Abstract
Maternal history for sporadic Alzheimer’s disease (AD) predisposes the offspring to the disease later in life. However, the mechanisms behind this phenomenon are still unknown. Lifestyle and nutrition can directly modulate susceptibility to AD. Herein we investigated whether gestational high fat diet influences the offspring susceptibility to AD later in life. Triple transgenic dams were administered high-fat diet or regular chow throughout 3 weeks gestation. Offspring were fed regular chow throughout their life and tested for spatial learning and memory, brain amyloidosis, tau pathology and synaptic function. Gestational high fat diet attenuated memory decline, synaptic dysfunction, amyloid-β and tau neuropathology in the offspring by transcriptional regulation of BACE-1, CDK5 and tau gene expression via the up-regulation of FOXP2 repressor. Gestational high fat diet protects offspring against the development of the AD phenotype. In-utero dietary intervention could be implemented as preventative strategy against AD.
Keywords: Alzheimer’s disease, maternal, high fat diet, transgenic mice, memory, amyloid beta, tau, synapse
INTRODUCTION
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder characterized by progressive memory loss and cognitive impairment due to neuronal cells death secondary to accumulation of toxic misfolded proteins in the brain parenchyma. (1, 2). Today, approximately 5.7 million Americans live with AD and the economic burden on society is extremely heavy, accounting for an estimate of 277 billion dollars for 2018 in the United States alone (3). Moreover, since the mechanisms responsible for its most common sporadic form remain elusive, despite an extensive research effort, no treatment is currently available to block or slow down AD progression.
Sporadic or late-onset AD is probably due to a combination of both genetic and environmental risk factors (4). After age, a maternal history of AD is the strongest risk factor for developing the disease. Indeed, individuals from mother with late-onset AD have a 3 times higher chance to develop the disease when compared to the ones who have a father affected by it (5–8). However, the mechanisms governing the maternal influence and transmissibility of AD to the offspring are unknown. Among the most credited hypothesis, a potential unidentified genetic mutation in either genomic or mitochondrial DNA has been invoked (9, 10), but so far no evidence has been produced in its support. Given the crucial contribution of lifestyle to AD pathogenesis, it is possible that a specific maternal diet during pregnancy will determine the susceptibility of the offspring to develop AD later in life. Several reports have shown that nutrition could play a direct role in predisposing individuals to AD susceptibility later in life through epigenetic modifications (11–14). Experimental studies have demonstrated that feeding a high fat diet to different AD mouse models exacerbates their neuropathology in the exposed animals (15–17). Others have shown that feeding a high fat diet during the gestation and lactation period worsens brain pathology of the offspring (18–20). However, no study has ever tested whether administration of the high fat diet restricted to the gestation period alone directly affects the susceptibility of the offspring to develop AD. To this end, we treated pregnant triple transgenic (3TG) dams with high fat diet throughout gestation and then assessed memory, synaptic function and brain pathology in their offspring later in life. To our surprise, gestational high fat diet rescued the cognitive decline and memory impairments, improved synaptic dysfunction, and significantly reduced brain amyloidosis and tau neuropathology in the offspring.
MATERIALS AND METHODS
Animals
All procedures were approved by the Animal Care and Usage Committee in accordance with the National Institutes of Health guidelines. Triple transgenic (3TG) mice harboring APP (KM670/671NL) mutation, human PS1 (M146V) mutation and human MAPT (P301L) transgene were used in this study together with their wild type counterpart (21). Mice were kept in a pathogen-free environment on a 12-hour light/dark cycle and had access to food and water ad libitum. Ten wild type (WT) dams and ten 3TG dams were used for breeding with one male per dam in each cage. After pregnancy was assessed by examining vaginal plug, dams were randomized to receive regular chow (PicoLab 5053: 13% calories from fat, 0.05 calories from cholesterol) (5 dams per strain), or high fat diet (Harlan TD88137: 42% calories from fat, 0.2% calories from cholesterol) (5 dams per strain) throughout the 3 weeks of gestation. After, delivery, all dams were put back on regular chow during lactation. No difference in litter size was observed between strains or diet regimens. No difference in diet compliance was observed between regular chow and high fat diet. An average of 4 to 8 pups per pregnancy was delivered. A total of 8 to 12 offspring (4 to 6 males and 4 to 6 females) was randomized for each experimental condition (WT control: WT CTR; WT high fat diet: WT HF; 3TG control: 3TG CTR, 3TG high fat diet: 3TG HF). Offspring were kept on regular chow from birth until euthanasia. Throughout their life offspring from all experimental conditions were assessed for their body weight at 2, 6 and 12 months of age.
Glucose tolerance test
Offspring from all experimental condition underwent glucose tolerance test at 2, 6 and 12 months of age. The evening before the test, offspring were housed in a new cage with paper bedding. Food was removed from 5:00 pm of the evening before until 9:00 am of the test day. During this time mice had access to fresh water at libitum. The test day, animals were placed in a restrainer device and the tail vein was punctured with a 23–27-gauge needle. Blood drops were collected onto a glucose testing strip (One Touch Ultra Blue strips) and tested for basal glucose concentration at fasting using a glucose meter (One Touch Ultra 2). Subsequently, animals were injected 2mg/g body weight glucose in the intraperitoneal cavity. At 30 minutes and 2 hours after the injection, glucose concentration was assessed again as described above. After the procedure, animals were returned their homecage with regular bedding, fresh chow and water.
Plasma lipids
Blood was collected after euthanasia by left intraventricular puncture and immediately mixed with 77mM EDTA, then centrifuged at 3.0 g and 4° C to obtain plasma. Plasma lipids levels were measured at the Vanderbilt Metabolic Regulation Core, Vanderbilt University, Nashville, TN. Total plasma cholesterol and triglyceride were measured by standard enzymatic assays. HDL cholesterol was measured with the enzymatic method after precipitation of VLDL and LDL using polyethylene glycol reagent.
Cognitive behavioral test
Mice were pre-handled for 3 days before the test. All tests were conducted by an experimenter who was blinded to the treatment or genotype. They were tested in a randomized order in the Morris Water Maze (MWM) paradigm at 6 and 12 months of age as previously described (22, 23). Briefly, the test was conducted in a white circular plastic tank filled with opaque water. Mice were trained to find a submerged Plexiglas platform starting from the 4 cardinal points, every day for a total of 4 days. The fifth day, mice were tested in the probe trial upon reaching the training criterion of 20 seconds (escape latency). The probe consists in a free 60 seconds swim in the pool without platform, to assess the number of entries in the zone of the platform and other parameters of interest.
Protein samples preparation
After behavioral tests, mice were euthanized and brains immediately harvested after intraventricular perfusion with PBS buffer, EDTA, protease and phosphatase inhibitor cocktail. Brains were immediately dissected in two halves: one for biochemistry and the other for immunohistochemistry. For whole protein lysate preparation, 20 to 40 mg of frozen brain tissue was cut and sonicated for 20 seconds in 200 to 400 ul of Radioimmunoprecipitation assay buffer (RIPA buffer). Lysates were centrifuged in Beckman Coulter Optima MAX Ultracentrifuge at 100,000 g for 45 minutes at 4° C. Supernatant was used for western blot or ELISA analysis of RIPA soluble protein fraction. Pellets were resuspended in 30 to 60 ul of 70 % formic acid and neutralized in 6N NaOH to analyze RIPA insoluble (formic acid soluble) protein fraction by western blot and ELISA. For subcellular fractionation (cytoplasmic and nuclear lysate), 20 to 40 mg of frozen brain tissue was cut and processed with NE-PER™ Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Philadelphia PA) according to manufacturer instructions. Protein lysates were assessed for protein concentration with BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
RNA samples preparation
Aliquots (20 mg) of frozen brain cortex tissue were sonicated for 20 seconds in 700 μl of Qiazol reagent (Qiagen, Germany). RNA was isolated utilizing miRNeasy isolation kit (Qiagen, Germany) according to manufacturer protocol. RNA concentration was assessed utilizing Nanodrop system.
