Abstract
Background
This is an updated version of the original Cochrane review published in 2007. Traditionally, after major abdominal gynaecologic surgery postoperative oral intake is withheld until the return of bowel function. There has been concern that early oral intake would result in vomiting and severe paralytic ileus with subsequent aspiration pneumonia, wound dehiscence, and anastomotic leakage. However, evidence‐based clinical studies suggest that there may be benefits from early postoperative oral intake.
Objectives
To assess the effects of early versus delayed (traditional) initiation of oral intake of food and fluids after major abdominal gynaecologic surgery.
Search methods
We searched the Menstrual Disorders and Subfertility Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), electronic databases (MEDLINE, EMBASE, CINAHL), and the citation lists of relevant publications. The most recent search was conducted 1 April 2014. We also searched a registry for ongoing trials (www.clinicaltrials.gov) on 13 May 2014.
Selection criteria
Randomised controlled trials (RCTs) were eligible that compared the effect of early versus delayed initiation of oral intake of food and fluids after major abdominal gynaecologic surgery. Early feeding was defined as oral intake of fluids or food within 24 hours post‐surgery regardless of the return of bowel function. Delayed feeding was defined as oral intake after 24 hours post‐surgery and only after signs of postoperative ileus resolution.
Data collection and analysis
Two review authors selected studies, assessed study quality and extracted the data. For dichotomous data, we calculated the risk ratio (RR) with a 95% confidence interval (CI). We examined continuous data using the mean difference (MD) and a 95% CI. We tested for heterogeneity between the results of different studies using a forest plot of the meta‐analysis, the statistical tests of homogeneity of 2 x 2 tables and the I² value. We assessed the quality of the evidence using GRADE methods.
Main results
Rates of developing postoperative ileus were comparable between study groups (RR 0.47, 95% CI 0.17 to 1.29, P = 0.14, 3 RCTs, 279 women, I² = 0%, moderate‐quality evidence). When we considered the rates of nausea or vomiting or both, there was no evidence of a difference between the study groups (RR 1.03, 95% CI 0.64 to 1.67, P = 0.90, 4 RCTs, 484 women, I² = 73%, moderate‐quality evidence). There was no evidence of a difference between the study groups in abdominal distension (RR 1.07, 95% CI 0.77 to 1.47, 2 RCTs, 301 women, I² = 0%) or a need for postoperative nasogastric tube placement (RR 0.48, 95% CI 0.13 to 1.80, 1 RCT, 195 women). Early feeding was associated with shorter time to the presence of bowel sound (MD ‐0.32 days, 95% CI ‐0.61 to ‐0.03, P = 0.03, 2 RCTs, 338 women, I² = 52%, moderate‐quality evidence) and faster onset of flatus (MD ‐0.21 days, 95% CI ‐0.40 to ‐0.01, P = 0.04, 3 RCTs, 444 women, I² = 23%, moderate‐quality evidence). In addition, women in the early feeding group resumed a solid diet sooner (MD ‐1.47 days, 95% CI ‐2.26 to ‐0.68, P = 0.0003, 2 RCTs, 301 women, I² = 92%, moderate‐quality evidence). There was no evidence of a difference in time to the first passage of stool between the two study groups (MD ‐0.25 days, 95% CI ‐0.58 to 0.09, P = 0.15, 2 RCTs, 249 women, I² = 0%, moderate‐quality evidence). Hospital stay was shorter in the early feeding group (MD ‐0.92 days, 95% CI ‐1.53 to ‐0.31, P = 0.003, 4 RCTs, 484 women, I² = 68%, moderate‐quality evidence). Infectious complications were less common in the early feeding group (RR 0.20, 95% CI 0.05 to 0.73, P = 0.02, 2 RCTs, 183 women, I² = 0%, high‐quality evidence). In one study, the satisfaction score was significantly higher in the early feeding group (MD 11.10, 95% CI 6.68 to 15.52, P < 0.00001, 143 women, moderate‐quality evidence).
Authors' conclusions
Early postoperative feeding after major abdominal gynaecologic surgery for either benign or malignant conditions appeared to be safe without increased gastrointestinal morbidities or other postoperative complications. The benefits of this approach include faster recovery of bowel function, lower rates of infectious complications, shorter hospital stay, and higher satisfaction.
Plain language summary
Early versus delayed feeding for reducing complications after gynaecologic surgery
Review Question
What are the risks and benefits of eating food early, versus delaying food for at least 24 hours after abdominal gynaecologic surgery?
Background
Physicians often delay giving food and drink to women after abdominal gynaecologic surgery (uterine fibroids, endometriosis, ovarian cysts, uterine and ovarian cancer) until bowel function recommences (typically 24 hours after surgery). This is to reduce the risk of complications such as vomiting, gastrointestinal disruptions and wound rupturing or leakage. However, it has been suggested that some women may recover more quickly if food is introduced earlier. We reviewed evidence from randomised controlled trials of early and delayed feeding after abdominal gynaecologic surgery.
Study Characteristics
We assessed evidence on the following outcomes:
1. Nausea, vomiting, cramping abdominal pain, bloating, abdominal distension, wound complication, deep venous thrombosis, urinary tract infection, pneumonia.
2. Time to first: bowel sound, gas, stool, start of regular diet.
3. Length of hospital stay
Early feeding was defined as having fluids or food within 24 hours of surgery. Delayed feeding was defined as having fluids or food 24 hours after surgery, and only if there are bowel sounds, passage of gas or stool, and a feeling of hunger.
The evidence is current to April 2014
Key Results
We included five published studies of 631 women, mainly with gynaecologic cancer.
Recovery of bowel function was faster in those with early feeding. There was no difference in rates of nausea or vomiting, abdominal distension, need for a postoperative nasogastric tube or time to first bowel movement, but early feeding was associated with a shorter time to bowel sounds and onset of gas. The early feeding group resumed a solid diet 1½ days sooner than those having delayed feeding and the hospital stay was one day shorter. Also, the early feeding group were more satisfied with the feeding schedule, although only one study reported this.
Early feeding appeared safe, without increased postoperative complications and with fewer infectious complications overall.
Quality of the Evidence
Most of the evidence was moderate quality. The main limitation was lack of blinding, which could influence the findings for subjective outcomes such as self‐reported symptoms, hospital stay, patients' satisfaction and quality of life.
Conclusions
The evidence suggests that eating and drinking on the first day after abdominal gynaecologic surgery is safe and could reduce the length of hospital stay.
Summary of findings
Summary of findings for the main comparison. Early oral feeding compared to delayed oral feeding for women who had major abdominal gynaecologic surgery.
| Early oral feeding compared to delayed oral feeding for women who had major abdominal gynaecologic surgery | ||||||
| Patient or population: Women who had major abdominal gynaecologic surgery Settings: University hospital/cancer centre Intervention: Early oral feeding Comparison: Delayed (traditional) oral feeding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed oral feeding | Early oral feeding | |||||
| Postoperative ileus | 77 per 1000 | 36 per 1000 (13 to 99) | RR 0.47 (0.17 to 1.29) | 279 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Nausea or vomiting or both | 352 per 1000 | 363 per 1000 (225 to 588) | RR 1.03 (0.64 to 1.67) | 484 (4 studies) | ⊕⊕⊕⊝ moderate2 | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) |
| Time to bowel sounds [days] | The mean time to the presence of bowel sound [days] in the intervention groups was 0.32 lower (0.61 to 0.03 lower) | 338 (2 studies) | ⊕⊕⊕⊝ moderate3 | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| Time to the passage of flatus [days] | The mean time to the passage of flatus [days] in the intervention groups was 0.21 lower (0.4 to 0.01 lower) | 444 (3 studies) | ⊕⊕⊕⊕ high | |||
| Time to the first solid diet [days] | The mean time to the first solid diet [days] in the intervention groups was 1.47 lower (2.26 to 0.68 lower) | 301 (2 studies) | ⊕⊕⊕⊝ moderate3 | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| Time to first passage of stool [days] | The mean time to first passage of stool [days] in the intervention groups was 0.25 lower (0.58 lower to 0.09 higher) | 249 (2 studies) | ⊕⊕⊕⊝ moderate3 | |||
| Hospital stay [days] | The mean hospital stay [days] in the intervention groups was 0.92 lower (1.53 to 0.31 lower) | 484 (4 studies) | ⊕⊕⊕⊝ moderate4 | Random effects model, deployed because of substantial heterogeneity between studies (I2> 50%) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 For the three studies contributing data, all were at high risk of performance bias, two were at unclear risk of detection bias, and one was at high risk of selection bias (no allocation concealment). 2 For the four studies contributing data, all were at high risk of performance bias and unclear risk of detection bias. 3 This outcome may be influenced by the high risk of performance bias in the two studies that contributed data.
4 This outcome may be influenced by the high risk of performance bias in all studies that provided data.
Background
This review is an update of a previously published review in The Cochrane Library (Issue 4, 2007) on 'Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery'
Description of the condition
Abdominal surgery plays a major role in the treatment of various benign and malignant gynaecologic disorders, including uterine fibroids, endometriosis, ovarian cyst, uterine cancer, and ovarian cancer. In this regard, hysterectomy and removal of ovarian cysts or ovarian tumours are commonly performed procedures. Women, especially those with cancer, frequently undergo multiple procedures during surgery.
There is a widespread belief that intestinal stasis (paralytic ileus), a temporary inhibition of bowel motility, follows all abdominal surgery. The exact cause of this clinical phenomenon is unknown, but proposed mechanisms include stimulation of pain fibres, excessive sympathetic tone, and the release of inhibitory neurotransmitters from the gut wall (Kelly 1997). Gynaecologists have traditionally withheld postoperative oral intake until the return of bowel function as evidenced by the presence of bowel sounds, a passing of flatus or stool or both, and a feeling of hunger. There has been concern that early oral intake would result in vomiting and severe paralytic ileus with subsequent aspiration pneumonia, wound dehiscence (break down), and anastomotic leakage (leakage of surgically‐created connections between parts of the intestine) (Fanning 2001). This belief has become surgical dogma, unsupported by scientific evidence.
