic treatment for FMF attacks. It is recommended in all patients regardless of the frequency and intensity of attacks [75]; however, the mechanism of action is still unclear. Because colchicine has been shown to prevent TNF-induced toxicity in vivo,[76] it can be speculated that blocking chronic TNF signaling could be more effective in limiting inflammation in FMF.
In addition to the mutations that cause FMF, 2 distinct, dominant MEFV mutations that cause a different autoinflammatory syndrome termed pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND) have been identified (pS242R and pE244K).[77, 78] These mutations abolish or reduce protein kinase N-1/2–mediated phosphorylation of pyrin on serine 242 and impair the binding of 14–3-3 proteins, leading to constitutive activation of the pyrin inflammasome. Interestingly, PAAND-associated mutations do not map to the B30.2 domain, where most pathogenic FMF-associated mutations are found. Furthermore, FMF-associated MEFV mutations do not seem to affect pyrin phosphorylation levels or 14–3-3 binding. [70, 79, 80]
Although both autoinflammatory syndromes described above are caused by direct MEFV mutations, there are other monogenic syndromes that indirectly affect pyrin inflammasome activation. For example, PAPA syndrome is defined by symptoms of sterile, purulent arthritis; pyoderma gangrenosum; and cystic acne, as summarized by its acronym (Table 1) [54]. PAPA syndrome is a dominantly inherited disease associated with missense mutations in CD2-binding protein 1, more commonly described by its murine ortholog Pstpip1 which encodes the proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP-1).[81] PSTPIP-1 interacts with pyrin and promotes its ability to interact with ASC and to trigger its oligomerization. In PAPA syndrome, PSTPIP1 mutations decrease the ability of PSTPIP-1 to interact with proline-, glutamic acid-, serine-and threonine-rich phosphatase, leading to hyperphosphorylated PSTPIP-1 variants that have increased avidity for pyrin [82]. Although there is clear evidence of inflammasome deregulation in PAPA syndrome [83], the syndrome’s pathophysiology is complex and probably involves functions of PSTPIP-1 other than its regulation of the pyrin inflammasome.[84] These studies demonstrate that pyrin, although known for its association with FMF, can also modulate other autoinflammatory skin disorders.
2.3. RHEUMATOID ARTHRITIS (RA)
RA is a chronic inflammatory/autoimmune disease that carries a substantial burden for both the individual and society.[85] This disease is characterized by persistent synovial inflammation and causes multi-joint destruction and cartilage degradation with limited treatment options leading to poor patient outcomes.[86–88] Since RA is the inflammatory disease with the highest worldwide incidence of 0.5% to 1%,[89] the role of inflammasomes in RA has been widely studied.
The gene expression of NLRP3 inflammasome components, including apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), full-length NLRP3, short-length NLRP3, and caspase-1, is increased in peripheral blood mononuclear cells (PBMCs) of RA patients compared with those of healthy controls, and there is some evidence of an association between single-nucleotide polymorphisms (SNPs) at the NLRP3 locus and susceptibility for RA.[90, 91] Another study suggested that polymorphism in the NLRP1 gene is associated with susceptibility to RA in the Han Chinese population. Individuals with an NLRP1 polymorphism associated with RA risk had significantly higher NLRP1 mRNA levels than those with the non-risk genotype.[92] A subsequent study showed that inhibiting mRNA expression of P2X4, a purinergic receptor, suppresses joint inflammation and damage in collagen-induced arthritic (CIA) mice and affects synovial cells of patients with RA by suppressing the activation of the NLRP1 inflammasome and effectively reducing the serum level of IL-1β.[93] Collectively, these studies clearly indicate that inflammasomes and their polymorphisms are strongly associated with RA pathogenesis.
Recent studies also indicate that different methods of arthritis induction in animal models may have differential effects on immune cells and effector molecules. These differential effects could lead to different experimental outcomes.[94, 95] The CIA model showed that Nlrp3–/– and Caspase1–/– mice were susceptible to arthritis, whereas Asc–/– mice were protected.[94] However, arthritis induced by the K/BxN transgenic mice serum transfer model containing autoantibodies recognizing glucose-6-phosphate isomerase (GPI) [96] did not affect immune cells in Caspase1–/– mice.[95] Animal model studies focused on RA have shown that induction of chronic arthritis (characterized by minimal numbers of neutrophils) in Caspase1–/– mice led to reduced joint inflammation and less cartilage damage. Alternatively, in a model of acute (neutrophil-dominated) arthritis, Caspase1–/– mice developed joint inflammation similar to that in wild-type control mice.[95] These findings suggest that caspase-1 inhibitors may be of therapeutic value only in inflammatory conditions in which limited numbers of neutrophils are present.
Myeloid-cell-specific deletion of the RA susceptibility gene Tnfaip3/A20 in mice triggers a spontaneous erosive polyarthritis resembling RA in patients. This destructive polyarthritis was TNF independent but relies on the NLRP3 inflammasome and IL-1R signaling. Deletion of NLRP3 or caspase-1/11 or IL-1R significantly rescues arthritis in myeloid-cell-specific A20–/– mice [97], indicating that myeloid cell-specific A20–/– mice can be used as a suitable pre-clinical model to validate any experimental therapies for RA targeting inflammasomes and/or IL-1 signalling. Further studies demonstrated enhanced activity of the NLRP3 inflammasome in peripheral blood cells of patients with active RA.[98] These patients expressed higher basal intracellular levels of NLRP3, ASC, active caspase-1, and pro–IL-1β [98], suggesting that targeting NLRP3 or downstream caspases may be of benefit in suppressing IL-1β production in RA.
The efficacy of anakinra treatment in RA has been evaluated in several controlled studies.[99] Although no direct comparison is available between the efficacy of IL-1 blockade and the overwhelming number of competing biologic agents, including TNFα blockers, based upon indirect comparisons, anakinra seem to be modestly efficacious biologic therapy for RA.[100] Similar to anakinra, the anti-IL-1β monoclonal antibody canakinumab led to reduced disease severity in patients with RA, including those who were unresponsive to anti-TNFα therapies[101]; however, unlike anakinra, long-term preservation of joint function with canakinumab remains unstudied. Taken together, these data suggest that inflammasome activation and IL-1R signaling are risk factors for RA pathogenesis, and their selective inhibition reduces RA symptoms and progression.