DNA samples preparation
Aliquots (20 mg) of frozen brain cortex tissue were incubated overnight with lysis buffer PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific, Philadelphia PA). The following day, genomic DNA was isolated utilizing PureLink Genomic DNA Mini Kit (Thermo Fisher Scientific, Philadelphia PA) according to manufacturer protocol. DNA concentration was assessed utilizing Nanodrop system.
Amyloid beta 40 and 42 ELISA
For the 12 month-old 3TG, RIPA soluble and formic acid soluble protein lysates were utilized to assess soluble and insoluble human Amyloid beta (Aβ) 1–40 and Aβ 1–42 levels. For the 2 month-old 3TG animals, aliquots of brain cortex samples were sonicated in DEA buffer (0.2 % diethylamine in 50mM NaCl) with protease inhibitor cocktail, spun down at 100,000 g for 60 minutes and assayed for total human Aβ 1–40 levels. Human Aβ 1–40 and Aβ 1–42 ELISA Kit (Wako, Japan) were used according to manufacturer protocol and previously described (22, 23).
Western blot analyses
Western blot analyses were executed as previously described (22, 23). Briefly, RIPA soluble and formic acid soluble protein lysates were separated on sodium dodecyl sulfate (SDS)-PAGE by using a 10% Bis-Tris gel and then transferred onto nitro-cellulose membranes (Bio-Rad, Richmond, CA, USA). Membranes were blocked with Odyssey blocking buffer for 1 hour at room temperature and incubated with primary antibodies overnight at 4 °C. After 3 washing cycles in T-TBS, membranes were incubated with IRDye 800CW labeled secondary antibody (LI-COR Bioscience, Lincoln, NE, USA) for 1 hour at room temperature and developed with Odyssey Infrared Imaging System (LI-COR Bioscience). Primary antibodies used are summarized in Table 1.
TABLE 1.
Antibodies used in the study.
| ANTIBODY | CAT # | COMPANY | MW(kDa) | DILUTION | APPLICATION |
|---|---|---|---|---|---|
| Anti-Aβ (4G8) | SIG-39220 | Covance | N.A. | 1:400 | IHC |
| Anti-APP A4 | MAB348 | Millipore | 120 | 1:200 | WB |
| Anti-APH-1 | AB9214 | Millipore | 29 | 1:400 | WB |
| Anti-Pen2 | 36–7100 | Invitrogen | 14 | 1:200 | WB |
| Anti-PS1 | 3622S | CST | 22 | 1:100 | WB |
| Anti-Nicastrin | 3632 | CST | 120 | 1:500 | WB |
| Anti-ADAM10 | AB19026 | Millipore | 85 | 1:200 | WB |
| Anti-BACE1 | MAB5308 | Millipore | 50–70 | 1:200 | WB |
| Anti-sAPPα | 11088 | IBM | 95 | 1:100 | WB |
| Anti-sAPPβ | 10321 | IBM | 95 | 1:100 | WB |
| Anti-CD10 | sc-46656 | SCB | 100 | 1;200 | WB |
| Anti-IDE | sc-393887 | SCB | 118 | 1:200 | WB |
| Ant-APOE | sc-390925 | SCB | 36 | 1:200 | WB |
| Anti-HT7 | MN1000 | TFS | 50 | 1:400 | WB, IHC |
| Anti-AT180 | P10636 | TFS | 50 | 1:200 | WB, IHC |
| Anti-AT8 | MN1020 | TFS | 50 | 1:200 | WB, IHC |
| Anti-PHF13 | 9632 | CST | 50 | 1:200 | WB, IHC |
| Anti-PHF1 | sc-515013 | SCB | 50 | 1:200 | WB, IHC |
| Anti-MC1 | gift | Dr. Davies | 50 | 1:200 | WB, IHC |
| Anti-CDK5 | sc-6247 | SCB | 35 | 1:400 | WB |
| Anti-P35/25 | sc-518009 | SCB | 35/25 | 1:200 | WB |
| Anti-GSK3α/β | sc-7291 | SCB | 5¼6 | 1:200 | WB |
| Anti-pGSK3α/β | 9331 | CST | 5¼6 | 1:200 | WB |
| Anti-SYP | sc-17750 | SCB | 38 | 1:500 | WB, IHC |
| Anti-PSD95 | MA1–045 | TFS | 95 | 1:200 | WB, IHC |
| Anti-GFAP | sc-33673 | SCB | 50 | 1:200 | WB, IHC |
| Anti-IBA1 | MABN92 | Millipore | 17 | 1:200 | WB |
| Anti-REST | 07–579 | Millipore | 120 | 1:200 | WB |
| Anti-FOXP2 | 5337 | CST | 85 | 1:200 | WB |
| Anti-CREM | sc-390426 | SCB | 39 | 1:200 | WB |
| Anti-actin-beta | sc-47778 | SCB | 42 | 1:1000 | WB |
| Anti-GAPDH | 2128 | CST | 37 | 1:1000 | WB |
WB = Western blot; IHC = immunohistochemistry; CST = Cell signaling Technology, SCB = Santa Cruz Biotechnology; TFS = Thermo Fisher Scientific.
Immunohistochemistry
Mouse brains were prepared for immunohistochemistry as previously described (22, 23). Briefly, serial brain sections were cut throughout each brain and mounted on 3-aminopropyl-triethoxysaline-coated slides. Sections were deparaffinized, hydrated, rinsed with PBS, and pretreated with citric acid for 5 minutes for antigen retrieval, then with 3% H2O2 in methanol for 30 minutes to eliminate endogenous peroxidase activity in the tissue and with blocking solution (5% normal serum in Tris buffer, pH 7.6). Subsequently, sections were incubated overnight at 4 °C with primary antibody. The next morning, sections were incubated with secondary antibody and developed using the avidin-biotin complex method (Vector Laboratories, Burlingame, CA, USA) with 3,3diaminobenzidine as chromogen. Primary antibodies used are summarized in Table 1.
Long-term potentiation (LTP) electrophysiology on hippocampal slices
LTP electrophysiology studies were executed as previously described (24). Briefly, 12 month-old WT CTR, 3TG CTR and 3TG HF offspring were sacrificed by rapid decapitation and brains were put into ice-cold artificial cerebral spinal fluid (ACSF) in which sucrose (248 mmol/L) was substituted for NaCl. Six to ten transverse hippocampal slices (400 μm thick) per animal were cut using a Vibratome 3000 plus (Vibratome, Bannockburn, Illinois) and placed in ACSF (124 mM NaCl, 2.5 mmol/L KCl, 2 mmol/L NaH2PO4, mmol/L CaCl2, 2 mmol/L MgSO4, 10 mmol/L dextrose, and 26 mmol/L NaHCO3) at room temperature to recover for 1 hour bubbled with 95% O2/5% CO2. Slices were transferred to a recording chamber (Warner Instruments, Hamden, Connecticut) and constantly perfused with ACSF at 1.5 to 2.0 mL/min flow, bubbled with 95% O2/5% CO2, and maintained by an inline solution heater (TC-324; Warner Instruments) at 32° to 34°C. Field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 stratum radiatum by using an extracellular glass pipette (3–5 MΩ) filled with ACSF. Schaffer collateral/commissural fibers in the stratum radiatum were stimulated with a bipolar tungsten electrode placed 200 to 300 μm from the recording pipette. Stimulation intensities were chosen to produce a fEPSP that was one-third of the maximum amplitude, based on an input–output curve using stimulations of 0 to 300 μA, in increments of 20 μA. Paired-pulse facilitation experiments were performed using a pair of stimuli of the same intensity delivered 20, 50, 100, 200, and 1000 milliseconds apart. Baseline was recorded for 20 minutes before tetanization with pulses every 30 seconds. Long-term potentiation (LTP) at CA3 to CA1 synapses was induced by four trains of 100-Hz stimulation delivered in 20-second intervals. Recordings were made every 30 seconds for 2 hours following tetanization. The fEPSP rise/slope (mV/msec) between 30% and 90% was measured offline using Clampfit 10.3 (Molecular Devices, Sunnyvale, California) and normalized to the mean rise/slope of the baseline.