Description of the intervention
Recently, the practice of delayed postoperative oral intake has been challenged by evidence from several gastrointestinal physiologic studies that examine contractile activity of the intestine. Gastric emptying and small intestinal absorptive capacity resume on the first postoperative day while colonic activity normally returns within 48 hours after surgery (Wells 1964; Wilson 1975; Woods 1978). These data suggest that postoperative ileus may not occur as a paralysis of the entire bowel with complete absence of any functional contractile activity, as is conventionally assumed (Pearl 1998). If postoperative ileus takes place, it is usually transient and not clinically significant. It is also known that typically the stomach and pancreas secrete one to two litres of fluid daily which are readily absorbed in the small intestine (Bufo 1994). Women after surgery without a nasogastric tube are therefore tolerating high volumes of fluid even though nothing is given orally. In addition, there are studies demonstrating that physical signs suggestive of resolution of postoperative ileus are not well correlated with the incidence of nausea and vomiting (Nachlas 1972; Bufo 1994). Based on these findings, withholding oral intake until resolution of postoperative ileus is not an evidence‐based practice, and may also be unnecessary.
How the intervention might work
Several clinical benefits of giving food and fluids soon after surgery have been proposed in the literature. Following surgery, optimal nutritional status and maintenance of bowel function contribute significantly to wound healing (Windsor 1988; Deitch 1991). Early oral intake has also been suggested to be an effective alternative in postoperative stress ulcer prophylaxis as it helps to maintain strength of bowel mucosa. In patients receiving early oral intake, the risk of sepsis is reduced because of decreased bacterial colonisation and reduced migration through defects on the bowel mucosa into blood circulation (Deitch 1991). Furthermore, an improved sense of well‐being was observed in patients who ate sooner (Schilder 1997). This psychological aspect contributes considerably to the entire postoperative recovery process. Cost effectiveness is another potential advantage of early feeding scheme, as those who begin eating sooner tend to have a shorter length of hospital stay.
Regarding the safety of early postoperative oral intake, evidence from general surgical studies (Reissman 1995; Singh 1998) and a systematic review in women undergoing caesarean delivery (Mangesi 2002) have shown that early oral intake is safe and does not result in any significant increase in complications. Another systematic review on feeding after gastrointestinal surgery has confirmed that there is no clear benefit in keeping patients "nil by mouth", and that early feeding might be beneficial (Lewis 2001).
Why it is important to do this review
Given the lack of a clear evidence‐based rationale for the traditional practice of delaying oral intake after major abdominal gynaecologic surgery, a systematic review of relevant studies will assess potential benefits and harms from early postoperative oral intake. The scope of this review is focused on a specific group of patients, so that results may be more directly applicable. A larger, heterogeneous group of patients (e.g. gynaecology and general surgery patients combined) could potentially complicate results and delay applicability, due to different courses of diseases and operative characteristics.
Objectives
To assess the effects of early versus delayed (traditional) initiation of oral intake of food and fluids after major abdominal gynaecologic surgery.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were eligible that compared the effect of early versus delayed initiation of oral intake of food and fluids after major abdominal gynaecologic surgery on clinically meaningful postoperative outcomes. We excluded quasi‐randomised controlled trials. We did not accept studies with significant violations of allocation procedure and exclusions after allocation.
Types of participants
The study participants were women who had had major open abdominal gynaecologic surgery, regardless of the type of the incision (midline or transverse). 'Major open abdominal gynaecologic surgery' excludes any operations performed mainly for tubal sterilisation. We also excluded studies of women who received gynaecologic laparoscopic surgery (major or minor) or vaginal hysterectomy.
Types of interventions
Main intervention
Early postoperative oral intake of fluids and food. 'Early postoperative oral intake' was defined as having oral intake of fluids or food within the first 24 hours after surgery, regardless of the presence or absence of the signs that indicate the return of bowel function.
Comparison intervention
'Delayed postoperative oral intake' was defined as the introduction of oral fluids or food after the first 24 hours following surgery, and only after clinical signs of resolution of postoperative ileus were evident ‐ most commonly a presence of bowel sound, a passing of flatus or stool, and a feeling of hunger.
We considered that having only ice chips or sips of water within the first 24 hours after surgery but nothing else orally until clinical signs of resolution of postoperative ileus counted as part of the 'delayed' group.
Types of outcome measures
We recorded the following outcomes if the information was available.
Primary outcomes
Development of symptoms and signs of postoperative ileus: rate of nausea, vomiting, cramping abdominal pain, bloating, abdominal distension [dichotomous data]
Time interval: time to the presence of bowel sound, time to the first passage of flatus, time to the first passage of stool, time to the start of regular diet, length of postoperative hospital stay [continuous data]
Secondary outcomes
Other major postoperative complications: rate of infectious complication, wound complication, deep venous thrombosis (DVT), urinary tract infection, pneumonia [dichotomous data]
Satisfaction and health‐related quality of life [continuous data]
Search methods for identification of studies
We searched for all published and unpublished RCTs, without language restriction and in consultation with the Cochrane Menstrual Disorders and Subfertility Group (CMDSG) Trials Search Co‐ordinator:
Electronic searches
We searched the following electronic databases to find reports of relevant RCTs:
Cochrane Menstrual Disorders and Subfertility Group's Specialised Register (searched 1 April 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (1 April 2014);
Ovid MEDLINE (1946 to 1 April 2014);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 1 April 2014);
Ovid EMBASE (1980 to 2014 Week 13);
EBSCO CINAHL (1982 to 1 April 2014)
The registry for ongoing trials [www.clinicaltrials.gov; a service of the US National Institutes of Health] (searched 13 May 2014)]
The search strategies for these databases can be found in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
Searching other resources
We checked the citation lists of relevant publications, systematic reviews, review articles, abstracts of scientific meetings and included studies.
We conducted personal communications with experts, specialists in the field, and the authors of relevant publications in an attempt to identify unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (KC and EM) undertook study selection, screening the titles and abstracts of articles found in the search. We discarded those studies that were clearly ineligible, but the aim was to be overly inclusive rather than risk losing relevant studies. KC obtained copies of the full‐text articles and made copies for EM. Both review authors independently assessed whether the studies met the inclusion criteria, resolving disagreements by discussion. We sought further information from the study authors where papers contained insufficient information to make a decision about eligibility.
Data extraction and management
The review authors independently extracted information using the pro forma designed by the Review Group, resolving discrepancies by discussion. For each included trial, we collected information regarding the study location, study methods (as per quality assessment checklist), participants (age range, eligibility criteria), the nature of the interventions, and data relating to the outcomes specified above. Where possible, we sought missing data from the study authors.
Assessment of risk of bias in included studies
The two review authors independently assessed study quality, with discrepancies resolved by discussion. We explored the risk of bias in the included studies, using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). The domains of bias assessed included selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessors), attrition (incomplete outcome data), and selective reporting.
We present risk of bias assessments for the included studies in tables within the Characteristics of included studies tables, which provide a context for discussing the reliability of the results.
Measures of treatment effect
For dichotomous data, we express results for each study as a risk ratio (RR) with a 95% confidence interval (CI). For continuous data, we express results from each study as a mean difference (MD) with a 95% confidence interval. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements was normal. Hence, skewed data were to be reported in the publication separately as the median and range with non‐parametric tests of significance.
Unit of analysis issues
The primary analysis was by the individual woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible, and sought missing data by contacting investigators from the original trials. If data were still unobtainable, we planned to undertake imputation of individual values for the primary outcomes only.
Assessment of heterogeneity
We examined heterogeneity (variation) between results from different studies by inspecting the forest plot of a meta‐analysis for variation in effects. We also considered formal statistical tests, such as tests of homogeneity of 2 x 2 tables and the I² value (Higgins 2003) in conjunction with graphical approaches, to determine between‐study differences.
When there was substantial unexplained heterogeneity between studies (I2>50%), we used the random‐effects model, and compared pooled results from the fixed‐effect and random‐effects models.
Assessment of reporting biases
For the included studies for which the study protocols were published in www.clinicaltrials.gov, we compared the planned and the reported outcomes to examine their consistency.
Data synthesis
If the studies were sufficiently similar, we combined the data for meta‐analysis with Review Manager 5 software, using a fixed‐effect model (unless there was substantial unexplained heterogeneity) in the following comparison:
Early versus delayed oral fluids and food after major abdominal gynaecologic surgery
An increase in the risk of a particular outcome, which may be beneficial or detrimental, are displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the risk of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We did not plan to perform any subgroup analyses.
Sensitivity analysis
We planned to undertake a sensitivity analysis to see whether excluding trials at high risk of bias affected the results; these include trials at high risk of selection bias.
Results
Description of studies
Results of the search
At the time of the original review, we identified 13 trials providing data comparing different feeding schedules or bowel management strategies following gynaecologic surgery (Finan 1995; Griffenberg 1997; Schilder 1997; Pearl 1998; Cutillo 1999; Fanning 1999; Kraus 2000; MacMillan 2000; Amatyakul 2001; Taguchi 2001; Pearl 2002; Steed 2002; Delaney 2005). We reviewed the full‐text reports of these trials. Three randomised controlled studies (RCTs), two published ( Pearl 1998; Steed 2002) and one unpublished study (Amatyakul 2001) met the inclusion criteria and were included in the final analysis. Ten studies failed to meet the inclusion criteria for reasons outlined in the table of Characteristics of excluded studies. We excluded Schilder 1997 because of its quasi‐randomised design. We considered three RCTs to be closely relevant but we excluded them: Cutillo 1999 compared early feeding with nasogastric decompression after major gynaecologic oncology surgery; MacMillan 2000 included women undergoing vaginal gynaecologic surgery (48% of participants); Pearl 2002 compared a regular diet with clear liquid as the first meal after intra‐abdominal gynaecologic oncology surgery. The remaining six studies in the area of postoperative management to reduce intestinal ileus (Finan 1995; Griffenberg 1997; Fanning 1999; Kraus 2000; Taguchi 2001; Delaney 2005) reported comparisons which were clearly not within the scope of this review.
For this updated version of the review, we identified five additional eligible studies (Feng 2008; Minig 2009a; Minig 2009b; Fanning 2011; Terzioglu 2013), and examined the full‐text reports. Two studies (Minig 2009a; Minig 2009b) met the inclusion criteria and are included in the updated analyses. For the three excluded studies: Feng 2008 compared a semiliquid diet with clear feeds, both started at six hours after major abdominal gynaecologic oncology surgery; Fanning 2011 is a non‐comparative study that examined the effects of immediate postoperative feeding and bowel stimulation in 707 women who had major gynaecologic operations over a five‐year period; Terzioglu 2013 compared eight different combinations of postoperative interventions, including gum chewing, early oral hydration, and early mobilisation following abdominal gynaecologic surgery.
See: Study flow diagram (Figure 1), Characteristics of included studies, and Characteristics of excluded studies.
1.