3. OTHER INFLAMMASOME-RELATED AUTOINFLAMMATORY DISORDERS
3.1. AIM2 in autoinflammation
AIM2 had not been linked to inherited autoinflammatory diseases, and no gain‐of function mutations have been identified within the Aim2 gene. This could be because mutations in the oligomerization domains of NLRP3 and pyrin are associated with autoinflammatory disorders, whereas AIM2 does not possess known oligomerization domains, which may be surrogated by its multivalent ligand dsDNA.[102] Mutations in the receptors that diminish DNA binding may result in reduced oligomerization and signaling and a compromised immune response to microbial infections, whereas mutations that enhance DNA binding or destabilize the autoinhibited state may be linked to autoimmune disorders.[102]
Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates innate immune responses through production of the second messenger cGAMP, which activates the adaptor molecule STING. Aberrant activation of the cGAS pathway by self DNA can also lead to autoimmune and inflammatory disease. In a mouse model of polyarthritis‐like disease caused by DNase II deficiency, both the cGAS‐STING pathway and the AIM2 inflammasome pathway contribute to autoinflammation caused by the increased release of self DNA within the cytosol.[103, 104] The inability to degrade self DNA also contributes to the pathogenesis of autoimmune polyarthritis. Lacking the lysosomal endonuclease DNase II (DnaseII−/−) is embryonically lethal in mice due to the impaired ability of macrophages to degrade self DNA.[105] Genomic deletion of type I IFN receptor (IFNAR) rescues DnaseII−/− mice from embryonic lethality [106]; however, the mice lacking both IFNAR and DNase II (DnaseII−/−Ifnar−/−) eventually develop polyarthritis.[107] Intriguingly, genomic deletion of AIM2 prevents inflammasome activation, inflammatory cytokine production, macrophage infiltration in the joint, and arthritis development in DnaseII−/−Ifnar−/− mice.[103, 104] Genomic deletion of STING also protects DnaseII−/−Ifnar−/− mice from joint inflammation,[104] indicating that multiple DNA sensors might contribute to the inappropriate DNA recognition driving clinical manifestation. In psoriatic lesions, abundant cytosolic DNA in keratinocytes acts as a DAMP that can elicit the AIM2 inflammasome, leading to IL‐1β maturation and the autoinflammation signs observed in patients with psoriasis.[108] Overall, aberrant AIM2 activation by self DNA is a key driver of inflammatory and autoimmune diseases.
3.2. NLRC4 in autoinflammation
Gain-of-function mutations in the NLRC4 gene are associated with early-onset autoinflammation with enterocolitis or recurrent macrophage activation syndrome (MAS), depending on the mutation.[67, 109] Patients with such alterations are characterized by mutations in the NBD region of NLRC4 and benefit from recombinant human IL-18–binding protein therapy.[110] The autoinflammatory-associated NLRC4 mutation H443P can constitutively activate caspase-8 and induce apoptosis by interacting with the suppressor of Gal 1 component of the 26S proteasome and with ubiquitinated cellular proteins.[111] Additionally, a macrophage-intrinsic defect can drive the MAS phenotype in the absence of a primary cytotoxic defect, thus providing a new paradigm for the pathogenesis of MAS.[67]
Activating mutations in NLRC4 cause a new autoinflammatory disease presenting as a periodic fever syndrome with MAS;, distinct from CAPS and partially responsive to IL-1 inhibition.[67] The persistence of high serum IL-18 levels and macrophage hyper-responsiveness even after the initiation of IL-1–blocking treatment suggests a role for IL-18 in MAS and warrants an exploration of IL-18 as a therapeutic target. Physical manifestations in patients with NLRC4 mutations include rash and splenomegaly; laboratory findings include increased levels of inflammatory markers, including ferritin, increased levels of lactate dehydrogenase, and pancytopenia.[66] Leukocytes from some of these patients also displayed increased production of IL-1β, IL-18, or both, with increased caspase-1 cleavage and the appearance of ASC specks, consistent with inflammasome activation.[66, 67, 109] A mouse with a similar mutation observed in one of the patient families had dermatitis and arthritis that was worsened when exposed to cold. The mutation in NLRC4 increased its oligomerization and resulted in hyperactivation of caspase-1. Bone marrow-derived cells from these mice also produce increased levels of IL-1β and neutrophilic inflammation that is dependent on IL-17.[66] Thus, NLRC4 has finally provided a link between autoinflammation and the systemic inflammatory response syndrome (SIRS) via a hyperinflammatory SIRS subtype known as MAS.
Collectively, mutations in genes encoding inflammasome sensor proteins, such as NLRP1, NLRP3, NLRC4, or pyrin, contribute to the development and progression of a number of autoinflammatory disorders due to uncontrolled activation of caspase-1 and the aberrant release of inflammation-inducing cytokines (Fig. 3).
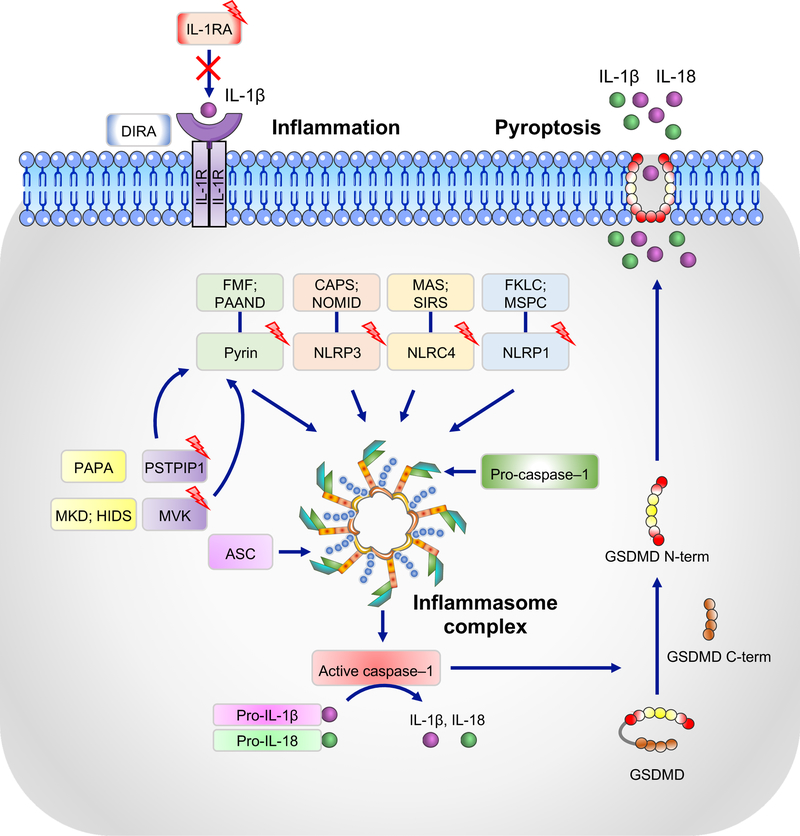
Figure 3. Inflammasomes and their association with autoinflammatory syndromes.
