Abstract
BACKGROUND
Sodium level is an important clinical predictor of complex biliary disease. Hyponatremia has been observed in conjunction with biliary disease, however the nature of this association remains unclear.
AIM
To investigate the association between serum sodium and severe biliary disease.
METHODS
Of 920 patients with gallstone disease treated at the SFVA Hospital from 1989-2019 were studied. We conducted multivariate analyses of correlation between sodium level and biliary disease severity, the presence/location of biliary bacteria, and other factors. Minimum sodium level pre-intervention was collected. Gallstones, bile, and blood (as relevant) were cultured. Illness severity was characterized: (1) None (no infectious manifestations); (2) Systemic inflammatory response syndrome; (3) Severe illness (gangrenous cholecystitis, cholangitis, necrotizing pancreatitis); and (4) Multiple organ dysfunction syndrome (bacteremia, hypotension, organ failure). Comorbidity was defined using the Charlson Comorbidity Index (CCI).
RESULTS
Decreased sodium level significantly correlated with worsening illness severity, ascending bacterial infection, gangrenous changes, elevated CCI score, increasing age, male sex, and glucose. On multivariate analysis, all factors, except age, gender and glucose, independently correlated with sodium level and factors were additive.
CONCLUSION
This unique study is the first to explore, with such granularity, the relationship between biliary disease and sodium. No prior studies have examined specific culture and clinical data. It illustrates an inverse, independent correlation between illness severity and sodium. Culture data demonstrate that sodium decreases as infection ascends from gallstone colonization to bactibilia to bacteremia. Patient comorbidity and gangrenous changes also independently correlate with sodium on multivariate analysis. Sodium level is an important clinical indicator of disease severity for patients with biliary disease.
Keywords: Hyponatremia, Biliary disease, Cholecystitis, Cholangitis, Sodium, Sepsis
Core tip: This unique study is the first to explore, with such granularity, the relationship between biliary disease and sodium. No prior studies have examined specific culture and clinical data. It demonstrates an inverse, independent correlation between illness severity and sodium. Culture data demonstrate that sodium decreases as infection ascends from gallstone colonization to bactibilia to bacteremia. Patient comorbidity and gangrenous changes also independently correlate with sodium on multivariate analysis. Sodium level is an important clinical indicator of disease severity for patients with biliary disease.
INTRODUCTION
According to nationwide estimates, approximately 20.5 million Americans between ages 20-74 years have gallstone disease[1]. Other sources estimate a prevalence in the general population of 10%-15%, however these values may still underestimate the extent of disease. Surgical procedures to address symptomatic cholelithiasis are very common with over 500000 cholecystectomies performed annually[2]. The spectrum of gallstone disease ranges from simple biliary colic and chronic cholecystitis to severe, life-threatening illnesses such as gangrenous cholecystitis, cholangitis, and biliary sepsis. Despite the many laboratory and radiographic studies available, efficiently identifying patients with a serious underlying biliary infection remains a clinical challenge. Early diagnosis is crucial to prevent rapid clinical deterioration. Selecting reliable and predictive admission variables can facilitate recognizing patients with more severe biliary illness, allowing clinicians to optimize the treatment approach[3]. One such routinely obtained variable is sodium. Hyponatremia, defined as sodium less than 135 mEq/L, has previously been recognized in the context of a variety of inflammatory and infectious disease states. The pathophysiologic mechanism is likely multifactorial and may be related to the effects of inflammatory cytokines[4,5]. Hyponatremia has similarly been described in association with intra-abdominal infectious processes, including appendicitis, diverticulitis, and gangrenous cholecystitis[6-8]. Importantly, no previous study has described hyponatremia over the spectrum of biliary disease. We hypothesized that markers of more severe biliary illness were associated with a predictable decrease in sodium level.
MATERIALS AND METHODS
Using a prospectively collected, comprehensive quality outcomes database containing detailed patient demographics, clinical information, and outcomes spanning from March 1989 to October 2019, we studied 920 patients with gallstone disease (892 at San Francisco VA Hospital, 28 at UCSF Medical Center). Patients were identified by general surgery consultation. The lowest sodium level during the initial acute phase of illness, prior to therapeutic intervention (surgery, endoscopic retrograde cholangiopancreatography, percutaneous transhepatic cholangiography, percutaneous cholecystostomy tube drainage) was recorded. These values were, in most cases, within 24-48 h of acute presentation or preoperative values. Coincident glucose values were also recorded. Operative report, pathology reports, and radiology imaging studies, were reviewed for the presence of gangrenous cholecystitis, abscess, perforation, and/or necrotizing pancreatitis.
