Abstract
Abstract
Gingerol, a biologically active component in ginger, has shown antiemetic properties. Our study aimed to explore the underlying mechanisms of gingerol on protecting rats and minks from chemotherapy-induced nausea and vomiting. The preventive impact of gingerol was evaluated in the pica model of rats and the vomiting model of minks induced by cisplatin at every 6 h continuously for a duration of 72 h. Animals were arbitrarily separated into blank control group, simple gingerol control group, cisplatin control group, cisplatin + metoclopramide group, cisplatin + three different doses gingerol group (low-dose; middle-dose; high-dose). The area postrema as well as ileum damage were assessed using H&E stain. The levels of 5-TH, 5-HT3 receptor, TPH, SERT, SP, NK1 receptor, PPT, NEP, DA, D2R, TH, and DAT were determined using immunohistochemistry or qRT-PCR in rats and minks. All indicators were measured in the area postrema along with ileum. The kaolin intake by rats and the incidence of CINV of minks were significantly decreased after pretreatment with gingerol in a dosage-dependent way for the duration of 0–24-h and 24–72-h. Gingerol markedly decreased the levels of 5-TH, 5-HT3 receptor, TPH, SP, NK1 receptor, PPT, DA, D2R, TH, alleviated area postrema as well as ileum damage, and increased the accumulation of SERT, NEP, DAT in the area postrema along with ileum of rats and minks. Gingerol alleviates cisplatin-induced kaolin intake of rats and emesis of minks possibly by regulating central and peripheral 5-HT system, SP system and DA system.
Graphic abstract
Keywords: Gingerol, Vomiting, Serotonin, Substance P, Dopamine
Introduction
Nausea as well as vomiting (emesis) are indications of numerous diseases and adverse effects of several medicines [1]. Chemotherapy-induced nausea and vomiting (CINV) continue to be a problem for patients with cancer [2]. Even though the antiemetic therapies have advanced, as many as 90% of patients receiving highly emetogenic chemotherapies (HEC) experience CINV [3]. CINV can restrict clinical application of chemotherapy, lower the quality of patients' life and even result in termination of therapy [4, 5]. Cisplatin, one effective agent of chemotherapy, often results into both acute and delayed emesis [6, 7]. Some patients who received cisplatin therapy (chemotherapy) regard CINV as one of their greatest feared and troubling events [8]. However, the existing antiemetic drugs have problems such as single target and poor safety, and hence sometimes need combination drugs [9, 10]. Therefore, the exploration of drugs with multiple targets and high safety to prevent and treat nausea and vomiting has become a priority while using chemotherapy to maintain the quality of patients' life.
The acute and delayed emesis are two phases of CINV, which involve central mechanism and peripheral gastrointestinal mechanism. The acute emesis happens during first 24 h of the initial administration of chemotherapeutic agent, whereas the delayed emesis transpires after this period [11]. 5-hydroxytryptamine (5-HT) is one of the vital causative agents in acute emesis [12]. 5-hydroxytryptamine type 3 (5-HT3) receptors, which facilitate the quick and transitory membrane-depolarizing influence of 5-HT in the central and peripheral nervous system, have imperative roles in nausea as well as vomiting [13]. The production of 5-HT is initiated by the enzyme tryptophan hydroxylase (TPH). 5-HT from enterochromaffin cells could either be released into the lumen from the apical part or to the circulation and local surroundings from the basal part [14, 15]. Extracellular 5-HT is deactivated and reprocessed via reuptake into adjacent cells by a selective serotonin transporter (SERT) [14, 16]. More recently, the neuropeptide substance P (SP) and dopamine (DA) systems in addition to the 5-HT3 system are involved in CINV [17–20]. The homeostasis of SP transmission is dominated essentially by three control originals: neurokinin-1 (NK1) receptor, preprotachykinin (PPT) and neutral endopeptidase (NEP). Substance P is cleaved from preprotachykinin A (PPTA) encoded by Tac1, which plays a neurotransmitter or neuroregulatory role in central and peripheral nervous systems and is involved in a variety of physiological and disease processes, including vomiting, nociception, inflammation and depression [17, 21]. NK1 receptors, as receptor of SP, are also existent in the gastrointestinal (GI) tract and might contribute to acute-phase CINV [22, 23]. NEP is a cell membrane-bound metallopeptidase enzyme, which controls SP activity [24]. As a traditional neurotransmitter of vomiting, DA has an imperative involvement in the pathogenesis of vomiting [25, 26]. DA is secreted in gastrointestinal and central nervous system, and its effect is mainly by binding to DA receptors (DR) [27]. Tyrosine hydroxylase (TH) and dopamine transporters (DATs) regulate DA neurotransmission at the biosynthesis and reuptake steps, respectively [28]. Despite all the exploration of the mechanism of vomiting over the years, the mechanism of 5-HT, SP and DA systems in CINV is not fully understood.
Ginger has been used as a household medicinal agent in China for more than 2000 years to treat colds, headaches, respiratory, fever, asthmatic disorders and other ailments [29]. Ginger and the traditional Chinese recipe Xiao-Ban-Xia-Tang that is made from ginger and pinellia, are widely used for nausea and vomiting induced by different stimuli. Gingerol is a principle bioactive ingredients of ginger. Due to the advances in the purification technique, gingerol has shown the possibility of its use in clinical trials and has received much attention due to its antiemetic, antioxidant, anti-inflammatory, prevention of motion sickness and relief of nausea during pregnancy [30–33]. Our previous studies have proved that gingerol and ginger-containing Xiao-Ban-Xia-Tang can significantly alleviate nausea as well as vomiting triggered by cisplatin [31, 34], but the specific mechanism is still unclear.
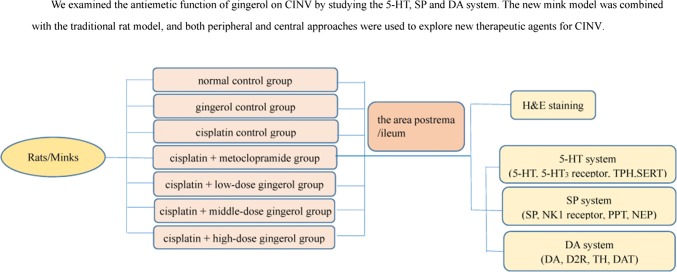
The goal of our study was to examine the antiemetic function of gingerol on CINV by studying the 5-HT, SP and DA system. Two chemotherapy-induced vomiting animal models were used in this study for the first time. The new mink model was combined with the traditional rat model, and both peripheral and central approaches were used to explore new therapeutic agents for CINV.
Materials and methods
Reagents
Gingerol was purchased from Xi'an Biotechnology and was liquefied by 1% tragacanth. Cisplatin from Jinan Qilu Pharmaceutical was prepared using NS at 70 ℃ then cooled stably to 40 ℃ and was given instantly. Kaolin was prepared according to the previously published method [35] with minor modifications. Kaolin (Tianjin Kemiou Chemical Reagent, China) and with 7% gum arabic (Tianjin Guangfu Fine Chemical Research Institute, China) were combined together in distilled water to prepare a thick paste. This paste was kept in a tube and partly dried using a dryer. The paste was removed from the tube, and was cut into the same size as the normal feed pellets. Next, it was dried entirely in a dryer. Metoclopramide was obtained from Qilu Pharmaceutical, Jinan, China. Anti-HT, anti-5-HT3 receptor, anti-TPH, anti-SP, anti-NK1 receptor, anti- NEP, anti-DA, anti-D2R, anti-TH, anti-DAT and goat anti-rabbit antibody were obtained from Beijing Bioss Biological Technology, China.
Animals
Adult male Wistar rats weighing 180 ~ 220 g were provided by Shandong University Laboratory Animal Shelter (Shandong, China). They were accommodated by separate cages with temperature 22 ± 2 ℃ and relative humidity 40–60%, 12 h dark/light cycles, and food as well as water free.