mRNA expression analysis
Extracted RNA samples were converted to cDNA utilizing RT2 First Strand Kit (Qiagen, Germany) according to manufacturer instructions. Genes expression was measured by SYBR-green qPCR technology using RT2 qPCR primer assay (Qiagen, Germany) according to manufacturer specification with Applied Biosystems Step One Plus rt-PCR system. The following commercially available primers were used: BACE1 mouse cat # PPM26538A, MAPT human cat # PPH05972F, CDK5 mouse cat # PPM05037C, CDK6 mouse cat # PPM02912F, APP human cat # PPH05947A, GSK3alpha mouse cat # PPM05437A and GSK3beta mouse cat # PPM03380C (Qiagen, Germany).
Methylation analysis
500 ng of genomic DNA were converted to bisulfite DNA utilizing EZ DNA Methylation-Gold kit (Zymo Research, Irvine, CA) as manufacturer specifications. Bisulfite DNA was then utilized for global and promoter specific DNA methylation assay. In particular, global DNA methylation was assessed by measuring methylation at DNA repetitive long interspersed nuclear elements, using LINE-1 assay as a surrogate marker as in (25, 26). Promoter specific DNA methylation of candidate genes was screened by bisulfite sequencing as in (25, 26).
LINE-1 assay
DNA repetitive long interspersed nuclear elements (LINE-1 elements) were amplified by PCR. After PCR amplification, PCR product was run on agarose gel to verify correct size. Subsequently, PCR products underwent pyrosequencing and DNA methylation at LINE-1 element measured as percent of T to C conversion. The following primers were used for 1st PCR amplification:
TGGGATTTTAAGATTTTTGGTGAG (forward primer);
CTTCCCTATTTACCACAATCTCAA (reverse primer);
The following primers were used for pyrosequencing: TTTTTGGTGAGTGGAATATA. UNIV-reverse primer and Biotin-UNIV primer were used for 2nd PCR. The following conditions were used for both PCRs: 95°C for 3 minutes denaturation step followed by 30 cycles of 95°C/15 seconds, 60°C/30 seconds and 68°C/30 seconds.
Promoter specific bisulfite methylation assay
We designed bisulfite PCR primers for candidate genes (BACE1 and CDK5) to analyze CpG islands at transcription start sites. Several primer pairs were tested for optimal results. The following primers were used: TGGGAGTTGGATTATGGTGGTTT (forward primer 1st step BACE1), ACTACAAAATCTACAAACCCCTC (reverse primer 1st step BACE1), TGGGAGTTGGATTATGGTGGTTT (forward primer 2nd step BACE1), ACTACAAAATCTACAAACCCCTC (reverse primer 2nd step BACE1), GGAGTTGGGATTGTAAGTAGGG (forward primer CDK5) and ACTATAAATACCACCTCCTCTACAA (reverse primer CDK5). BACE-1 assay was performed as semi-nested approach with two step PCR. CDK5 assay was performed as single PCR step. Following PCR conditions were used for both assays and PCR steps: 94°C for 3 minutes denaturation step followed by 40 cycles of 94°C/15 seconds, 60°C/30 seconds and 68°C/30 seconds. PCR products were run in agarose gel to assess correct size. PCR products were ligated in cloning vector utilizing TOPO TA cloning kit (Invitrogen) as manufacturer specifications and transformed in bacteria by using competent cells. 20 colonies for each transformation were harvested and run on agarose gel to assess right size of insert. Finally, products were sequenced at TACGEN (San Pablo, CA) and methylation was measured as CG to TG conversion at CpG sites.
In vitro experiments
Neuro-2 A neuroblastoma cells stably expressing human sweAPP mutation (N2a-swe) were cultured in DMEM medium, 50% fetal bovine serum, 100 U/mL streptomycin (Cellgro, Herdon, VA) and 0.002 % Geneticin (Life Technologies, CA) at 37ºC in the presence of 5% CO2. Cells were plated in 6-well plate and treated with vehicle or 2 ug / well human-FOXP2-GFP plasmid (Origene, Rockville MD). Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, MA) was used as transfection agent. Cells were harvested 24 hours later and processed for protein and RNA extraction. Supernatant was collected for Aβ measurement.
Statistical analysis
All data are expressed as mean ± SD. The two-tailed Student t test was used to compare up to two groups. One-way ANOVA was used to compare more than two groups whether only one independent variable was present. Two-way ANOVA was used to compare more than two groups whether two independent variables were present. Bonferroni correction test was used after ANOVA. Fisher’s exact test with contingency 2×2 table was utilized to analyze bisulfite sequencing data. In vitro studies were repeated 2 to 4 times in duplicate. Statistical significance was set at p value < .05.
RESULTS
Gestational high fat diet does not affect metabolic parameters in the offspring
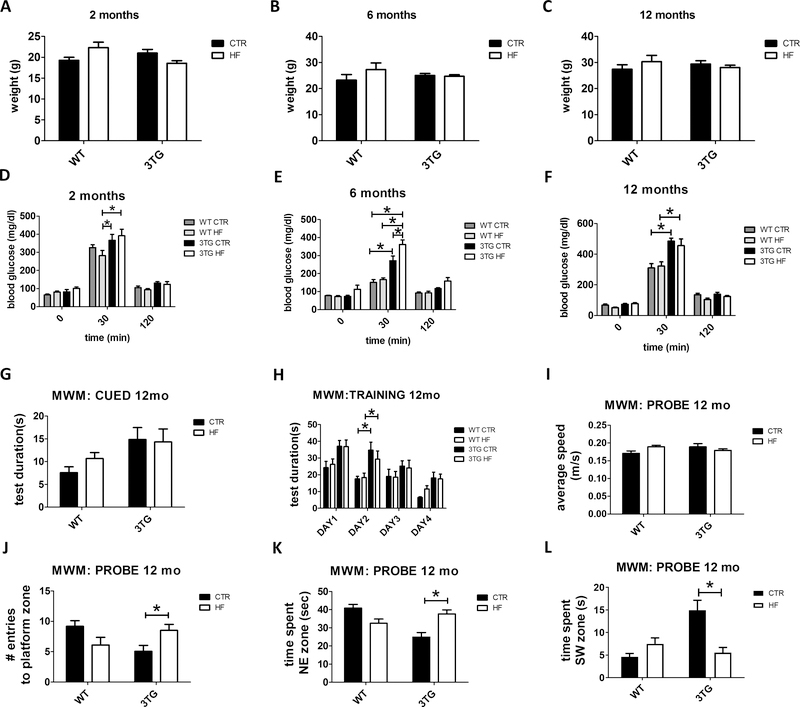
Offspring from WT and 3TG mothers fed either regular chow or high fat diet throughout gestation (WT regular chow: WT CTR; WT high fat diet: WT HF; 3TG regular chow: 3TG CTR; 3TG high fat diet: 3TG HF) were assessed for body weight at 2, 6 and 12 months of age. No significant differences in body weight were observed among the four groups at any of the age time-points considered (Figure 1 A–C). To investigate the effect of the gestational HF diet on blood glucose levels and insulin sensitivity in the offspring, mice were assessed in the glucose tolerance test at 2, 6 and 12 months of age. As shown in figure 1 D–F, no differences in the baseline blood glucose levels were detected at fasting among the four groups at any of the 3 considered age time-points (Figure 1 D–F). However, 3TG CTR and 3TG HF offspring showed higher blood glucose peak 30 minutes after glucose administration at 2, 6 and 12 months of age compared to their WT counterparts (Figure 1 D–F). Finally, no significant differences among the four groups of mice were observed after 120 minutes post-glucose injection at any of the three age time-points considered (Figure 1 D–F).
