Abstract
Venetoclax with high‐dose methotrexate and rituximab seem effective and safe to treat central nervous system involvement of chronic lymphocytic leukemia.
Keywords: central nervous system involvement, chronic lymphocytic leukemia, venetoclax
Venetoclax with high‐dose methotrexate and rituximab seem effective and safe to treat central nervous system involvement of chronic lymphocytic leukemia.
1. INTRODUCTION
Chronic lymphocytic leukemia (CLL) with central nervous system (CNS) involvement is a rare and severe condition.1, 2 There is currently no consensus regarding optimal management.3, 4 Venetoclax, an oral BCL‐2 inhibitor, has recently proven to be effective for refractory/relapsed CLL when administered with or without rituximab.5, 6 Venetoclax is a small molecule which is also able to cross the blood‐brain barrier as reported in a recent case study demonstrating its clinical and radiological efficacy in a patient with CNS involvement.7
2. CASE PRESENTATION
We report the case of a seventy‐one‐year‐old patient diagnosed with CLL in 2006, initially without any indication for treatment (as per IwCLL2008 criteria). He received one cycle of fludarabine‐cyclophosphamide‐rituximab in April 2014 which was discontinued due to profound cytopenia. This was followed by two cycles of rituximab‐bendamustine in April‐May 2016 which had to be discontinued despite bulky splenomegaly because of transfusion‐dependant anemia, grade 4 neutropenia, and grade 3 thrombocytopenia. At this point, the patient was considered chemo‐refractory and was referred to our hospital. The initial molecular biology and cytogenetic work‐up detected a complex karyotype associated with an 11q deletion (24%) and one mutation in the TP53 gene (exon 5, variant allele frequency (VAF) = 11%). IgHV was not mutated. Ibrutinib 420 mg per day was started on June 2016 and led to a partial response (normal physical examination and abdominal ultrasound with persistent hyperlymphocytosis).
After 24 months of therapy, the patient was readmitted to the Hospital's emergency department due to a transient epileptic seizure. Laboratory tests detected thrombocytopenia (grade 2), anemia (grade 1), but no hyperlymphocytosis. The physical examination confirmed the progression of CLL under ibrutinib with an enlarged spleen, palpable axillary, and cervical adenopathies. The neurological examination was also abnormal with decreased left upper limb strength (with pyramidal syndrome), left hemianopsia, and visual hallucinations. Bone marrow aspiration detected 12% lymphocytes (including 25% with CLL phenotype, TP53 exon 5 mutation VAF = 1.5%, considered as a minor bone marrow infiltration). A lumbar puncture found no evidence of CLL or infection. The spleen biopsy confirmed the relapse diagnosis and “accelerated CLL” features but did not support a Richter transformation (PET/CT showed no additional FDG avid lesions). The brain MRI confirmed an increased signal in the right parietotemporal leptomeninx in FLAIR and diffusion sequences. The electroencephalogram (EEG) showed generalized periodic epileptiform discharges, which were more prominent in the right cerebral hemisphere, suggestive of cerebral injury. Additional tests were performed to attribute a differential diagnosis: bacterial, mycobacterial, and fungal cultures on cerebrospinal fluid (CSF); virological tests for HSV, BKV, and JCV on CSF; TPHA test on CSF; Borrelia serology on CSF; QuantiFERON®; nucleic acid amplification (NAA) test for Mycobacterium tuberculosis on CSF; NAA test for Toxoplasma gondii on CSF and serum; galactomannan antigen test on CSF and serum; cryptococcal antigen test on CSF and serum; testing for oligoclonal synthesis of immunoglobulin in CSF. All these tests were either normal or negative.
These results confirmed the diagnosis of CLL relapse with nodal, splenic, and CNS involvement after two years of ibrutinib treatment. This is the first such case encountered in our hospital among 150 patients treated with ibrutinib. Ibrutinib is indeed reported to show efficacy in the management of mantle cell lymphoma, Waldenström disease, and CLL progression in the CNS. Ibrutinib was discontinued at the beginning of the patient's hospitalization and corticotherapy promptly initiated and then progressively tapered. The patient received two cycles of high‐dose IV methotrexate (2 g/m2 day 1‐15) and rituximab (375 mg/m2 day 1 of the first cycle, then 500 mg/m2 day 1 of the second cycle). On day 5 of cycle 1 the patient was started on venetoclax, according to the following protocol:
20 mg per day: 1 day
50 mg per day: 5 days
100 mg per day: 13 days
200 mg per day: continuously
Initially, no significant adverse events occurred more specifically no clinical or biological tumor lysis syndrome was observed. After 3 weeks, a persistent grade 3 neutropenia partially due to drug‐drug interactions with antiepileptic agents (lacosamide, levetiracetam, and valpromide) prevented us from achieving the recommended 400 mg/day threshold. The grade 2 thrombocytopenia remained stable.
Three weeks after starting treatment, despite an initial improvement of all symptoms, the patient suddenly relapsed presenting with the previously described neurological deficiencies. EEG was deemed unchanged. The brain MRI showed local modifications of the previously identified parietotemporal leptomeninx lesion suggestive of a necrotizing lesion. There was no radiological argument in favor of CLL progression nor of ischemic stroke. Figure 1 illustrates the evolution of CNS involvement on the brain MRI during the first three months of treatment. It highlights the paradoxical response after three weeks of treatment with sudden worsening of neurological deficiencies and laminar necrosis of the tumor on cerebral MRI scans.
Figure 1.