Adult castrated male minks (1.0 ~ 1.8 kg in weight) were acquired from Qingdao University Guide for the Care and Use of Laboratory Animals. Minks were kept separately in iron cages which were 75 cm × 50 cm × 50 cm in size with free water as well as food.
Rat experiments were conducted in the laboratory of Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Since only Qingdao has the special animal mink breeding center, the mink was raised, observed and collected in Qingdao University laboratory as per the guidelines of the Qingdao University Institutional Animal Care and Use Committee, and the follow-up experiment was conducted in the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. All actions were approved by the ethics committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (No. 2016096).
Groups and treatment
Animals were arbitrarily separated into seven groups (rats: n = 5, minks: n = 6): blank control group (C), simple gingerol control group (CG), cisplatin control group (V), cisplatin + metoclopramide group (M), cisplatin + three different doses gingerol group (low-dose, GL; middle-dose, GM; high-dose, GH). The Group C and V received pretreatment with sterile saline. Animals in Group CG received pretreatment with gingerol (rats: 40 mg/kg, i.g.; minks: 200 mg/kg, i.g.). The Group M were preprocessed with metoclopramide (rats: 2.5 mg/kg, i.g.; minks: 4 mg/kg, i.g.). The GL, GM and GH groups received pretreatment with gingerol (rats: 10 mg/kg, i.g., 20 mg/kg, i.g., 40 mg/kg, i.g.; minks: 50 mg/kg, i.g., 100 mg/kg, i.g., 200 mg/kg, i.g.). Gingerol doses were selected based on our previous studies [31, 36]. The above operations were carried out for three days. Gingerol was dissolved with 1% tragacanth. On the third day, cisplatin (rats: 3 mg/kg, i.p.; minks: 6 mg/kg, i.p.) was given 30 min after each process with metoclopramide or gingerol excluding the Groups C and CG. After treatment of cisplatin, rats and minks were perceived at every 6 h uninterruptedly for 72 h for the consumption of kaolin of rats and emetic reactions of minks. The emetic reaction involved the start time of vomiting as well as the sum of retching plus vomiting.
H&E staining
Specimens of the area postrema in addition to ileum were fixed by 10% formaldehyde for 24 h and then dehydrated by means of 50% xylene and 100% xylene for 1 h and 2 h, respectively. The specimens were immersed in paraffin and then sliced using a microtome at room temperature. After dewaxed using paraffin I and II each for 15 min, specimens were hydrated with pure ethanol, 90% ethanol and 70% ethanol for 5 min, 2 min, and 2 min, respectively. They were subsequently stained with hematoxylin for 10 min, distinguished with hydrochloric acid alcohol solution for 35 s and eosin for 1 min. Then the specimens were dehydrated in an alcohol gradient with xylene. Using a light microscope, pathological changes in the area postrema along with ileum following gingerol treatment were observed.
Immunohistochemistry
Tissues from the area postrema along with the ileum of rats and minks were used for immunohistochemical examination. The ileum was excised at a distance of 15 cm from the pylorus in rats and 20 cm in minks. Briefly, these tissue were dewaxed and dehydrated, and then were sectioned (4 μm thick) by means of a cold microtome (ASpZr35), followed by usual immunohistochemical processes to visualize 5-HT, 5-HT3 receptor, TPH, SP, NK1 receptor, NEP, DA, D2R, TH and DAT protein. Next, at room temperature, wash the sections four times (10 min each time) with PBS (pH 7.4) and block using 10% normal goat serum.
Next, they were cultured with anti-HT, anti-HT3 receptor, anti-TPH, anti-SP, anti-NK1 receptor, anti-NEP, anti-DA, anti-D2R, anti-TH and anti-DAT at 4 °C overnight. After the washing using PBS, the sections were cultured for 2 h with goat anti-rabbit antibody and reacted with 3′3′-diaminobenzidine (DAB, Beijing Zhongshan Jinqiao Biotechnology Co. Ltd.) for 10 min. Finally, the sections were counterstained with Neutral Red (0.5%). Sections were examined under light microscope (Olympus CKX41-32PH) comprising an imaging system (Olympus Optical, Japan).
The optical densities were detected by Image-Pro Plus v 6.0 (Media Cybernetics, USA).
Real-time quantitative PCR (qRT-PCR)
Total RNA from the frozen area postrema in addition to ileum tissues of rats or minks were isolated by means of TRIZOL (Sigma-Aldrich, USA) as per the supplier’s protocol. DNase (DNAfree, Ambion, USA) was used to eliminate contaminated genomic DNA, tailed by phenol, chloroform extraction as well as ethanol precipitation. The purity of the total RNA was assessed by the NanoDrop system. Primer Premier 5.0 (Premier Biosoft, USA) was utilized to design the primers for SERT, PPT, NEP genes of rat, and the primers for SERT, PPT genes of mink. The primer sequences are mentioned in Table 1. Primers were manufactured by BioAsia Corp (Shanghai, China). qRT-PCR was done on the LightCycler device (Roche Diag Diagnostics, Germany) by means of Power SYBR Green PCR Master Mix kits (Applied Biosystems). The effectiveness of qRT-PCR was evaluated with sequential dilutions of cDNA sample from the normal control group. All experimentations were done in duplicates and data were evaluated by LightCycler Software 4.0 (Roche Diagnostics). GAPDH was utilized as the reference gene.
Table 1.
List of primers utilized in qRT-PCR
| Animal | Primer | Forward | Reverse |
|---|---|---|---|
| Rat | SERT | 5′-CCCTCTAAGCCAAGCCTGATG-3′ | 5′-GGGAGATTCGGTTCGGACTAC-3′ |
| Mink | 5′-GAGATGCGGAACGAGGATGTG-3′ | 5′-CTCTACGCCTTGCTCCTACAC-3′ | |
| Rat | PPT | 5′-AGAGGCATATCAATGAGTCCTAC-3′ | 5′-TCTCCGTATAGTTACTCAGGATG-3′ |
| Mink | 5′-GGGGAGTCAATGAGTCCTACAA-3′ | 5′-CCCCTCAGTTACTCAGGATGTT-3′ | |
| Rat | NEP | 5′-ACCGTTCACTTCTGGTTCTCA-3′ | 5′-TGGCAAGTGAAGACCAAGAGT-3′ |
| GAPDH | 5′-GCAAGTTCAACGGCACAGTCA-3′ | 5′-TGGTGGTGAAGACGCCAGTAG-3′ |
Statistical analysis
Investigational outcomes were mentioned as mean ± standard deviation. Analysis was done using Student’s t test as well as one-way analysis of variance by SPSS 20 (New York, USA). P < 0.05 was regarded to indicate a statistical significance.
Results
Cisplatin induces pica in rats and emesis in minks
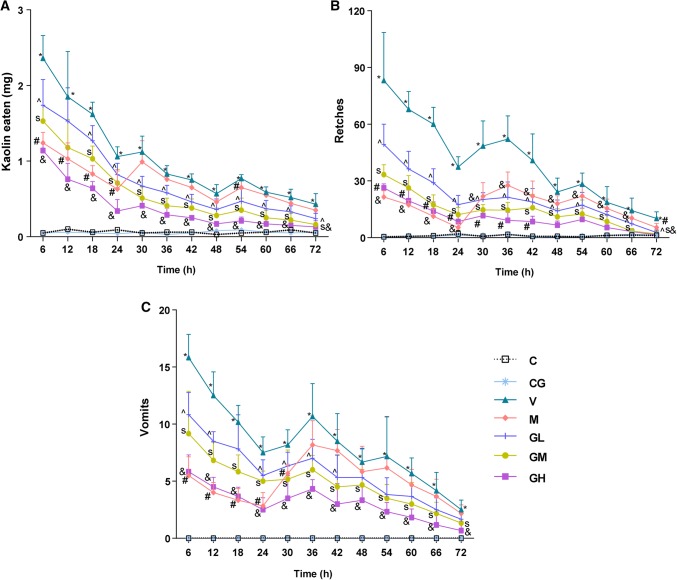
As shown in Fig. 1, rats had an initial acute phase of pica behavior and minks had an acute retching and vomiting within 24 h of the administration of cisplatin. Metoclopramide significantly inhibited the pica behavior of rats and emesis response of minks throughout 24 h after cisplatin administration (P < 0.05). Nevertheless, the content of kaolin as well as the number of retching as well as vomiting did not decrease significantly during 24–72 h. Pretreatment with gingerol significantly decreased the consumption of kaolin of rats and the number of retching as well as vomiting of minks prompted by cisplatin in a dosage-dependent manner throughout the duration of 72 h (P < 0.05). As in Group CG and C, there were no effect on pica behavior in rats and vomiting frequency in minks.