Illness severity, based on pre-intervention findings, was classified into four groups: (1) None: No clinical infection or inflammatory manifestations; (2) Systemic inflammatory response syndrome (SIRS): One or more of temperature ≥ 100F; white blood cell > 10 K/cm2, respiratory rate > 20 breaths/minute, or heart rate > 90 beats/minute; (3) Severe illness: Gallbladder empyema, gangrenous cholecystitis, necrotizing pancreatitis, abdominal or hepatic abscess formation, and/or cholangitis; and (4) Sepsis/multiple organ dysfunction syndrome (MODS): Bacteremia, hypotension, or organ dysfunction/failure. Patients were placed into the most severe group for which they met criteria.
The level of bacterial infection was determined using culture results from gallstones, bile, and blood. Patients were stratified by the highest level of bacterial infection detected. When bacteria were present at multiple levels, the highest bacterial level detected was ascribed. For example, patients with positive gallstone and bile cultures were stratified in the bactibilia group, while cases with positive bile and blood cultures were stratified in the bacteremia group.
The Charlson Comorbidity Index (CCI) was calculated for each patient to assess underlying medical morbidity. The CCI, developed in 1987, assigns a score based on the presence and severity of 19 underlying comorbid conditions[9]. Using this index, an estimated 10-year survival can be calculated. CCI was analysed as a continuous variable and also in three groups, CCI 0-2, 3-4, and ≥ 5.
The primary measures assessed were sodium level correlation with illness severity and the presence/location of biliary bacteria. Hyponatremia is defined as serum sodium less than 135.0 mEq/L. Secondary measures determined whether other clinical factors correlated, including: The presence of gangrene (gangrenous cholecystitis, abscess, perforation, or necrotizing pancreatitis), CCI score, age, gender, and/or glucose level.
Statistical analysis
Statistical analysis was performed using analysis of variance for interval parametric data, Chi-squared test for variables on a nominal scale (rates and proportions), and Spearman’s correlation for bivariate comparisons of ordinal and interval data. A probability of < 0.05 was considered significant. Multivariate analysis was performed using SPSS (IBM, Version 21). The study was approved by UCSF and San Francisco VA IRB.
RESULTS
Of 920 patients with gallstone disease were studied. The population was predominantly male (n = 806, 88%) with mean age of 63 (range 17-104 years). Of 267 (29%) patients had preoperative sodium < 135 mEq/L. Most patients underwent a procedure during their admission and some underwent multiple procedures: 847 (92%) underwent laparoscopic or open cholecystectomy, 113 (12%) underwent cholecystostomy tube placement, 204 (22%) had an endoscopic retrograde cholangiopancreatography (or PTC), and 12 (1%) underwent endoscopic ultrasonography. There were no post-operative deaths. Three patients (two who were DNR), treated non-operatively, died following a sudden cardiac event and/or aspiration pneumonia.
Correlation between sodium levels and age, CCI, illness severity, location of biliary bacteria, presence of gangrenous changes, and glucose is shown in Table 1. Included in this table are results of both bivariate and multivariate statistical analyses.
Table 1.
Bivariate and multivariate analysis of sodium level and clinical factors
| n (%) | Na (mEq/L) | Bivariate (P value) | Multivariate (P value) | ||
| Age1 | < 50 yr | 150 (16) | 138.3 | 0.0001 | 0.821 |
| 50-69 yr | 487 (53) | 136.6 | |||
| ≥ 70 yr | 283 (31) | 135.8 | |||
| Glucose1 | Normal (< 115 mg/dL) | 392 (44) | 137.6 | 0.0001 | 0.127 |
| Elevated (≥ 115 mg/dL) | 501 (56) | 135.7 | |||
| Illness severity | No infectious manifestations | 403 (44) | 138.7 | 0.0001 | 0.0001 |
| SIRS | 196 (21) | 136.7 | |||
| Severe | 154 (17) | 134.7 | |||
| Sepsis/MODS | 167 (18) | 133.2 | |||
| Bacterial location | None/sterile | 396 (45) | 138.3 | 0.0001 | 0.023 |
| Gallstone only | 60 (7) | 137.2 | |||
| Bile | 338 (38) | 135.4 | |||
| Blood | 85 (10) | 133.1 | |||
| Gangrenous changes | None | 732 (80) | 137.3 | 0.0001 | 0.002 |
| Gangrene | 188 (20) | 133.8 | |||
| Charlson Co-Morbidity index1 | 0-2 | 518 (57) | 137.4 | 0.0001 | 0.001 |
| 3-4 | 261 (29) | 136.2 | |||
| 5 or more | 140 (14) | 134.6 |
Analyzed as a continuous variable. MODS: Multisystem organ dysfunction syndrome; SIRS: Systemic inflammatory response syndrome; Gangrenous changes: Gangrenous cholecystitis, gallbladder perforation, abscess, necrotizing pancreatitis.
Age and gender
Age was studied both as a continuous variable and in groups. Three age groups were examined: < 50 years (n = 150, 16%), 50-69 years (n = 487, 53%), and ≥ 70 years (n = 283, 31%). Mean preoperative sodium for each group was 138.3 mEq/L, 136.6 mEq/L, 135.8 mEq/L, respectively (P < 0.0001, AVOVA). However, on multivariate analysis, age did not independently correlate with hyponatremia (P = 0.821).
