Abstract
Introduction
Neuromuscular Junctions (NMJs) are the synapses between motor neurons and skeletal muscle fibers, and they are responsible for voluntary motor function. NMJs are affected at early stages of numerous neurodegenerative and neuroimmunological diseases. Due to the difficulty of systematically studying and manipulating NMJs in live subjects, in vitro systems with human tissue models would provide a powerful complement to simple cell cultures and animal models for mechanistic and drug development studies.
Areas covered
The authors review the latest advances in in vitro models of NMJs, from traditional cell co-culture systems to novel tissue culture approaches, with focus on disease modeling and drug testing.
Expert opinion
In recent years, more sophisticated in vitro models of human NMJs have been established. The combination of human stem cell technology with advanced tissue culture systems has resulted in systems that better recapitulate the human NMJ structure and function, and thereby allow for high-throughput quantitative functional measurements under both healthy and diseased conditions. Although they still have limitations, these advanced systems are increasingly demonstrating their utility for evaluating new therapies for motoneuron and autoimmune neuromuscular diseases, and we expect them to become an integral part of the drug discovery process in the near future.
1. INTRODUCTION
The neuromuscular junction (NMJ) is the chemical synapse between a motoneuron and a skeletal muscle fiber that enables muscle contraction and allows for voluntary motor movement. NMJ formation is controlled by interactions between motoneurons, skeletal muscle fibers, and glial cells [1]. In vertebrates, the presynaptic motor nerve terminal dictates the synthesis, storage, and release of the neurotransmitter acetylcholine (ACh) and agrin[2]. When an action potential reaches the presynaptic terminal and activates voltage-gated calcium channels, calcium enters the neuron and triggers the diffusion of ACh across the synaptic cleft to the acetylcholine receptors (AChRs) on the postsynaptic muscle membrane. Activation of these receptors leads to opening of cation channels in the muscle membrane, producing its depolarization. Meanwhile, acetylcholinesterases located within the synaptic cleft degrade the acetylcholine, allowing the NMJ to return to its resting state.
The NMJ is a structure of interest in a number of processes with biomedical relevance. For instance, the NMJ is the target of numerous poisons and neurotoxins such as curare, botulinum toxin, or nervous agents such as sarin gas. Furthermore, changes in NMJ structure have been detected at early stages in several motoneuron diseases, such as spinal muscular atrophy (SMA) [3–5] and amyotrophic lateral sclerosis (ALS)[6,7], suggesting that the NMJ could be a relevant therapeutic target. The NMJ is also affected in autoimmune diseases such as myasthenia gravis (MG), Lambert-Eaton myasthenic syndrome (LEMS) and Guillain-Barré Syndrome (GBS), causing muscle weakness. Similarly, structural and functional changes of the NMJ have been reported in healthy individuals as well as part of the normal aging process[8,9].
Historically, animal models have been used to study the NMJ and have led to many discoveries regarding its formation and function[10]. However, there are differences between the human and other mammalian synapses that interfere with the transferability of results from animal models to humans. Jones et al. [11] showed there is a distinct anatomical difference between the human NMJ and the mouse NMJ; morphologically, the human NMJ is significantly smaller and more fragmented than its mouse counterpart, yet its active zone density is also higher than that of mice They also found differences in protein expression levels between human and mouse in crucial molecular pathways impacting NMJ form and function.
Furthermore, despite the undeniable value of animal systems in preclinical drug discovery, there is a disconnect between drug responses measured in animal systems and those in human clinical trials. As a result, only a small fraction of drugs that enter the clinical trial pipeline ends up being approved. This unacceptably low success rate can be attributed, at least in part, to the failure of animal disease models in replicating human disease, which often results in differential drug responses and toxicities relatively to the human data they would need to emulate. In vitro human physiological models have the potential to help bridge this gap and to greatly increase the safety and decrease the cost of drug development by complementing animal studies during the preclinical phases of drug development. Furthermore, from a basic science standpoint, systematically studying and manipulating NMJs in vivo presents an enormous technical challenge. Robust in vitro human tissue systems would allow for controllable and systematic exploration of NMJ formation, maturation and response to toxic agents and drugs.
An important stepping stone for the development of human in vitro systems over the past decade has been the development of protocols for the differentiation of human stem cell lines into multiple cell types such as cardiomyocytes [12–14], hepatocytes [15,16], skeletal myocytes [17,18], and motoneurons [19–21], among others. The ability to genetically reprogram easily obtainable adult cells such as skin fibroblasts into induced pluripotent stem cells (iPSCs) through the induction of transcription factors [22,23] has opened the door to the development of donor-specific in vitro models, including the genetic disease models. For instance, recent studies have successfully created iPSC cell lines from patients with SMA [24–26] and ALS [27–32] and subsequently differentiated these cells into motoneurons, providing a human platform for disease modeling and drug screening in two diseases that have historically lacked treatment options.
Here, we review the development of in vitro NMJ models, starting from the organotypic and co-culture systems that have been initially created, and progressing to the later and more sophisticated microfluidic and tissue-engineered models, with a focus on human systems for personalized medicine and disease modeling. We then highlight the progress made towards developing robust, reproducible and quantifiable systems and their potential for use in predictive drug screening and development.
2. HEALTHY in vitro NMJ MODELS
The first step of mimicking NMJ development and functionality in vitro is the establishment of healthy NMJ models. These models allow for mechanistic studies of NMJ formation and maturation, aging, toxins and antitoxins, and drug effects. Furthermore, they lay the foundation for the development of disease models. In this section we summarize the advances made in healthy NMJ models, starting from traditional 2D cultures of different cell types (Table 1) to advanced culture systems (Table 2).
Table 1.