Fig. 1.
Pica induced by cisplatin in rats and emesis prompted by cisplatin in minks. Antiemetic impact of gingerol a on pica prompted by cisplatin in rats (n = 5), b on retches prompted by cisplatin in minks (n = 6) and c on vomits prompted by cisplatin in minks. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05: Group V vs. Group C; #P < 0.05: Group M vs. Group V; ^P < 0.05: Group GL vs. Group V; SP < 0.05: Group GM vs. Group V; &P < 0.05: Group GH vs. Group V
Representative H&E staining in the area postrema along with ileum in rats and minks
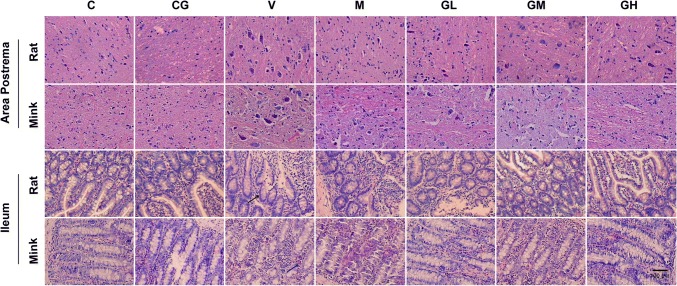
As shown in Fig. 2, H&E staining revealed that after cisplatin treatment, the nerve cells in the area postrema were swollen and disorderly arranged. Some nerve cells shrank and stained deeply, and were even broken both in rats and minks. However, the number of abnormal neurons was suggestively reduced in the groups treated with gingerol in a dosage-dependent way. Compared to the model group, the protective effect of metoclopramide was stronger than two low-dose groups, and weaker than that of high-dose gingerol group.
Fig. 2.
Representative H&E staining in area postrema along with ileum of rats and minks (100 × magnification). The figures show the H&E staining in area postrema along with ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. The red arrow shows the nerve cell, black arrow shows the epithelial cell, and blue arrow shows the inflammatory cell
Similarly, H&E staining revealed that after cisplatin treatment, the arrangement of ileum villi was irregular, the subepithelial space was obviously widened, some epithelial cells were lost, accompanied by a large number of inflammatory cells infiltration in rats and minks. Exposure to different doses of gingerol alleviated the injury of ileum mucosa caused by cisplatin. And the protective effect was positively correlated with the dose. The protective effect of metoclopramide was superior to that of two low-dose groups, and inferior to that of high-dose gingerol group.
5-HT, 5-HT3 receptor, TPH and SERT levels in the area postrema in addition to ileum in rats and minks
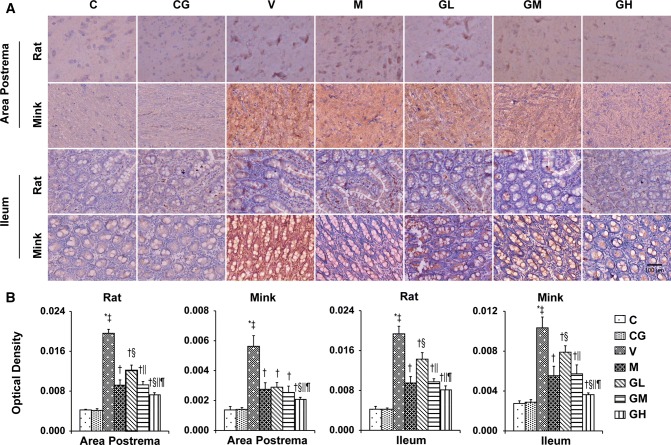
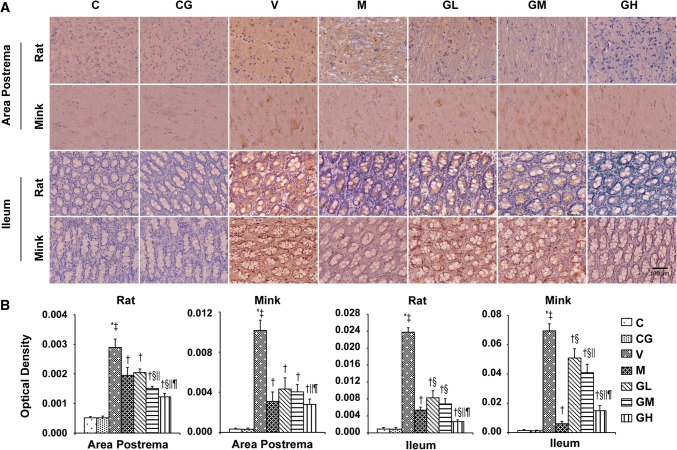
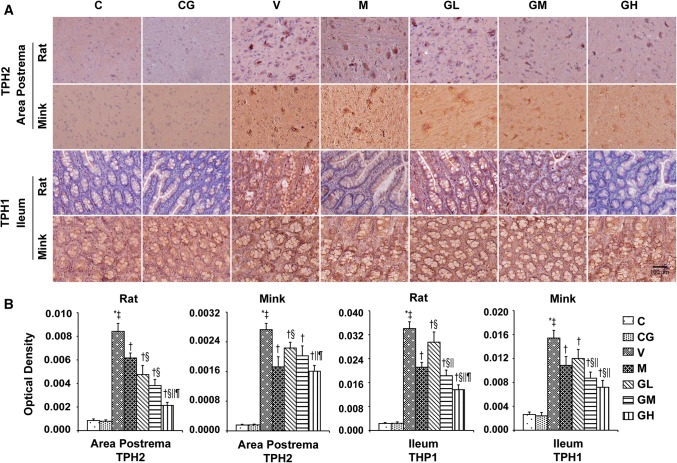
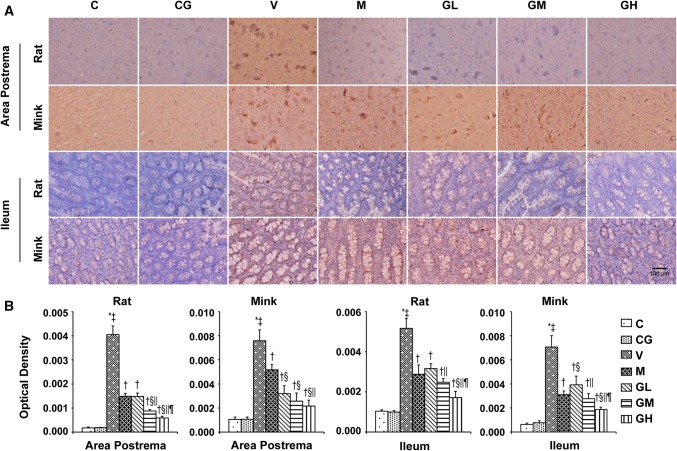
As shown in Figs. 3, 4 and 5, we analyzed the expression patterns of 5-HT, 5-HT3 receptor and TPH in the area postrema plus ileum of rats and minks by immunohistochemical staining. TPH1 is one of the major isomers of TPH, mainly expressed in intestinal chromaffin cells. While, TPH2, as the other one of the major isomers of TPH, mainly expressed in brain cells. Thus, we also examined the expression of TPH1 in the ileum and the manifestation of TPH2 in the area postrema by immunohistochemical staining both in rats and minks. The immunohistochemical analysis showed 5-HT and 5-HT3 receptor staining intensities (Red-brown deposits indicate positive staining) were mostly located in the mucosa as well as submucosa of the ileum along with the neurons of the area postrema.