NLRP1, NLRP3, NLRC4, and pyrin form canonical inflammasomes with ASC and caspase-1 that process pro–IL-1β and pro–IL-18 into their active forms, IL-1β and IL-18. Boxes with lightening bolts denote proteins that have been reported to be mutated in the respective diseases. Gain-of-function mutations in these proteins lead to constitutively active inflammasomes. Diseases associated with corresponding mutations are: NLRP1, multiple self-healing palmoplantar carcinoma (MSPC) and familial keratosis lichenoides chronica (FKLC) [153]; NLRP3, cryopyrin-associated periodic syndrome (CAPS) and neonatal onset multisystem inflammatory disease (NOMID); NLRC4, macrophage activation syndrome (MAS) and systemic inflammatory response syndrome (SIRS); pyrin, familial Mediterranian fever (FMF) and pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND). Mutations in mevalonate kinase (MVK) and proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) associated with mevalonate kinase deficiency (MKD) and/or hyper IgD syndrome (HIDS) and purulent arthritis, pyoderma gangrenosum, and cystic acne (PAPA), respectively, affect the activity of the pyrin inflammasome through various mechanisms and lead to increased IL-1β secretion. Deficiency in the IL-1 receptor antagonist (IL-1RA), which is associated with deficiency of IL-1 receptor antagonist (DIRA), leads to unchecked IL-1 signaling. Activated caspase-1 can also cleave gasdermin D (GSDMD), and its released N-terminal domain (N-term) forms cytotoxic pores in the plasma membrane, thus inducing pyroptosis, an inflammatory form of cell death.
4. INFLAMMASOME-MEDIATED AUTOIMMUNE DISEASES
4.1. SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
SLE is a systemic autoimmune disorder characterized by increased type I IFN production and immune complex deposition within the tissues, leading to varied inflammatory responses and often devastating organ involvement.[112] Dysregulation of both the innate and adaptive immune responses is central to SLE development. Recent evidence from human and murine studies indicates that inflammasomes are central contributors to the development of lupus nephritis and the associated organ damage.[113]
The NLRP3 and AIM2 inflammasome have received a significant amount of attention as contributors to SLE in murine models and have been actively explored. Upregulation of inflammasome components in lupus nephritis biopsies has been reported,[114] suggesting a priming effect for inflammasome activation. Most studies focused on the upregulation of inflammasome components were conducted using human monocytes and associated with neutrophil extracellular trap (NET) formation. NETs are extracellular structures composed of chromatin, with specific proteins and enzymes from the neutrophilic granules critical for host defense [115] that play a key role in SLE development.[116] NETs can activate the NLRP3 inflammasome in macrophages derived from patients with SLE, resulting in release of active IL-1β and IL-18. This activation of the NLRP3 inflammasome utilizes P2X7 receptor-mediated potassium efflux.[117] Importantly, IL-18 can induce NETosis, a form of cell death, suggesting that inflammasome-activated IL-18 stimulates a perpetual cycle of NET formation and inflammasome activation.[117] This mechanism yields an intriguing possibility suggesting a feed-forward inflammatory loop that could potentially lead to disease flares and/or organ damage.
A hallmark of SLE is the production of autoantibodies that recognize nuclear antigens. Circulating double-stranded DNAs (dsDNAs) and anti-dsDNA antibodies are commonly found in the sera of patients with SLE.[118, 119] These immune complexes can activate the NLRP3 inflammasome, and SLE-derived macrophages are hyper-responsive to innate immune stimuli, leading to enhanced activation of the inflammasome and production of inflammatory cytokines.[120]
The results of murine lupus model studies further suggest a role for the inflammasome in lupus pathogenesis. The pristane-induced lupus mouse model pointed to an association between the hyperactivation of the NLRP3 inflammasome and severe lupus-like symptoms. In mice with the Nlrp3R258W gain-of-function mutation, a more severe lupus-like syndrome was developed, and these mice exhibited significantly higher mortality upon pristane challenge as compared with wild-type mice.[121] Interestingly, the phenotype observed in this mouse strain was largely due to the myeloid cell-specific Nlrp3R258W mutation, as depletion of this mutation from myeloid cells rescued mice from experimental lupus.[121] Inhibiting the P2X7 receptor, an activator of NLRP3 inflammasome assembly, also benefits mice with lupus nephritis.[122] Additionally, caspase-1 deficiency provided protection from autoantibody generation and lupus nephritis in the pristane-induced mouse model of lupus. Other investigations have reported that in a mild model of spontaneous lupus-like autoimmunity (C57BL/6-lpr/lpr mice), NLRP3 or ASC deficiency increased dendritic cell and macrophage activation, the expression of numerous proinflammatory mediators, necrosis of lymphocytes, and the expansion of most T-cell and B-cell subsets with no defect in plasma cells and autoantibody production. This unexpected immunosuppressive effect of NLRP3 and ASC could be attributed to their role in transforming growth factor (TGF)-β receptor signaling [123], as NLRP3 or ASC deficiency significantly suppressed the expression of numerous TGF-β target genes in C57BL/6-lpr/lpr mice.[123] Taken together, these studies demonstrate a critical role for NLRP3 in the development of SLE, although an inflammasome-independent function from NLRP3 or ASC likely accounts for the more severe disease; these findings suggest that modulating the inflammasome signal may help to control the inflammatory damage in SLE.
Overexpression of inflammasome scaffolds such as IFI16 and AIM2 in leukocytes from patients with SLE has been reported.[124] Immune complexes containing DNA or RNA antigens can prime the inflammasome for activation, subsequently triggering assembly of NLRP3 inflammasome complexes.[125, 126] The activated complement component, C3a, promotes adenosine triphosphate secretion, thus serving as a trigger for inflammasome activation.[127] The complement component C1q is a risk factor for SLE development,[128] and its deficiency suppresses NLRP3 expression in human macrophages.[129] Interestingly, C1q-mediated phagocytosis of apoptotic lymphocytes inhibited NLRP3 inflammasome activity,[129] suggesting that activation of NLRP3 inflammasomes due to lack of C1q plays a role in SLE pathogenesis. Anakinra treatment proved beneficial in a very small, non-placebo–controlled trial of patients with lupus and refractory arthritis.[130]
The inflammasome scaffold AIM2 has also been studied for its role in SLE pathogenesis. Repeated injections of apoptotic DNA in Balb/c mice results in a lupus-like syndrome, and the disease progression in this model correlates well with the elevated levels of AIM2 expression in different organs. Furthermore, these mice have a diminished lupus-like phenotype in the absence of AIM2.[131] Others, however, have demonstrated that inhibiting AIM2 may promote lupus pathogenesis. In mice, another IFN-inducible p200 family member, p202, is upregulated in many lupus-prone strains [132, 133] and negatively regulates AIM2 inflammasome activation, consequently decreasing cell death and promoting prolonged type I IFN production in response to cytosolic DNA.[134] Inhibiting AIM2 results in p202 upregulation.[135] Importantly, in T and B cells, type I IFN exposure resulted in AIM2 suppression and p202 upregulation.[136] This could lead to a repeating cycle of type I IFN production. Thus, inhibiting AIM2 may have both positive and negative effects on lupus pathogenesis, necessitating careful assessment of this strategy for disease management.