Overview of the in vitro co-culture models of NMJ and their use for drug testing.
| Muscle source | Neuron source | Achievement | Validation of NMJ function | Disease model | Drug testing | Ref |
|---|---|---|---|---|---|---|
| Rat/mouse | Rat/mouse | First in vitro NMJs | No | No | No | Peterson et al., 1972 [33] |
| Rat | Rat | long-term culture, defined system | No | No | No | Das et al., 2010 [34] |
| Mouse ESCs | Chicken | stem cell derived | Drug response |
No | ATX,TC, dynasore, nifedipine, TTX | Chipman et al, 2014 [35] |
| Mouse, transdifferentiated fibroblasts | Mouse ESCs | electrical stimulation | Glutamate Stim, drug response | No | BoNT, neostigmine, MEChMAz, TTX, vesamicol | Charoensook et al., 2017 [68] |
| Mouse C2C12 | Human ESCs | human MN | No | No | No | Li et al., 2005 [36] |
| Rat | Human spinal cord stem cells | human MN, defined system | Glutamate Stim, drug response | No | TC | Guo et al., 2010 [37] |
| Human | Human spinal cord stem cells | patterned surface for myotube alignment | Drug response | No | TC | Guo et al., 2011[38] |
| Mouse C2C12 | Human iPSCs | First disease NMJ model | No | SMA | VPA, ASO | Yoshida et al., 2015 [65] |
| Human iPSCs | Human iPSCs | all iPSC-derived | No | No | No | Demestre et al., 2015 [39] |
ASO: antisense oligonucleotides, ATX: Agatoxin, BoNT: Botulinum toxin, TC: Tubocurarine, ESC: embryonic stem cells, MEChMAz: Acetylethylcholine mustard hydrochloride, iPSCs: induced pluripotent stem cells, TTX: Tetrodotoxin., VPA: valproic acid.
Table 2.
Overview of the advanced in vitro models of NMJ and their applications for drug testing and disease modeling.
| Muscle source | Neuron source | Special features | Validation of NMJ function | Disease model | Drug testing | Ref |
|---|---|---|---|---|---|---|
| Rat | Rat | Compartmentalized, glial cells | No | No | No | Southam et al., 2013 [43] |
| Mouse C2C12 | Mouse | Compartmentalized | No | No | No | Park et al., 2013 [44] |
| Mouse | Mouse | Compartmentalized | Glutamate stimulation, drug response | No | TTX | Zahavi et al., 2015 [45] |
| Human | human NSC, iPSCs | Compartmentalized, BioMEMs | Electrical stimulation, drug response | No | TC, BoNT, BTX | Santhanam et al., 2018 [46] |
| Mouse C2C12 | Mouse, ESCs | 3D skeletal tissue, cantilevers | Glutamate stimulation, drug response | No | TC | Morimoto et al., 2013 [54] |
| Rat | Rat | Cantilevers, photodetector | Glutamate stimulation, drug response | No | TC | Smith et al., 2013 [56] |
| Human | Human ESCs | 3D culture | Glutamate stimulation | MG | TC, BoT, WTX | Afshar Bakooshli et al., 2019 [57] |
| Human | Human ESCs | Optogenetic | Optical stimulation | MG | PYR | Steinbeck et al., 2016 [59] |
| Mouse C2C12 | Mouse ESCs | Compartmentalized, 3D, optogenetic | Optical stimulation, drug response | No | BTX | Uzel et al., 2016 [60] |
| Human | Human iPSCs | Compartmentalized, 3D, optogenetic, automated | Optical stimulation, drug response | MG | BTX | Vila et al., 2019 [62] |
| Human iPSCs | Human iPSCs | Compartmentalized, optogenetic | Optical stimulation, drug response | ALS | BTX, rapamycin, bosutinib | Osaki et al., 2018 [61] |
ALS: amyotrophic lateral sclerosis, ATX: Agatoxin, BoNT: Botulinum toxin, TC: Tubocurarine, ESC: embryonic stem cells, iPSCs: induced pluripotent stem cells, MG: myasthenia gravis, NSC: Neural stem cells, PYR: pyridostigmine; TTX: Tetrodotoxin.
2. 1. Co-culture in vitro NMJ models
The very first in vitro NMJ models consisted of organotypic cultures of neuromuscular junctions generated from explanted cells. In 1972, Peterson et al. cultured adult rodent and human skeletal fibers with fetal rodent spinal cord explant, and demonstrated for the first time that innervation was necessary for muscle regeneration [33].
The next step in the development of in vitro NMJs models was the cultivation of isolated motoneurons and skeletal muscle, which allowed for a better control of the cultures. Das et al. [34] used rat embryonic neurons and rat primary muscle to establish a long-term co-culture in a serum-free system, demonstrating myotube maturation by expression of neonatal myosin heavy chain. In these studies, the formation of NMJs was demonstrated through immunocytochemistry, but no functional tests were performed.
A major step towards the development of human in vitro NMJ models was the incorporation of stem cell-derived components into the co-cultures, to eliminate the need for repeated harvesting of primary cells. The first stem-cell models used the cells of murine origin, allowing for direct comparisons between NMJs formed from primary cells and those derived from stem cells. In 2014, Chipman et al. used co-cultures of murine embryonic stem cell (ESC)-derived motoneurons (MN) and primary chick myotubes to study the role of the neural cell adhesion molecule (NCAM) in NMJs maturation [35]. They recorded spontaneous action potentials in the myotubes that stopped upon blockage of the NMJs. Their model was able to replicate the structural and functional deficits presented in NCAM−/− mice. Furthermore, their results showed that chronic treatment with a synaptic vesicle blocker improved synapse formation and function in the NCAM deficient cultures, demonstrating the potential of their model to test new therapeutic strategies. Importantly, these animal stem cell-derived models demonstrated the feasibility of the approach, leading the way for future human stem cell-derived models.
In 2005, Li et al. reported a new method for differentiation of human embryonic stem cells into motoneurons with capacity to form NMJs when co-cultured with the murine C2C12 myoblast cell line [36]. However, the validation of these NMJs was limited to immunocytochemistry and visualization of spontaneous contractions in the myotubes cultured with motoneurons, which could happen by other mechanisms other than NMJ formation. In 2010, Guo et al successfully demonstrated NMJ function in a co-culture between human spinal cord stem cell-derived motoneurons and rat skeletal muscle in a chemically defined culture system. Formation of NMJs was confirmed with the staining of AChR clusters, and NMJ function was demonstrated by MN stimulation with glutamate in the presence or absence of an AChR antagonist [37]. Mammalian NMJs, unlike insect NMJs, are cholinergic synapses, not glutaminergic; meaning that glutamate is not able induce muscle contractions by direct stimulation of the myotubes. Instead, the neurotransmitter glutamate is used here to trigger an action potential by depolarizing the MNs. As a result, if functional NMJs are present, ACh is released to the synaptic cleft and reaches the muscle AChRs to induce contraction.
The following year, the same team reported the first entirely human NMJ model, using motoneurons derived from spinal cord stem cells and primary skeletal myoblasts seeded on to a patterned non-biological surface. The NMJs characterized by immunocytochemistry and video analysis of motoneuron-induced muscle contractions showed that spontaneous contractions stopped upon treatment with an NMJ antagonist [38].