Figure 1: Effect of gestational high fat diet on metabolic parameters, and cognitive function.
Body weight of WT CTR, WT HF, 3TG CTR and 3TG HF offspring at 2 (A), 6 (B) and 12 (C) months old. Glucose tolerance test (GTT) in WT CTR, WT HF, 3TG CTR and 3TG HF offspring at 2 (D), 6 (E) and 12 (F) months old. Cued phase (G), training phase (H) and probe phase (I-L) of the Morris Water Maze test (MWM) in 12 months old WT CTR, WT HF, 3TG CTR and 3TG HF offspring. Results are mean ± sem. * = p < 0.05.
To assess blood circulating lipid levels in these mice, total cholesterol, HDL cholesterol, VLDL cholesterol and triglycerides were measured by standard enzymatic assay in samples from 12 months old WT CTR, WT HF, 3TG CTR and 3TH HF offspring. As shown in table 2, no significant differences were detected for any of these lipid parameters among the four groups.
TABLE 2.
Blood lipids profile in the 4 groups of mice at 12 months of age.
| Total cholesterol (mg/dL) | HDL cholesterol (mg/dL) | VLDL cholesterol (mg/dL) | Triglycerides (mg/dL) | |
|---|---|---|---|---|
| WT CTR | 67.2 +/− 23.9 | 73.0 +/− 33.9 | 9.2 +/− 3.1 | 48.8 +/− 15.3 |
| WT HF | 93.7 +/− 35.7 | 101.2 +/− 46.0 | 9.2 +/− 1.7 | 45.7 +/− 5.2 |
| 3TG CTR | 44.8 +/− 2.3 | 52.2 +/− 4.1 | 6.4 +/− 1.5 | 32.2 +/− 6.4 |
| 3TG HF | 27.7 +/− 10.7 | 34.5 +/− 13.0 | 7.6 +/− 2.2 | 38.5 +/− 11.7 |
Data are expressed as means ± standard deviation.
Gestational high fat diet rescues spatial learning and memory deficits in the offspring
To assess cognitive function, offspring from all groups were tested in the MWM behavioral paradigm at 6 and 12 months of age. No differences were detected in the cued phase (Figure S1 A) and average swimming speed (Figure S1 C) of the MWM test among the 4 groups at 6 months of age. An increase in the training time was detected in the training phase of the MWM in both 3TG CTR and 3TG HF compared to WT CTR and WT HF respectively at 6 months of age (Figure S1 B). Moreover, no differences were detected in the probe phase of the same test among the 4 groups at 6 months of age (Figure S1 D–F). At 12 months of age, we observed an increase in the time during the training phase of the MWM in both 3TG CTR and 3TG HF compared to WT CTR and WT HF respectively (Figure 1 H). However, when compared with WT controls, 12 month-old 3TG CTR offspring showed reduced spatial learning and memory as indicated by lower number of entries in the platform zone, lower time spent in the NE quadrant and increased time spent in the SW quadrant (Figure 1 J–L). By contrast, at this age 3TG HF offspring showed restoration of cognitive function to WT levels in the same parameters (Figure 1 J–L). No significant differences were detected in the cued phase and average swimming speed of the MWM at 12 months of age among the four groups (Figure 1 G, I).
Gestational high fat diet reduces brain amyloid pathology in the offspring
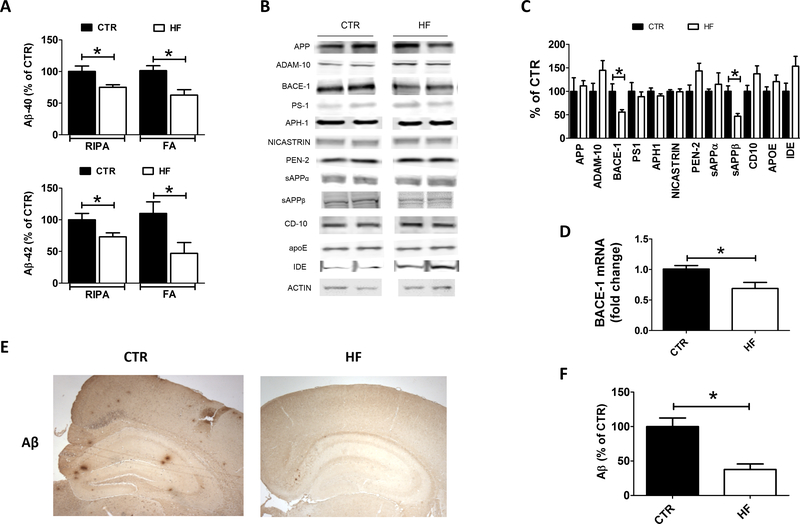
To investigate the effect of the HF diet on brain amyloidosis in the offspring, 12 months old 3TG CTR and 3TG HF brain cortices were used to assess Aβ1–40 and Aβ1–42 levels in both the RIPA and the formic acid soluble fractions. Compared with controls, 3TG HF offspring showed a statistically significant decrease in the levels of Aβ1–40 and Aβ1–42 in both fractions (Figure 2 A). To confirm these findings, brain sections from the two groups were assayed for Aβ (4G8) immunoreactivity as a measure of brain amyloid burden, which reflect Aβ peptides deposited in their parenchyma. As shown in figure 2E and F, we observed that compared with controls, 12 month-old 3TG HF offspring had a significant lower amyloid plaque burden.
Figure 2: Effect of gestational high fat diet on brain amyloidosis.
Aβ−40 and Aβ−42 levels in both RIPA soluble and formic acid soluble fractions in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (A). Protein levels of enzymes involved in production and clearance of Aβ measured by western blot analysis in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (B). Densitometry of previous panel (C). BACE-1 mRNA levels measured by rt-PCR in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (D). Aβ plaques burden measured as Aβ (4G8) immunoreactivity in brain sections of 12 months old 3TG HF and 3TG CTR offspring (E). Quantification of immunoreactivity shown in previous panel (F). Results are mean ± sem. * = p < 0.05. Western blot molecular weight: see Table 1.
To dissect the potential molecular mechanisms by which gestational HF diet resulted in reduced brain amyloidosis, protein levels of the enzymes involved in the production and clearance of Aβ were assayed by western blot analysis. No differences were observed between the two groups of mice when the steady state levels of APP, ADAM-10 and the four components of the γ-secretase complex were assayed (Figure 2 B, C). By contrast, beta secretase 1 (BACE-1) protein levels were significantly decreased in the brain of 3TG HF compared to control offspring (Figure 2 B, C). BACE-1 cleavage product sAPPβ was also reduced in the same group, confirming an effect of the diet on BACE-1 pathway (Figure 2 B, C). BACE-1 mRNA levels were significantly reduced in brain cortex of 3TG HF offspring compared to controls, suggesting a translational regulation of BACE-1 gene expression by gestational HF diet (Figure 2 D). Finally, no changes in the steady state levels of CD-10 and IDE, two major Aβ degrading enzymes, or in apoE levels, an Aβ chaperone, were noted between the two groups of mice (Figure 2 B, C).