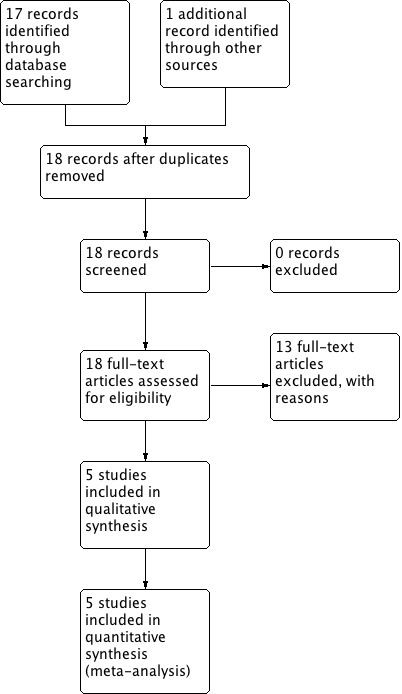
Study flow diagram.
Included studies
Study design and setting
All of the five included studies were parallel‐design randomised controlled trials.
Participants The updated review includes a total of 631 women, increased from the previous total of 413 women. The size of the included studies was: 200 (Pearl 1998), 106 (Amatyakul 2001), 107 (Steed 2002), 51 (Minig 2009a), and 167 (Minig 2009b). Each study was conducted in a single university‐based hospital. The countries represented were the United States (Pearl 1998), Canada (Steed 2002), Thailand (Amatyakul 2001), and Italy (Minig 2009a; Minig 2009b). Three studies (Pearl 1998; Minig 2009a; Minig 2009b) were conducted essentially in gynaecologic oncology patients. In Steed 2002, the majority of women were gynaecologic oncology patients. Almost all (95%) women in the remaining study (Amatyakul 2001) had benign or pre‐invasive gynaecological diagnoses. The women in Amatyakul 2001 were younger (Mean age: 40.8 years in early group, 41.1 years in delayed group) than those in Pearl 1998 (Mean age: 56.5 years in early group, 57.7 years in delayed group), Steed 2002 (Mean age: 50.0 years in early group, 52.0 years in delayed group), Minig 2009a (Median age: 54 years in early group, 58 years in delayed group), and Minig 2009b (Mean age: 54 years in early group, 57 years in delayed group). Body mass index (BMI) was reported in Steed 2002 (Mean BMI: 28.5 kg/m² in early group, 28.7 kg/m² in delayed group), Minig 2009a (Median BMI: 23.0 kg/m2 in early group, 24.0 kg/m2 in delayed group), and Minig 2009b (Mean BMI: 25.0 kg/m2 in both groups). In three studies (Pearl 1998; Amatyakul 2001; Minig 2009b), baseline characteristics of the women were comparable between the two groups. In Steed 2002, there were significantly more women in the early group who received epidural analgesia for pain treatment. However, there was no effect on the primary outcome of the feeding regimen and length of hospital stay when this potential confounder was factored into the final statistical analysis model. In Minig 2009a, estimated operative blood loss was significantly higher in the delayed feeding group (Median blood loss: 800 ml vs. 300 ml). Interventions
The definitions of early and delayed feeding schedule varied among included studies in that:
For the early feeding group, the diet schedule applied in Amatyakul 2001, Minig 2009a, and Minig 2009b, appeared to be more aggressive. In Amatyakul 2001, women were started on a soft diet in the morning of the first postoperative day and proceeded to a regular solid diet on the second postoperative day. In Minig 2009a and Minig 2009b, participants were offered liquids, mineral water (still), tea, chamomile infusion, or apple juice during the first 24 hours. If no nausea and vomiting, a regular diet of boiled or grilled beef, chicken, or fish was given starting on day 1 and continued for the entire hospital stay. In the remaining studies the participants began a clear liquid diet on the first postoperative day and then advanced to a regular diet as tolerated.
For the delayed feeding group, the schedule used in Amatyakul 2001 was slightly more conservative than others. After signs of the return of bowel function, women were allowed to have only sips of water before advancing to a liquid diet in the evening of the same day, while in the other studies women were readily started on a liquid diet after the presence of those signs. We note that the criteria for a return of bowel function were similar in all studies.
Outcomes
Steed 2002 reported the incidence of postoperative ileus, which was defined as more than two episodes of vomiting of at least 100 ml each within a 24‐hour time period, with associated abdominal distension and no bowel sounds. The other studies indirectly assessed the occurrence of postoperative ileus through the incidence of related postoperative gastrointestinal morbidity. Pearl 1998 reported the incidence of nausea, vomiting, abdominal distension, and nasogastric tube use. Amatyakul 2001 reported the incidence of vomiting and abdominal distension. Minig 2009a and Minig 2009b reported intensity of abdominal pain and presence of nausea and emesis. Regarding data on postoperative time intervals to the return of bowel function, time to the presence of bowel sound was reported in Pearl 1998, Minig 2009a and Minig 2009b. Time to the first passage of flatus and time to the start or tolerance of solid food were reported in Pearl 1998, Amatyakul 2001, Minig 2009a, and Minig 2009b. Amatyakul 2001, Minig 2009a, and Minig 2009b reported time to the first passage of stool.
Length of postoperative hospital stay was reported as means in Pearl 1998, Amatyakul 2001, Minig 2009a, and Minig 2009b. However, these data were reported as medians in Steed 2002, because of the skewed distribution.
For the two studies that assessed wound complications, the reported outcomes vary; Pearl 1998 reported as overall wound complications while Steed 2002, Minig 2009a, and Minig 2009b as wound infection. Meta‐analysis results for these dissimilar wound complication outcomes should therefore be interpreted with caution.
Risk of bias in included studies
See: 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
2.
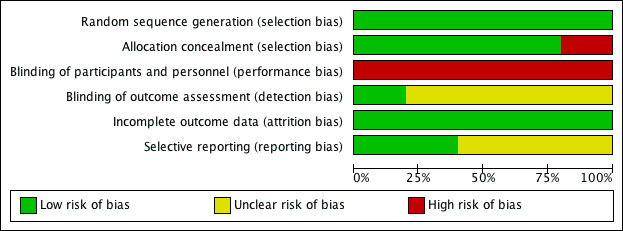
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
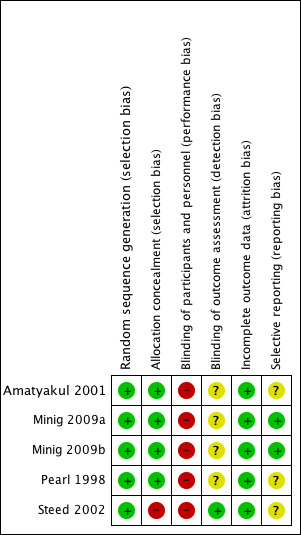
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods of random sequence generation were appropriate in all five included studies. Computer‐generated sequences were employed in four studies (Pearl 1998; Steed 2002; Minig 2009a; Minig 2009b). Amatyakul 2001 used a random number table.
Allocation concealment appeared clearly adequate in four studies (Pearl 1998; Amatyakul 2001; Minig 2009a; Minig 2009b), in which randomisation was performed by using sequentially numbered, sealed, opaque assignment envelopes according to the random number list generated by computer (Pearl 1998), by a random number table (Amatyakul 2001), or by a web‐based Tenalea randomisation system (Minig 2009a; Minig 2009b). In Steed 2002, there appeared to be no concealment of allocation, given the fact that randomisation was performed by the clinic nurses according to an open random number list generated by computer. In Pearl 1998 and Steed 2002, randomisation was performed before the start of the operation. In three studies (Amatyakul 2001; Minig 2009a; Minig 2009b), women were randomised at the end of the operation.
Blinding
Because of the nature of this research question, women and attending physicians were not blinded to the intervention received in any of the studies, and were therefore all at high risk of performance bias.
The outcome assessors in Steed 2002 were blinded, so the risk of detection bias was low. In Amatyakul 2001, Pearl 1998, Minig 2009a, and Minig 2009b, the outcome assessors were aware of participants' study allocation (information from the study authors). However, the influence of the lack of blinding on study outcomes was unclear.
Incomplete outcome data
The follow‐up rate of women in the included studies was: 100% in Amatyakul 2001, 97.5% in Pearl 1998, 90% in Steed 2002, 86% in Minig 2009b, and 78% in Minig 2009a. In Amatyakul 2001, a full intention‐to‐treat analysis was applied. The studies with participant withdrawals used available case analyses.
Selective reporting
In Minig 2009a and Minig 2009b, the study protocols were published in www.clinicaltrials.gov, and the reported outcomes corresponded with those listed in the registered protocols. We therefore judged that these studies were at low risk of reporting bias. In other studies, the study protocols were not published in a protocol registry. However, the report included the expected outcomes. We deemed the risk of reporting bias in these studies to be unclear.
Other potential sources of bias
A power analysis was performed in all trials. With the numbers of women recruited, there was a reasonable probability of detecting a significant effect (should one exist) in Amatyakul 2001, Steed 2002, Minig 2009a, and Minig 2009b, but not in Pearl 1998.
Effects of interventions
See: Table 1
For outcomes with data available, the number of studies contributing usable data ranged from one to four (Table 1).
Early versus delayed oral fluids and food after major abdominal gynaecologic surgery
Primary outcomes
1.1 Postoperative ileus
Rates of developing postoperative ileus were comparable between the study groups (risk ratio (RR) 0.47, 95% confidence interval (CI) 0.17 to 1.29, P = 0.14, 3 RCTs, 279 women, I² = 0%, moderate‐quality evidence) (Analysis 1.1.1). This suggests that in women with an 8% chance of developing postoperative ileus with delayed oral feeding, the chance of developing postoperative ileus with early feeding will be between 1% and 10%. When we specifically considered the rates of nausea or vomiting or both, there was no evidence of a difference between the study groups (RR 1.03, 95% CI 0.64 to 1.67, P = 0.90, random‐effects model, 4 RCTs, 484 women, I² = 73%, moderate‐quality evidence) (Analysis 1.1.2; Analysis 1.5). Considering each symptom separately, early commencement of oral fluids and food was associated with increased nausea (RR 1.79, 95% CI 1.19 to 2.71, P = 0.006, 1 RCT, 195 women, moderate‐quality evidence) (Analysis 1.1.3). However, there was no increase in vomiting (RR 0.86, 95% CI 0.37 to 2.00, P = 0.73, 2 RCTs, 301 women, I² = 2%, moderate‐quality evidence) (Analysis 1.1.4).