Finally, in 2015, Demestre et al. reported an NMJ model where both motoneurons and myotubes were derived from human iPSCs. However, the formation of NMJs was validated only by immunocytochemistry, and no functional assay was performed[39].
2.2. Advanced in vitro NMJ models
When engineering a predictive pre-clinical drug testing platform, it is crucial for the cells to endure long-term culture and reach maturity. However, the platform should also mimic the in vivo mechanical and biochemical environment and allow for relevant measurements. Due to these reasons, advanced culture systems have a number of advantages over traditional monolayer cultures. The development of more sophisticated models of NMJs has largely occurred along three axes: (i) microfluidics for compartmentalization, (ii) tissue engineering for better physiologic recapitulation, and (iii) optogenetics as a vector for controlled actuation of the NMJ (Fig.1).
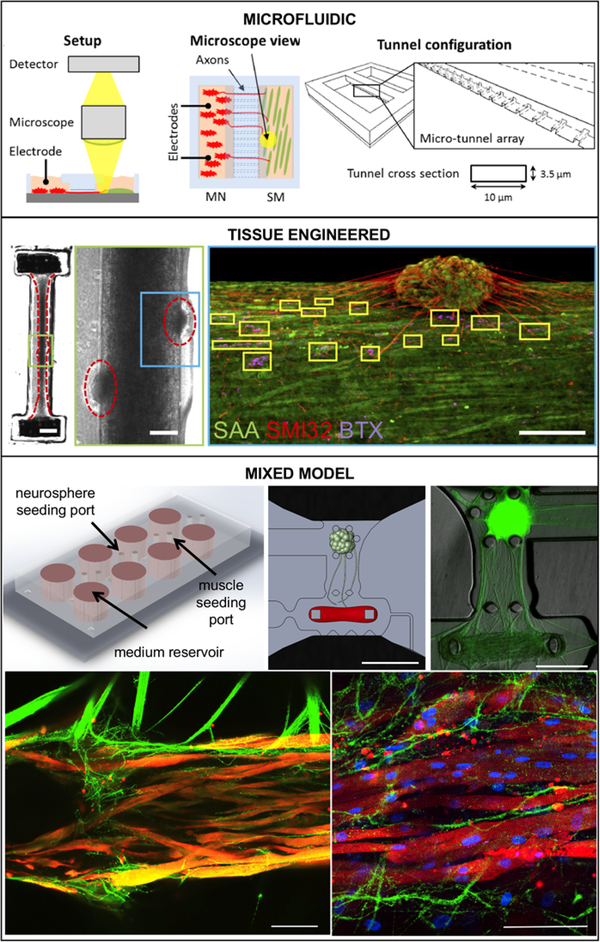
Figure 1. Examples of Advanced NMJ In vitro Models.
(A) Microfluidic system, Adapted from Santhanam et al., 2018 [46] with permission. Co-culture chip with separate populations of motoneurons and skeletal muscle connected through micro-tunnels. A microscope was used to image the co-culture and detect skeletal muscle contractions upon electrical stimulation. Stimulation is provided by two silver electrodes dipped into the bath of either chamber. Electrical pulses on the motoneuron-side evoked action potentials that traveled down the axons to the neuromuscular junction. (B) Tissue-engineered model, Reproduced from Afshar et al., 2019 [57]. 3D skeletal muscle-motor neuron (MN) co-culture at two weeks of culture. Neuromuscular tissue outlined with red dashed line in left panel. Region outlined in green box is magnified in the image to the immediate right. Red dashed lines in center panel outline motor neuron clusters. Right panel shows a representative confocal image of a two-week old neuromuscular co-culture immunostained for sarcomeric α-actinin (SAA; green), α-bungarotoxin (BTX; magenta), and neurofilament heavy SMI-32 (red). AChR clusters co-localized with neurites are outlined with yellow boxes. Scale bars, 2 mm (left panel) and 200 μm (center and right panels) (C) Mixed NMJ model, Reproduced from Vila et al., 2019 [62]. The microfluidic platform features channels for compartmentalized cultured, and pillars for tissue formation. Neurites extend from optogenetic a motoneuron neurosphere to innervate a skeletal microtissue. Bottom row shows confocal images showing innervation of the skeletal micro tissues and muscle striation after 10 (left) and 20 days in co-culture (right) (green = ChR2-YFP, red = α-actinin, blue = DAPI). Scale bars: 1mm (top center), 500 μm (top left), 100 μm (bottom).
2.2.1. Microfluidics
Compartmentalization is of special interest when modeling NMJs since they involve two cell types residing in very different environments in the body. By isolating the motoneuron and muscle cultures, allowing only the axons to grow into the muscle compartment, it is possible to tightly control culture conditions as well as stimulation of both cell types.
The first compartmentalized culture systems for neuronal cultures were the Campenot chambers, consisting of a Teflon divider attached to a glass coverslip [40]. These chambers have been used to study NMJs using mouse primary cells [41,42] but they were very tedious to manufacture. Microfluidic chambers manufactured from polydimethylsiloxane (PDMS) provide a modern alternative for the design and fabrication of custom-designed compartmentalized culture systems. Their small size makes microfluidic devices are ideal for high throughput analysis since they require small number of cells and culture reagents.
The first NMJ models that used microfluidics were reported in 2013 by Southam et al. [43]and Park et al.[44]. Both systems consisted of two chambers separated by an array of microchannels and supported the rat primary [43] and mouse ESC-derived motoneurons [44] to extend axons through the microchannels and innervate muscle cells. This process of axon extension mimics the physiologic process of NMJ formation and maturation in vivo.
Zahavi et al. demonstrated the relevance of compartmentalized cultures not just for NMJ formation but also for the study of NMJ biology, by using a similar microfluidic system to investigate the differential spatial effects of glial-derived growth factor (GDNF), and show that they facilitate the growth and muscle innervation at the axon terminals, but not at the soma [45].
The relevance of compartmentalization was further emphasized in 2018, when Santhanam et al. reported the first all-human NMJ microfluidic model. In addition to its all-human biology, the platform was designed to provide electrical stimulation of motoneurons without affecting the muscle compartment [46] (Fig. 1, top panel). This feature allowed for the first-ever selective electrical activation of motoneurons while without simultaneous stimulation of the muscle, thereby avoiding any interference with the measurement of muscle contraction as a proxy for NMJ function. Using this capability, the authors demonstrated dose-dependent responses to pre- and post-synaptic inhibitors.