Gestational high fat diet reduces brain tau pathology in the offspring
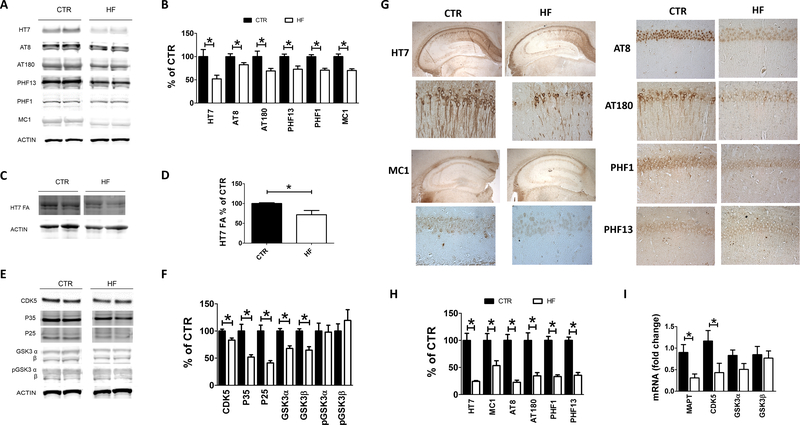
To assess the effect of the gestational diet on tau levels and phosphorylation, 12 months old 3TG CTR and 3TG HF offspring brain cortices were assessed for total soluble tau (HT7 antibody) and its phosphorylated isoforms at Ser202/Thr205, Thr231, Ser396/Ser404 and Ser396, as recognized by the antibodies AT8, AT180, PHF1 and PHF13, respectively. Compared with controls, 3TG HF had significant lower levels of total soluble tau and its phosphorylated isoforms (Figure 3 A, B). These findings were confirmed by immunohistochemistry analyses showing that brain sections of 3TG HF mice had lower immunoreactivity to tau and the phosphorylated tau epitopes (Figure 3 G, H). Additionally, 3TG HF offspring had a significant decrease in the levels of formic acid-soluble tau (insoluble tau) when compared with control mice (Figure 3 C, D). In agreement with this finding we observed that in the same mice the immunoreactivity to tau aggregation-prone isoforms, as recognized by MC1 antibody (27), was also significantly reduced (Figure 3 G, H). Having observed a reduction in the state steady levels of tau protein, next we assessed its mRNA levels. Compared with controls, brains from 3TG HF offspring had a significant decrease in tau mRNA levels (Figure 3 I), suggesting a transcriptional regulation of this gene by the diet.
Figure 3: Effect of gestational high fat diet on tau brain pathology.
Protein levels of RIPA soluble and phosphorylated tau measured by western blot analysis in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (A). Densitometry of previous panel (B). Protein levels of formic acid soluble tau measured by western blot analysis in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (C). Densitometry of previous panel (D). Protein levels of CDK5, P25, P35, GSK3α, GSK3β, pGSK3α, pGSK3β measured by western blot analysis in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (E). Densitometry of previous panel (F). Immunoreactivity to tau, phosphorylated tau and aggregation prone tau measured in brain sections of 12 months old 3TG HF and 3TG CTR offspring (G). Quantification of immunoreactivity shown in previous panel (H). Human tau (MAPT), CDK5 and GSK3α/β mRNA levels measured by rt-PCR in brain cortex of 12 months old 3TG HF and 3TG CTR offspring (I). Results are mean ± sem. * = p < 0.05. Western blot molecular weight: see Table 1.
To investigate potential mechanisms involved in the diet-dependent effects on tau phosphorylation, levels of tau kinases and phosphatases were measured in the brains of these mice. Levels of the kinase CDK5 along with its active forms p35 and p25 were reduced in 3TG HF when compared with controls (Figure 3 E, F). Additionally, the same group had a significant decrease in the levels of total GSK3α and GSK3β, but no changes were detected in their phosphorylated isoforms (Figure 3 E, F). Interestingly, while compared with controls 3TG HF mice showed significant reduction in the mRNA levels for CDK5, no changes were observed in the mRNA levels for GSK3α and GSK3β (Figure 3 I).
Gestational high fat diet rescues synaptic dysfunction in the offspring
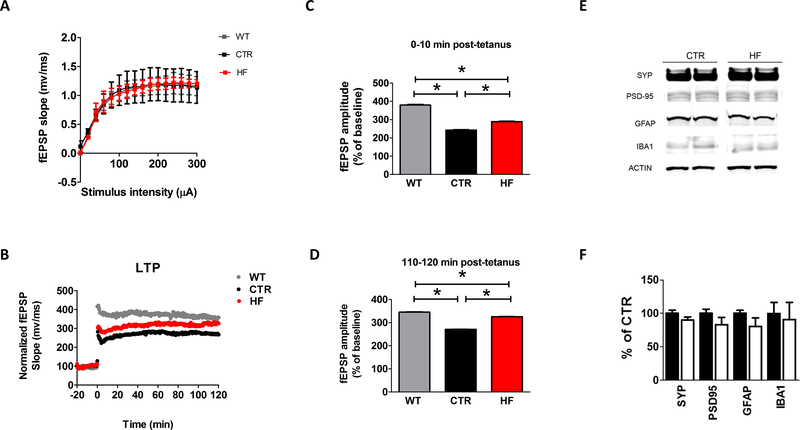
Since gestational high fat diet restored the behavioral impairments in the 3TG offspring, we then assessed synaptic function by electrophysiology of hippocampal slices in the same group of mice. Basal synaptic transmission was assessed by generating input/output (I/O) curves and measuring field excitatory postsynaptic potentials (fEPSPs) elicited in CA1 by stimulation of the Schaffer collaterals at increasing strength of stimulus intensities. As shown in Figure 4 A, no significant differences were detected in I/O curves among the groups. Next, short-term plasticity was measured by examining paired pulse facilitation, displaying an activity-dependent presynaptic modulation of transmitter release (28). Long-term potentiation (LTP) in the CA1 region of the hippocampus was examined as a measure of neuronal plasticity and in vitro representation of memory function (29). Consistent with their genotype 3TG CTR offspring displayed significantly reduced fEPSP slope when compared to WT CTR. By contrast, 3TG HF offspring showed a partial restoration of the fEPSP slope at 10 minutes post-tetanus, and complete restoration to the WT CTR levels at 120 minutes post-tetanus (Figure 4 B–D).
Figure 4: Effect of gestational high fat diet on synaptic integrity and neuroinflammation.
Input/Output (I/O) curves and representative field excitatory postsynaptic potentials (fEPSPs) at increasing stimulus strengths (0–300 A) in 12 months old WT CTR, 3TG CTR and 3TG HF offspring (A). 2 hours fEPSP slopes recordings expressed as percent pretetanus baseline in 12 months old WT CTR, 3TG CTR and 3TG HF offspring (B). Long-term potentiation (LTP) magnitudes expressed as percent of baseline for 0 to 10 minutes post-tetanus (C) and long-term potentiation (LTP) magnitudes expressed as percent of baseline for 110 to 120 minutes post-tetanus (D) in 12 months old WT CTR, 3TG CTR and 3TG HF offspring. Protein levels of synaptic integrity and neuroinflammatory markers measured by western blot in brain cortex of in 12 months old 3TG CTR and 3TG HF offspring (E). Densitometry of the immune-reactivities presented in the previous panel (F). Results are mean ± sem. * = p < 0.05. Western blot molecular weight: see Table 1.
Synaptic integrity was also assessed by measuring brain cortex levels of pre and post-synaptic protein markers synaptophysin (SYP) and post-synaptic density protein 95 (PSD-95) in the two groups of mice. No significant differences were detected in any of the synaptic integrity markers between them (Figure 4 E, F). Since neuroinflammation can also influence synaptic health in the contest of AD, markers for astrocytes and microglia activation such as glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (IBA1) respectively were measured in brain cortex of the two groups of offspring. No significant differences were detected in any of the neuroinflammatory markers between them (Figure 4 E, F).