On bivariate analysis, sodium significantly correlated with gender (P = 0.0001, Spearman’s rho). Sodium levels were significantly lower in men than in women, 136.4 mEq/L vs 137.9 mEq/L, respectively (P = 0.0001, ANOVA). However, on multivariate analysis, gender did not independently correlate with hyponatremia (P = 0.452).
Glucose, triglycerides, and cirrhosis
Blood glucose values were available in 899 (98%) cases. On bivariate analysis, hyponatremia was associated with higher mean glucose (160 vs 130 mg/dL, P = 0.0001, ANOVA), but glucose was below 200 mg/dL in 82% of cases with hyponatremia. Diabetic patients had lower sodium levels compared to non-diabetics (135 mEq/L vs 137 mEq/L, respectively, P < 0.0001, AVOVA). On multivariate analysis, glucose did not independently correlate with hyponatremia (P = 0.127).
Triglyceride levels were available in 819 cases (89%). Triglyceride levels were not different among cases with and without hyponatremia (148.6 mg/dL vs 153 mg/dL, respectively, P = 0.534, ANOVA).
Cirrhosis was uncommon in this series, only 64 (7%) patients had cirrhosis. Average sodium levels were lower in cases with cirrhosis (136.7 mEq/L vs 135.9 mEq/L, none vs cirrhosis, respectively), but differences were not significant (P = 0.120, ANOVA). Contributions from cirrhosis were reflected in the CCI calculation, which was included in the multivariate analysis.
Charlson Comorbidity Index
Comorbidity was studied as both a continuous variable and in groups. Patients were stratified into three groups based on CCI: 0-2 (n = 518, 57%), 3-4 (n = 261, 29%), and ≥ 5 (n = 140, 14%). Based on CCI, the estimated 10-year survival for each group was 90%-98%, 53%-77%, and ≤ 21%, respectively. The mean sodium for each group was 137.4 mEq/L, 136.2 mEq/L, and 134.6 mEq/L, respectively (P = 0.0001, ANOVA). CCI also independently correlated with hyponatremia on multivariate analysis (P = 0.001).
Clinical biliary illness
Among the 618 cases with stones confined to the gallbladder, 257 (42%) had biliary colic, 182 (30%) had uncomplicated acute cholecystitis, and 179 (28%) had complicated acute cholecystitis (empyema, gangrenous cholecystitis, gallbladder perforation, and/or abscess formation). Mean sodium among these three groups was 139 mEq/L, 137.3 mEq/L, and 133.9 mEq/L, respectively (P < 0.0001, ANOVA).
Among the 302 cases with common bile duct stones, 84 (28%) had uncomplicated choledocholithiasis, 89 (29%) had pancreatitis, and 129 (43%) had cholangitis. Mean sodium among these three groups were 137.7 mEq/L, 136.4 mEq/L, and 134.2 mEq/L, respectively (P < 0.0001, ANOVA).
Illness severity
Patients were categorized into one of four groups (None, SIRS, Severe, MODS) based on illness severity prior to intervention (surgery, endoscopic retrograde cholangiopancreatography, cholecystostomy tube, etc.). The distribution of illness severity was: None [403 (44%) cases], SIRS [198 (22%) cases], Severe [151 (16%) cases], and sepsis/MODS [168 (18%) cases]. Mean preoperative sodium for each group was 138.7 mEq/L, 136.7 mEq/L, 134.7 mEq/L, and 133.2 mEq/L, respectively (P < 0.0001, ANOVA). On multivariate analysis, illness severity independently correlated with hyponatremia (P = 0.0001).
Bacterial culture
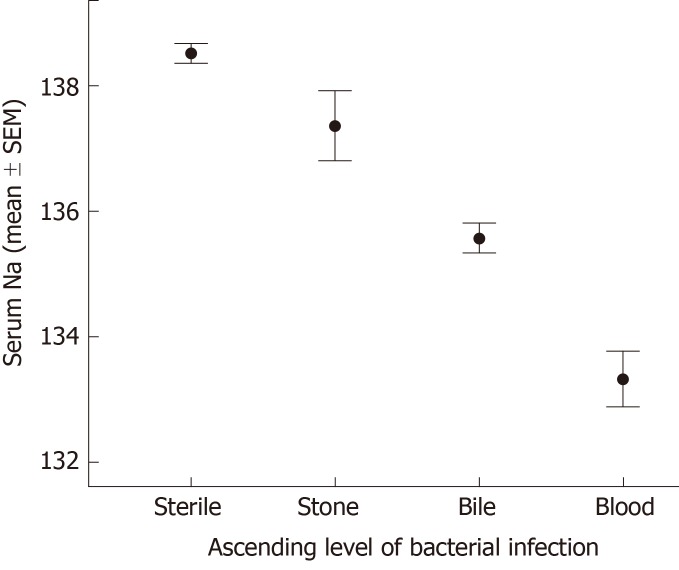
Cultures were obtained from gallstones and bile, as well as blood when clinically appropriate. Bacterial cultures were available in 879 cases, while blood cultures were obtained in 359 cases. One or more bacterial cultures were positive in 483 (55%) cases. The presence of biliary bacterial correlated with hyponatremia (135.2 mEq/L vs 138.3 mEq/L, biliary bacteria vs sterile, P < 0.0001, ANOVA) (Figure 1).