7. IL-1–RELATED BUT INFLAMMASOME-INDEPENDENT AUTOINFLAMMATORY DISORDERS
A spontaneous mutation in Shank-associated RH domain-interacting protein (Sharpin) causes chronic proliferative dermatitis (cpdm) in C57BL/6 mice.[137, 138] Sharpincpdm mice have a single base pair deletion in exon 1, resulting in early termination of the transcript and the complete absence of Sharpin protein mimicking Sharpin-deficient mice.[138] These mice are runted and develop spontaneous systemic inflammation with apparent inflammation of the skin as early as 3–4 weeks after birth.[138] Nlrp3, Caspase1, Caspase11, Il1r, and Il1b deficiency all delay the onset of dermatitis in Sharpincpdm mice.[139, 140] Sharpincpdm inflammasome component-deficient mice still develop a full spectrum of disease, suggesting that the IL-1 signaling pathway may play only a minor role in disease progression. Another recent study has determined important roles for RIPK-1, TNF, and myeloid differentiation primary response protein 88 (MyD88) signaling in the complete rescue of skin inflammation in Sharpincpdm mice.[141–143] Together, these studies show that Sharpin is a crucial regulator of both apoptotic and necroptotic cell death pathways in epidermal cells. Moreover, these findings have been further strengthened by the demonstration that Sharpincpdm mice lacking TNFR1 specifically in epidermal cells (SharpincpdmTnfr1fl/flK14Cre) are completely protected from disease.[144] Although mutations in Sharpin have not been identified in human patients so far, Sharpincpdm mice have been very helpful in understanding the basic biology of how several immune components are involved in the progression of an autoinflammatory skin disease resembling dermatitis.
A spontaneous mutation in the protein tyrosine phosphatase non-receptor type 6 (Ptpn6) gene encoding the Src homology region 2 domain-containing phosphatase 1 (SHP-1) protein causes skin lesions in mice that are heavily infiltrated with neutrophils.[145] These mice harbor a Y208N missense mutation in the Ptpn6 gene (Ptpn6spin), and their lesions closely resemble those of neutrophilic dermatoses in humans.[146] Gene analyses from patients with neutrophilic dermatoses have suggested that there are PTPN6 splice variants or heterozygous mutations in affected individuals.[147] Compared to SHP-1–deficient mice, Ptpn6spin mice have 70% less SHP-1 phosphatase activity and present with a more mild phenotype and no disease-associated mortality.[148] Most Ptpn6spin mice develop spontaneous footpad swelling at 8–16 weeks of age with signs of neutrophilia, intra-epidermal pustules, and cutaneous tissue damage, all of which are characteristics of neutrophilic dermatoses.[148, 149] Interestingly, Ptpn6spin disease symptoms can be completely rescued with Il1r and MyD88 deficiency.[148] Recent results from our group have shown that this disease progresses independent of inflammasome regulators. Crossing Ptpn6spin mice with Nlrp3–/–, Asc–/–, or Caspase1–/– mice did not protect them from the footpad inflammation.[149] Interestingly, disease induction in Ptpn6spin mice is mediated by RIPK1 and IL-1α but not by IL-1β.[149] Furthermore, we have shown the involvement of TAK1, ASK1/2, SYK, and CARD9 signaling pathways in mediating the footpad inflammation in Ptpn6spin mice.[150–152] Because pro–IL-1α is fully biologically active and constitutively expressed in a wide variety of cell types, understanding IL-1α cytokine biology, which remains significantly obscure, will shed more light on the pathogenesis of this inflammatory disorder and could provide much-needed targeted therapy for these rare diseases.
8. CONCLUDING REMARKS
During the past two decades, remarkable advancements have greatly increased our understanding of innate immunity, inflammasomes, and their related disorders through a growing number of murine models and advances in the patient-centered research. Despite these impressive strides, the clinical applications of these findings are still in their infancy. The genetic characterization of autoinflammatory disorders with respect to inflammasomes has substantially increased our understanding of NLR biology and has highlighted the inflammasome’s ability to contribute to many inflammatory diseases without major provocation of the adaptive immune system. This recognition coupled with insight into the central role of IL-1β in mediating inflammation in these disorders has led to markedly improved patient care through the successful therapeutic use of IL-1 inhibition. Further characterization of the high-resolution structure of inflammasome proteins will improve our understanding of the mechanisms governing inflammasome regulation. As this knowledge continues to evolve, additional targets and therapies to regulate inflammasome activity during disease will be discovered. Future research should also focus on whether inhibition of inflammasome components may serve as a viable target for therapeutic development in SLE and other autoinflammatory/autoimmune diseases. Challenges for the future include understanding the clinical significance of low-penetrance variants, the genetics and pathophysiology of different autoinflammatory diseases, and the long-term safety and efficacy of anti–IL-1 and anti–IL-18 therapies.
ACKNOWLEDGMENTS
The authors acknowledge many investigators in the field whose primary data could not be cited in this review because of space limitations. We would like to thank the members of Kanneganti lab for their comments and suggestions and Rebecca Tweedell, PhD, for scientific editing. Research in the Kanneganti laboratory was supported by the National Institutes of Health grants CA163507, AR056296, AI124346, and AI101935 and by the American Lebanese Syrian Associated Charities.