2.2.2. Tissue-Engineered models
Tissue engineering aims to grow cells in architectures that recapitulates human physiology by incorporating extracellular matrices in form of three-dimensional scaffolds. One of the more common problems faced when trying to culture NMJs is the detachment of skeletal myotubes after fusion. To solve this problem, and to help with alignment, some models incorporated patterned substrates [47] or electrospun fibers in their monolayer cultures [48], resulting in better survival, increased fusion and formation of larger NMJs.
A more sophisticated solution is the growth of myoblasts as microtissues attached to pillars or wires. The resulting uniaxial mechanical tension improved not only cell alignment, but also cell attachment, fusion, and maturation, as has been shown in skeletal [49–51] and cardiac [52,53] tissue-engineered models.
Morimoto et al. used PDMS stamps and Matrigel to generate free-standing muscle bundles derived from murine C2C12 cells attached to glass only in their extremes. They later immobilized neurospheres composed from murine neural stem cells atop of muscle bundles and induced differentiation to motoneurons to promote the formation of NMJs. Their platform included cantilevers that bent with muscle contractions, allowing for the measurement of muscle force as a function of cantilever displacement [54].
Smith et al. used a similar system to establish 3D cultures primary rat myocytes and motoneurons. Their co-cultures showed increased expression of muscle and NMJ maturation markers when compared to muscle cultures only [55], recapitulating the results showed by Peterson in the first organotypic NMJ cultures [33]. A slightly different approach was used by Smith et al. to grow skeletal tissues on top of individual cantilevers that bent upon muscle contraction. Cantilever deflection was measured using a laser photodetector, allowing for easy quantification of the muscle responses [56].
Afshar Bakooshli et al. recently reported a human tissue-engineered NMJ model consisting of primary myoblast-derived skeletal muscle tissue surrounded by iPSC-derived motoneuron spheroids [57] (Fig. 1, middle panel). As seen in the murine NMJ systems, the presence of motoneurons improved maturation of the muscle tissue. Furthermore, they demonstrated that their tissue-engineered NMJ model achieved greater levels of maturity than a co-culture of the same cell types, by showing replacement of the embryonic AChR gamma subunit with the adult epsilon subunit and greater enrichment of laminin β2 at the AChR clusters.
2.2.3. Optogenetics
Several investigators have explored alternative, optically based methods to create controllable NMJs. Electrical stimulation has classically been used for the controlled stimulation of excitable cell types but cannot easily target only the specific cell types in culture - such as motoneurons - without affecting muscle cells. An elegant solution to this problem can be achieved through the use of optogenetics, defined as the creation of transgenic cell lines expressing light-actuated proteins. ChR2 is a light-activated ion channel that opens upon stimulation with blue light, allowing for the precise spatial-temporal control of neural activation in neurons expressing this protein [58]. With the aim to achieve a controllable NMJ model, Steinbeck et al. combined human ESC-derived motoneurons expressing the optogenetic protein channelrhodopsin-2 (ChR2) with non-optogenetic skeletal myotubes in a co-culture system. The resulting muscle contractions in response to photostimulation of the motoneurons provided concrete evidence of controllable NMJ function. The authors used their system to model MG and showed the ability to detect a reduction in light responsiveness in their disease samples. However, due to the disorganized nature of their 2D co-culture, this quantification was based on the recording of random optical fields, hindering reproducibility and automatization [59] (Fig. 1, bottom panel).
2.2.4. Mixed in vitro NMJ models
Microfluidic systems allow for compartmentalized culture of different cell types, as well as close control of culture conditions and manipulation, improving reproducibility and making them ideal for high throughput studies. Tissue engineered models, on the other hand, more closely resemble physiological conditions and achieve greater levels of maturity. They also make it easy to detect muscle contraction and even quantify muscle force as a function of pillar displacement. A few studies in the past years have combined the best of both worlds to grow mouse [60] or human [61,62] three-dimensional skeletal muscle microtissues inside a microfluidic platform. Furthermore, they incorporated optogenetic motoneurons to allow for the precise activation of the NMJs.
In our studies, a custom-made optical stimulation platform was developed in conjunction with custom video processing software for the automated, high-throughput, quantitative evaluation of NMJ function in a human system comprised of skeletal tissues and motoneurons derived from the same donor. While some of studies use average muscle force as a proxy to measure NMJ function [61], the fatigable nature of the NMJ and the skeletal muscle makes force a potentially noisy parameter to work with, since it can be affected by factors other than neurotransmission efficiency. Instead, we use a scoring system based muscle responsiveness to a series of stimuli at increasing frequencies, which takes into account the presence of muscle contraction but not its force. Using this system, we were able to detect functional changes in response to NMJ maturation, pharmacological intervention and disease [62].
3. In vitro NMJ MODELS OF DISEASE
Since in vitro NMJ models have only recently attained the necessary levels of sophistication and reliability, few teams have ventured into the realm of disease modeling. However, these initial reports demonstrate the potential of human in vitro systems for the study of neuromuscular diseases (Table 2).
Myasthenia gravis is the most common disorder of neuromuscular transmission. It is an autoimmune condition caused by autoantibodies that bind the AChR or associated proteins in the NMJ, resulting in their destruction through complement-induced damage and receptor endocytosis [63]. Impairment of NMJ function results in muscular weakness of different severities. MG is estimated to affect 15 to 200 per million people, with an incidence of 2–22 new diagnoses per million people each year [64]. Due to its autoimmune nature, the myasthenic phenotype can be recapitulated in these in vitro models by simply adding patient serum to the system. Steinbeck et al. [59] were the first to do this, using an optogenetic monolayer coculture system. Their results show reduced light responsiveness after treatment with immunoglobulins G (IgG) from MG patients and serum containing complement. Normal function was recovered after washing of the MG IgG, recapitulating the therapeutic effect of plasmapheresis during myasthenic crises. Afshar Bakooshli et al. [57] also incorporated MG IgGs in their tissue-engineered model, showing localized deposition of complement on NMJs. Our group [62] has recently reported the use of an automated optical stimulation system to demonstrate that in vitro NMJ models responded heterogeneously to serum from different patients, recapitulating the known variability in phenotype severity.
While less common, the various genetic NMJ diseases further showcase the capabilities of human iPSC-derived in vitro models, which recapitulate the genotype of the patient from whom the iPSCs are derived from. So far, no model has incorporated both skeletal muscle and motoneurons derived from a patient.