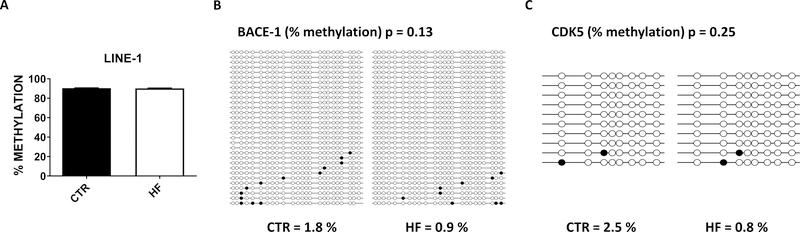
Gestational high fat diet does not affect brain DNA methylation in the offspring
To investigate possible mechanism(s) by which gestational HF diet affected gene expression in the 3TG offspring, we examined brain DNA methylation status given prior data linking gestational diets with epigenetic changes (30). To this end, we assessed the effect on both repetitive element methylation as a surrogate for global methylation (25, 26, 31), and promoter specific methylation of two of the genes we found downregulated in the offspring from mothers exposed to HF diet: BACE-1 and CDK5. As shown in figure 5, we did not observe any significant difference in methylation of LINE-1 repeats in the two groups (Figure 5 A). Next, we assessed methylation of the BACE-1 and CDK5 promoters by bisulfite-cloning and sequencing in the same samples. Compared with control mice, no significant effect of the HF diet was observed on the methylation status of BACE-1 and CDK5 promoter in the 3TG HF offspring (Figure 5 B, C).
Figure 5: Effect of gestational high fat diet on global and promoter specific DNA methylation.
Methylation levels at LINE-1 repeats analyzed by bisulfite pyrosequencing in brain cortex of 12 months old 3TG CTR and 3TG HF offspring (A). BACE-1 promoter (B) and CDK5 promoter (C) methylation levels assessed by bisulfite sequencing in brain cortex of 12 months old 3TG CTR and 3TG HF offspring. Results are mean ± sem. * = p < 0.05.
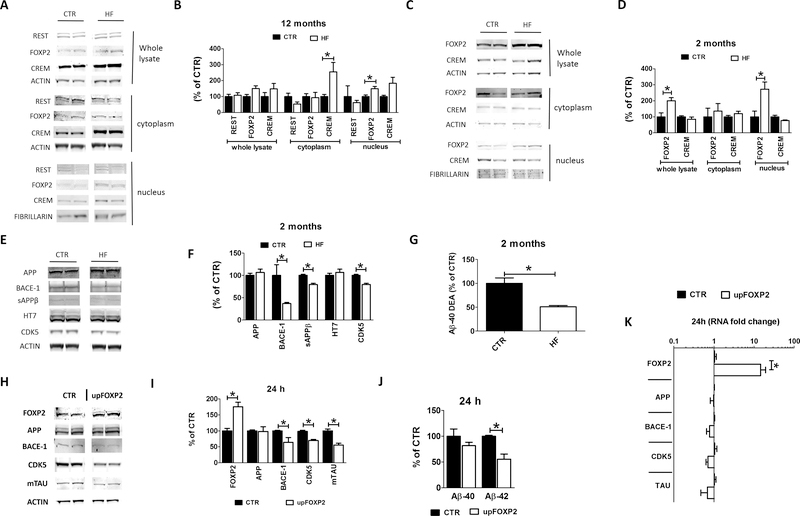
Gestational high fat diet upregulates brain FOXP2 in the offspring
In search for other mechanism(s) whereby the HF diet could have modulated the expression levels of our candidate genes, next we focused on transcription factors predicted to bind to them at either promoter or enhancer sites, thus potentially capable of regulating their expression simultaneously. We selected 3 transcription factors reported to be dysregulated in AD and other tauopathies: repressor element 1 silencing transcription factor (REST), fork head box P2 (FOXP2) and cAMP responsive element modulator (CREM) (32–34). In particular, we measured their protein levels in total cell lysate, cytoplasm and nuclear fractions from brain cortices of 12 months old 3TG CTR and 3TG HF offspring. No differences between the 2 groups of mice were observed when the whole lysate was probed for any of these factors (Figure 6 A, B). In the cytoplasm fraction we observed that compared with controls 3TG HF mice had a significant increase only for CREM (Figure 6 A, B). On the other hand, compared with controls, we found that the nuclear fraction from the 3TG HF mice had a significant increase in the levels of FOXP2 and a trend towards increase in the levels of CREM (Figure 6 A, B). By contrast, no significant changes were found in REST levels between the two groups in the nuclear fraction (Figure 6 A, B). To assess whether the observed dysregulation of these transcription factors was an early event, their levels were also assessed in 2 month-old mice. At this age, compared with controls 3TG HF offspring had significantly higher FOXP2 nuclear levels, but no changes were observed for CREM levels (Figure 6 C, D). Given the early upregulation of FOXP2 under our experimental condition, we measured protein levels of our candidate genes at this age in the two groups of mice. As show in figure 6, we found a significant reduction of steady state levels of BACE-1, its cleavage product sAPP-β, and CDK5 in 2 months old 3TG HF offspring compared to controls (Figure 6 E, F). While no changes were observed for total APP and tau protein levels in the same animals, 3TG HF offspring had a significant decrease in the total Aβ−40 levels (Figure 6 G).
Figure 6: Gestational high fat diet reduces BACE-1, tau and CDK5 expression by upregulating transcriptional repressor FOXP2 in the offspring.
Protein levels of REST, FOXP2 and CREM measured by western blot in whole lysate, cytoplasm and nuclear fraction from brain cortex of 12 months old 3TG CTR and 3TG HF offspring (A). Densitometry of previous panel (B). Protein levels of REST, FOXP2 and CREM measured by western blot in whole lysate, cytoplasm and nuclear fraction from brain cortex of 2 months old 3TG CTR and 3TG HF offspring (C). Densitometry of previous panel (D). Protein levels of APP, BACE-1, sAPPβ, tau (HT7) and CDK5 measured by western blot in in brain cortex of 2 months old 3TG CTR and 3TG HF offspring (E). Densitometry of previous panel (F). Aβ−40 levels measured by ELISA in diethylamine (DEA) lysates from brain cortex of 2 months old 3TG CTR and 3TG HF offspring (F). FOXP2, APP, BACE-1, mouse tau (mTAU) and CDK5 protein levels measured by western blot in N2A cells treated with FOXP2-GFP plasmid or vehicle for 24 hours (H). Densitometry of previous panel (I). Aβ−40 and −42 levels in the supernatant of N2A cells from panel H (J). FOXP2, APP, BACE-1, mouse tau (mapt) and CDK5 mRNA levels measured by rt-PCR in N2A cells from panel H (K). Results are mean ± sem. * = p < 0.05. Western blot molecular weight: see Table 1.
FOXP2 regulates of BACE-1, CDK5 and tau gene expression
To prove that FOXP2 specifically influenced the expression levels of BACE-1, tau and CDK5, we treated N2A cells carrying the APPswe mutation with FOXP2-GFP plasmid and assessed FOXP2, BACE-1, tau and CDK5 protein and mRNA levels 24 hours after treatment. As expected, we observed a robust upregulation of FOXP2 protein and mRNA levels, which was associated with a downregulation of BACE-1, tau and CDK5 at both mRNA and protein levels (Figure 6 H–K). However, FOXP2 upregulation did not affect APP protein and mRNA levels suggesting a specific effect of this factor on the candidate genes (Figure 6 H–K). Finally, in line with the FOXP2-dependent downregulation of BACE-1 pathway we observed a significant reduction in Aβ1–42 peptide levels in the conditioned media from the same cells (Figure 6 J).
DISCUSSION
In this study we demonstrate that HF diet administered to dams during their gestation period protects the offspring of an AD mouse model from spatial learning and memory impairments, synaptic dysfunction, brain amyloidosis, and tau neuropathology later in life. Additionally, we provide experimental evidence showing that these diet-dependent effects are mediated by transcriptional regulation of three major AD relevant genes, BACE-1, tau, and CDK5, via the activation of the transcription repressor FOXP2.