Fig. 3.
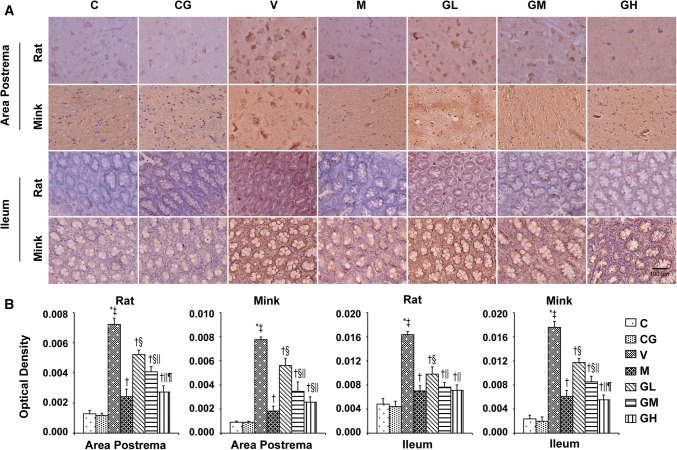
5-HT immunostaining expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of 5-HT in area postrema in addition to ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of 5-HT. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. 5-HT 5-tyrosine hydroxylase
Fig. 4.
5-HT3 receptor immunostaining expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of 5-HT3 receptor in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of 5-HT3 receptor. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. 5-HT3receptor 5-hydroxytryptamine type 3 receptor
Fig. 5.
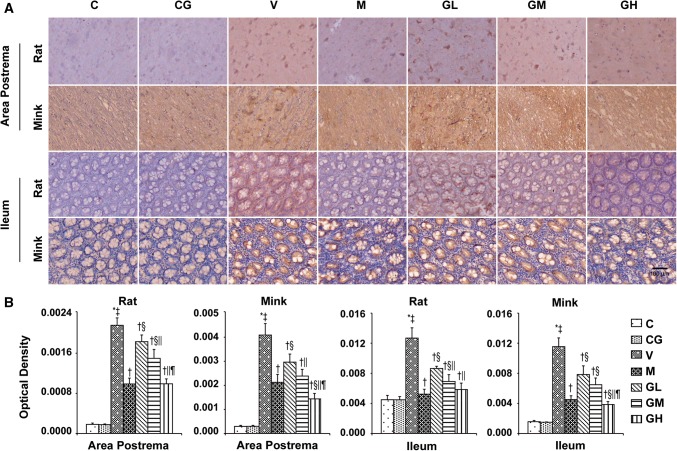
TPH immunostaining manifestation in area postrema as well as ileum of rats and minks. a Immunohistochemistry manifestation of TPH2 in area postrema of rats plus minks, and TPH1 in ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of TPH2 and TPH1. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. TPH1 tryptophan hydroxylase 1, TPH2 tryptophan hydroxylase 2
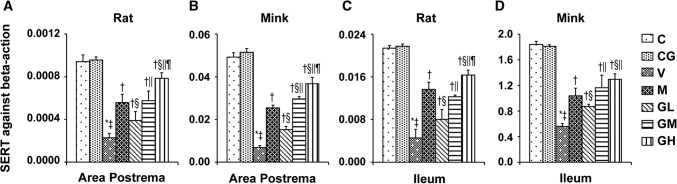
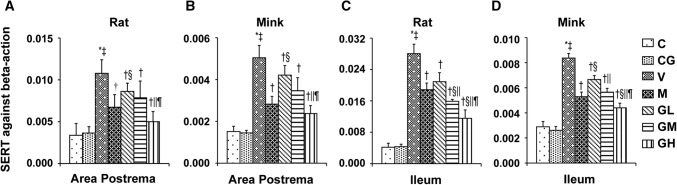
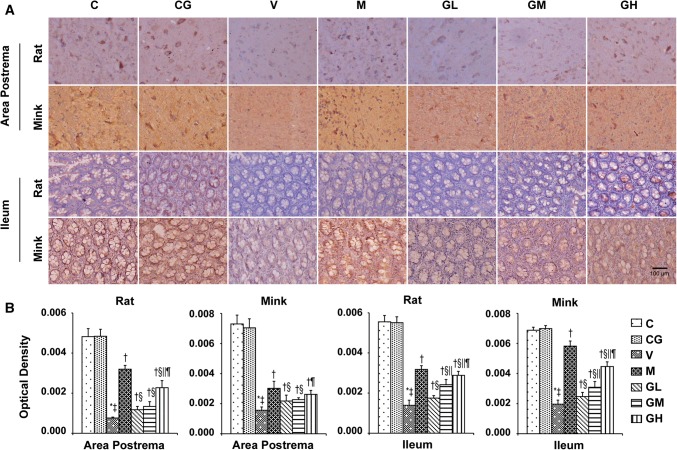
The immunohistochemical analysis showed the positive staining of 5-HT, 5-HT3 receptor and TPH proteins were increased in Group V (P < 0.05). After gingerol treatment, the 5-HT, 5-HT3 receptor and TPH proteins levels were dosage-dependently lower (P < 0.05). However, there was not any substantial change among the Group C and Group CG. qRT-PCR was done to identify SERT (Fig. 6). The results showed that gingerol could dosage-dependently reverse the decreasing trend of SERT prompted by cisplatin in the area postrema along with ileum of rats plus minks (P < 0.05).
Fig. 6.
SERT mRNA manifestation in area postrema in addition to ileum of rats and minks. The mRNA manifestation of SERT were investigated by qRT-PCR in area postrema and ileum of rats as well as minks (rats: n = 5, minks: n = 6). C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. SERT serotonin transporter
SP, NK1 receptor, PPT and NEP levels in the area postrema in addition to ileum in rats and minks
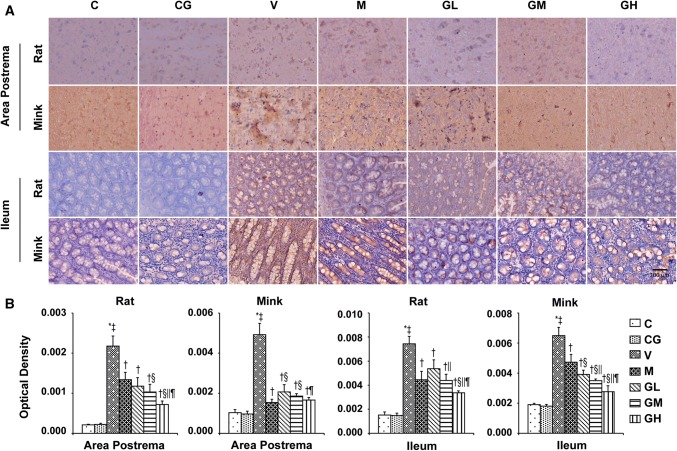
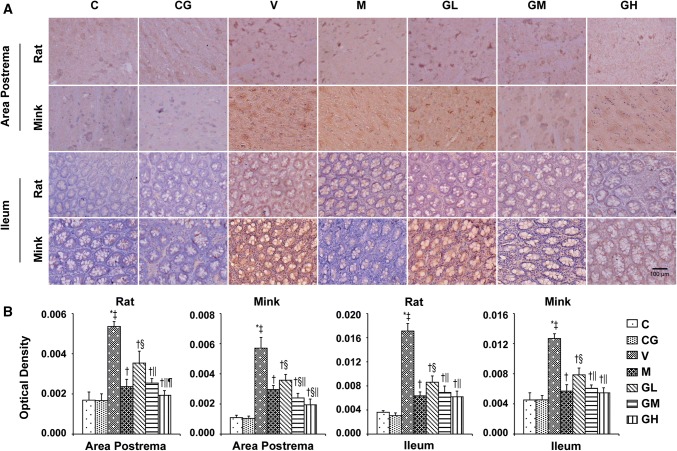
Next, we investigated whether SP, NK1 receptor, PPT and NEP are involved in the antiemetic effect of gingerol in the area postrema in addition to ileum in rats and minks. As shown in Fig. 7, SP staining was significantly increased in Group V of rats and minks. Gingerol treatment reduced the increasing tendency of SP significantly in a dosage-dependent way (P < 0.05). As shown in Fig. 8, after gingerol treatment, we found that the increasing trend of NK1 receptor prompted by cisplatin decreased significantly in rats (P < 0.05). The changes of NK1 receptor in minks were as same as in rats (P < 0.05). PPT is known to be the necessary precursor for the synthesis of SP. As shown in Fig. 9, the manifestation of PPT in both the area postrema plus ileum in rats and minks were suggestively augmented due to cisplatin (P < 0.05), and gingerol treatment showed opposite effect on PPT manifestation compared to the Group V (P < 0.05).