Abbreviations:
- AIM2
absent in melanoma 2
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- ATD
acidic transactivation domain
- BIR
Baculoviral inhibition of apoptosis protein repeat domain
- CAPS
cryopyrin-associated periodic syndromes
- CARD
caspase association and recruitment domain
- cGAMP
cyclic GMP-AMP
- cGAS
cyclic GMP-AMP synthase
- cpdm
chronic proliferative dermatitis
- DAMP
damage-associated molecular pattern
- DIRA
deficiency of IL-1 receptor antagonist
- FCAS
familial cold autoinflammatory syndrome
- FIIND
function to find domain
- FMF
familial Mediterranean fever
- GSDMD
gasdermin D
- HIDS
hyper IgD syndrome
- IFI16
interferon-γ–inducible protein 16
- IFN
interferon
- IFNAR
type I interferon receptor
- IL
interleukin
- IL-1RA
interleukin 1 receptor antagonist
- KI
knock-in
- LRR
leucine-rich repeats
- MAS
macrophage activation syndrome
- MKD
mevalonate kinase deficiency
- MyD88
myeloid differentiation primary response protein 88
- NBD
nucleotide-binding domain
- NET
neutrophil extracellular trap
- NF-κB
nuclear factor kappa B
- NLR
nucleotide-binding oligomerization domain-like receptor
- NOD
nucleotide-binding oligomerization domain
- NOMID
neonatal-onset multisystem inflammatory disease
- NSAIDs
non-steroidal anti-inflammatory drugs
- PAAND
pyrin-associated autoinflammation with neutrophilic dermatosis
- PAMP
pathogen-associated molecular pattern
- PAPA
purulent arthritis, pyoderma gangrenosum, and cystic acne
- PRR
pattern recognition receptor
- PSTPIP-1
proline-serine-threonine phosphatase-interacting protein 1
- PTPN6
protein tyrosine phosphatase non-receptor type 6
- PYD
pyrin domain
- RA
rheumatoid arthritis
- Sharpin
Shank-associated RH domain-interacting protein
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- SIRS
systemic inflammatory response syndrome
- SLE
systemic lupus erythematosus
- TGF-β
transforming growth factor-β
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TNFSFR1A
tumor necrosis family receptor superfamily 1A
- TRAPS
tumor necrosis factor receptor-associated periodic syndrome
Footnotes
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Medzhitov R. (2008) Origin and physiological roles of inflammation. Nature 454, 428–35. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA Jr. (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1, 1–13. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O. and Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–20. [DOI] [PubMed] [Google Scholar]
- 4.Akira S. and Takeda K. (2004) Toll-like receptor signalling. Nat Rev Immunol 4, 499–511. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T. and Akira S. (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–50. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801. [DOI] [PubMed] [Google Scholar]
- 7.Tartey S. and Takeuchi O. (2017) Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol 36, 57–73. [DOI] [PubMed] [Google Scholar]
- 8.Inohara Chamaillard, McDonald C, Nunez G. (2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74, 355–83. [DOI] [PubMed] [Google Scholar]
- 9.Kanneganti TD, Lamkanfi M, Nunez G. (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–59. [DOI] [PubMed] [Google Scholar]
- 10.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M Jr., Akira S, Yonehara S, Kato A, Fujita T. (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 175, 2851–8. [DOI] [PubMed] [Google Scholar]
- 11.Zelensky AN and Gready JE (2005) The C-type lectin-like domain superfamily. FEBS J 272, 6179–217. [DOI] [PubMed] [Google Scholar]
- 12.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11, 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA (2008) The NLR gene family: a standard nomenclature. Immunity 28, 285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartey S. and Kanneganti TD (2019) Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology 156, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinon F, Burns K, Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–26. [DOI] [PubMed] [Google Scholar]
- 17.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356, 768–74. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–71. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–5. [DOI] [PubMed] [Google Scholar]
- 20.Lamkanfi M. and Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157, 1013–22. [DOI] [PubMed] [Google Scholar]
- 21.Man SM and Kanneganti TD (2015) Regulation of inflammasome activation. Immunol Rev 265, 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287, 36617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang I, Yang J, Hong S, Ju Lee E, Lee SH, Fernandes-Alnemri T, Alnemri ES, Yu JW (2015) Non-transcriptional regulation of NLRP3 inflammasome signaling by IL-4. Immunol Cell Biol 93, 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. (2007) Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282, 18810–8. [DOI] [PubMed] [Google Scholar]
- 25.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–43. [DOI] [PubMed] [Google Scholar]
- 26.Kesavardhana S. and Kanneganti TD (2017) Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol 29, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. (2013) K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, McCarthy J, Frucht DM, Aringer M, Torosyan Y, Teppo AM, Wilson M, Karaarslan HM, Wan Y, Todd I, Wood G, Schlimgen R, Kumarajeewa TR, Cooper SM, Vella JP, Amos CI, Mulley J, Quane KA, Molloy MG, Ranki A, Powell RJ, Hitman GA, O’Shea JJ, Kastner DL (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–44. [DOI] [PubMed] [Google Scholar]
- 29.Masters SL, Simon A, Aksentijevich I, Kastner DL (2009) Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu Rev Immunol 27, 621–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamkanfi M. and Dixit VM (2012) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28, 137–61. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman HM, Wanderer AA, Broide DH (2001) Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol 108, 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 29, 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, Stein L, Russo R, Goldsmith D, Dent P, Rosenberg HF, Austin F, Remmers EF, Balow JE Jr., Rosenzweig S, Komarow H, Shoham NG, Wood G, Jones J, Mangra N, Carrero H, Adams BS, Moore TL, Schikler K, Hoffman H, Lovell DJ, Lipnick R, Barron K, O’Shea JJ, Kastner DL, Goldbach-Mansky R. (2002) De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum 46, 3340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott MF and Aksentijevich I. (2002) The autoinflammatory syndromes. Curr Opin Allergy Clin Immunol 2, 511–6. [DOI] [PubMed] [Google Scholar]
- 35.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aksentijevich I, Putnam CD, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL (2007) The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum 56, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. (2004) Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem 279, 21924–8. [DOI] [PubMed] [Google Scholar]
- 38.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, Soroosh P, Watford WT, O’Shea JJ, Kastner DL, Hoffman HM (2009) Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosengren S, Mueller JL, Anderson JP, Niehaus BL, Misaghi A, Anderson S, Boyle DL, Hoffman HM (2007) Monocytes from familial cold autoinflammatory syndrome patients are activated by mild hypothermia. J Allergy Clin Immunol 119, 991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, Hoffman HM (2005) IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol 175, 2630–4. [DOI] [PubMed] [Google Scholar]
- 41.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP (2007) Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A 104, 8041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. (2006) Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281, 36560–8. [DOI] [PubMed] [Google Scholar]