In 2015, Yoshida et al. [65] showed that by incorporating human SMA motoneurons in conjunction with healthy murine muscle in their microfluidic system, they achieved a diseased phenotype characterized by deficient AChR clustering. Treatment with valproic acid (VPA) and an antisense oligonucleotide rescued the healthy phenotype. However, no functional testing was performed, and their observations were based on immunocytochemistry analysis only.
In 2018, Osaki et al. reported an ALS-on-a-chip model that incorporates ALS motoneurons in conjunction with healthy human muscle in a microfluidic system [61]. Their system allowed for 3D culture and incorporates optogenetics for easily activation of the NMJ. Their results show that the NMJ formed by the ALS motoneurons presented a diseased phenotype characterized by increased presence of caspase3/7-positive cells and reduced muscle force generation. Furthermore, treatment with rapamycin, an immunosuppressant, or a combination of rapamycin and bosutinib, a drug developed for the treatment of chronic myelogenous leukemia, increased the contraction force and decreasing caspase activity in the treated motor units. They also demonstrated the excitotoxicity of excess of glutamic acid (5mM) in the human motor units, widely recognized as one of the major pathways contributing to ALS pathogenesis [66,67].
4. In vitro NMJ MODELS FOR DRUG TESTING
One of the main advantages of advanced human in vitro models is in the potential for their use as an adjunct to animal studies to better prescreen drugs for clinical trials, which could greatly reduce the high costs associated with drug development. The physiology of the NMJ is very well known as drugs that affect its function at different molecular levels are very well characterized. As such, most of the drug testing performed so far in human in vitro NMJs has focused on model validation using known drugs (Table 3).
Table 3.
In vitro models of NMJ for drug testing
| Drug | Role | Refs |
|---|---|---|
| Tetrodotoxin (TTX) | Sodium channel blocker | Zahavi et al., 2015 [45]; Charoensook et al., 2017 [68]; Chipman et al., 2014 [35] |
| Nifedipine | Calcium channel blocker | Chipman et al., 2014 [35] |
| Acetylethylcholine mustard hydrochloride | Choline acteyltransferase inhibitor | Charoensook et al., 2017 [68] |
| Neostigmine | ACh esterase inhibitor | Charoensook et al., 2017 [68] |
| Pyridostigmine (PYR) | ACh esterase inhibitor | Steinbeck et al., 2016 [59] |
| Vesamicol | ACh transporter inhibitor | Charoensook et al., 2017 [68] |
| Dynasore | Endocytosis inhibitor | Chipman et al., 2014 [35] |
| Agatoxin (ATX) | Calcium channel blocker | Chipman et al., 2014 [35] |
| Botulinum neurotoxin A (BoNT) | ACh vesicle fusion inhibitor | Charoensook et al., 2017 [68]; Santhanam et al., 2018 [46]; Afshar Bakooshli et al., 2019 [57] |
| Tubocurarine (TC) | AChR antagonist | Guo et al., 2010 [37]; Guo et al., 2011 [38]; Morimoto et al.,2013 [54]; Smith et al., 2013 [56]; Chipman et al., 2014 [35] Asfhar Bakooshli et al., 2019 [57]. |
| Bungarotoxin (BTX) | AChR antagonist | Santhanam et al., 2018 [46]; Uzel et al., 2016 [60]; Osaki et al., 2018 [61]; Vila et al., 2019 [62] |
| Waglerin-1 (WTX) | AChR epsilon subunit blocker | Asfhar Bakooshli et al., 2019 [57]. |
| Valproic Acid (VPA) | Yoshida et al., 2015 [65]. | |
| Phosphorodiamidate morpholino | Antisense oligonucleotide | Yoshida et al., 2015 [65]. |
| Rapamycin | neuroprotective drug | Osaki et al., 2018 [61]. |
| Bosutinib | neuroprotective drug | Osaki et al., 2018 [61]. |
ACh: acetylcholine
4.1. Presynaptic blockers
Presynaptic blockers are drugs that act on the neuronal side of the neuromuscular junction, preventing initiation of synaptic neurotransmission. This category includes ion channel blockers that prevent the propagation of the action potential through the axon, drugs that interfere with ACh recycling, and drugs that prevent ACh from reaching its receptors.
Tetrodotoxin (TTX) is a potent neurotoxin found in multiple aquatic animals, such as the pufferfish, that causes muscle paralysis. TTX is a voltage-gated sodium channel antagonist that inhibits the nerve action potentials. Charoensook et al. showed that treatment with TTX suppressed the beneficial effects of electrical stimulation in the maturation of skeletal myotubes and motoneurons in their coculture system [68]. Chipman et al., on the other hand, used TTX to show that the spontaneous contractions in their motoneuron-muscle cultures were driven by neural action potentials [35].
In terms of acetylcholine recycling, Charoensook and colleagues demonstrated the dose-dependent effect of various drugs that alter ACh recycling such as vesamicol, that inhibits the vesicular ACh transporter, acetylethylcholine mustard hydrochloride, that inhibits the choline acetyltransferase, and neostigmine an ACh esterase inhibitor, demonstrating the potential of their model to be used in pharmacological screening [68]. Steinbeck et al. also used an ACh esterase inhibitor, pyridostigmine, to improve NMJ function in their optogenetic MG model [59].
Chipman et al. [35] studied the role of a synapse vesicle inhibitor, dynasore, in their NMJ model. Dynasore inhibits dynamin, a GTPase essential to endocytic function [69]. Their results showed that dynasore treatment blocked activity-dependent endocytosis in their healthy but not in cultures lacking NCAM, suggesting that NCAM has role in synaptic vesicle endocytosis. Further, this demonstrated that their stem cell-based NMJ model respond to pharmacological interventions in an analogous manner to primary cell-based and animal models. Similarly, ω−Agatoxin IVA (ATX) is a toxin present in the venom of various spiders that blocks the voltage-gated CaV2.1 calcium channels of presynaptic terminals. Chipman et al. showed that treatment with ATX inhibited the activity-dependent endocytosis in their NMJ co-culture system [35].
Finally, botulinum neurotoxin (BoNT) is a neurotoxic protein produced by the bacterium Clostridium botulinum. This toxin prevents the fusion of ACh vesicles to the membrane, thus stopping their release [70]. Charoensook et al. and Santhanam et al. performed dose response-curves to BoNT in human co-culture NMJ models [46,68]. Their results showed that BoNT was able to block NMJ function at much lower concentrations that those used by the current methods for evaluation of botulinum toxin preparations, consisting on lethal dose assays in mice [71]. Thus, in vitro NMJ could be a better alternative to these methods. Afshar Bakooshli et al. also showed decreased NMJ function in their human tissue engineered system after BoNT treatment [57]. We note the absence of some commonly used presynaptic drugs, such as 3,4-diaminpyridine (3,4-DAP) from these drug assays.