Fig. 7.
SP immunostaining expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of SP in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of SP. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. SP substance P
Fig. 8.
NK1 receptor immunostaining expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of NK1 receptor in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of NK1 receptor. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. NK1 receptor neurokinin-1 receptor
Fig. 9.
PPT mRNA manifestation in area postrema in addition to ileum of rats and minks. The mRNA manifestation of PPT were examined by qRT-PCR in area postrema and ileum of rats and minks (rats: n = 5, minks: n = 6). C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM: cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. PPT preprotachykinin
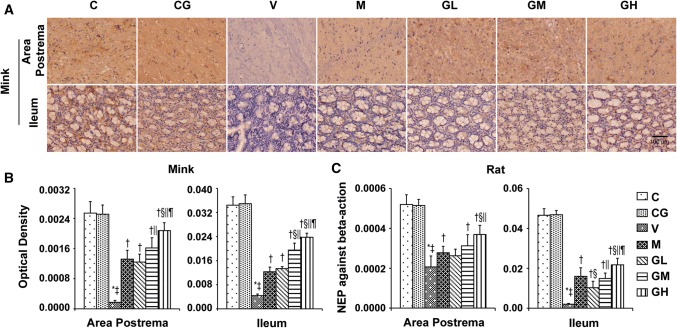
Furthermore, the results of NEP from qRT-PCR in rats and immunohistochemical in minks (Fig. 10) showed that gingerol induced an increase in a dosage-dependent manner in the area postrema plus ileum. However, NEP was suggestively lower in cisplatin treated group compared with that in the normal control group.
Fig. 10.
NEP expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of NEP in area postrema plus ileum of minks (n = 6). Bar indicates 100 µm. b Mean optical density values of NEP of minks. The images were quantified by Image-Pro Plus. c The mRNA manifestation of NEP was examined by qRT-PCR in area postrema and ileum of rats (n = 5). C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. NEP neutral endopeptidase
DA, D2R, TH as well as DAT levels in the area postrema plus ileum in rats and minks
To determine whether the antiemetic impact of gingerol is correlated to the DA system, DA, D2R, TH plus DAT were evaluated in the area postrema in addition to ileum in rats and minks. As shown in Figs. 11, 12 and 13, the manifestation of DA, D2R, TH in the area postrema along with ileum were significantly alleviated in a dosage-dependent manner after gingerol treatment, which were increased due to cisplatin. Subsequently, the expression of DAT was also investigated. The DATs are functional proteins that serve to shift DA from the synapse back into the presynaptic terminals to dismissing or gating DA signals [28]. Figure 14 shows that cisplatin treatment resulted in the decreasing tendency of DAT, while gingerol increased the levels dramatically in a dosage-dependent manner in rats and minks (P < 0.05).
Fig. 11.
DA immunostaining expression in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of DA in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of DA. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. DA dopamine
Fig. 12.
D2R immunostaining manifestation in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of D2R in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of D2R. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. D2R dopamine D2 receptor
Fig. 13.
TH immunostaining manifestation in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of TH in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of TH. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. TH tyrosine hydroxylase
Fig. 14.
DAT immunostaining manifestation in area postrema in addition to ileum of rats and minks. a Immunohistochemistry manifestation of DAT in area postrema plus ileum of rats and minks (rats: n = 5, minks: n = 6). Bar indicates 100 µm. b Mean optical density values of DAT. The images were quantified by Image-Pro Plus. C normal control group, CG simple gingerol control group, V cisplatin control group, M cisplatin + metoclopramide group, GL cisplatin + low-dose gingerol group, GM cisplatin + middle-dose gingerol group, GH cisplatin + high-dose gingerol group. *P < 0.05 vs. Group C, ‡P < 0.05 vs. Group CG, †P < 0.05 vs. Group V, §P < 0.05 vs. Group M, ||P < 0.05 vs. Group GL, ¶P < 0.05 vs. Group GM. DAT dopamine transporter
To further confirm the roles of DA system in the antiemetic effects of gingerol, rats and minks were treated with metoclopramide. Metoclopramide is a powerful D2 receptor antagonist. The results showed that metoclopramide could prevent the upsurge of DA, TH, D2R and the decrease of DAT induced by cisplatin in the area postrema plus ileum in rats and minks which was similar to gingerol (P < 0.05). Metoclopramide had better effect than low-dose gingerol (P < 0.05).
Discussion
The feeling of nausea in addition to the capability to vomit are main constituents of human defenses against unpremeditated absorption of harmful substances and are part of a structured defensive method [10]. Vomiting is initiated when afferent impulses from the cerebral cortex, chemoreceptor trigger zone as well as vagal afferent fibers of the GI tract travel to the vomiting center, situated in the medulla. Efferent impulses then travel from the vomiting center to the abdominal muscles, cranial nerves, salivation center, as well as respiratory center, leading to vomiting [13, 37]. Clinical antitumor therapy (chemotherapy, molecular targeted drug therapy, analgesic therapy, surgery and so on) can lead to nausea as well as vomiting, among which chemotherapy drugs are the furthermost common and serious. Cisplatin, one effective agent of chemotherapy, damages the GI tract and promotes the release of serotonin from enterochromaffin cells, thereby stimulating 5-HT3 receptors on vagal afferents and activates the chemoreceptor trigger zone and vomiting center. When chemoreceptor trigger zone is activated, it also releases various neurotransmitters such as dopamine, serotonin, or neurokinin, which in turn stimulate the vomiting center and cause emesis [38]. The bioactive compounds in rhizome of ginger, particularly the gingerol and shogaol class of compounds, has a good effect on CINV. This is closely related to the properties include 5-HT3, substance P, and acetylcholine receptor antagonism; antiinflammatory properties; modulation of cellular redox signaling, vasopressin release, gastrointestinal motility, and gastric emptying rate [31, 32, 36]. To explore the mechanism, we established cisplatin-induced rats and minks as vomiting models. As rats do not vomit, we observed the amount of kaolin ingested by rats as an index of the degree of nausea and vomiting [39]. The other vomiting model is mink, which has been used as a reliable vomiting model because of its similar vomiting performance to that of ferrets after cisplatin, and is less expensive and more available compared with ferret [40, 41]. According to the report, cisplatin, a chemotherapeutic drug, can induce pica in rats, while antiemetic agents can inhibit the consumption of kaolin [39]. In this study, compared to the model group, the number of rats gnawing kaolin was significantly decreased after the application of gingerol during the entire 72 h. Similarly, the retching and vomiting responses of minks during whole 72 h were also evidently reduced. It was suggested that gingerol can effectively inhibit CINV not only in rats but also in minks.