1.1. Analysis.
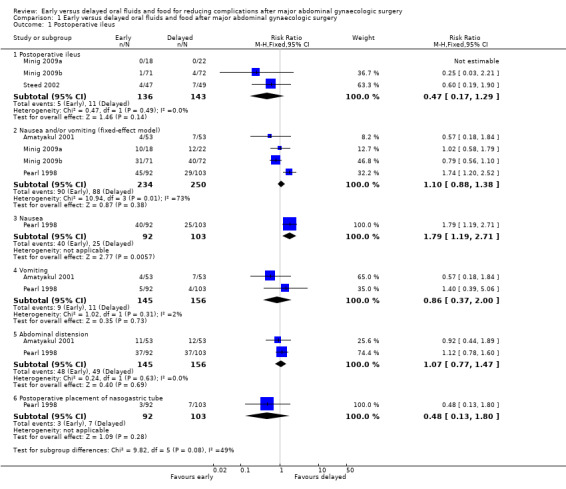
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 1 Postoperative ileus.
1.5. Analysis.
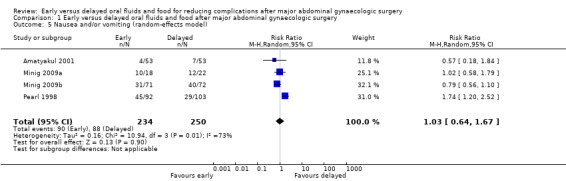
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 5 Nausea and/or vomiting (random‐effects model).
There was no evidence of a difference in abdominal distension (RR 1.07, 95% CI 0.77 to 1.47, 2 RCTs, 301 women, I² = 0%, moderate‐quality evidence) or in the need for postoperative nasogastric tube placement (RR 0.48, 95% CI 0.13 to 1.80, 1 RCT, 195 women, moderate‐quality evidence) between the study groups (Analysis 1.1.5, Analysis 1.1.6).
1.2 Time to recovery of bowel function
Early feeding was associated with shorter time to the presence of bowel sounds (MD ‐0.32 days, 95% CI ‐0.61 to ‐0.03, P = 0.03, random‐effects model, 2 RCTs, 338 women, I² = 52%, moderate‐quality evidence) (Analysis 1.2.1; Analysis 1.6) and faster onset of flatus (MD ‐0.21 days, 95% CI ‐0.40 to ‐0.01, P = 0.04, 3 RCTs, 444 women, I² = 23%, moderate‐quality evidence) (Analysis 1.2.2). In addition, women in the early feeding group resumed a solid diet sooner (MD ‐1.47 days, 95% CI ‐2.26 to ‐0.68, P = 0.0003, random‐effects model, 2 RCTs, 301 women, I² = 92%, moderate‐quality evidence) (Analysis 1.2.3; Analysis 1.7). In Minig 2009b, the proportions of women in the early feeding and delayed feeding groups who tolerated a solid diet were 89% versus 0% on day 1, 10% versus 6% on day 2, and 1% versus 94% on day 3, respectively. However, there was no evidence of a difference in time to the first passage of stool between the two study groups (MD ‐0.25 days, 95% CI ‐0.58 to 0.09, P = 0.15, 2 RCTs, 249 women, I² = 0%, moderate‐quality evidence) (Analysis 1.2.4). The time to the first passage of stool was reported in median in Minig 2009a. They were also comparable between the study groups (5.0 days in the early feeding group and 5.5 days in the delayed feeding group).
1.2. Analysis.
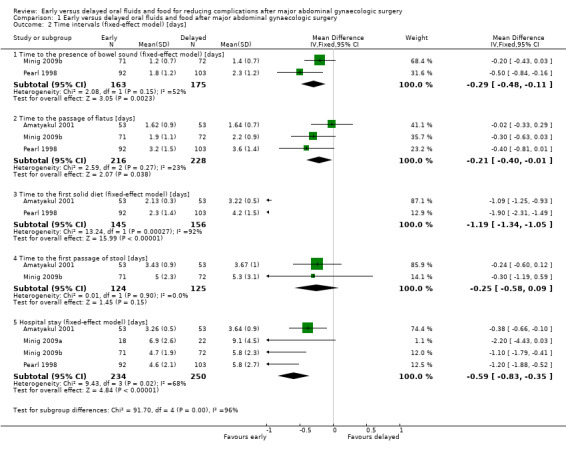
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 2 Time intervals (fixed‐effect model) [days].
1.6. Analysis.
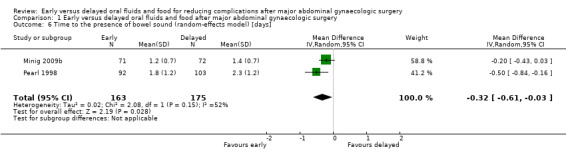
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 6 Time to the presence of bowel sound (random‐effects model) [days].
1.7. Analysis.
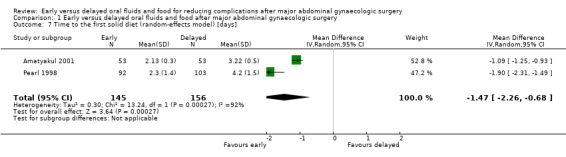
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 7 Time to the first solid diet (random‐effects model) [days].
Hospital stay was shorter in the early feeding group (MD ‐0.92 days, 95% CI ‐1.53 to ‐0.31, P = 0.003, random‐effects model, 4 RCTs, 484 women, I² = 68%, moderate‐quality evidence) (Analysis 1.2.5; Analysis 1.8). The shorter hospital stay associated with early postoperative feeding was further evident in the only study (Steed 2002) that reported length of hospital stay in median days (‐2 days, 4.0 days in the early feeding group and 6.0 days in the delayed feeding group).
1.8. Analysis.
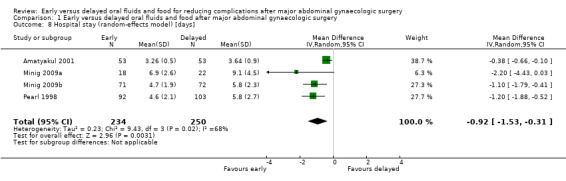
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 8 Hospital stay (random‐effects model) [days].
Sensitivity analyses
Some outcomes had substantial heterogeneity: nausea or vomiting or both, time to the presence of bowel sounds, time to the first solid diet, and hospital stay. In those cases, we also produced a random‐effects model and compared the pooled results from the fixed‐ and random‐effects models. Generally, the two models provided similar results.
Exclusion from analyses of the study at high risk of selection bias (Steed 2002) did not substantially influence any of the findings.
Secondary outcomes
1.3 Other postoperative complications
While the rates of febrile morbidity were similar between the group (RR 0.98, 95% CI 0.76 to 1.27, P = 0.89) in Pearl 1998 (Analysis 1.3.1), infectious complications were less common in the early feeding group (RR 0.20, 95% CI 0.05 to 0.73, P = 0.02, 2 RCTs, 183 women, I² = 0%, high‐quality evidence). (Analysis 1.3.2). The rates of wound complications and pneumonia were comparable between the groups. (Analysis 1.3.3, Analysis 1.3.4)
1.3. Analysis.
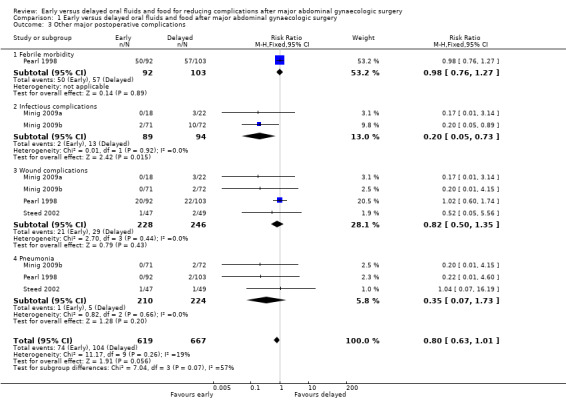
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 3 Other major postoperative complications.
1.4 Satisfaction and health‐related quality of life
Participant satisfaction and quality of life outcomes related to different postoperative feeding schedules were addressed for the first time in the two newly included studies (Minig 2009a; Minig 2009b).
Satisfaction was assessed before hospital discharge by using a visual analogue scale (VAS). In Minig 2009b, the satisfaction score was higher in the early feeding group (MD 11.10, 95% CI 6.68 to 15.52, P < 0.00001, 143 women, moderate‐quality evidence) (Analysis 1.4). In Minig 2009a, the satisfaction score was reported as a median (Q₁ ‐ Q₃). The scores were 80 (80 ‐ 90) in the early feeding group and 75 (60 ‐ 90) in the delayed feeding group (P = 0.07). For women who had delayed feeding, 65% and 58% wished to eat sooner in Minig 2009a and Minig 2009b, respectively.
1.4. Analysis.
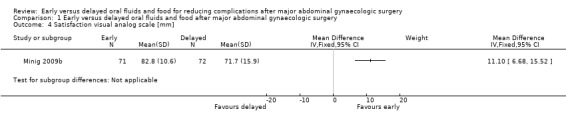
Comparison 1 Early versus delayed oral fluids and food after major abdominal gynaecologic surgery, Outcome 4 Satisfaction visual analog scale [mm].
Quality of life was assessed at 30 days after surgery by using validated questionnaires designed by the European Organization for Research and Treatment of Cancer (EORTC) to measure overall health status in people with cancer in general (EORTC QLQ‐C30), and in those with ovarian cancer (EORTC QLQ‐OV28). The questionnaires focus on global function, physical function, cognitive function, social function, cancer‐related symptoms, and financial impact. There were no statistically significant difference in any of the health‐related quality of life domains between the two study groups.
Discussion
Summary of main results
Early postoperative feeding after major abdominal gynaecologic surgery appeared to be safe without increased gastrointestinal morbidities and other postoperative complications. From the pooled analysis of ileus‐related outcomes, the rates of postoperative ileus (three trials, 279 women), nausea or vomiting or both (four trials, 484 women), and abdominal distention (two trials, 301 women) were comparable between the early and delayed feeding groups. In addition, the rates of overall infectious complications were significantly lower in the participants who had early feeding (two trials, 183 women).
Furthermore, the recovery of bowel function was faster in those with early feeding. These include shorter time to the presence of bowel sounds (two trials, 338 women), shorter time to the passage of flatus (three trials, 444 women), and shorter time to starting a solid diet (two trials, 301 women). Importantly, early feeding was associated with reduced length of hospital stay by one day (four trials, 484 women). It also appeared that women with early feeding were more satisfied with the feeding schedule (one trial, 143 women).