Figure 1.
Sodium level - influence of ascending level of biliary bacteria. A higher level of bacterial infection is an independent predictor of lower sodium level, P < 0.01.
In many cases, cultures from multiple sites demonstrated bacterial growth, so cases were stratified by the highest level of bacterial infection detected. 396 (43%) cases had sterile cultures, gallstone culture (alone) was positive in 60 (7%) cases, and bactibilia was detected in 338 (40%) cases. Among 359 cases where blood cultures were obtained, bacteremia was present in 85 (24%) cases. Mean sodium levels from each group were 138.3 mEq/L, 137.2 mEq/L, 135.4 mEq/L, and 133.1 mEq/L, (sterile, gallstone bacteria, bactibilia, and bacteremia, respectively, P = 0.0001, ANOVA). On multivariate analysis, ascending level of biliary bacterial correlated with hyponatremia (P = 0.023); sodium significantly decreased as bacteria ascended from gallstone colonization to bactibilia to bacteremia. The simple presence of biliary bacteria demonstrated a trend (P = 0.058) with hyponatremia.
Presence of gangrenous changes
Patients were included in this group based on the presence of the following: Gangrenous cholecystitis (intraoperative or pathologic diagnosis), gallbladder perforation, pericholecystic abscess, and/or necrotizing pancreatitis. Of the 189 (20.5%) patients with gangrenous changes, 61% had preoperative hyponatremia. Gangrenous changes were associated with significantly lower mean sodium (133.8 mEq/L vs 137.3 mEq/L, P = 0.0001, ANOVA). On multivariate analyses, gangrenous changes independently correlated with hyponatremia (P = 0.002).
Additive effects of clinical factors
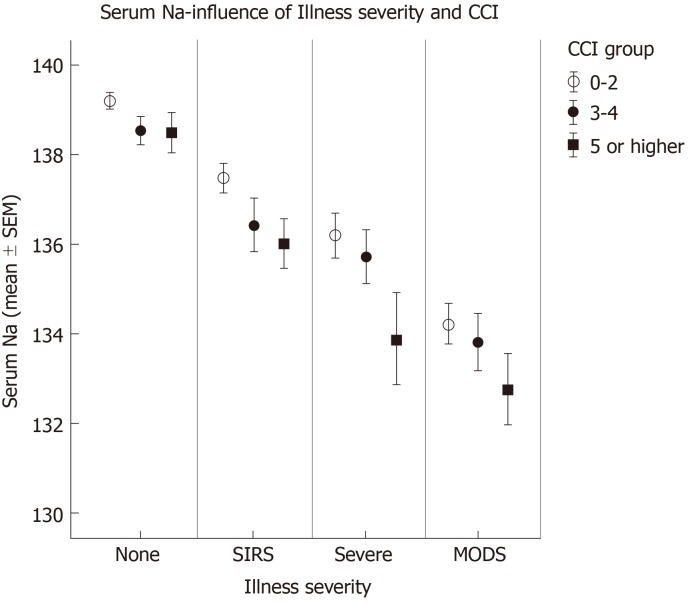
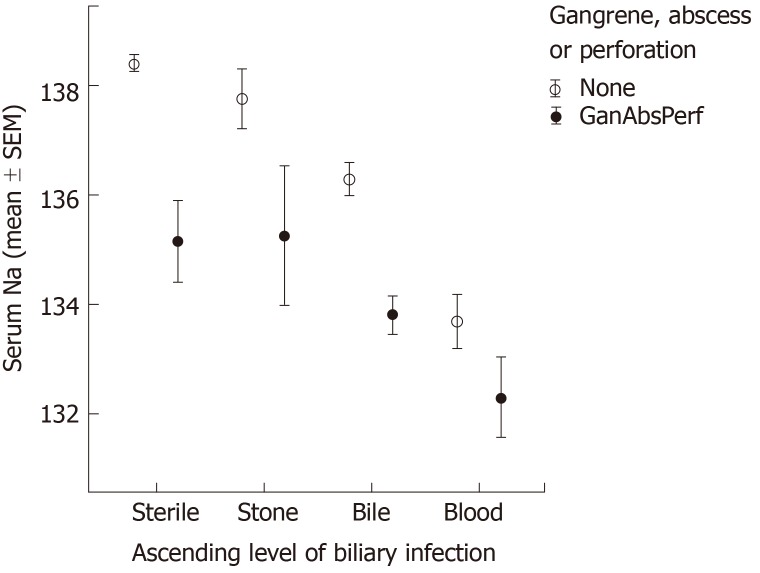
Clinical factors that were independently associated with sodium level on multivariate analyses included: Co-morbidity (CCI), illness severity, ascending location of biliary bacteria, and the presence of gangrenous changes. The effects of these factors on sodium level seemed to be additive. As shown in Figure 2, for each level of illness severity, mean sodium is even lower as CCI increased. In addition, as shown in Figure 3, mean sodium was lower for patients with a higher level of biliary bacteria and even lower for those patients who also demonstrated gangrenous changes. Note that even among cases with a sterile biliary tree, gangrenous changes (e.g., necrotizing pancreatitis) correlated with decreased sodium level.