4.2. Postsynaptic blockers
Postsynaptic blockers bind to the AChR in the myoblast membrane, thus preventing their binding to ACh and subsequent activation.
Tubocurarine chloride pentahydrate, a toxic alkaloid and muscle relaxant that was used with anesthetics in the mids-1900s, is one of the postsynaptic blockers most commonly used in NMJ studies. Treatment with tubocurarine has been shown to dim or stop both spontaneous [38] and glutamate-induced [35] contractions in motoneuron-muscle co-cultures, as well as in mouse microfluidics [54] and human [57] tissue-engineered systems. Smith et al. also showed that tubocurarine was able to block the glutamate-induced contractions in their cantilever system [56]. In addition to these qualitative studies, a dose-response curve performed in a compartmentalized microfluidic system demonstrated an IC50 in agreement with reported results for human AChRs [72,73].
Alpha-bungarotoxin (BTX), a 74-amino acid polypeptide purified from the venom of the snake Bungarus multicinctus originally found in Taiwan [74], is another postsynaptic blocker frequently used to validate NMJ models. Santhanam et al. showed a dose-response curve to BTX treatment in their microfluidic NMJ system [46] whereas Uzel et al., and Vila et al. showed its ability to irreversibly stop both spontaneous and light-induced contractions in their mouse [60] and human [62] optogenetic NMJ models.
Finally, Waglerin-1 (WTX) is a peptide that selectively binds and blocks the epsilon subunit of the muscle AChR [75], characteristic of adult NJMs. Afshar Bakooshli et al. showed that their 3D model, but not a traditional co-culture, was affected by WTX treatment, suggesting the presence of adult-like AChR in at least a fraction of their engineered NMJs [57].
4.3. Drug discovery
The validation summarized above was a necessary preliminary step to show that these in vitro models can recapitulate the drug responses we see in patients. However, the end goal has always been drug discovery. While most studies have focused on model validation, Osaki et al. [61] were the first group to go a step further than the previous studies, using an ALS-on-a-chip system to test two drug candidates for the first time: rapamycin and bosutinib.
Rapamycin is a macrolide compound originally developed as an immunosuppressant that has shown beneficial effects in several ALS cell lines and animal models, and is currently being tested in a phase 2 clinical trial [76]. Rapamycin accelerates the removal of abnormal accumulation of aggregated proteins [77]. Osaki et al. showed that rapamycin has a neuroprotective effect in their ALS-on-a-chip model, increasing muscle contraction force, reducing the expression of caspase 3/7 positive cells, and reducing the missed muscle contractions in response to optical stimulation of the motoneurons [61].
Bosutinib is an ALS drug candidate currently undergoing a phase 1 clinical trial [78]. Using bosutinib in combination with rapamycin resulted in a further reduction of caspase3/7-positive cells and fewer missed contractions when compared to tissues treated with rapamycin alone. Osaki et al. also studied the ability of rapamycin and bosutinib to penetrate an endothelial barrier that emulates the effect of the blood-brain barrier. Their results show that while the endothelial barrier had a dimming effect on the drug effectiveness, the drug was still able to diffuse through the barrier and achieve therapeutic effect.
This study represents the first application of a human in vitro NMJ model to drug discovery, highlighting the potential of these systems to improve the drug discovery pipeline.
5. CONCLUSION
Over the past several years, numerous in vitro NMJ models have been reported, achieving unprecedented levels of complexity. On the one hand, advances in the derivation and differentiation of stem cells has led to the development of single-donor human models, allowing for genetic disease modeling and the potential for personalized medicine applications down the line. On the other hand, technologies such as microfluidics, tissue engineering, optogenetics and video processing have made these systems more representative of human physiology and easier to control and evaluate. Together, these advances have facilitated the development of the first quantitative systems for drug testing in both healthy and diseased human in vitro NMJ models, demonstrating the great potential of these systems to improve the drug discovery process.
6. EXPERT OPINION
While important steps have been taken in recent years towards the establishment of human, patient-specific NMJ models, further efforts are required to achieve the levels of reproducibility and maturity required for predictive high throughput drug screening. The ability to derive multiple cell types for iPSCs has opened a wide range of possibilities in terms of personalized medicine and disease modeling, but the maturity of the generated cells is a concern, especially when aiming to model neurodegenerative diseases. While advanced tissue engineered models have demonstrated the ability to form mature NMJ, evidenced by the switch from embryonic gamma AChR subunits to adult epsilon subunits, this switch occurs only in a fraction of the synapses, suggesting that further maturation is necessary to achieve adult-like synapses.
Furthermore, the human NMJ is a complex system that incorporates multiple cell types. For the most part, current NMJs models use a simplified model where only skeletal cells and motoneurons are present. However, it has been demonstrated that the presence of glial cells is necessary for the formation of mature NMJs [79]. Further efforts to incorporate other cell types such as Schwann cells will result in more realistic models that more closely resemble the human NMJs. This is especially relevant for disease modeling of pathologies that affect these cell types, such as Charcot-Marie-Tooth disease.
Finally, clinical trials fail not only due to the insufficient on-target effects, but also due to unforeseen off-target effects. Regardless of the target tissue, drug recalls are largely due to cardiotoxicity, hepatotoxicity and drug-drug interactions [80]. Most of these off-target effects are only detected after causing patient death, such as in the case of cisapride, which had unintended interactions with K+ channels in the heart, leading to acquired long-QT syndrome and sudden cardiac death from arrhythmias. These off-target effects are equally important in determining the utility of drugs in clinical trials. The current iterations of human in vitro NMJ models are all organ-specific. However, the next generation of body-on-a-chip technology promises to recapitulate large swathes of human (patho)physiology in a dish to test for not only the curative effects in target tissues, but also for potential side effects in other tissues [81,82].
Despite of these challenges, the continuous advances in stem cell technology, fabrication and 3D printing, and gene editing, predict a great future for the human in vitro models of NMJ. It is not difficult to envision a future where complex NMJ models not only incorporate glial cells but also interneurons and sensory neurons, and are interconnected with other tissue models to allow for cross-talk and the study of off-target effects of therapeutic treatments. Systems as such will tremendously increase the power of preclinical testing, reducing the need of animal and clinical studies, and improving the drug development pipeline yield.
Highlight box.