- 43.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–6. [DOI] [PubMed] [Google Scholar]
- 44.Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, Civitelli R, Abu-Amer Y, Kanneganti TD, Link DC, Mbalaviele G. (2018) Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol 16, e3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Torre-Minguela C, Mesa Del Castillo P, Pelegrin P. (2017) The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Front Immunol 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. (2015) The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol 10, 395–424. [DOI] [PubMed] [Google Scholar]
- 47.Aksentijevich I. and Kastner DL (2011) Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol 7, 469–78. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Q, Aksentijevich I, Wood GM, Walts AD, Hoffmann P, Remmers EF, Kastner DL, Ombrello AK (2015) Brief Report: Cryopyrin-Associated Periodic Syndrome Caused by a Myeloid-Restricted Somatic NLRP3 Mutation. Arthritis Rheumatol 67, 2482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL (2017) The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol 18, 832–842. [DOI] [PubMed] [Google Scholar]
- 50.Meng G, Zhang F, Fuss I, Kitani A, Strober W. (2009) A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30, 860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonar SL, Brydges SD, Mueller JL, McGeough MD, Pena C, Chen D, Grimston SK, Hickman-Brecks CL, Ravindran S, McAlinden A, Novack DV, Kastner DL, Civitelli R, Hoffman HM, Mbalaviele G. (2012) Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS One 7, e35979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu C, Bonar SL, Hickman-Brecks CL, Abu-Amer S, McGeough MD, Pena CA, Broderick L, Yang C, Grimston SK, Kading J, Abu-Amer Y, Novack DV, Hoffman HM, Civitelli R, Mbalaviele G. (2015) NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J 29, 1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM (2013) Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest 123, 4695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins PN, Lachmann HJ, McDermott MF (2003) Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med 348, 2583–4. [DOI] [PubMed] [Google Scholar]
- 55.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL (2006) Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 355, 581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Church LD and McDermott MF (2009) Rilonacept in cryopyrin-associated periodic syndromes: the beginning of longer-acting interleukin-1 antagonism. Nat Clin Pract Rheumatol 5, 14–5. [DOI] [PubMed] [Google Scholar]
- 57.Savic S. and McDermott MF (2009) Inflammation: canakinumab for the cryopyrin-associated periodic syndromes. Nat Rev Rheumatol 5, 529–30. [DOI] [PubMed] [Google Scholar]
- 58.Chae JJ, Aksentijevich I, Kastner DL (2009) Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol 146, 467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-Chetrit E. and Levy M. (1998) Familial Mediterranean fever. Lancet 351, 659–64. [DOI] [PubMed] [Google Scholar]
- 60.(1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 90, 797–807. [DOI] [PubMed] [Google Scholar]
- 61.French FMFC (1997) A candidate gene for familial Mediterranean fever. Nat Genet 17, 25–31. [DOI] [PubMed] [Google Scholar]
- 62.Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer HD, Grutter C, Grutter M, Tschopp J. (2007) The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ 14, 1457–66. [DOI] [PubMed] [Google Scholar]
- 63.Tidow N, Chen X, Muller C, Kawano S, Gombart AF, Fischel-Ghodsian N, Koeffler HP (2000) Hematopoietic-specific expression of MEFV, the gene mutated in familial Mediterranean fever, and subcellular localization of its corresponding protein, pyrin. Blood 95, 1451–5. [PubMed] [Google Scholar]
- 64.Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, Katz SI, Kastner DL (2011) Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 34, 755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarrauste de Menthiere C, Terriere S, Pugnere D, Ruiz M, Demaille J, Touitou I. (2003) INFEVERS: the Registry for FMF and hereditary inflammatory disorders mutations. Nucleic Acids Res 31, 282–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. (2014) An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med 211, 2385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, Chapelle D, Plass N, Huang Y, Villarino AV, Biancotto A, Fleisher TA, Duncan JA, O’Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, Goldbach-Mansky R. (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46, 1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, Frouin E, Seyger M, Girard M, Puechberty J, Costes V, Rodiere M, Carbasse A, Jeziorski E, Portales P, Sarrabay G, Mondain M, Jorgensen C, Apparailly F, Hoppenreijs E, Touitou I, Genevieve D. (2017) A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis 76, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 69.Jamilloux Y, Magnotti F, Belot A, Henry T. (2018) The pyrin inflammasome: from sensing RhoA GTPases-inhibiting toxins to triggering autoinflammatory syndromes. Pathog Dis 76. [DOI] [PubMed] [Google Scholar]
- 70.Van Gorp H, Saavedra PH, de Vasconcelos NM, Van Opdenbosch N, Vande Walle L, Matusiak M, Prencipe G, Insalaco A, Van Hauwermeiren F, Demon D, Bogaert DJ, Dullaers M, De Baere E, Hochepied T, Dehoorne J, Vermaelen KY, Haerynck F, De Benedetti F, Lamkanfi M. (2016) Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc Natl Acad Sci U S A 113, 14384–14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omenetti A, Carta S, Delfino L, Martini A, Gattorno M, Rubartelli A. (2014) Increased NLRP3-dependent interleukin 1beta secretion in patients with familial Mediterranean fever: correlation with MEFV genotype. Ann Rheum Dis 73, 462–9. [DOI] [PubMed] [Google Scholar]
- 72.Sharma D, Sharma BR, Vogel P, Kanneganti TD (2017) IL-1beta and Caspase-1 Drive Autoinflammatory Disease Independently of IL-1alpha or Caspase-8 in a Mouse Model of Familial Mediterranean Fever. Am J Pathol 187, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanneganti A, Malireddi RKS, Saavedra PHV, Vande Walle L, Van Gorp H, Kambara H, Tillman H, Vogel P, Luo HR, Xavier RJ, Chi H, Lamkanfi M. (2018) GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J Exp Med 215, 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma D, Malik A, Guy C, Vogel P, Kanneganti TD (2019) TNF/TNFR axis promotes pyrin inflammasome activation and distinctly modulates pyrin inflammasomopathy. J Clin Invest 129, 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldfinger SE (1972) Colchicine for familial Mediterranean fever. N Engl J Med 287, 1302. [DOI] [PubMed] [Google Scholar]
- 76.Tiegs G, Freudenberg MA, Galanos C, Wendel A. (1992) Colchicine prevents tumor necrosis factor-induced toxicity in vivo. Infect Immun 60, 1941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masters SL, Lagou V, Jeru I, Baker PJ, Van Eyck L, Parry DA, Lawless D, De Nardo D, Garcia-Perez JE, Dagley LF, Holley CL, Dooley J, Moghaddas F, Pasciuto E, Jeandel PY, Sciot R, Lyras D, Webb AI, Nicholson SE, De Somer L, van Nieuwenhove E, Ruuth-Praz J, Copin B, Cochet E, Medlej-Hashim M, Megarbane A, Schroder K, Savic S, Goris A, Amselem S, Wouters C, Liston A. (2016) Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med 8, 332ra45. [DOI] [PubMed] [Google Scholar]