Figure 2.
Sodium level - influence of illness severity and Charlson Comorbidity Index score. Illness severity and Charlson Comorbidity Index score (CCI) are both independent predictors of sodium level (Open circles - CCI 0-2; Closed circles - CCI 3-4; Closed squares - CCI 5 or higher). P values significant (< 0.01) for all. CCI: Charlson Comorbidity Index.
Figure 3.
Sodium level - Influence of the level of bacterial infection and presence of gangrene. A higher level of biliary infection and the presence of gangrene are both independent predictors of sodium level (Open circles - absence of gangrene; Closed circles - presence of gangrene). P values significant (< 0.01) for all parings.
DISCUSSION
This study is the first of such size to describe the relationship between hyponatremia and complex biliary disease. Specifically, it illustrates a progressive decrease in sodium level with worsening disease severity. Patients presenting with more severe infections and illness severity demonstrated a significantly reduced serum sodium. In addition, availability of bacterial culture data allowed us to identify associations between the presence of bacteria and hyponatremia, as well as the influence of ascending levels of bacterial infection (gallstone, bile, or blood) on the degree of sodium reduction. This association could be used clinically at the time of presentation to identify patients at greatest risk of decompensation and to guide treatment[3].
The importance of hyponatremia as a marker of disease severity has been documented in other studies[10-22]. Hyponatremia has been reported in the context of medical illnesses and inflammatory disease including congestive heart failure, chronic kidney disease, pneumonia, acute respiratory distress syndrome, meningitis, encephalitis, tuberculosis, human immunodeficiency virus infection, and pediatric malignancies and inflammatory illnesses. These reported associations between medical illness with decreased sodium level are consistent with the results of this study - that of decreasing sodium with increasing CCI. But this numerical straightforward association between CCI and sodium level has not previously been reported.
The importance of acute hyponatremia in surgical outcomes has also been recently reported, with some surgical studies reporting a significantly increased mortality risk with hyponatremia[4-8,10,23-27]. Sakr et al[23] studied nearly 11000 surgical intensive care unit patients, and demonstrated that hyponatremia at the time of intensive care unit admission was independently associated with increased risk of in-hospital mortality (16.5% vs 7.4%, P < 0.001). Using multivariate analysis, Mc Causland et al[24] examined patients undergoing orthopedic surgery and demonstrated an increase in the risk of 30-d perioperative mortality rate associated with decreasing sodium. Leung et al[10] using the ACS NSQIP database, compared 75423 patients with preoperative hyponatremia to 888,840 patients with normal baseline sodium, and noted a higher risk of 30-d mortality (5.2% vs 1.3%) in cases with hyponatremia. These findings are consistent with our results and demonstrate the clinical utility of utilizing sodium preoperatively. The results of these studies reflect both the contribution from underlying morbidity and acute illness to surgical outcomes. Our study is unique in that it identified that both of these parameters (co-morbidity and illness severity) are reflected in the sodium level. Further, our study revealed that the sodium level seems to reflect additive contributions from both of these factors. This is an important distinction when analysing the significance of a decreased sodium level.
There is an increasing body of literature to explain potential associations between sodium level and inflammatory/infectious states. Currently, lipopolysaccharide and the inflammatory cytokines TNF-α, IL-1, and IL-6, which are elevated in inflammatory states, are believed to play a key role[5,28-33]. Studies have demonstrated that increased levels, or intravenous injection, of IL-6 leads to increased release of vasopressin from the hypothalamus[28]. The antidiuretic effect of vasopressin acting on renal tubule V2 receptors could explain the subsequent hyponatremia. A possible limitation of this theory is a lack of clarity regarding the passage of IL-6 through the blood-brain barrier, but mechanisms have been described[29]. Multiple studies have also documented the powerful effect of TNF-α on sodium channels, which may explain its role in developing hyponatremia[30-33]. Schmidt, studying mechanisms of lipopolysaccharide-induced renal dysfunction, demonstrated that injection of TNF-α or IL-1β increased the production of renal cytokines, decreased expression of renal sodium transporters, and decreased renal function overall[30]. In other studies, TNF-α has also been shown to interact with amiloride-sensitive, sodium ion channels on the surface of vertebrate cells via N,N’-diacetylchitobiose lectin-like activity, inducing an increase of membrane conductance, causing membrane depolarization[31-33]. Taken together, these studies explore potential mechanisms for hyponatremia in association with significant inflammatory responses and acute illness caused by complex biliary disease.