The chemoreceptor trigger zone in the area postrema of the fourth ventricle floor is one of the parts of the central nervous system closely related to vomiting [31]. It is rich in dopamine, 5-HT and substance P receptors [42]. Endo et al. [43] have suggested that 5-HT release from the enterochromaffin cells in ileum, accompanied by histopathological changes of the ileum mucosa, is involved in the onset of delayed emesis after administration of cisplatin. Our previous studies [36] also showed that gingerol significantly inhibited cisplatin-induced vomiting by down regulating 5-HT, DA and SP expression of ileum in minks. In this study, we chose the area postrema along with the ileum as our focus. We selected the ileum at a distance of 15 cm from the pylorus in rats [34] and 20 cm in minks as reported in the study of Zhang et al. [40]. The histopathological tissues of the area postrema in rats and minks showed that cisplatin could significantly reduce the number of normal neurons and the content of Nissl granules compared with normal group. However, gingerol treatment in this study displayed a decrease area postrema damage through a markedly increase in the Nissl granules content of the normal neurons in a dosage-dependent way. Histopathological studies of the ileum in rats and minks indicated that cisplatin could induce an obvious decrease in the number of the normal ileum villi and epithelial cells and an obvious increase of inflammatory cell infiltrates compared with normal group. By contrast, gingerol can reduce the loss of villi, epithelial cells and inflammatory cell infiltration in a dosage-dependent manner. This indicated that gingerol could protect against cisplatin -induced area postrema and ileum tissue damage.
5-HT, as an important neurotransmitter of vomiting, exists widely in GI tract and nervous system [44, 45]. Peripherally, 5-HT is predominantly stored and secreted by gastrointestinal chromaffin cells; in the center, 5-HT is mainly concentrated in the raphe nucleus, posterior region, solitary tract nucleus as well as dorsal vagal nucleus. In the acute phase, an emetogenic mediator releases serotonin (5-HT) from the enterochromaffin cells in the GI tract that successively activates 5-HT3 receptors on the vagus afferents to start the vomiting reaction [46]. The biosynthesis of 5-HT is regulated by TPH [47]. There are two isoforms of TPH: TPH1, mainly manifested in the enterochromaffin cells of the GI tract, plus TPH2, manifested entirely in neuronal cells [48]. Chemotherapeutic drugs can stimulate gastrointestinal mucosa and cause mucosal damage, occasioning the release of 5-HT by gastrointestinal chromaffin cells. 5-HT3 receptors mediate fast excitatory depolarizing reactions in pre- as well as post-synaptic neurons in the central plus peripheral nervous system [13]. SERT retrieve 5-HT from the extracellular space and dismiss their action at the pre- and post-synaptic receptors [49]. Ullah et al. [20] have reported that pigeons vomiting after the application of cisplatin, accompanied by an increase in serotonin and 5-Hydroxy Indole Acetic Acid in area postrema and intestine. However, this phenomenon was suppressed after taking Zingiber officinale. Our experiments have verified that cisplatin can augment the expressions of 5-HT, TPH as well as 5-HT3 receptor in the area postrema in addition to ileum of rats and minks. The manifestation of SERT was decreased with the application of cisplatin. However, gingerol could reverse this trend. The expression of 5-HT, TPH and 5-HT3 receptor in the area postrema plus ileum of rats and minks were decreased, and the manifestation of SERT was augmented by gingerol in a dosage-reliant manner.
SP is another neurotransmitter, which has been implicated in the control of the vomiting reflex that has been found in the area postrema in addition to ileum [10, 50]. Experiments have shown that SP can cause vomiting in ferrets and shrews [51].The plasma SP levels in cancer patients treated with cisplatin (˃75 mg/m2) continued to increase, which explained close relationship between SP and CINV [52]. SP is a neurokinin, which belongs to the tachykinin family together with neurokinin A and neurokinin B. There are three kinds of tachykinin receptors: NK1 receptor, NK2 receptor plus NK3 receptor. SP comes from the PPT and applies its biological effects on target cells by interrelating mostly with the NK1 receptor [53], since SP binds preferentially to NK1 receptor [21]. NEP, as a major cell surface proteolytic enzyme, can rapidly degrade and inactivate SP. In this study, we found that cisplatin upregulated the manifestation of SP, NK1 receptor and PPT and decreased the accumulation of NEP in the area postrema in addition to ileum of rats and minks. Of note, the quantity of SP, NK1 receptor and PPT reduced significantly, the expression of NEP enhanced with the application of gingerol in a dosage-dependent way. In a clinical trial, NK1 receptor antagonist was used to prevent acute and delayed CINV in patients with well toleration [54]. This indicated that gingerol has similar effect as an NK1 receptor antagonist in the treatment of CINV.
DA is another focus of our research. Quantitative analysis by high performance liquid chromatography and electrochemical detector showed that not only 5-HT and its metabolite 5-hydroxyindoleacetic acid (5HIAA) but also DA and its metabolite Di-hydroxy Phenyl Acetic acid along with Homovanillic acid were mainly distributed in specific brain areas (area postrema and brain stem) and intestine in pigeons [20, 26, 42]. As one of the several neurotransmitters, DA theaters its involvement in the beginning of vomiting by specific stimulation of DRs, especially D2R [27]. This specific stimulation of D2 receptors leads to the vomiting response [42]. DA synthesis and metabolism are affected by many factors, among which the regulation of TH and DATs are very important. TH is the rate-limiting step in the synthesis of catecholamines, which regulate the transformation of l-tyrosine to l-3, 4-dihydroxyphenylalanine (l-DOPA); L-DOPA is the precursor for the neurotransmitters, DA, norepinephrine, as well as epinephrine [55]. Even though the concentration of extracellular DA is predominantly controlled by dispersion, the kinetics as well as the volume of extra synaptic DA is chiefly controlled by DAT [56]. DAT, a presynaptic transmembrane Na+/Cl− symporter, acts by regulating duration of the dopaminergic response by reuptake of released DA [57]. Metoclopramide is a regulator of DA as well as serotonin receptors and commonly used for treating in addition to avoiding nausea and vomiting [58]. Metoclopramide at low dose could block dopamine receptors while at high doses it act as a 5-HT3 receptor antagonist [10, 59]. Ullah et al. [42] found that 5-HT, DA and their metabolites accumulated in the area postrema and intestine of the pigeons after cisplatin, and metoclopramide showed activity against reserpine or cisplatin induced emesis in the pigeon. In our previous study [31], metoclopramide, which was chosen as standard drug, significantly inhibited the vomiting of mink caused by cisplatin. Our results found that cisplatin increased the concentrations of DA, D2R and TH; and decreased DAT level in the area postrema along with ileum of rats as well as minks. Very importantly, we further found that gingerol treatment could significantly reverse the motivation effect of cisplatin on the expression of DA, D2R plus TH, in addition to the inhibitory effect of DAT.
Conclusion
In summary, the antiemetic efficacy of gingerol was evaluated using two vomiting models of cisplatin-induced nausea as well as vomiting. Both the models were identified in the area postrema in addition to ileum. The outcomes of this study clearly indicated that treatment with gingerol reduced cisplatin-induced consumption of kaolin in rats and the number of emesis and vomiting in minks. Furthermore, the antiemetic mechanism of gingerol might be correlated to the simultaneous regulation of 5-HT system, SP system and DA system.
Abbreviations
- CINV
Chemotherapy-induced nausea and vomiting
- 5-HT
5-Hydroxytryptamine
- 5-HT3 receptor
5-Hydroxytryptamine type 3 receptor
- TPH
Tryptophan hydroxylase
- SERT
Serotonin transporter
- SP
Substance P
- NK1 receptor
Neurokinin-1 receptor
- PPT
Preprotachykinin
- NEP
Neutral endopeptidase
- DA
Dopamine
- D2R
Dopamine D2 receptor
- TH
Tyrosine hydroxylase
- DAT
Dopamine transporter
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 81904143, 81704071 and 81273702), Key Research and Development Plan of Shandong province (Grant No. 2018GSF119027), the Taishan Scholars Program of Shandong Province in China of Pulmonary disease of traditional Chinese Medicine (Grant no. ts201712096), the Taishan Scholars Youth Expert Program of Shandong Province in China (tsqn201812146), Young Elite Scientists Sponsorship Program by CAST (CACM-2018-QNRC2-B01), China Postdoctoral Science Foundation Grant (Grant nos. 2019T120608 and 2019M652462), Postdoctoral Innovation Project of Shandong Province (Grant no. 201901014).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Tian and Weibin Qian are contributed equally.