- 78.Park YH, Wood G, Kastner DL, Chae JJ (2016) Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17, 914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao W, Yang J, Liu W, Wang Y, Shao F. (2016) Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A 113, E4857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moghaddas F, Llamas R, De Nardo D, Martinez-Banaclocha H, Martinez-Garcia JJ, Mesa-Del-Castillo P, Baker PJ, Gargallo V, Mensa-Vilaro A, Canna S, Wicks IP, Pelegrin P, Arostegui JI, Masters SL (2017) A novel Pyrin-Associated Autoinflammation with Neutrophilic Dermatosis mutation further defines 14–3-3 binding of pyrin and distinction to Familial Mediterranean Fever. Ann Rheum Dis 76, 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, Lovett M. (2002) Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet 11, 961–9. [DOI] [PubMed] [Google Scholar]
- 82.Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, Kastner DL (2003) Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A 100, 13501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Omenetti A, Carta S, Caorsi R, Finetti M, Marotto D, Lattanzi B, Jorini M, Delfino L, Penco F, Picco P, Buoncompagni A, Martini A, Rubartelli A, Gattorno M. (2016) Disease activity accounts for long-term efficacy of IL-1 blockers in pyogenic sterile arthritis pyoderma gangrenosum and severe acne syndrome. Rheumatology (Oxford) 55, 1325–35. [DOI] [PubMed] [Google Scholar]
- 84.Starnes TW, Bennin DA, Bing X, Eickhoff JC, Grahf DC, Bellak JM, Seroogy CM, Ferguson PJ, Huttenlocher A. (2014) The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood 123, 2703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388, 2023–2038. [DOI] [PubMed] [Google Scholar]
- 86.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH, National Arthritis Data W. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58, 15–25. [DOI] [PubMed] [Google Scholar]
- 87.Scott DL, Symmons DP, Coulton BL, Popert AJ (1987) Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet 1, 1108–11. [DOI] [PubMed] [Google Scholar]
- 88.Pincus T, Brooks RH, Callahan LF (1994) Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med 120, 26–34. [DOI] [PubMed] [Google Scholar]
- 89.Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376, 1094–108. [DOI] [PubMed] [Google Scholar]
- 90.Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Biologics in Rheumatoid Arthritis G., Genomics Study S., Eyre S, Churchman SM, Wilson AG, Isaacs JD, Hyrich K, Barton A, Plant D, Savic S, Cook GP, Sarzi-Puttini P, Emery P, Barrett JH, Morgan AW, McDermott MF (2014) Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis 73, 1202–10. [DOI] [PubMed] [Google Scholar]
- 91.Jenko B, Praprotnik S, Tomsic M, Dolzan V. (2016) NLRP3 and CARD8 Polymorphisms Influence Higher Disease Activity in Rheumatoid Arthritis. J Med Biochem 35, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B, Yang F, Zou Q, Wu Y. (2012) NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in han chinese. Arthritis Rheum 64, 647–54. [DOI] [PubMed] [Google Scholar]
- 93.Li F, Guo N, Ma Y, Ning B, Wang Y, Kou L. (2014) Inhibition of P2X4 suppresses joint inflammation and damage in collagen-induced arthritis. Inflammation 37, 146–53. [DOI] [PubMed] [Google Scholar]
- 94.Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R, Van de Veerdonk FL, Netea MG, Joosten LA, Lamkanfi M, Kanneganti TD (2010) Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem 285, 12454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB (2009) Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum 60, 3651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. (2002) Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med 196, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M. (2014) Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choulaki C, Papadaki G, Repa A, Kampouraki E, Kambas K, Ritis K, Bertsias G, Boumpas DT, Sidiropoulos P. (2015) Enhanced activity of NLRP3 inflammasome in peripheral blood cells of patients with active rheumatoid arthritis. Arthritis Res Ther 17, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mertens M. and Singh JA (2009) Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 36, 1118–25. [DOI] [PubMed] [Google Scholar]
- 100.Cavalli G. and Dinarello CA (2018) Anakinra Therapy for Non-cancer Inflammatory Diseases. Front Pharmacol 9, 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alten R, Gomez-Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, Preiss R, Arulmani U, Widmer A, Gitton X, Kellner H. (2011) Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study. BMC Musculoskelet Disord 12, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, Xiao TS (2012) Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jakobs C, Perner S, Hornung V. (2015) AIM2 Drives Joint Inflammation in a Self-DNA Triggered Model of Chronic Polyarthritis. PLoS One 10, e0131702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baum R, Sharma S, Carpenter S, Li QZ, Busto P, Fitzgerald KA, Marshak-Rothstein A, Gravallese EM (2015) Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J Immunol 194, 873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, Nagata S. (2001) Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 292, 1546–9. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. (2005) Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol 6, 49–56. [DOI] [PubMed] [Google Scholar]
- 107.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. (2006) Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443, 998–1002. [DOI] [PubMed] [Google Scholar]
- 108.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. (2011) Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 3, 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, West B, Podoltsev NA, Boggon TJ, Kazmierczak BI, Lifton RP (2014) Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet 46, 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, Gabay C, Goldbach-Mansky R, Behrens EM (2017) Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 139, 1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raghawan AK, Sripada A, Gopinath G, Pushpanjali P, Kumar Y, Radha V, Swarup G. (2017) A Disease-associated Mutant of NLRC4 Shows Enhanced Interaction with SUG1 Leading to Constitutive FADD-dependent Caspase-8 Activation and Cell Death. J Biol Chem 292, 1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lisnevskaia L, Murphy G, Isenberg D. (2014) Systemic lupus erythematosus. Lancet 384, 1878–1888. [DOI] [PubMed] [Google Scholar]
- 113.Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365, 2110–21. [DOI] [PubMed] [Google Scholar]
- 114.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ (2011) Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol 187, 6143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brinkmann V. and Zychlinsky A. (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5, 577–82. [DOI] [PubMed] [Google Scholar]
- 116.Knight JS and Kaplan MJ (2012) Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol 24, 441–50. [DOI] [PubMed] [Google Scholar]
- 117.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ (2013) Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 190, 1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sano H, Takai O, Harata N, Yoshinaga K, Kodama-Kamada I, Sasaki T. (1989) Binding properties of human anti-DNA antibodies to cloned human DNA fragments. Scand J Immunol 30, 51–63. [DOI] [PubMed] [Google Scholar]
- 119.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD (2005) Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 115, 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kahlenberg JM and Kaplan MJ (2014) The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol 26, 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu A, Li H, Niu J, Wu S, Xue G, Yao X, Guo Q, Wan N, Abliz P, Yang G, An L, Meng G. (2017) Hyperactivation of the NLRP3 Inflammasome in Myeloid Cells Leads to Severe Organ Damage in Experimental Lupus. J Immunol 198, 1119–1129. [DOI] [PubMed] [Google Scholar]