Our group has previously shown an association between TNF-α and severe biliary infections. We have had a long interest in the contribution of biliary bacteria to the severity of illness in patients with gallstone disease[34-43]. We previously identified several bacterial factors that facilitate biliary infection and influence the severity of disease. One such observation was that bacteria present in the biliary tree of patients with more severe biliary illness, produced greater levels of TNF-α in in vitro and in vivo models[41-43]. The most virulent bacterial species (from patients with sepsis) were associated with increased TNF-α production. We also noted that elderly patients harbored bacteria with a heightened ability to produce TNF-α, suggesting an important contribution from inflammatory cytokines[41-43]. This agrees well with the current study given the well-described effect of TNF-α on sodium channels. Reduced sodium level is likely a feature of both acute and chronic inflammatory states, and may reflect an elevated production of inflammatory cytokines. From the observations of this study, these effects are both independent and additive.
We also examined the effect of glucose on sodium levels, to determine if pseudohyponatremia, or dilutional hyponatremia, was a confounding factor. While glucose was significantly increased in patients with preoperative hyponatremia on univariate analysis, the mean glucose was below 200 mg/dL in 80% of cases, indicating there was likely little effect on sodium levels. In addition, glucose did not independently correlate with hyponatremia on multivariate analysis. While glucose remains a useful parameter, it did not appear to be the primary reason for hyponatremia in this study population. Additionally, possible influences from cirrhosis or elevated triglycerides on sodium levels, did not appear to be a factor in this study.
There are some limitations of this study. First, most patients in this study were veterans, a generally older, male-predominant population. While we did note an association between male gender and hyponatremia, male gender did not independently correlate with hyponatremia on multivariate analysis. It remains unclear whether the study population limited our ability to examine contributions made by gender. It is unlikely this would greatly change our findings since women more commonly have cholesterol gallstones, which are generally sterile[37,40].
On the other hand, men and elderly patients are more likely to harbor biliary bacteria, so our population is ideal for studying the effects of biliary infection on sodium levels. Furthermore, this elderly population is more medically complex, facilitating our investigation into the role of co-morbid disease and hyponatremia. While the study time period was long (reflecting our long-term interest in biliary infections), there was continuity assured by the senior author and methods for managing biliary disease were consistent throughout the period.
In conclusion, this unique, large study is the first to explore, with such granularity, the relationship between complicated biliary disease and sodium levels. No prior studies have examined specific culture and clinical data, as these results are often not readily available in administrative databases. While other studies identified an association between chronic and acute medical illness on sodium levels, this study revealed that both underlying medical morbidity and acute illness severity contribute to decreased sodium levels, and that the effects are additive. This unique observation has not been previously reported. The study illustrates an inverse, independent correlation between illness severity and sodium level. Culture data demonstrate that sodium decreases as bacterial infection ascends from gallstone colonization to bactibilia to bacteremia. Patient comorbidity and the presence of gangrenous changes also independently correlate with sodium on multivariate analysis. Sodium is an important clinical indicator of underlying disease severity for patients with biliary disease.
ARTICLE HIGHLIGHTS
Research background
While hyponatremia has been studied in the context of other infectious and inflammatory disease states, sodium level has not yet been studied in a large-scale clinical study of patients with severe biliary disease. Serum sodium a common initial laboratory test for patients presenting with biliary disease, may serve as an important diagnostic tool for assessing the underlying severity of illness.
Research motivation
Despite advances in both laboratory and radiographic technologies, identifying patients with severe biliary disease remains a challenge for health care providers in the acute setting. We felt that serum sodium could serve as a critical tool in identifying patients with severe illness, accelerating their care, and improving patient outcomes.
Research objectives
We sought to clarify the relationship between serum sodium and severe biliary disease. Specifically, our goals were to determine if a correlation existed between serum sodium and both illness severity and the presence/location of biliary bacteria. We also sought to determine if such a correlation existed with other clinical factors including the presence of gangrenous changes, Charlson comorbidity score, and glucose level.
Research methods
We utilized a prospectively collected, comprehensive quality outcomes database containing detailed patient demographics, clinical information, and outcomes spanning from March 1989 to October 2019 with 920 patients with gallstone disease. The lowest sodium level during the initial acute phase of illness, prior to therapeutic intervention was recorded. We determined illness severity based on pre-intervention findings and classified patients accordingly. The level of bacterial infection was determined using culture results from gallstones, bile, and blood; patients were stratified by the highest level of bacterial infection detected. Patients were also stratified by the Charlson Comorbidity Index.
Research results
We observed a progressive, statistically significant decrease in sodium level with worsening disease severity. We also observed an incremental decrease in serum sodium with ascending bacterial infection. Charlson Comorbidity Index and the presence of gangrenous changes were also independent predictors of decreased sodium on multivariate analysis.