Our work is the first to report that in utero exposure to a specific dietary regimen can modulate AD pathophysiology in the offspring later in life. This new knowledge could help elucidate the mechanisms underlying the maternal influence on the risk of AD onset and development as well as provide insights on novel preventative strategies against the disease.
Early-onset familial AD is due to specific autosomal dominant mutations inherited from one of the two parents (4). By contrast, the most common late-onset or sporadic variant of the disease stems from the interaction of both genetic risk alleles and environmental risk factors (3). Epidemiological studies have demonstrated that some sporadic AD cases follow a maternal inheritance pattern putting offspring of mothers with late-onset AD at three times higher risk to develop the disease later in life compared to offspring from fathers with sporadic AD (9). However, the mechanism(s) governing this form of inheritance are still elusive. We know that nutrition and lifestyle can play a crucial and direct role in AD pathophysiology (11–14). Midlife hypercholesterolemia and dyslipidemia are predisposing risk factors for AD later in life (35). Several reports have shown that direct exposure to HF diet exacerbates cognitive decline and brain pathology in both AD and WT mice (15–17, 36–38). Also, in mice HF diet during gestation and then lactation exacerbates memory decline and brain pathology in the offspring (18–20). However, the effect that maternal exposure to a HF diet restricted only to the gestational period may have on the risk to develop AD later in life in the offspring has never been tested.
Addressing this important biological question could ultimately shed some new lights into the molecular and cellular mechanisms involved into the AD maternal transmission and inheritance. With this goal in mind, we selected the 3TG mice which recapitulate the main hallmarks of AD such as memory impairments, brain amyloidosis, tau pathology and synaptic dysfunction (21) and fed them HF diet during gestation only. In particular, we investigated the effect that this diet had on the AD-like phenotype of the offspring which were never exposed to it during their life time. First, we tested their metabolic profile by assessing body weight, glucose levels, insulin sensitivity and a panel of the most common circulating plasma lipids. In utero exposure to HF diet did not influence body weight, fasting blood glucose, insulin sensitivity and plasma lipid levels in the offspring, suggesting that this diet did not have any effect on the general metabolic profile of the animals.
To measure cognitive function, we assessed the offspring in the Morris water maze paradigm at 6 and 12 months of age. Despite no change was observed at 6 months of age, gestational HF diet restored cognitive function of the 12-month-old 3TG offspring back to WT mice levels in this test demonstrating a protective effect on the age-dependent cognitive deficits. Interestingly, we did not observe any gender difference in the effect that the gestational HF diet had on the offspring’s behavior. Previous studies reported that administering HF diet during both gestation and lactation has a deleterious effect of 3xTg offspring’s cognition in the Morris water maze test (19). The discrepancy between these findings and our results could be justified by the different exposure time, with gestation only in our study, versus gestation and lactation interval exposure in the other one. We hypothesize that the timing of the in utero exposure to the HF diet is crucial in terms of offspring’s cognitive performance later in life. Moreover, previous studies (19) implemented a more aggressive type of HF diet with 60% calories from fats, compared with our own diet which had 42% calories from fats. It is reasonable to hypothesize that a higher fat intake could promote a deleterious effect compared to a moderate exposure.
Having observed a positive effect on the behavioral impairments in the 3TG mice secondary to the in utero exposure to a HF diet, next we were interested to assess whether brain pathology in these mice would follow the same pattern of improvement for both the classical Aβ as well as the tau pathologic signatures in this model. As expected for their age, 3TG CTR offspring had discrete levels of both Aβ 1–40 and Aβ 1–42 peptides in both the soluble and insoluble fractions. However, these levels were significantly decreased in the 3TG offspring from mothers that during gestation received the HF diet. Importantly, these results were mirrored by a dramatic reduction in the amount of Aβ peptides deposited in the brains of the same mice. In search for the molecular mechanisms whereby the diet has resulted in a reduction in brain amyloidosis, we investigated a possible effect on the Aβ precursor protein, APP, as well as the different proteolytic enzymes that are known to proteolytically metabolize it in order to generate its final products Aβ 1–40 and Aβ 1–42 peptides. While we did not observe any significant changes in the levels of APP, ADAM-10 and the γ-secretase complex, we saw that compared with controls, brains from 3TG HF offspring had a significant decrease in BACE-1 protein levels and its cleavage product sAPPβ. The same offspring displayed reduced BACE-1 mRNA, suggesting that gestational HF regulated BACE-1 expression at the transcriptional level. Our results for this aspect of the phenotype are in contrast with a previous study in which the authors showed that when maternal HF diet was administered to AD mice starting at 4 weeks before mating up to lactation, the effect on brain amyloidosis was instead detrimental (20). Once again, we believe that this apparent discrepancy further highlights the crucial importance of timing for the exposure to maternal HF diet in terms of offspring’s brain health and susceptibility to AD pathophysiology.
In addition to lower amyloidosis, compared with controls 3TG HF offspring displayed significantly lower levels of tau neuropathology as assessed by decreased tau phosphorylation at different epitopes involved in tangles formation, together with lower aggregation prone tau isoforms, as assessed with MC1 immunoreactivity, and a significant decrease in the insoluble tau fraction. These observations correlated with lower expression of both human tau and CDK5, an important kinase for tau phosphorylation, at the protein and mRNA levels, suggesting a transcriptional regulation of these gene pathways secondary to gestational HF diet. No gender differences were observed in the effects that the gestational HF diet had on the offspring for both Aβ as well as tau neuropathology.
Another important aspect of the AD phenotype is an alteration of synaptic function, which is not only considered as one of the earliest changes occurring in the pathophysiology of the disease, but also the biologic substrate for the memory and cognitive impairments in the patients affected by it (29). To test this function, we implemented an electrophysiological approach by measuring long-term potentiation in hippocampal slices. As expected from the behavioral data, we observed that compared with control WT offspring 3TG had a significant impairment of their LTP responses at both 10 and 120 minutes. However, the deficit in the synaptic response was rescued in the 3TG HF offspring, which at 120 minutes of recording reached a level that was almost indistinguishable from the WT mice controls.
One of the most common mechanisms associating in utero exposure with a later in life effect are epigenetic modifications which are typically mediated by changes in methylation levels (13, 30). Having observed significant changes in some AD relevant genes, we were interested to assess whether gestational HF diet had an influence on brain global methylation or gene promoter specific DNA methylation in these animals. Under our experimental conditions, no changes were observed both in global DNA methylation and methylation of both BACE-1 and CDK5 promoter between the 2 groups of mice. Although we cannot completely rule out the possibility that additional DNA methylation changes other than those (i.e., BACE-1 and CDK5) that we investigated might be present in the animals, taken together our results would exclude methylation as the potential primary mechanism whereby the HF diet modulated the expression levels of our genes of interest.
In search for other potential mechanisms, we assessed levels of three major transcriptional repressors (REST, FOXP2 and CREM) all of which were predicted to modulate simultaneously the expression of the three genes of interest. Among them, we found that compared with controls FOXP2 was upregulated in brain nuclear fraction of 12 months old 3TG HF offspring. To assess whether the dysregulation of this transcription factors was an early event before the development of the neuropathology and behavioral impairments, we investigated its levels also in 2 month-old mice. Interestingly, we observed that a similar FOXP2 nuclear fraction upregulation together with lower expression levels of our candidate genes and reduced total brain amyloidosis were already present in the brains of these very young mice, suggesting not only an early effect of the in utero exposure to the HF diet, but also a primary role of this effect in modulating the development of the AD-like phenotype later in life. The direct influence and functional role of FOXP2 on our candidate genes was further corroborated in our vitro experiments where we showed that transient over-expression of this factor resulted in downregulation of BACE-1, tau and CDK5 together with a significant reduction in the amount of Aβ peptide released in the conditioned media.