- 122.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM (2013) P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum 65, 3176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lech M, Lorenz G, Kulkarni OP, Grosser MO, Stigrot N, Darisipudi MN, Gunthner R, Wintergerst MW, Anz D, Susanti HE, Anders HJ (2015) NLRP3 and ASC suppress lupus-like autoimmunity by driving the immunosuppressive effects of TGF-beta receptor signalling. Ann Rheum Dis 74, 2224–35. [DOI] [PubMed] [Google Scholar]
- 124.Kimkong I, Avihingsanon Y, Hirankarn N. (2009) Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RT-PCR. Lupus 18, 1066–72. [DOI] [PubMed] [Google Scholar]
- 125.Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, Lazova R, Kang I. (2013) Self double-stranded (ds)DNA induces IL-1beta production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies. J Immunol 190, 1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, Kang I. (2012) U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. J Immunol 188, 4769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C. (2013) C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122, 3473–81. [DOI] [PubMed] [Google Scholar]
- 128.Manderson AP, Botto M, Walport MJ (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22, 431–56. [DOI] [PubMed] [Google Scholar]
- 129.Benoit ME, Clarke EV, Morgado P, Fraser DA, Tenner AJ (2012) Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol 188, 5682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. (2005) Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis 64, 630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang W, Cai Y, Xu W, Yin Z, Gao X, Xiong S. (2013) AIM2 facilitates the apoptotic DNA-induced systemic lupus erythematosus via arbitrating macrophage functional maturation. J Clin Immunol 33, 925–37. [DOI] [PubMed] [Google Scholar]
- 132.Panchanathan R, Xin H, Choubey D. (2008) Disruption of mutually negative regulatory feedback loop between interferon-inducible p202 protein and the E2F family of transcription factors in lupus-prone mice. J Immunol 180, 5927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haywood ME, Rose SJ, Horswell S, Lees MJ, Fu G, Walport MJ, Morley BJ (2006) Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Genes Immun 7, 250–63. [DOI] [PubMed] [Google Scholar]
- 134.Yin Q, Sester DP, Tian Y, Hsiao YS, Lu A, Cridland JA, Sagulenko V, Thygesen SJ, Choubey D, Hornung V, Walz T, Stacey KJ, Wu H. (2013) Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell Rep 4, 327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Panchanathan R, Duan X, Shen H, Rathinam VA, Erickson LD, Fitzgerald KA, Choubey D. (2010) Aim2 deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol 185, 7385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panchanathan R, Duan X, Arumugam M, Shen H, Liu H, Choubey D. (2011) Cell type and gender-dependent differential regulation of the p202 and Aim2 proteins: implications for the regulation of innate immune responses in SLE. Mol Immunol 49, 273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.HogenEsch H, Gijbels MJ, Offerman E, van Hooft J, van Bekkum DW, Zurcher C. (1993) A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am J Pathol 143, 972–82. [PMC free article] [PubMed] [Google Scholar]
- 138.Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, Roopenian DC, Sundberg JP (2007) Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun 8, 416–21. [DOI] [PubMed] [Google Scholar]
- 139.Douglas T, Champagne C, Morizot A, Lapointe JM, Saleh M. (2015) The Inflammatory Caspases-1 and −11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice. J Immunol 195, 2365–73. [DOI] [PubMed] [Google Scholar]
- 140.Gurung P, Sharma BR, Kanneganti TD (2016) Distinct role of IL-1beta in instigating disease in Sharpin(cpdm) mice. Sci Rep 6, 36634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ (2014) Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol 192, 5476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, Lalaoui N, Lawlor KE, Vanyai H, Hall C, Bankovacki A, Gangoda L, Wong WW, Corbin J, Huang C, Mocarski ES, Murphy JM, Alexander WS, Voss AK, Vaux DL, Kaiser WJ, Walczak H, Silke J. (2014) TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma BR, Karki R, Lee E, Zhu Q, Gurung P, Kanneganti TD (2019) Innate immune adaptor MyD88 deficiency prevents skin inflammation in SHARPIN-deficient mice. Cell Death Differ 26, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumari S, Redouane Y, Lopez-Mosqueda J, Shiraishi R, Romanowska M, Lutzmayer S, Kuiper J, Martinez C, Dikic I, Pasparakis M, Ikeda F. (2014) Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pao LI, Badour K, Siminovitch KA, Neel BG (2007) Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol 25, 473–523. [DOI] [PubMed] [Google Scholar]
- 146.Nesterovitch AB, Szanto S, Gonda A, Bardos T, Kis-Toth K, Adarichev VA, Olasz K, Ghassemi-Najad S, Hoffman MD, Tharp MD, Mikecz K, Glant TT (2011) Spontaneous insertion of a b2 element in the ptpn6 gene drives a systemic autoinflammatory disease in mice resembling neutrophilic dermatosis in humans. Am J Pathol 178, 1701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nesterovitch AB, Gyorfy Z, Hoffman MD, Moore EC, Elbuluk N, Tryniszewska B, Rauch TA, Simon M, Kang S, Fisher GJ, Mikecz K, Tharp MD, Glant TT (2011) Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am J Pathol 178, 1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Croker BA, Lawson BR, Rutschmann S, Berger M, Eidenschenk C, Blasius AL, Moresco EM, Sovath S, Cengia L, Shultz LD, Theofilopoulos AN, Pettersson S, Beutler BA (2008) Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc Natl Acad Sci U S A 105, 15028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, Kanneganti TD (2013) RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature 498, 224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gurung P, Fan G, Lukens JR, Vogel P, Tonks NK, Kanneganti TD (2017) Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1alpha-Mediated Inflammatory Disease. Immunity 46, 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tartey S, Gurung P, Samir P, Burton A, Kanneganti TD (2018) Cutting Edge: Dysregulated CARD9 Signaling in Neutrophils Drives Inflammation in a Mouse Model of Neutrophilic Dermatoses. J Immunol 201, 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tartey S, Gurung P, Dasari TK, Burton A, Kanneganti TD (2018) ASK1/2 signaling promotes inflammation in a mouse model of neutrophilic dermatosis. J Clin Invest 128, 2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverenyi I, Takeichi T, Balaji R, Lau A, Tye H, Roy K, Bonnard C, Ahl PJ, Jones LA, Baker PJ, Lacina L, Otsuka A, Fournie PR, Malecaze F, Lane EB, Akiyama M, Kabashima K, Connolly JE, Masters SL, Soler VJ, Omar SS, McGrath JA, Nedelcu R, Gribaa M, Denguezli M, Saad A, Hiller S, Reversade B. (2016) Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 167, 187–202 e17. [DOI] [PubMed] [Google Scholar]
- 154.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ (2005) Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 44, 406–8. [DOI] [PubMed] [Google Scholar]
- 155.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. (2009) Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol 161, 1199–201. [DOI] [PubMed] [Google Scholar]
- 156.Curtis CD and Fox CC (2015) Treatment of adult hyper-IgD syndrome with canakinumab. J Allergy Clin Immunol Pract 3, 817–8. [DOI] [PubMed] [Google Scholar]
- 157.Galon J, Aksentijevich I, McDermott MF, O’Shea JJ, Kastner DL (2000) TNFRSF1A mutations and autoinflammatory syndromes. Curr Opin Immunol 12, 479–86. [DOI] [PubMed] [Google Scholar]
- 158.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. (2009) An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 360, 2426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, Hessner MJ, Verbsky J. (2009) An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med 360, 2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]