Overall completeness and applicability of evidence
The two newly included studies (Minig 2009a and Minig 2009b, 183 women) have contributed data to most important outcomes of the review, including postoperative ileus, symptoms of nausea or vomiting or both, time to the presence of bowel sounds, time to the passage of flatus, time to the passage of stool, hospital stay, infectious complications, wound complications, pneumonia, and satisfaction. These studies considerably improved the power of the meta‐analysis in detecting the difference between study groups for some outcomes. From this updated version of the review, we can be more confident to suggest the safety of early feeding with its benefits for faster recovery of bowel function, shorter hospital stay, and higher satisfaction. This information would be readily applicable to women undergoing major abdominal gynaecologic surgery for benign or malignant conditions, and to healthcare professionals taking care of them.
It should be noted that the difference between study groups on rare complications might not be detected, given the total number of participants in all of the included studies. Also, there were no data on other outcomes that may also be of interest, such as costs and other physiological benefits of early feeding, e.g. the effects on fluid, electrolyte balance, and wound healing. These data would be useful additional information for patients and physicians deciding on individualised postoperative feeding approaches.
Quality of the evidence
The five included studies were randomised controlled trials with study groups directly relevant to the review question, and provided consistent outcome data. The allocation methods were generally appropriate. The follow‐up was acceptable. There were insufficient studies to assess the risk of publication bias. However, the main methodological concern was with lack of blinding. Because of the context and nature of the studies, it was not possible to blind study participants and difficult or impractical to blind attending physicians. Performance bias may therefore have affected all these studies. It is possible that some outcomes (subjective intestinal morbidities, hospital stay, participants' satisfaction and quality of life) were influenced by the lack of blinding. In addition, outcome assessors were blinded in only one of the five included studies. This raises a concern about detection bias. However, the influence of the lack of blinding of the outcome assessors on study outcomes is unclear. This leads to our rating of the evidence as of moderate quality for subjective outcomes and of high quality for more objective outcomes.
Potential biases in the review process
This review addressed an important clinical question with a clearly defined population, intervention, and outcomes. We were able to include directly relevant studies with randomised controlled designs. For this updated version of the review, we searched a registry for ongoing trials (www.clinicaltrials.gov) in addition to the electronic databases. We also contacted study authors directly for information on relevant studies and on individual study methodology. We explored the risk of bias in the included studies by using the updated Cochrane 'Risk of bias' assessment tool, with each domain of bias explicitly considered. We have clearly tabulated the results of the included studies. The results of the studies which contributed to the pooled analyses appeared similar for most outcomes. In the presence of statistically significant heterogeneity, we deployed appropriate statistical analytic tools, i.e. random‐effects model for the meta‐analyses .
Agreements and disagreements with other studies or reviews
In a randomised study comparing early oral feeding and nasogastric decompression followed by feeding at the first passage of flatus in women undergoing major surgery for gynaecologic malignancies (Cutillo 1999), early oral feeding was associated with a faster resolution of postoperative ileus, a faster return to a regular diet, an earlier passage of stool, and a shorter postoperative hospital stay. The rates of nausea and vomiting were comparable in both groups. Nearly 90% of women who had a nasogastric tube inserted reported discomfort related to difficulty in swallowing and nasal soreness.
The safety and benefits of early feeding for bowel recovery following major abdominal gynaecologic surgery was also generally supported by findings from studies that were closely relevant but excluded from this review (Schilder 1997; MacMillan 2000). In Schilder 1997, which was a quasi‐randomised study comparing early commencement of a clear liquid diet on postoperative day one to delayed feeding until the return of bowel function in gynaecologic oncology patients, those with early feeding tolerated the solid diet earlier (1.88 days versus 2.72 days, P < 0.0001) and had shorter hospital stay (3.12 days versus 4.02 days, P = 0.008). However, the incidence of emesis was higher in the early feeding group, but did not translate to significant adverse outcomes. In MacMillan 2000, early feeding (a low‐residue diet started six hours after surgery) was compared to delayed feeding (a clear liquid diet with the presence of normal bowel sound) in women who had major abdominal or vaginal surgery for benign gynaecologic conditions. While the incidence of postoperative ileus was comparable between the study groups (3% in the early group and 5.8% in the delayed feeding group), the incidence of nausea was higher in the delayed feeding group (23% versus 13%, P = 0.04). There were no differences in perioperative complications, gastrointestinal function, pain scores, pain medication requirement, and fluid and calorie intake between the two study groups.
Authors' conclusions
Implications for practice.
Results from this updated review strengthen the evidence supporting the safety of early postoperative feeding in women undergoing major abdominal gynaecologic surgery for either benign or malignant conditions. The benefits of this approach include faster recovery of bowel function, a lower rate of infectious complications, shorter hospital stay, and higher satisfaction.
Implications for research.
Further methodologically sound studies that examine cost effectiveness, participant satisfaction and preference, and other physiological changes (fluid and electrolyte balance, tissue response, wound healing) associated with different postoperative feeding approaches, would provide additional meaningful information.
What's new
| Date | Event | Description |
|---|---|---|
| 12 September 2014 | New citation required and conclusions have changed | The updated review has two new studies (Minig 2009a and Minig 2009b) included in the analysis. |
| 12 September 2014 | New search has been performed | This review has been updated. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Converted to new review format. |
| 10 November 2008 | Amended | Title edited "traditional" removed |
| 6 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thanks the Cochrane Menstrual Disorders and Subfertility Group (CMDSG) editorial group for their support. We would like to acknowledge the contribution of Greg Phillipson and Teraporn Vutyavanich for their contribution to the original version of this review.
Appendices
Appendix 1. MDSG search strategy
MDSG Search strategy for KC813 15.06.11
Keywords CONTAINS "gynecologic surgical procedure"or "salpingectomy"or"salpingo‐oopherectomy" or "*Salpingostomy‐" or "salpingotomy" or "surgery" or "surgery‐gynaecological" or"Surgical"or "myomectomy"or "Hysterectomy,abdominal"or "total abdominal hysterectomy" or "abdominal myomectomy"or"abdominal hysterectomy"or"abdominal myomectomy" or Title CONTAINS "gynecologic surgical procedure"or "salpingectomy"or"salpingo‐oopherectomy" or "*Salpingostomy‐" or "salpingotomy" or "surgery" or "surgery‐gynaecological" or"Surgical"or "myomectomy"or "Hysterectomy,abdominal"or "total abdominal hysterectomy" or "abdominal myomectomy"or"abdominal hysterectomy"or"abdominal myomectomy"
AND
Keywords CONTAINS "Food intake"or "early feeding"or"starved state" or Title CONTAINS "Food intake"or "early feeding"or"starved state" or"starved state"
Appendix 2. CENTRAL search strategy
1 (surgery or surgical).tw. (67324) 2 exp General Surgery/ (227) 3 exp Postoperative Complications/ (24734) 4 postoperative.tw. (35065) 5 exp Gynecologic Surgical Procedures/ (3154) 6 or/1‐5 (86713) 7 (gynecol$ or gynaecol$).tw. (4596) 8 exp Gynecology/ (87) 9 (abdomin$ or abdomen$).tw. (11988) 10 exp Abdomen/ (2194) 11 exp Pelvis/ (538) 12 pelvi$.tw. (4062) 13 intraabdominal.tw. (308) 14 hysterectom$.tw. (2321) 15 or/7‐14 (21207) 16 exp eating/ or exp gastrointestinal motility/ (4623) 17 exp Food/ (29667) 18 exp Feeding Methods/ or exp Feeding Behavior/ (7376) 19 feeding.tw. (5096) 20 food.tw. (9126) 21 fluid$.tw. (10387) 22 oral.tw. (55828) 23 water.tw. (10191) 24 solid$.tw. (3058) 25 eating.tw. (3105) 26 eat.tw. (725) 27 or/16‐26 (111186) 28 early.tw. (41225) 29 exp Time Factors/ (45655) 30 (time or timing).tw. (113194) 31 day one.tw. (762) 32 day two.tw. (740) 33 or/28‐32 (173700) 34 6 and 15 and 27 and 33 (606) 35 limit 34 to yr="2012 ‐Current" (51)
Appendix 3. MEDLINE search strategy
1 (surgery or surgical).tw. (1204744) 2 exp General Surgery/ (33397) 3 exp Postoperative Complications/ (411947) 4 postoperative.tw. (303965) 5 exp Gynecologic Surgical Procedures/ (63349) 6 or/1‐5 (1586874) 7 (gynecol$ or gynaecol$).tw. (71639) 8 exp Gynecology/ (13214) 9 (abdomin$ or abdomen$).tw. (244185) 10 exp Abdomen/ (80147) 11 exp Pelvis/ (18399) 12 pelvi$.tw. (98306) 13 intraabdominal.tw. (6783) 14 hysterectom$.tw. (26049) 15 or/7‐14 (460798) 16 exp eating/ or exp gastrointestinal motility/ (88490) 17 exp Food/ (1038694) 18 exp Feeding Methods/ or exp Feeding Behavior/ (152611) 19 feeding.tw. (134574) 20 food.tw. (248095) 21 fluid$.tw. (348392) 22 oral.tw. (419172) 23 water.tw. (491988) 24 solid$.tw. (218823) 25 eating.tw. (44448) 26 eat.tw. (12050) 27 or/16‐26 (2648763) 28 early.tw. (1029482) 29 exp Time Factors/ (989633) 30 (time or timing).tw. (2037094) 31 day one.tw. (2125) 32 day two.tw. (1070) 33 or/28‐32 (3546350) 34 6 and 15 and 27 and 33 (4855) 35 randomized controlled trial.pt. (368553) 36 controlled clinical trial.pt. (87954) 37 randomized.ab. (288824) 38 randomised.ab. (57687) 39 placebo.tw. (156289) 40 clinical trials as topic.sh. (168882) 41 randomly.ab. (209520) 42 trial.ti. (123979) 43 (crossover or cross‐over or cross over).tw. (59999) 44 or/35‐43 (931125) 45 exp animals/ not humans.sh. (3907798) 46 44 not 45 (858747) 47 34 and 46 (768) 48 (2012$ or 2013$ or 2014$).ed. (2212373) 49 (2012$ or 2013$ or 2014$).dp. (2164044) 50 48 or 49 (2713731) 51 47 and 50 (132)
Appendix 4. EMBASE search strategy
1 gynecologic surgery/ or uterine tube surgery/ or salpingoplasty/ or salpingostomy/ or uterus surgery/ or hysterectomy/ or abdominal hysterectomy/ or hysterotomy/ or radical hysterectomy/ (53972) 2 ovariectomy/ or salpingooophorectomy/ (32953) 3 ((gynecolog$ or gynaecolog$) adj5 surg$).tw. (11122) 4 ((gynecolog$ or gynaecolog$) adj5 operat$).tw. (2627) 5 (hysterectomy or ovariectomy or salpingostomy or salpingooophorectomy).tw. (41333) 6 exp Uterus Tumor/su [Surgery] (25970) 7 exp Ovary Tumor/su [Surgery] (14387) 8 or/1‐7 (116284) 9 food intake/ or drinking/ or eating/ (108665) 10 feeding/ (26586) 11 feeding behavior/ (52426) 12 (oral adj5 intak$).tw. (8436) 13 (intake adj5 (food or drink or fluid$)).tw. (50060) 14 (postoperat$ adj5 feed$).tw. (1071) 15 (tradition$ adj5 feed$).tw. (460) 16 (tradition$ adj5 intake$).tw. (306) 17 (early adj5 feed$).tw. (4248) 18 (early adj5 intake$).tw. (1386) 19 (delay$ adj5 feed$).tw. (2130) 20 (delay$ adj5 intake$).tw. (503) 21 (timing adj4 intake).tw. (242) 22 (timing adj4 feed$).tw. (403) 23 fasting.tw. (94474) 24 nil per mouth.tw. (7) 25 nil per os.tw. (100) 26 or/9‐25 (294313) 27 8 and 26 (1103) 28 Clinical Trial/ (829495) 29 Randomized Controlled Trial/ (338330) 30 exp randomization/ (61474) 31 Single Blind Procedure/ (18001) 32 Double Blind Procedure/ (112268) 33 Crossover Procedure/ (38288) 34 Placebo/ (236087) 35 Randomi?ed controlled trial$.tw. (95686) 36 Rct.tw. (13359) 37 random allocation.tw. (1288) 38 randomly allocated.tw. (19758) 39 allocated randomly.tw. (1894) 40 (allocated adj2 random).tw. (707) 41 Single blind$.tw. (13923) 42 Double blind$.tw. (137961) 43 ((treble or triple) adj blind$).tw. (351) 44 placebo$.tw. (193373) 45 prospective study/ (244428) 46 or/28‐45 (1338033) 47 case study/ (25023) 48 case report.tw. (253523) 49 abstract report/ or letter/ (882948) 50 or/47‐49 (1156030) 51 46 not 50 (1300837) 52 27 and 51 (137) 53 (2012$ or 2013$ or 2014$).em. (3244058) 54 52 and 53 (27)