Research conclusions
This study is the first to describe an inverse relationship between serum sodium and severity of biliary disease. Furthermore, it reveals that both underlying medical morbidity and acute illness severity contribute to decreased sodium levels, and that the effects are additive. This study justifies the use of serum sodium as a clinical predictor of the severity of biliary disease.
Research perspectives
The direction of future research regarding patients with low serum sodium and biliary disease should focus on the pathophysiologic cause of decreased serum sodium. The applications of this research have implications not only for patients with biliary disease, but with any inflammatory or infectious process.
Footnotes
Institutional review board statement: This study was exempt from IRB approval as patient data was anonymously collected and not associated with any identifying information.
Informed consent statement: No consent was needed for this study.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Peer-review started: September 5, 2019
First decision: October 13, 2019
Article in press: December 14, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kato J, Uhlmann D S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
Contributor Information
Michael John Zobel, Department of Surgery, University of California San Francisco, San Francisco, CA 94143, United States.
Lygia Stewart, Department of Surgery, University of California San Francisco, San Francisco, CA 94143, United States; Department of Surgery, San Francisco VA Medical Center, San Francisco, CA 94121, United States.
References
- 1.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/S0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 2.McClusky D. Laparoscopic Cholecystectomy-A SAGES Wiki Article. SAGES [cited 1 December 2017] Available from: https://www.sages.org/wiki/laparoscopic-cholecystectomy/
- 3.Miura F, Takada T, Kawarada Y, Nimura Y, Wada K, Hirota M, Nagino M, Tsuyuguchi T, Mayumi T, Yoshida M, Strasberg SM, Pitt HA, Belghiti J, de Santibanes E, Gadacz TR, Gouma DJ, Fan ST, Chen MF, Padbury RT, Bornman PC, Kim SW, Liau KH, Belli G, Dervenis C. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:27–34. doi: 10.1007/s00534-006-1153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SJ, Shin JI. Inflammation and hyponatremia: an underrecognized condition? Korean J Pediatr. 2013;56:519–522. doi: 10.3345/kjp.2013.56.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiol. 2011;118:45–51. doi: 10.1159/000322238. [DOI] [PubMed] [Google Scholar]
- 6.Falor AE, Zobel M, Kaji A, Neville A, De Virgilio C. Admission variables predictive of gangrenous cholecystitis. Am Surg. 2012;78:1075–1078. doi: 10.1007/s11695-012-0698-9. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Nassiri N, de Virgilio C, Ferebee MP, Kaji AH, Hamilton CE, Saltzman DJ. Association Between Hyponatremia and Complicated Appendicitis. JAMA Surg. 2015;150:911–912. doi: 10.1001/jamasurg.2015.1258. [DOI] [PubMed] [Google Scholar]
- 8.Käser SA, Furler R, Evequoz DC, Maurer CA. Hyponatremia is a specific marker of perforation in sigmoid diverticulitis or appendicitis in patients older than 50 years. Gastroenterol Res Pract. 2013;2013:462891. doi: 10.1155/2013/462891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Leung AA, McAlister FA, Rogers SO, Jr, Pazo V, Wright A, Bates DW. Preoperative hyponatremia and perioperative complications. Arch Intern Med. 2012;172:1474–1481. doi: 10.1001/archinternmed.2012.3992. [DOI] [PubMed] [Google Scholar]
- 11.Ohta M, Ito S. [Hyponatremia and inflammation] Rinsho Byori. 1999;47:408–416. [PubMed] [Google Scholar]
- 12.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170:294–302. doi: 10.1001/archinternmed.2009.513. [DOI] [PubMed] [Google Scholar]
- 13.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, Shorr AF. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin. 2008;24:1601–1608. doi: 10.1185/03007990802081675. [DOI] [PubMed] [Google Scholar]
- 15.Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–190. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 16.Bettari L, Fiuzat M, Felker GM, O'Connor CM. Significance of hyponatremia in heart failure. Heart Fail Rev. 2012;17:17–26. doi: 10.1007/s10741-010-9193-3. [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605–610. doi: 10.1016/S1590-8658(00)80844-0. [DOI] [PubMed] [Google Scholar]
- 19.Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med. 2011;124:77–84. doi: 10.1016/j.amjmed.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patwari AK, Singh BS, Manorama DE. Inappropriate secretion of antidiuretic hormone in acute bacterial meningitis. Ann Trop Paediatr. 1995;15:179–183. doi: 10.1080/02724936.1995.11747769. [DOI] [PubMed] [Google Scholar]