Neuromuscular junctions are affected in several neurodegenerative and neuroimmunological diseases for which there are currently very few treatment options.
In vitro models allow for systematic manipulation and testing of human systems.
Recent advances in stem cell technology, fabrication and tissue engineering have allowed for the development of in vitro NMJ models that recapitulate human physiology.
Incorporation of novel techniques such as optogenetics and automated optical stimulation and sensing allows for precise control and evaluation of NMJ function.
These models can be derived from patient cells for personalize medicine and human disease modeling.
The combination of all these advances will allow for the generation of high-throughput systems for novel drug screening and development.
Acknowledgments
FUNDING:
The authors gratefully acknowledge the funding support of the National Institutes of Health (grants EB025765 and EB027062 to G Vunjak-Novakovic), New York State Department of Health (NYSTEM) (grant C32606GG to G Vunjak-Novakovic) and the Department of Defense (grant PR171955 to OF Vila).
Footnotes
DECLARATION OF INTEREST:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REVIEWER DISCLOSURES:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES
- [1].Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Dev. Camb. Engl 2010;137:1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Punga AR, Ruegg MA. Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr. Opin. Pharmacol 2012;12:340–346. [DOI] [PubMed] [Google Scholar]
- [3].Cifuentes-Diaz C, Nicole S, Velasco ME, et al. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet 2002;11:1439–1447. [DOI] [PubMed] [Google Scholar]
- [4].Kariya S, Park G-H, Maeno-Hikichi Y, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum. Mol. Genet 2008;17:2552–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kong L, Wang X, Choe DW, et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. Off. J. Soc. Neurosci 2009;29:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moloney EB, de Winter F, Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murray LM, Talbot K, Gillingwater TH. Review: Neuromuscular synaptic vulnerability in motor neurone disease: amyotrophic lateral sclerosis and spinal muscular atrophy. Neuropathol. Appl. Neurobiol 2010;36:133–156. [DOI] [PubMed] [Google Scholar]
- [8].Gonzalez-Freire M, de Cabo R, Studenski SA, et al. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci 2014;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taetzsch T, Valdez G. NMJ maintenance and repair in aging. Curr. Opin. Physiol 2018;4:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Webster RG. Animal Models of the Neuromuscular Junction, Vitally Informative for Understanding Function and the Molecular Mechanisms of Congenital Myasthenic Syndromes. Int. J. Mol. Sci 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones RA, Harrison C, Eaton SL, et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017;21:2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chunhui Xu, Shailaja Police, Namitha Rao, et al. Characterization and Enrichment of Cardiomyocytes Derived From Human Embryonic Stem Cells. Circ. Res 2002;91:501–508. [DOI] [PubMed] [Google Scholar]
- [14].Jianhua Zhang, Wilson Gisela F, Soerens Andrew G, et al. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circ. Res 2009;104:e30–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hay DC, Fletcher J, Payne C, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc. Natl. Acad. Sci 2008;105:12301–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Si‐Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo X, Greene K, Akanda N, et al. In vitro Differentiation of Functional Human Skeletal Myotubes in a Defined System. Biomater. Sci 2014;2:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rao L, Qian Y, Khodabukus A, et al. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun 2018;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hu B-Y, Zhang S-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc 2009;4:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maury Y, Côme J, Piskorowski RA, et al. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat. Biotechnol 2015;33:89–96. [DOI] [PubMed] [Google Scholar]
- [21].Du Z-W, Chen H, Liu H, et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun 2015;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- [23].Takahashi K, Tanabe K, Ohnuki M, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- [24].Corti S, Nizzardo M, Simone C, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci. Transl. Med. 2012;4:165ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ebert AD, Yu J, Rose FF, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sareen D, Ebert AD, Heins BM, et al. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PloS One. 2012;7:e39113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. [DOI] [PubMed] [Google Scholar]
- [28].Sareen D, O’Rourke JG, Meera P, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci. Transl. Med 2013;5:208ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Serio A, Bilican B, Barmada SJ, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. U. S. A 2013;110:4697–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kiskinis E, Sandoe J, Williams LA, et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Devlin A-C, Burr K, Borooah S, et al. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat. Commun 2015;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sances S, Bruijn LI, Chandran S, et al. Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat. Neurosci 2016;19:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peterson ER, Crain SM. Regeneration and innervation in cultures of adult mammalian skeletal muscle coupled with fetal rodent spinal cord. Exp. Neurol 1972;36:136–159.* First in vitro NMJ system
- [34].Das M, Rumsey JW, Bhargava N, et al. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chipman PH, Zhang Y, Rafuse VF. A stem-cell based bioassay to critically assess the pathology of dysfunctional neuromuscular junctions. PloS One. 2014;9:e91643.* First stem cell NMJ model
- [36].Li X-J, Du Z-W, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol 2005;23:215–221. [DOI] [PubMed] [Google Scholar]
- [37].Guo X, Das M, Rumsey J, et al. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng. Part C Methods. 2010;16:1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guo X, Gonzalez M, Stancescu M, et al. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611.**First all-human NMJ model
- [39].Demestre M, Orth M, Föhr KJ, et al. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 2015;15:328–336.*First all iPSCs-derived NMJ model
- [40].Campenot RB. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. U. S. A 1977;74:4516–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jia M, Li M, Dunlap V, et al. The thrombin receptor mediates functional activity-dependent neuromuscular synapse reduction via protein kinase C activation in vitro. J. Neurobiol 1999;38:369–381. [PubMed] [Google Scholar]
- [42].Nelson PG, Fields RD, Yu C, et al. Synapse elimination from the mouse neuromuscular junction in vitro: a non-Hebbian activity-dependent process. J. Neurobiol 1993;24:1517–1530. [DOI] [PubMed] [Google Scholar]
- [43].Southam KA, King AE, Blizzard CA, et al. Microfluidic primary culture model of the lower motor neuron–neuromuscular junction circuit. J. Neurosci. Methods 2013;218:164–169. [DOI] [PubMed] [Google Scholar]
- [44].Park HS, Liu S, McDonald J, et al. Neuromuscular junction in a microfluidic device. 2013 35th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBC. 2013. p. 2833–2835.*One of the first applications of microfluidics for compartmentalized culture of motoneurons and skeletal muscle.
- [45].Zahavi EE, Ionescu A, Gluska S, et al. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J. Cell Sci 2015;128:1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Santhanam N, Kumanchik L, Guo X, et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials. 2018;166:64–78.**First human NMJ model based in microfluidics and allowing selective stimulation of motoneurons.