Appendix 5. CINAHL search strategy
KC813 CINAHL search strategy 01.04.14
| # | Query | Results |
| S34 | S18 AND S32 | 8 |
| S33 | S18 AND S32 | 72 |
| S32 | S19 OR S20 or S21 or S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | 880,148 |
| S31 | TX allocat* random* | 3,851 |
| S30 | (MH "Quantitative Studies") | 11,743 |
| S29 | (MH "Placebos") | 8,683 |
| S28 | TX placebo* | 31,261 |
| S27 | TX random* allocat* | 3,851 |
| S26 | (MH "Random Assignment") | 36,901 |
| S25 | TX randomi* control* trial* | 70,671 |
| S24 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 707,698 |
| S23 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 103 |
| S22 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S21 | TX clinic* n1 trial* | 161,901 |
| S20 | PT Clinical trial | 75,662 |
| S19 | (MH "Clinical Trials+") | 172,920 |
| S18 | S7 AND S17 | 288 |
| S17 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 200,933 |
| S16 | TX eat* | 32,854 |
| S15 | (MH "Food Intake+") | 5,373 |
| S14 | TX drink* | 28,560 |
| S13 | TX feed* | 47,112 |
| S12 | TX "food" | 98,420 |
| S11 | TX "oral intake" | 645 |
| S10 | TX "fasting" | 10,669 |
| S9 | TX "nil by mouth" | 62 |
| S8 | TX fluids | 5,646 |
| S7 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 | 9,613 |
| S6 | TX Hysterectomy | 4,816 |
| S5 | TX Oophorectomy | 1,898 |
| S4 | TX gyn?ecolog* surg* | 432 |
| S3 | (MM "Oophorectomy") | 590 |
| S2 | (MH "Hysterectomy+") | 3,860 |
| S1 | (MH "Surgery, Gynecologic+") | 8,310 |
Data and analyses
Comparison 1. Early versus delayed oral fluids and food after major abdominal gynaecologic surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative ileus | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Postoperative ileus | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.29] |
| 1.2 Nausea and/or vomiting (fixed‐effect model) | 4 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 1.3 Nausea | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.19, 2.71] |
| 1.4 Vomiting | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.00] |
| 1.5 Abdominal distension | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.47] |
| 1.6 Postoperative placement of nasogastric tube | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.80] |
| 2 Time intervals (fixed‐effect model) [days] | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Time to the presence of bowel sound (fixed‐effect model) [days] | 2 | 338 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.11] |
| 2.2 Time to the passage of flatus [days] | 3 | 444 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.01] |
| 2.3 Time to the first solid diet (fixed‐effect model) [days] | 2 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.34, ‐1.05] |
| 2.4 Time to the first passage of stool [days] | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.09] |
| 2.5 Hospital stay (fixed‐effect model) [days] | 4 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.83, ‐0.35] |
| 3 Other major postoperative complications | 4 | 1286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 3.1 Febrile morbidity | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 3.2 Infectious complications | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.73] |
| 3.3 Wound complications | 4 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 3.4 Pneumonia | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.73] |
| 4 Satisfaction visual analog scale [mm] | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Nausea and/or vomiting (random‐effects model) | 4 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.67] |
| 6 Time to the presence of bowel sound (random‐effects model) [days] | 2 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.61, ‐0.03] |
| 7 Time to the first solid diet (random‐effects model) [days] | 2 | 301 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.26, ‐0.68] |
| 8 Hospital stay (random‐effects model) [days] | 4 | 484 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.53, ‐0.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amatyakul 2001.
| Methods | A prospective randomised controlled study in a single institution. Participants were randomised by using consecutively‐numbered, sealed, opaque envelopes according to the list generated from a random number table. Power calculation was performed a priori. Number of participants randomised: 106 Number of participants analysed: 106 (53 in the early group, 53 in the delayed feeding group) Analysis: Full intention‐to‐treat analysis | |
| Participants | Inclusion criteria: Women scheduled for major abdominal gynaecologic surgery; mean age: 40.8 years (early group), 41.1 years (delayed feeding group) No significant difference in baseline characteristics between the two groups including age, weight, prior abdominal surgery, procedure, type of anaesthesia, operative time, estimated blood loss, and need for blood transfusion. Location: Chiang Mai University hospital, Chiang Mai, Thailand Enrolment period: September 1998 to January 1999 Exclusion criteria: Pregnancy, postoperative intensive care unit admission, endotracheal or nasogastric intubations in the immediate postoperative period, coincidental bowel surgery (excluding appendectomy), history of gastrointestinal diseases or gastrointestinal surgery (excluding appendectomy), history of pelvic or abdominal radiation, preoperative diagnosis of bowel obstruction or preoperative vomiting, preoperative bowel preparation, and history of peritonitis | |
| Interventions | Early group: Participants were allowed to have sips of water within 8 hours after surgery. They were started on a soft diet in the morning of the 1st postoperative day and proceeded to a regular solid diet on the 2nd postoperative day
Delayed feeding group: Participants received nothing by mouth until at least 2 of the following signs of return of bowel function were present: 1) presence of bowel sounds; 2) passage of stool or flatus; 3) subjective hunger, in the morning of the first postoperative day. They were then allowed to have sips of water, and advanced to a liquid diet in the evening of the same day. Participants were given a soft diet in the morning of the 2nd postoperative day and were started on a regular solid diet on the 3rd postoperative day. Discharge criteria included the tolerance of a solid diet, passing flatus, and the discontinuance of intravenous fluids and medications. Participants were not required to have had a bowel movement. |
|
| Outcomes | Hospital stay Gastrointestinal information Morbidity (vomiting, abdominal distention) Postoperative intervals (presence of bowel sounds, passage of flatus, passage of stool, start of regular diet) |
|
| Notes | Funding source: self‐funded Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by using consecutively numbered, sealed, opaque envelopes according to the list generated from a random number table." Comment: Random number table |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised by using consecutively numbered, sealed, opaque envelopes according to the list generated from a random number table." Comment: Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The outcome assessors were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding to study outcomes was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
Minig 2009a.
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. Number of participants randomised: 51 (27 in the early group, 24 in the delayed feeding group) Number of participants analysed: 40 (18 in the early group, 22 in the delayed feeding group), Analysis: Available case analysis | |
| Participants | Inclusion criteria: Gynecologic oncology patients aged 18 ‐ 75 years, undergoing laparotomy with associated intestinal resection; median age: 54 years (early group), 58 years (delayed feeding group). No significant difference in patient characteristics and surgical variables between the two groups except for a higher estimated blood loss in the delayed feeding group (median 800 ml vs. 300 ml). Location: European Institute of Oncology (IEO), Milan, Italy Enrolment period: January 1, 2007 to March 15, 2008 Exclusion criteria: Preoperative (infections, intestinal obstruction, severe malnutrition, American Society of Anesthesiologists score > 4), intraoperative (total or anterior pelvic exenteration, surgery without bowel resection), postoperative (admission to the intensive care unit for > 24 hours, final histopathologic diagnosis revealing nongynaecologic disease) | |
| Interventions | Early group: Participants were offered liquids, mineral water (no gas), tea, chamomile infusion, or apple juice during the first 24 hours. If no nausea and vomiting, a regular diet of boiled or grilled beef, chicken, or fish was given starting on day 1 and continued for the entire hospital stay
Delayed feeding group: Participants received nothing by mouth until the presence of bowel sound and the passage of flatus. Then, if no nausea and vomiting, an oral liquid diet was given for 24 hours. If well tolerated, a semisolid diet was given for 1 day before proceeding to regular diet
Discharge criteria included the tolerance of a regular diet for at least 24 hours with recovery of bowel function, normal clinical parameters and physical examination All participants underwent bowel preparation and preoperative antibiotics prophylaxis. In addition, a nasogastric tube was placed in all participants during surgery and was removed after the surgery finished. Postoperative analgesia was given via epidural catheter for 3 days (ropivacaine and fentanyl) or as intravascular continuous administration of ketorolac and tramadol in those without an epidural catheter. |
|
| Outcomes | Hospital stay Recovery of bowel activity (time to first passage of gas and stool, time to tolerance of a solid diet) Intestinal morbidities (presence of ileus, intensity of abdominal pain, presence of nausea and vomiting) Other morbidities (wound infection, abdominal abscess, pneumonia, urinary tract infection, bacteraemia, wound dehiscence, marked postoperative bleeding, anastomotic leak, respiratory failure, cardiovascular instability, renal dysfunction, thromboembolic complications) Participants' satisfaction level and quality of life Analgesic and antiemetic drug requirements |
|
| Notes | Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using the web‐based TENALEA randomization system (https://it.tenalea.net/ieo)." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized using the web‐based TENALEA randomization system (https://it.tenalea.net/ieo)." Comment: Central web‐based allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay, subjective intestinal morbidities, participants' satisfaction level and quality of life) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Nursing staff, the primary outcome assessors, were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding on study outcomes was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "11 were subsequently excluded (after randomisation) due to postoperative evidence of nongynecologic malignancy (n=3) and admission to ICU for more than 24 h (n=8)." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol was published in www.clinicaltrials.gov. The reported outcomes corresponded to those listed in the registered protocol. |
Minig 2009b.