Change history
1/17/2020
In the original publication of the article, Figures 2, 3, 5, 11 and 13 were published incorrectly. The corrections version of figures are given below.
Contributor Information
Wei Zhang, Email: doctorzwjn@126.com.
Xinrui Cai, Email: doctorcai@163.com.
References
- 1.Johnston KD, Lu Z, Rudd JA. Looking beyond 5-HT(3) receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol. 2014;722:13–25. doi: 10.1016/j.ejphar.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Wu F, Lin X, Yang Z, Sun Z, Zeng F, Heng J, Qu J, Zeng L, Yang N, Zhang Y. Phase III randomized trial of palonosetron and dexamethasone with or without aprepitant to prevent nausea and vomiting induced by full-dose single-day cisplatin-based chemotherapy in lung cancer. Clin Lung Cancer. 2018;19:e913–e918. doi: 10.1016/j.cllc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Botteman M, Nickel K, Corman S, Turini M, Binder G. Cost-effectiveness of a fixed combination of netupitant and palonosetron (NEPA) relative to aprepitant plus granisetron (APR + GRAN) for prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a trial-based analysis. Support Care Cancer. 2019;3:1–10. doi: 10.1007/s00520-019-04824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyatt G, Sikorskii A, Tesnjak I, Victorson D, Srkalovic G. Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Support Care Cancer. 2015;23:3183–3191. doi: 10.1007/s00520-015-2698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Zhao J, Cong W. Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Front Pharmacol. 2018;9:1442. doi: 10.3389/fphar.2018.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G, Yang E, Kim S, Park CS, Park YS, Jin YH. Parapheromones suppress chemotherapy side effects. J Pharmacol Exp Ther. 2018;367:215–221. doi: 10.1124/jpet.118.251363. [DOI] [PubMed] [Google Scholar]
- 7.Herrstedt J, Summers Y, Jordan K, von Pawel J, Jakobsen AH, Ewertz M, Chan S, Naik JD, Karthaus M, Dubey S, Davis R, Fox GM. Amisulpride prevents nausea and vomiting associated with highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled, dose-ranging trial. Support Care Cancer. 2019;27:2699–2705. doi: 10.1007/s00520-018-4564-8. [DOI] [PubMed] [Google Scholar]
- 8.Hesketh PJ, Palmas M, Nicolas P. Preventing chemotherapy-induced nausea and vomiting in patients with lung cancer: efficacy of NEPA (netupitant-palonosetron), the first combination antiemetic. Support Care Cancer. 2018;26:1151–1159. doi: 10.1007/s00520-017-3936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugino S, Janicki PK. Pharmacogenetics of chemotherapy-induced nausea and vomiting. Pharmacogenomics. 2015;16:149–160. doi: 10.2217/pgs.14.168. [DOI] [PubMed] [Google Scholar]
- 10.Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol. 2018;9:913. doi: 10.3389/fphar.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inui N. Antiemetic therapy for non-anthracycline and cyclophosphamide moderately emetogenic chemotherapy. Med Oncol. 2017;34:77. doi: 10.1007/s12032-017-0937-y. [DOI] [PubMed] [Google Scholar]
- 12.Kovac AL. Comparative pharmacology and guide to the use of the serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting. Drugs. 2016;76:1719–1735. doi: 10.1007/s40265-016-0663-3. [DOI] [PubMed] [Google Scholar]
- 13.Al Kury LT, Mahgoub M, Howarth FC, Oz M. Natural negative allosteric modulators of 5-HT(3) receptors. Molecules. 2018;23:3186. doi: 10.3390/molecules23123186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X, Voss U, Ekblad E. A novel serotonin-containing tuft cell subpopulation in mouse intestine. Cell Tissue Res. 2019;376:189–197. doi: 10.1007/s00441-018-02988-3. [DOI] [PubMed] [Google Scholar]
- 15.Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. Regional differences in nutrient-induced secretion of gut serotonin. Physiol Rep. 2017;5:e13199. doi: 10.14814/phy2.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz M, Covenas R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 2014;46:1727–1750. doi: 10.1007/s00726-014-1736-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Che T, Levit A, Shoichet BK, Wacker D, Roth BL. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature. 2018;555:269–273. doi: 10.1038/nature25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullah I, Subhan F, Lu Z, Chan SW, Rudd JA. Action of Bacopa monnieri to antagonize cisplatin-induced emesis in Suncus murinus (house musk shrew) J Pharmacol Sci. 2017;133:232–239. doi: 10.1016/j.jphs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Ullah I, Subhan F, Ayaz M, Shah R, Ali G, Haq IU, Ullah S. Anti-emetic mechanisms of Zingiber officinale against cisplatin induced emesis in the pigeon; behavioral and neurochemical correlates. BMC Complement Altern Med. 2015;15:34. doi: 10.1186/s12906-015-0556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin J, Chapman K, Clark LD, Shao Z, Borek D, Xu Q, Wang J, Rosenbaum DM. Crystal structure of the human NK1 tachykinin receptor. Proc Natl Acad Sci USA. 2018;115:13264–13269. doi: 10.1073/pnas.1812717115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZY, Wang J, Kansra V, Wang X. Absorption, metabolism, and excretion of the antiemetic rolapitant, a selective neurokinin-1 receptor antagonist, in healthy male subjects. Invest New Drugs. 2019;37:139–146. doi: 10.1007/s10637-018-0638-1. [DOI] [PubMed] [Google Scholar]
- 23.Navari RM. Management of chemotherapy-induced nausea and vomiting in pediatric patients. Paediatr Drugs. 2017;19:213–222. doi: 10.1007/s40272-017-0228-2. [DOI] [PubMed] [Google Scholar]
- 24.Scott JR, Muangman PR, Tamura RN, Zhu KQ, Liang Z, Anthony J, Engrav LH, Gibran NS. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 2005;115:1095–1102. doi: 10.1097/01.prs.0000156151.54042.da. [DOI] [PubMed] [Google Scholar]
- 25.May MB, Glode AE. Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res. 2016;8:49–55. doi: 10.2147/CMAR.S81425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah I, Subhan F, Rudd JA, Rauf K, Alam J, Shahid M, Sewell RD. Attenuation of cisplatin-induced emetogenesis by standardized Bacopa monnieri extracts in the pigeon: behavioral and neurochemical correlations. Planta Med. 2014;80:1569–1579. doi: 10.1055/s-0034-1383121. [DOI] [PubMed] [Google Scholar]
- 27.Osinski MA, Uchic ME, Seifert T, Shaughnessy TK, Miller LN, Nakane M, Cox BF, Brioni JD, Moreland RB. Dopamine D2, but not D4, receptor agonists are emetogenic in ferrets. Pharmacol Biochem Behav. 2005;81:211–219. doi: 10.1016/j.pbb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Salvatore MF, Calipari ES, Jones SR. Regulation of tyrosine hydroxylase expression and phosphorylation in dopamine transporter-deficient mice. ACS Chem Neurosci. 2016;7:941–951. doi: 10.1021/acschemneuro.6b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haniadka R, Rajeev AG, Palatty PL, Arora R, Baliga MS. Zingiber officinale (ginger) as an anti-emetic in cancer chemotherapy: a review. J Altern Complement Med. 2012;18:440–444. doi: 10.1089/acm.2010.0737. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Huang M, Wisniewski M, Li H, Zhang M, Tao X, Liu Y, Zou Y (2018) Transcriptome analysis provides insights into gingerol biosynthesis in ginger (Zingiber officinale). Plant Genome 11 [DOI] [PubMed]