- [47].Happe CL, Tenerelli KP, Gromova AK, et al. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol. Biol. Cell 2017;28:1950–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Luo B, Tian L, Chen N, et al. Electrospun nanofibers facilitate better alignment, differentiation, and long-term culture in an in vitro model of the neuromuscular junction (NMJ). Biomater. Sci 2018;6:3262–3272. [DOI] [PubMed] [Google Scholar]
- [49].Madden L, Juhas M, Kraus WE, et al. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife. 2015;4:e04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Okano T, Matsuda T. Tissue Engineered Skeletal Muscle: Preparation of Highly Dense, Highly Oriented Hybrid Muscular Tissues. Cell Transplant. 1998;7:71–82. [DOI] [PubMed] [Google Scholar]
- [51].Powell CA, Smiley BL, Mills J, et al. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol.-Cell Physiol 2002;283:C1557–C1565. [DOI] [PubMed] [Google Scholar]
- [52].Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Thavandiran N, Dubois N, Mikryukov A, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl. Acad. Sci 2013;110:E4698–E4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Morimoto Y, Kato-Negishi M, Onoe H, et al. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials. 2013;34:9413–9419.* First tissue engineered model of NMJs.
- [55].Smith AST, Passey SL, Martin NRW, et al. Creating Interactions between Tissue-Engineered Skeletal Muscle and the Peripheral Nervous System. Cells Tissues Organs. 2016;202:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Smith AST, Long CJ, Pirozzi K, et al. A functional system for high-content screening of neuromuscular junctions in vitro. Technology. 2013;1:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Afshar Bakooshli M, Lippmann ES, Mulcahy B, et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. eLife. 2019;8.**Human tissue-engineered model showing presence of adult NMJs.
- [58].Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 2005;8:1263–1268. [DOI] [PubMed] [Google Scholar]
- [59].Steinbeck JA, Jaiswal MK, Calder EL, et al. Functional Connectivity under Optogenetic Control Allows Modeling of Human Neuromuscular Disease. Cell Stem Cell. 2016;18:134–143.* First application of optogenetics to the study of NMJs. First in vitro model of myasthenia gravis.
- [60].Uzel SGM, Platt RJ, Subramanian V, et al. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci. Adv 2016;2.** Microfluidic platform for 3D culture of NMJs incorporating optogenetics.
- [61].Osaki T, Uzel SGM, Kamm RD. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv 2018;4:eaat5847.**First human ALS-on-a-chip model using microfluidics and optogenetics.
- [62].Vila OF, Uzel SGM, Ma SP, et al. Quantification of human neuromuscular function through optogenetics. Theranostics. 2019;9:1232–1246.**Single donor, human NMJs formed in a microfluidic device that allows for automated quantification using optogenetics.
- [63].Kusner LL, Kaminski HJ, Soltys J. Effect of complement and its regulation on myasthenia gravis pathogenesis. Expert Rev. Clin. Immunol 2008;4:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Carr AS, Cardwell CR, McCarron PO, et al. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yoshida M, Kitaoka S, Egawa N, et al. Modeling the Early Phenotype at the Neuromuscular Junction of Spinal Muscular Atrophy Using Patient-Derived iPSCs. Stem Cell Rep. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Blasco H, Mavel S, Corcia P, et al. The Glutamate Hypothesis in ALS: Pathophysiology and Drug Development 21(31):3551–75. [DOI] [PubMed] [Google Scholar]
- [67].Plaitakis A Glutamate dysfunction and selective motor neuron degeneration in amyotrophic lateral sclerosis: a hypothesis. Ann. Neurol 1990;28:3–8. [DOI] [PubMed] [Google Scholar]
- [68].Charoensook SN, Williams DJ, Chakraborty S, et al. Bioreactor model of neuromuscular junction with electrical stimulation for pharmacological potency testing. Integr. Biol. Quant. Biosci. Nano Macro 2017;9:956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Macia E, Ehrlich M, Massol R, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006;10:839–850. [DOI] [PubMed] [Google Scholar]
- [70].Dressler D, Saberi FA. Botulinum Toxin: Mechanisms of Action. Eur. Neurol 2005;53:3–9. [DOI] [PubMed] [Google Scholar]
- [71].Huber A, France RM, Riccalton-Banks L, et al. The Intercostal NMJ Assay: a new alternative to the conventional LD50 assay for the determination of the therapeutic potency of botulinum toxin preparations. Altern. Lab. Anim. ATLA 2008;36:141–152. [DOI] [PubMed] [Google Scholar]
- [72].Blount P, Merlie JP. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989;3:349–357. [DOI] [PubMed] [Google Scholar]
- [73].Sine SM. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc. Natl. Acad. Sci. U. S. A 1993;90:9436–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chang CC, Lee CY. Isolation of neurotoxins from the venom of bungarus multicinctus and their modes of neuromuscular blocking action. Arch. Int. Pharmacodyn. Ther 1963;144:241–257. [PubMed] [Google Scholar]
- [75].McArdle JJ, Lentz TL, Witzemann V, et al. Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther 1999;289:543–550. [PubMed] [Google Scholar]
- [76].Rapamycin Treatment for ALS - Full Text View - ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03359538.
- [77].Mandrioli J, D’Amico R, Zucchi E, et al. Rapamycin treatment for amyotrophic lateral sclerosis: Protocol for a phase II randomized, double-blind, placebo-controlled, multicenter, clinical trial (RAP-ALS trial). Medicine (Baltimore). 2018;97:e11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Phase 1 Dose Escalation Study of Bosutinib in Patients with Amyotrophic Lateral Sclerosis (ALS). Available from: https://rctportal.niph.go.jp/en/detail?trial_id=jRCT2051190001.
- [79].Mars T, Yu KJ, Tang XM, et al. Differentiation of glial cells and motor neurons during the formation of neuromuscular junctions in cocultures of rat spinal cord explant and human muscle. J. Comp. Neurol 2001;438:239–251. [DOI] [PubMed] [Google Scholar]
- [80].Piccini JP, Whellan DJ, Berridge BR, et al. Current challenges in the evaluation of cardiac safety during drug development: Translational medicine meets the Critical Path Initiative. Am. Heart J 2009;158:317–326. [DOI] [PubMed] [Google Scholar]
- [81].Boos JA, Misun PM, Michlmayr A, et al. Microfluidic Multitissue Platform for Advanced Embryotoxicity Testing In Vitro. Adv. Sci 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet 2018;33:43–48. [DOI] [PubMed] [Google Scholar]