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. Number of participants randomised: 167 (83 in the early group, 84 in the delayed feeding group) Number of participants analysed: 143 (71 in the early group, 72 in the delayed feeding group), In each group, 12 women were excluded after randomisation because of benign gynaecologic pathology, nongynaecologic pathology, and admission to the ICU for > 24 hours. Analysis: Available case analysis | |
| Participants | Inclusion criteria: Gynecologic oncology patients aged 18 ‐ 75 years, undergoing laparotomy; mean age: 54 years (early group), 57 years (delayed feeding group). The majority of participants had ovarian malignancy, 59% in the early group and 57% in the delayed feeding group.Pelvic and aortic lymphadenectomy were performed in > 70% and in almost 50% of participants, respectively. No significant difference in participant characteristics between the two groups. Location: European Institute of Oncology (IEO), Milan, Italy Enrolment period: January 1, 2007 to November 15, 2007 Exclusion criteria: Preoperative (infections, intestinal obstruction, severe malnutrition, American Society of Anesthesiologists score > 4), intraoperative (total or anterior pelvic exenteration, bowel resection), postoperative (admission to the intensive care unit for > 24 h, final histopathologic diagnosis revealing benign or nongynaecologic disease) |
|
| Interventions | Early group: Participants were offered liquids, mineral water (no gas), tea, chamomile infusion, or apple juice during the first 24 hours. If no nausea and vomiting, a regular diet of boiled or grilled beef, chicken, or fish was given starting on day 1 and continued for the entire hospital stay.
Delayed feeding group: Participants received nothing by mouth until the presence of bowel sound and the passage of flatus. Then, if no nausea and vomiting, an oral liquid diet was given for 24 hours. If well tolerated, a semisolid diet was given for 1 day before proceeding to a regular diet.
Discharge criteria included the tolerance of a regular diet for at least 24 hours with recovery of bowel function, normal clinical parameters and physical examination. All participants underwent bowel preparation and preoperative antibiotics prophylaxis. In addition, a nasogastric tube was placed in all participants during surgery and was removed after the surgery finished. All participants received general anaesthesia. Postoperative analgesia was given via epidural catheter for 3 days (ropivacaine and fentanyl) or as intravascular continuous administration of ketorolac and tramadol in those without an epidural catheter. |
|
| Outcomes | Hospital stay Recovery of bowel activity (time to first passage of gas and stool, time to tolerance for solid diet) Intestinal morbidities (presence of ileus, intensity of abdominal pain, presence of nausea and vomiting) Other morbidities (wound infection, abdominal abscess, pneumonia, urinary tract infection, bacteraemia, wound dehiscence, marked postoperative bleeding, anastomotic leak, respiratory failure, cardiovascular instability, renal dysfunction, thromboembolic complications) Participants' satisfaction level and quality of life Analgesic and antiemetic drug requirements |
|
| Notes | Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized by means of the Web‐based Tenalea randomization system (https://it.tenalea.net/ieo)." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized by means of the Web‐based Tenalea randomization system (https://it.tenalea.net/ieo)." Comment: Central web‐based allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation (information from the study's author). It is possible that some outcomes (hospital stay, subjective intestinal morbidities, participants' satisfaction level and quality of life) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Nursing staff, the primary outcome assessors, were aware of participants' study allocation (information from the study's author). However, the influence of the lack of blinding on study outcomes was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐four patients (12 for each group) were subsequently excluded as a result of benign gynecologic pathology, nongynecologic pathology, and admission to the intensive care unit for > 24 h." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol was published in www.clinicaltrials.gov. The reported outcomes corresponded to those listed in the registered protocol. |
Pearl 1998.
| Methods | A prospective randomised controlled study in a single institution. Power calculation was performed a priori. Number of participants randomised: 200 (95 in the early group, 105 in the delayed feeding group) Number of participants analysed: 195 (92 in the early group, 103 in the delayed feeding group); 5 participants were not measurable because of inoperable bowel obstructions and received gastrostomy tubes: (3 participants; 2 in the early group, 1 in the delayed feeding group) or died of multi‐organ system failure within 36 hours of surgery (1 in each group) Analysis: Available case analysis | |
| Participants | Inclusion criteria: All gynaecologic oncology patients undergoing non laparoscopic intra‐abdominal surgery Mean age: 56.5 years (early group), 57.7 years (delayed feeding group) Underlying diagnosis:‐ Early group: cervical cancer 8.7%, ovarian cancer 30.4%, uterine cancer 38.0%, benign 22.8% ‐ Delayed feeding group: cervical cancer 13.6%, ovarian cancer 33.9%, uterine cancer 24.3%, benign 28.1% No significant difference in baseline characteristics between the 2 groups including age, disease and surgical procedure distribution, operating time, and estimated blood loss Location: State University of New York at Stony Brook, Stony Brook, New York, USA Enrolment period: February 1996 to March 1997 Exclusion criteria were not specified. | |
| Interventions | Early group: Participants began a clear liquid diet on the 1st postoperative day and then advanced to a regular diet as tolerated. Delayed feeding group: Participants received nothing by mouth until return of bowel function (defined as the passage of flatus in the absence of vomiting or abdominal distention), then began a clear liquid diet, and advanced to a regular diet as tolerated. All participants had an orogastric tube placed intraoperatively and removed at the completion of surgery. Participants in either group who were unable to tolerate their diet were given nothing by mouth and received intravenous hydration until resolution of their symptoms, at which time they were restarted on a clear liquid diet and advanced as tolerated. A nasogastric tube was placed for intractable nausea, vomiting, or symptomatic abdominal distention. Standard criteria for discharge were used for all study participants. | |
| Outcomes | Gastrointestinal information
Morbidity (nausea, vomiting, abdominal distention, nasogastric tube use/duration)
Diet tolerance on 1st attempt of clear liquid and regular diet/ time to tolerance
Postoperative intervals (presence of bowel sounds, passage of flatus, start of clear liquid diet, start of regular diet) Other morbidities (febrile morbidity, pneumonia, wound complications, atelectasis) Hospital stay |
|
| Notes | Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using a computer‐generated random number list." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Comment: Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation. It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The outcome assessors were not blinded (information from the study's author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five patients were nonevaluable, three in the early feeding group and two in the delayed feeding group. Of these, one patient in each group died of multiorgan system failure within 36 hours of surgery. The remaining patients had inoperable bowel obstructions, received gastrostomy tubes, and were placed on hospice care." Comment: Reasons for missing outcome data unlikely to be related to true outcome. Also, the missing outcome data were balanced in numbers across groups, with similar reasons for missing data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
Steed 2002.
| Methods | A prospective randomised controlled study in a single institution. Participants were randomised by the clinic nurses according to a computer‐generated random number list. Power calculation was performed a priori. Number of participants randomised: 107 Number of participants analysed: 96 (47 in the early group, 49 in the delayed feeding group); 7 women were excluded because of intraoperative injury of the gastrointestinal tract, and 4 were excluded because of self withdrawal. Analysis: Available case analysis | |
| Participants | Inclusion criteria: Gynaecologic oncology and uro‐gynaecology patients undergoing laparotomy; mean age: 50 years (early group), 52 years (delayed feeding group) Underlying diagnosis of malignancy: 63.0% (early group), 54.0% (delayed feeding group) No significant difference in baseline characteristics between the 2 groups including age, body mass index (BMI), race, malignancy, bowel preparation, procedure, type of incision, operating time, blood loss, need for blood transfusion, and placement of a suprapubic catheter. However, there were significantly more women in early group who received epidural analgesia for pain treatment (55% vs 37%). Location: Royal Alexandra hospital, Edmonton, Alberta, Canada Enrolment period: October 2000 to June 2001 Exclusion criteria: Pregnancy, postoperative intensive care unit admission, endotracheal or nasogastric intubations in the immediate postoperative period, perioperative hyperalimentation, history of gastrointestinal surgery (excluding appendectomy) or bowel obstruction, history of pelvic or abdominal radiation, and history of peritonitis | |
| Interventions | Early group: Participants received clear fluids on the 1st postoperative day. After 500 ml of clear fluids was tolerated, a regular solid diet was given. Delayed feeding group: Participants received nothing by mouth until at least 2 of the following signs of return of bowel function were present: 1) presence of bowel sounds; 2) passage of stool or flatus; 3) subjective hunger. They were then started on clear fluids, and advanced to a regular diet in a stepwise fashion. If there was evidence of ileus (defined as > 2 episodes of emesis of at least 100 ml each within a 24‐hour time period, with associated abdominal distention and no bowel sounds), participants were treated with restriction of oral intake, intravenous fluids, and nasogastric suction, if necessary. Discharge criteria included the tolerance of a solid diet, passing flatus, and the discontinuance of intravenous fluids and medications. Participants were not required to have had a bowel movement. | |
| Outcomes | Hospital stay Gastrointestinal information Morbidity (incidence and episodes of postoperative ileus) Time to tolerance of a solid diet Other morbidities (wound infection, deep venous thrombosis, urinary tract infection, pneumonia, pulmonary oedema) |
|
| Notes | Funding source: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were prospectively randomized with a computer‐generated random number list." Comment: Computer‐generated sequence |
| Allocation concealment (selection bias) | High risk | Comment: Open random number list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the study's context, the participants could not be blinded. The physicians taking care of the participants were aware of their study allocation. It is possible that some outcomes (hospital stay and subjective intestinal morbidities) were influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seven women were excluded because of intraoperative injury of the gastrointestinal tract, and 4 patients were excluded because of self‐withdrawal." Comment: Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not published in a protocol registry. However, the report included expected outcomes. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cutillo 1999 | This study compared early feeding with nasogastric decompression after major gynaecologic oncology surgery, not early versus delayed (traditional) feeding schedule. |
| Delaney 2005 | This study compared different doses of alvimopan and placebo after partial colectomy or simple/radical hysterectomy, not early versus delayed (traditional) feeding schedule. |
| Fanning 1999 | This is a non‐comparative study on effects of aggressive postradical hysterectomy bowel stimulation, which consisted of milk of magnesia and bis colic suppositories. |
| Fanning 2011 | This is a non‐comparative study that examined the effects of immediate postoperative feeding and bowel stimulation in 707 women who had major gynaecologic operations over a 5‐year period. |
| Feng 2008 | This is a randomised controlled study that compared a semiliquid diet with clear feeds, both started at 6 hours after major abdominal gynaecological oncology surgery. The types of diet for early feeding were compared, not the timing. |
| Finan 1995 | This study compared administration of water‐soluble, hyperosmolar, radio‐opaque contrast material and conventional management after gynaecologic surgery, not early versus delayed (traditional) feeding schedule. |
| Griffenberg 1997 | This study compared a high‐fibre diet plan or their usual diet after radical hysterectomy, not early versus delayed (traditional) feeding schedule. |
| Kraus 2000 | This is a non‐comparative study on effects of aggressive postradical hysterectomy bowel stimulation with oral 66% sodium phosphate solution. |
| MacMillan 2000 | Participants had several different types of major gynaecologic surgeries, including abdominal and vaginal approaches, and were randomly allocated to feeding groups regardless of approach. |
| Pearl 2002 | This study compared a regular diet with clear liquid as the first meal after intra‐abdominal gynaecologic oncology surgery, not early versus delayed (traditional) feeding schedule. |
| Schilder 1997 | Although this prospective study compared early versus delayed (traditional) feeding after gynaecological surgery, the design was quasi‐randomisation. |
| Taguchi 2001 | This study compared different doses of ADL 8‐2698, an investigational opioid antagonist and placebo after partial colectomy or simple hysterectomy, not early versus delayed (traditional) feeding schedule. |
| Terzioglu 2013 | This is a randomised controlled study that compared 8 different combinations of postoperative interventions including gum chewing, early oral hydration, and early mobilisation following abdominal gynaecologic surgery. |
Contributions of authors
Kittipat Charoenkwan: Took the lead in writing the protocol. For the review took the lead in writing, selected trials for inclusion, performed independent data extraction, statistical analysis and interpretation of data.