In summary, in the current paper we provide the first experimental evidence that in utero exposure to a HF diet rescues spatial learning and memory impairments, synaptic dysfunction, Aβ and tau neuropathology in the offspring of an AD mouse model. The protective effect of the HF maternal diet against the development of the AD phenotype later in life is mediated by a transcriptional regulation of key genes relevant to the pathophysiology of the disease.
Taken together our findings, besides shedding light into a novel mechanism underlying the maternal influence on the risk of AD onset and development, provide insights on the design of possible in utero dietary interventional approaches as a novel preventative strategy against the disease onset later in life.
Supplementary Material
Figure S1: Effect of gestational high fat diet on cognitive function at 6 months old. Cued phase (A), training phase (B) and probe phase (C-F) of the Morris Water Maze test (MWM) in 6 months old WT CTR, WT HF, 3TG CTR and 3TG HF offspring. Results are mean ± sem. * = p < 0.05.
ACKNOWLEDGEMENT
Special thanks to Dr. Margaret Sperow and Dr. Lynn Kirby for technical assistance in the electrophysiology experiments, and to Dr. Peter Davies for providing the MC1 antibody. Domenico Praticò is the Scott Richards North Star Charitable Foundation Chair for Alzheimer’s Research. This study was supported in part by grants from the National Institute of Health (AG060711), and the Scott Richards North Star Charitable Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interest.
REFERENCES
- 1.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. (2002): Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 16:203–212. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA (2012): The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging. 27:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association As (2016): 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12:459–509. [DOI] [PubMed] [Google Scholar]
- 4.Querfurth HW, LaFerla FM (2010): Alzheimer’s disease. N Engl J Med. 362:329–344. [DOI] [PubMed] [Google Scholar]
- 5.Duara R, Lopez-Alberola RF, Barker WW, Loewenstein DA, Zatinsky M, Eisdorfer CE, et al. (1993): A comparison of familial and sporadic Alzheimer’s disease. Neurology. 43:1377–1384. [DOI] [PubMed] [Google Scholar]
- 6.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. (1997): Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 278:1349–1356. [PubMed] [Google Scholar]
- 7.Gómez-Tortosa E, Barquero MS, Barón M, Sainz MJ, Manzano S, Payno M, et al. (2007): Variability of age at onset in siblings with familial Alzheimer disease. Arch Neurol. 64:1743–1748. [DOI] [PubMed] [Google Scholar]
- 8.Heyman A, Wilkinson WE, Hurwitz BJ, Schmechel D, Sigmon AH, Weinberg T, et al. (1983): Alzheimer’s disease: genetic aspects and associated clinical disorders. Ann Neurol. 14:507–515. [DOI] [PubMed] [Google Scholar]
- 9.Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M (2010): Maternal transmission of Alzheimer’s disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 4:170–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, et al. (2011): Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J Alzheimers Dis. 27:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arab L, Sabbagh MN (2010): Are certain lifestyle habits associated with lower Alzheimer’s disease risk? J Alzheimers Dis. 20:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creegan R, Hunt W, McManus A, Rainey-Smith SR (2015): Diet, nutrients and metabolism: cogs in the wheel driving Alzheimer’s disease pathology? Br J Nutr. 113:1499–1517. [DOI] [PubMed] [Google Scholar]
- 13.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. (2008): Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 105:17046–17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J (2011): Epigenetic mechanisms in Alzheimer’s disease. Neurobiol Aging. 32:1161–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B, Hasegawa Y, Takane K, Koibuchi N, Cao C, Kim-Mitsuyama S (2016): High-Fat-Diet Intake Enhances Cerebral Amyloid Angiopathy and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease, Independently of Metabolic Disorders. J Am Heart Assoc. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sah SK, Lee C, Jang JH, Park GH (2017): Effect of high-fat diet on cognitive impairment in triple-transgenic mice model of Alzheimer’s disease. Biochem Biophys Res Commun. 493:731–736. [DOI] [PubMed] [Google Scholar]
- 17.Thériault P, ElAli A, Rivest S (2016): High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget. 7:67808–67827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SW, Yu HR, Sheen JM, Tiao MM, Tain YL, Lin IC, et al. (2017): A maternal high-fat diet during pregnancy and lactation, in addition to a postnatal high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: beneficial effects of resveratrol. Oncotarget. 8:111998–112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SA, Jameson CH, Allan SM, Lawrence CB (2014): Maternal high-fat diet worsens memory deficits in the triple-transgenic (3xTgAD) mouse model of Alzheimer’s disease. PLoS One. 9:e99226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizari S, Carare RO, Hawkes CA (2016): Increased Aβ pathology in aged Tg2576 mice born to mothers fed a high fat diet. Sci Rep. 6:21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. (2003): Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 39:409–421. [DOI] [PubMed] [Google Scholar]
- 22.Di Meco A, Joshi YB, Praticò D (2014): Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 35:1813–1820. [DOI] [PubMed] [Google Scholar]
- 23.Di Meco A, Lauretti E, Vagnozzi AN, Praticò D (2014): Zileuton restores memory impairments and reverses amyloid and tau pathology in aged Alzheimer’s disease mice. Neurobiol Aging. 35:2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannopoulos PF, Chu J, Joshi YB, Sperow M, Li JG, Kirby LG, et al. (2013): 5-lipoxygenase activating protein reduction ameliorates cognitive deficit, synaptic dysfunction, and neuropathology in a mouse model of Alzheimer’s disease. Biol Psychiatry. 74:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, et al. (2010): Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 20:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang S, et al. (2017): Caloric restriction delays age-related methylation drift. Nat Commun. 8:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jicha GA, Bowser R, Kazam IG, Davies P (1997): Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 48:128–132. [DOI] [PubMed] [Google Scholar]
- 28.Zucker RS, Regehr WG (2002): Short-term synaptic plasticity. Annu Rev Physiol. 64:355–405. [DOI] [PubMed] [Google Scholar]
- 29.Bliss TV, Collingridge GL (1993): A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361:31–39. [DOI] [PubMed] [Google Scholar]
- 30.Waterland RA, Jirtle RL (2003): Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 23:5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang AS, Estécio MR, Doshi K, Kondo Y, Tajara EH, Issa JP (2004): A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 32:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein HG, Kirches E, Bogerts B, Lendeckel U, Keilhoff G, Zempeltzi M, et al. (2013): Wide distribution of CREM immunoreactivity in adult and fetal human brain, with an increased expression in dentate gyrus neurons of Alzheimer’s as compared to normal aging brains. Amino Acids. 45:1373–1383. [DOI] [PubMed] [Google Scholar]
- 33.López-González I, Palmeira A, Aso E, Carmona M, Fernandez L, Ferrer I (2016): FOXP2 Expression in Frontotemporal Lobar Degeneration-Tau. J Alzheimers Dis. 54:471–475. [DOI] [PubMed] [Google Scholar]
- 34.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, et al. (2014): REST and stress resistance in ageing and Alzheimer’s disease. Nature. 507:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA (2009): Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busquets O, Ettcheto M, Pallàs M, Beas-Zarate C, Verdaguer E, Auladell C, et al. (2017): Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mech Ageing Dev. 162:38–45 [DOI] [PubMed] [Google Scholar]
- 37.Kessler AR, Kessler B, Yehuda S (1986): In vivo modulation of brain cholesterol level and learning performance by a novel plant lipid: indications for interactions between hippocampal-cortical cholesterol and learning. Life Sci. 38:1185–1192. [DOI] [PubMed] [Google Scholar]
- 38.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. (2008): High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 106:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effect of gestational high fat diet on cognitive function at 6 months old. Cued phase (A), training phase (B) and probe phase (C-F) of the Morris Water Maze test (MWM) in 6 months old WT CTR, WT HF, 3TG CTR and 3TG HF offspring. Results are mean ± sem. * = p < 0.05.