- 21.Riikonen P, Saarinen UM, Perkkiö M, Hovi L, Siimes MA. Changing pattern of treatment policies invalidates the use of C-reactive protein level and hyponatremia as indicators of sepsis in children with malignancies. Pediatr Hematol Oncol. 1992;9:365–372. doi: 10.3109/08880019209016609. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Oh YS, Choi MJ, Shin JI, Kim KH. Hyponatremia may reflect severe inflammation in children with febrile urinary tract infection. Pediatr Nephrol. 2012;27:2261–2267. doi: 10.1007/s00467-012-2267-9. [DOI] [PubMed] [Google Scholar]
- 23.Sakr Y, Rother S, Ferreira AM, Ewald C, Dünisch P, Riedemmann N, Reinhart K. Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit Care Med. 2013;41:133–142. doi: 10.1097/CCM.0b013e318265f576. [DOI] [PubMed] [Google Scholar]
- 24.Mc Causland FR, Wright J, Waikar SS. Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9:297–302. doi: 10.1002/jhm.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, Luketic VA, Shiffman ML, Maluf DG, Cotterell AH, Posner MP, Stravitz RT. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int. 2009;29:1071–1077. doi: 10.1111/j.1478-3231.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 26.Yun BC, Kim WR, Benson JT, Biggins SW, Therneau TM, Kremers WK, Rosen CB, Klintmalm GB. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. 2009;49:1610–1615. doi: 10.1002/hep.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg. 2011;54:433–439. doi: 10.1016/j.jvs.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994;79:934–939. doi: 10.1210/jcem.79.4.7962300. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt C, Höcherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol. 2007;18:1072–1083. doi: 10.1681/ASN.2006050454. [DOI] [PubMed] [Google Scholar]
- 31.Hribar M, Bloc A, van der Goot FG, Fransen L, De Baetselier P, Grau GE, Bluethmann H, Matthay MA, Dunant Y, Pugin J, Lucas R. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. Eur J Immunol. 1999;29:3105–3111. doi: 10.1002/(SICI)1521-4141(199910)29:10<3105::AID-IMMU3105>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- 33.Hazemi P, Tzotzos SJ, Fischer B, Andavan GS, Fischer H, Pietschmann H, Lucas R, Lemmens-Gruber R. Essential structural features of TNF-α lectin-like domain derived peptides for activation of amiloride-sensitive sodium current in A549 cells. J Med Chem. 2010;53:8021–8029. doi: 10.1021/jm100767p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart L, Smith AL, Pellegrini CA, Motson RW, Way LW. Pigment gallstones form as a composite of bacterial microcolonies and pigment solids. Ann Surg. 1987;206:242–250. doi: 10.1097/00000658-198709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg. 2002;6:891–903; discussion 903-4. doi: 10.1016/S1091-255X(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 36.Smith AL, Stewart L, Fine R, Pellegrini CA, Way LW. Gallstone disease. The clinical manifestations of infectious stones. Arch Surg. 1989;124:629–633. doi: 10.1001/archsurg.1989.01410050119023. [DOI] [PubMed] [Google Scholar]
- 37.Stewart L, Griffiss JM, Way LW. Spectrum of gallstone disease in the veterans population. Am J Surg. 2005;190:746–751. doi: 10.1016/j.amjsurg.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Stewart L, Ponce R, Oesterle AL, Griffiss JM, Way LW. Pigment gallstone pathogenesis: slime production by biliary bacteria is more important than beta-glucuronidase production. J Gastrointest Surg. 2000;4:547–553. doi: 10.1016/S1091-255X(00)80100-6. [DOI] [PubMed] [Google Scholar]
- 39.Stewart L, Griffiss JM, Jarvis GA, Way LW. Gallstones containing bacteria are biofilms: bacterial slime production and ability to form pigment solids determines infection severity and bacteremia. J Gastrointest Surg. 2007;11:977–83; discussion 983-4. doi: 10.1007/s11605-007-0168-1. [DOI] [PubMed] [Google Scholar]
- 40.Stewart L, Griffiss JM, Jarvis GA, Way LW. Bacteria entombed in the center of cholesterol gallstones induce fewer infectious manifestations than bacteria in the matrix of pigment stones. J Gastrointest Surg. 2007;11:1298–1308. doi: 10.1007/s11605-007-0173-4. [DOI] [PubMed] [Google Scholar]
- 41.Stewart L, Oesterle AL, Griffiss JM, Jarvis GA, Aagaard B, Way LW. Gram-negative bacteria killed by complement are associated with more severe biliary infections and produce more tumor necrosis factor-alpha in sera. Surgery. 2002;132:408–414. doi: 10.1067/msy.2002.127423. [DOI] [PubMed] [Google Scholar]
- 42.Stewart L, Oesterle AL, Grifiss JM, Jarvis GA, Way LW. Cholangitis: bacterial virulence factors that facilitate cholangiovenous reflux and tumor necrosis factor-alpha production. J Gastrointest Surg. 2003;7:191–8; discussion 198-9. doi: 10.1016/S1091-255X(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 43.Stewart L, Grifiss JM, Jarvis GA, Way LW. Elderly patients have more severe biliary infections: influence of complement-killing and induction of TNFalpha production. Surgery. 2008;143:103–112. doi: 10.1016/j.surg.2007.06.035. [DOI] [PubMed] [Google Scholar]