Elizabeth Matovinovic: For the updated review, selected articles for inclusion, performed independent data extraction, assessed risk of bias for included articles, data interpretation, and contributed to writing.
Sources of support
Internal sources
-
Faculty of Medicine, Chiang Mai University, Thailand.
Funding
External sources
-
National Research University Project under Thailand’s Office of the Higher Education Commission, Thailand.
Funding
Declarations of interest
Kittipat Charoenkwan: None Elizabeth Matovinovic: None
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Amatyakul 2001 {unpublished data only}
- Amatyakul P, Suprasert P. Length of hospital stay after major gynecologic operation: a comparison between traditional and early oral feeding. Dissertation thesis for the diploma in Obstetrics and Gynecology, The Royal Thai College of Obstetricians and Gynecologists 2001.
Minig 2009a {published data only}
- Minig L, Biffi R, Zanagnolo V, Attanasio A, Beltrami C, Bocciolone L, et al. Early oral versus "traditional" postoperative feeding in gynecologic oncology patients undergoing intestinal resection: a randomized controlled trial. Annals of Surgical Oncology 2009;16(6):1660‐8. [DOI] [PubMed] [Google Scholar]
Minig 2009b {published data only}
- Minig L, Biffi R, Zanagnolo V, Attanasio A, Beltrami C, Bocciolone L, et al. Reduction of postoperative complication rate with the use of early oral feeding in gynecologic oncologic patients undergoing a major surgery: a randomized controlled trial. Annals of Surgical Oncology 2009;16(11):3101‐10. [DOI] [PubMed] [Google Scholar]
Pearl 1998 {published data only}
- Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intra‐abdominal surgery. Obstetrics and Gynecology 1998;92(1):94‐7. [DOI] [PubMed] [Google Scholar]
Steed 2002 {published data only}
- Steed HL, Capstick V, Flood C, Schepansky A, Schulz J, Mayes DC. A randomized controlled trial of early versus "traditional" postoperative oral intake after major abdominal gynecologic surgery. American Journal of Obstetrics and Gynecology 2002;186(5):861‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cutillo 1999 {published data only}
- Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti‐Panici P. Early feeding compared with nasogastric decompression after major oncologic gynecologic surgery: a randomized study. Obstetrics and Gynecology 1999;93(1):41‐5. [DOI] [PubMed] [Google Scholar]
Delaney 2005 {published data only}
- Delaney C, Weese J, Hyman N, Bauer J, Techner L, Gabriel K, Du W, Schmidt W, Wallin B. Phase III trial of alvimopan, a novel, peripherally acting, Mu opioid antagonist, for postoperative ileus after major abdominal surgery. Diseases of the Colon and Rectum 2005;48(6):1114‐29. [DOI] [PubMed] [Google Scholar]
Fanning 1999 {published data only}
- Fanning J, Yu‐Brekke S. Prospective trial of aggressive postoperative bowel stimulation following radical hysterectomy. Gynecologic Oncology 1999;73(3):412‐4. [DOI] [PubMed] [Google Scholar]
Fanning 2011 {published data only}
- Fanning J, Hojat R. Safety and efficacy of immediate postoperative feeding and bowel stimulation to prevent ileus after major gynecologic surgical procedures. Journal of the American Osteopathic Association 2011;111(8):469‐72. [DOI] [PubMed] [Google Scholar]
Feng 2008 {published data only}
- Feng S, Chen L, Wang G, Chen A, Qiu Y. Early oral intake after intra‐abdominal gynecological oncology surgery. Cancer Nursing 2008;31(3):209‐13. [DOI] [PubMed] [Google Scholar]
Finan 1995 {published data only}
- Finan M, Barton D, Fiorica J, Hoffman M, Roberts W, Gleeson N, et al. Ileus following gynecologic surgery: management with water‐soluble hyperosmolar radiocontrast material. Southern Medical Journal 1995;88(5):539‐42. [PubMed] [Google Scholar]
Griffenberg 1997 {published data only}
- Griffenberg L, Morris M, Atkinson N, Levenback C. The effect of dietary fiber on bowel function following radical hysterectomy: a randomized trial. Gynecologic Oncology 1997;66(3):417‐24. [DOI] [PubMed] [Google Scholar]
Kraus 2000 {published data only}
- Kraus K, Fanning J. Prospective trial of early feeding and bowel stimulation after radical hysterectomy. American Journal of Obstetrics and Gynecology 2000;182(5):996‐8. [DOI] [PubMed] [Google Scholar]
MacMillan 2000 {published data only}
- MacMillan SL, Kammerer‐Doak D, Rogers RG, Parker KM. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstetrics and Gynecology 2000;96(4):604‐8. [DOI] [PubMed] [Google Scholar]
Pearl 2002 {published data only}
- Pearl ML, Frandina M, Mahler L, Valea FA, DiSilvestro PA, Chalas E. A randomized controlled trial of a regular diet as the first meal in gynecologic oncology patients undergoing intraabdominal surgery. Obstetrics and Gynecology 2002;100(2):230‐4. [DOI] [PubMed] [Google Scholar]
Schilder 1997 {published data only}
- Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, et al. A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecologic Oncology 1997;67(3):235‐40. [DOI] [PubMed] [Google Scholar]
Taguchi 2001 {published data only}
- Taguchi A, Sharma N, Saleem R, Sessler D, Carpenter R, Seyedsadr M, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. New England Journal of Medicine 2001;345(13):935‐40. [DOI] [PubMed] [Google Scholar]
Terzioglu 2013 {published data only}
- Terzioglu F, Şimsek S, Karaca K, Sariince N, Altunsoy P, Salman MC. Multimodal interventions (chewing gum, early oral hydration and early mobilisation) on the intestinal motility following abdominal gynaecologic surgery. Journal of Clinical Nursing 2013;22(13‐14):1917‐25. [DOI] [PubMed] [Google Scholar]
Additional references
Bufo 1994
- Bufo AJ, Feldman S, Daniels GA, Lieberman RC. Early postoperative feeding. Dis Colon Rectum 1994;37:1260‐5. [DOI] [PubMed] [Google Scholar]
Deitch 1991
- Deitch EA, Andrassy RJ, Booth FVM, Moore FA. Current concepts in postoperative feeding. Contemporary Surgery 1991;39:37‐55. [Google Scholar]
Fanning 2001
- Fanning J, Andrews S. Early postoperative feeding after major gynecologic surgery: evidence‐based scientific medicine. American Journal of Obstetrics and Gynecology 2001;185(1):1‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kelly 1997
- Kelly DG, Stanhope CR. Postoperative enteral feeding: myth or fact?. Gynecol Oncol 1997;67:233‐4. [DOI] [PubMed] [Google Scholar]
Lewis 2001
- Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus "nil by mouth" after gastrointestinal surgery: systematic review and meta‐analysis of controlled trials. BMJ 2001;323(7316):773‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangesi 2002
- Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section (Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nachlas 1972
- Nachlas MM, Younis MT, Roda CP, Wityk JJ. Gastrointestinal motility studies as a guide to postoperative management. Annals of Surgery 1972;175(4):510‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reissman 1995
- Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery?. Annals of Surgery 1995;222(1):73‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1998
- Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. Journal of the American College of Surgeons 1998;187(2):142‐6. [DOI] [PubMed] [Google Scholar]
Wells 1964
- Wells C, Tinckler LF, Rawlinson K, Jones H, Saunders J. Post‐operative gastrointestinal motility. Lancet 1964;I(7323):4‐10. [DOI] [PubMed] [Google Scholar]
Wilson 1975
- Wilson JP. Post‐operative motility of the large intestine in man. Gut 1975;16(9):689‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Windsor 1988
- Windsor JA, Knight GS, Hill GL. Wound healing response in surgical patients: recent food intake is more important than nutritional status. British Journal of Surgery 1988;75(2):135‐7. [DOI] [PubMed] [Google Scholar]
Woods 1978
- Woods JH, Erickson LW, Condon RE, Schulte WJ, Sillin LE. Post‐operative ileus. A colonic problem?. Surgery 1978;84(4):527‐33. [PubMed] [Google Scholar]
References to other published versions of this review
Charoenkwan 2003
- Charoenkwan K, Phillipson G, Tongsong T. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004508] [DOI] [PubMed] [Google Scholar]
Charoenkwan 2007
- Charoenkwan K, Phillipson G, Vutyavanich T. Early versus delayed oral fluids and food for reducing complications after major abdominal gynaecologic surgery. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD004508.pub3] [DOI] [PubMed] [Google Scholar]