- 31.Qian W, Cai X, Wang Y, Zhang X, Zhao H, Qian Q, Yang Z, Liu Z, Hasegawa J. Effect of gingerol on cisplatin-induced pica analogous to emesis via modulating expressions of dopamine 2 receptor, dopamine transporter and tyrosine hydroxylase in the vomiting model of rats. Yonago Acta Med. 2016;59:100–110. [PMC free article] [PubMed] [Google Scholar]
- 32.Marx W, Ried K, McCarthy AL, Vitetta L, Sali A, McKavanagh D, Isenring L. Ginger-Mechanism of action in chemotherapy-induced nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2017;57:141–146. doi: 10.1080/10408398.2013.865590. [DOI] [PubMed] [Google Scholar]
- 33.Lindblad AJ, Koppula S. Ginger for nausea and vomiting of pregnancy. Can Fam Physician. 2016;62:145. [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Q, Chen W, Guo C, Wu W, Qian W, Li S. Xiao-Ban-Xia-Tang inhibits cisplatin-induced pica by down regulating obestatin in rats. J Ethnopharmacol. 2011;135:186–193. doi: 10.1016/j.jep.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Saito R, Takano Y. Easy method for emesis using rats. Nihon Yakurigaku Zasshi. 2006;127(6):461–466. doi: 10.1254/fpj.127.461. [DOI] [PubMed] [Google Scholar]
- 36.Qian Q, Yue W, Wang Y, Yang Z, Liu Z, Chen W. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res. 2009;32:565–573. doi: 10.1007/s12272-009-1413-9. [DOI] [PubMed] [Google Scholar]
- 37.Navari RM. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim Biophys Acta. 2015;1848:2738–2746. doi: 10.1016/j.bbamem.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Ranganath P, Einhorn L, Albany C. Management of chemotherapy induced nausea and vomiting in patients on multiday cisplatin based combination chemotherapy. Biomed Res Int. 2015;2015:943618. doi: 10.1155/2015/943618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, Wang L, Yang ZH, Liu ZT, Yue W. Value of mink vomit model in study of anti-emetic drugs. World J Gastroenterol. 2006;12:1300–1302. doi: 10.3748/wjg.v12.i8.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian Q, Chen W, Yue W, Yang Z, Liu Z, Qian W. Antiemetic effect of Xiao-Ban-Xia-Tang, a Chinese medicinal herb recipe, on cisplatin-induced acute and delayed emesis in minks. J Ethnopharmacol. 2010;128:590–593. doi: 10.1016/j.jep.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 42.Ullah I, Subhan F, Alam J, Shahid M, Ayaz M. Suppression of cisplatin-induced vomiting by cannabis sativa in pigeons: neurochemical evidences. Front Pharmacol. 2018;9:231. doi: 10.3389/fphar.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo T, Hamaue N, Ihira E, Teramoto Y, Liu Y, Hirafuji M, Minami M. Effects of granisetron, a 5-HT3 receptor antagonist, on 5-hydroxytryptamine (5-HT) release from the isolated ileum in a delayed-emesis rat model. Res Commun Mol Pathol Pharmacol. 2002;111(1–4):55–68. [PubMed] [Google Scholar]
- 44.Xia Z, Zhang Y, Li C, Xu Y, Dong J, Wang L, He Q, Zou X, Wu H, Han J, Cai M, Du Y, Wei L, Shang J. Traditional Tibetan medicine Anzhijinhua San attenuates Ovalbumin-induced diarrhea by regulating serotonin signaling system in mice. J Ethnopharmacol. 2019;236:484–494. doi: 10.1016/j.jep.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Taciak PP, Lysenko N, Mazurek AP. Drugs which influence serotonin transporter and serotonergic receptors: pharmacological and clinical properties in the treatment of depression. Pharmacol Rep. 2018;70:37–46. doi: 10.1016/j.pharep.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Spartinou A, Nyktari V, Papaioannou A. Granisetron: a review of pharmacokinetics and clinical experience in chemotherapy induced - nausea and vomiting. Expert Opin Drug Metab Toxicol. 2017;13:1289–1297. doi: 10.1080/17425255.2017.1396317. [DOI] [PubMed] [Google Scholar]
- 47.Swami T, Weber HC. Updates on the biology of serotonin and tryptophan hydroxylase. Curr Opin Endocrinol Diabetes Obes. 2018;25:12–21. doi: 10.1097/MED.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 48.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, Hu Z, Yu W, Jonas B, Pineda R, Calderon-Gay V, Germann M, O'Neill E, Brommage R, Cullinan E, Platt K, Wilson A, Powell D, Sands A, Zambrowicz B, Shi ZC. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 49.Mayer FP, Cintulova D, Pittrich DA, Wimmer L, Luethi D, Holy M, Jaentsch K, Tischberger S, Gmeiner G, Hoener MC, Liechti ME, Mihovilovic MD, Sitte HH. Stereochemistry of phase-1 metabolites of mephedrone determines their effectiveness as releasers at the serotonin transporter. Neuropharmacology. 2019;148:199–209. doi: 10.1016/j.neuropharm.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 50.Obara Y, Machida T, Takano Y, Shiga S, Suzuki A, Hamaue N, Iizuka K, Hirafuji M. Cisplatin increases the number of enterochromaffin cells containing substance P in rat intestine. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:847–858. doi: 10.1007/s00210-018-1493-5. [DOI] [PubMed] [Google Scholar]
- 51.Rudd JA, Ngan MP, Lu Z, Higgins GA, Giuliano C, Lovati E, Pietra C. Profile of antiemetic activity of netupitant alone or in combination with palonosetron and dexamethasone in Ferrets and Suncus murinus (House Musk Shrew) Front Pharmacol. 2016;7:263. doi: 10.3389/fphar.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higa GM, Auber ML, Altaha R, Piktel D, Kurian S, Hobbs G, Landreth K. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. J Oncol Pharm Pract. 2006;12:201–209. doi: 10.1177/1078155206072080. [DOI] [PubMed] [Google Scholar]
- 53.Polidoro G, Giancola F, Fracassi F, Piktel D, Kurian S, Hobbs G, Landreth K. Substance P and the neurokinin-1 receptor expression in dog ileum with and without inflammation. Res Vet Sci. 2017;114:297–307. doi: 10.1016/j.rvsc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Trigg ME, Higa GM. Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical practice. J Oncol Pharm Pract. 2010;16(4):233–244. doi: 10.1177/1078155209354655. [DOI] [PubMed] [Google Scholar]
- 55.Mapanao R, Chang CC, Cheng W, Liu KF. Silencing tyrosine hydroxylase retards depression of immunocompetence of Litopenaeus vannamei under hypothermal stress. Fish Shellfish Immunol. 2018;72:519–527. doi: 10.1016/j.fsi.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 56.Julku UH, Panhelainen AE, Tiilikainen SE, Svarcbahs R, Tammimäki AE, Piepponen TP, Savolainen MH, Myöhänen TT. Prolyl oligopeptidase regulates dopamine transporter phosphorylation in the nigrostriatal pathway of mouse. Mol Neurobiol. 2018;55:470–482. doi: 10.1007/s12035-016-0339-8. [DOI] [PubMed] [Google Scholar]
- 57.Campbell NG, Shekar A, Aguilar JI, Peng D, Navratna V, Yang D, Morley AN, Duran AM, Galli G, O'Grady B, Ramachandran R, Sutcliffe JS, Sitte HH, Erreger K, Meiler J, Stockner T, Bellan LM, Matthies HJG, Gouaux E, Mchaourab HS, Galli A. Structural, functional, and behavioral insights of dopamine dysfunction revealed by a deletion in SLC6A3. Proc Natl Acad Sci USA. 2019;116:3853–3862. doi: 10.1073/pnas.1816247116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Kim W, Kim D, Jeong S, Yoo JW, Jung Y. A colon-specific prodrug of metoclopramide ameliorates colitis in an experimental rat model. Drug Des Devel Ther. 2019;13:231–242. doi: 10.2147/DDDT.S185257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanger GJ. Translating 5-HT receptor pharmacology. Neurogastroenterol Motil. 2009;21:1235–1238. doi: 10.1111/j.1365-2982.2009.01425.x. [DOI] [PubMed] [Google Scholar]