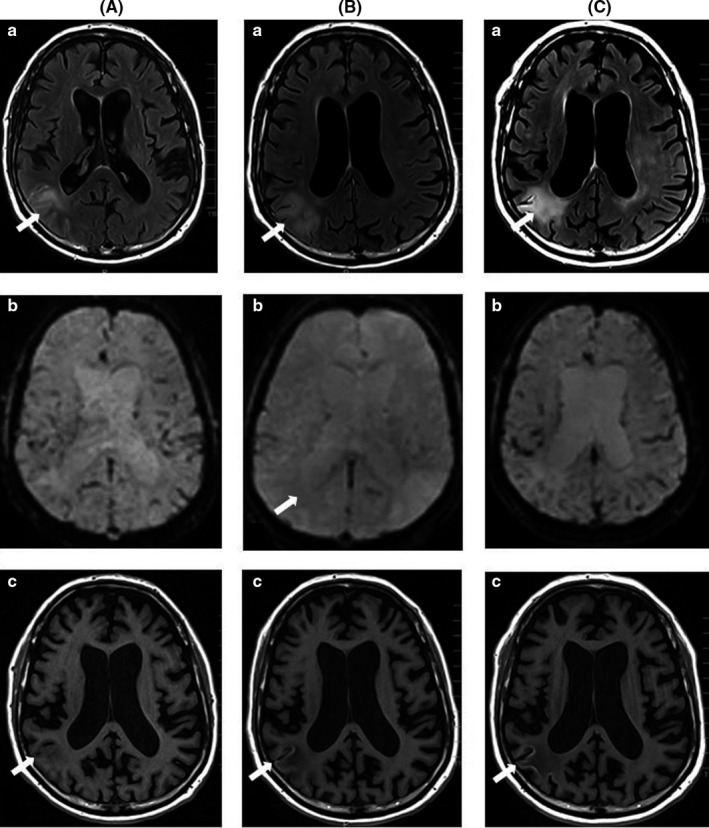
Brain magnetic resonance imaging (MRI) of central nervous system involvement (CNSi) became apparent during ibrutinib therapy and was treated with high‐dose methotrexate, rituximab, and venetoclax. A, Brain MRI scans at diagnosis of the CNSi, showing increased signal intensity in the right parietotemporal leptomeninx and parenchyma on FLAIR and diffusion sequences, with no restriction in ADC sequence, no vascular enhancement in postcontrast T1‐weighted sequence, and no perfusion abnormality, favoring a meningoencephalitis diagnosis. (a) Axial fluid‐attenuated inversion recovery (FLAIR) sequence, (b) perfusion sequence (normal), and (c) axial brain MRI T1‐weighted image. B, After 3 wk of venetoclax treatment, neurologic symptoms suddenly worsened. Repeat brain MRIs showed an increase in right parietotemporal edema, associated with cortex increased signal intensity in T1‐weighted sequence and an increase of vascular perfusion, favoring a laminar necrosis, without any supporting argument favoring tumor progression nor an ischemic stroke apparent by (a) FLAIR sequence, (b) perfusion sequence, and (c) T1‐weighted images. C, After 3 mo of treatment, a systematic brain MRI was performed. The edema linked to cortex laminar necrosis persisted, vascular perfusion normalized, with no sign of tumor progression apparent by (a) FLAIR sequence, (b) perfusion sequence (normal), and (c) T1‐weighted images
The patient's neurological condition improved and allowed him to be discharged from hospital indefinitely. The patient continues to take venetoclax at the dose of 200 mg/day. He has received six cycles of rituximab in total and continues to take oral prednisone (0.5 mg/kg per day). Clinically, a left‐inferior hemianopsia is still apparent at 6 months and appears to be the only sequelae. The evolution of the patient's response and the timing of treatment is illustrated in Figure 2.
Figure 2.
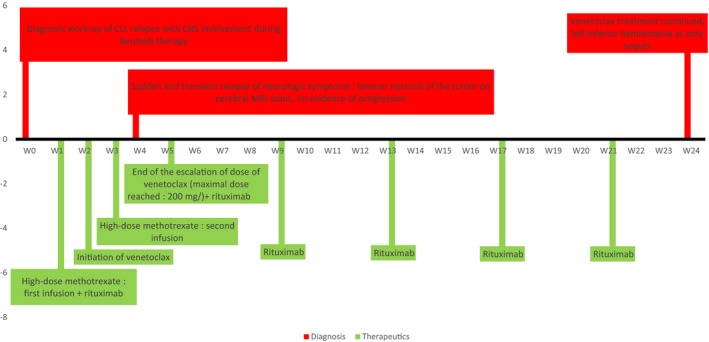
Timeline graph of the treatment and results
3. DISCUSSION
Current guidelines do not provide recommendations for the treatment of CLL patients with CNS involvement.3, 8 Reda et al first demonstrated the efficacy of venetoclax in combination with intrathecal chemotherapy. Intrathecal injections may contribute to rapid antitumoral effect on CNS dissemination, particularly in the early phase of treatment. Our report confirms the potency of venetoclax + rituximab to efficiently cross the blood‐brain barrier for the management of CLL with CNS involvement. Nevertheless, the initial contribution of methotrexate infusions and continuous oral corticosteroids cannot be ruled out to explain the clinical improvement. Indeed, previously reported cases show that intrathecal chemotherapy (methotrexate and cytarabine) is the most frequently given treatment for CLL with CNS involvement.9, 10 Our patient's previous medical history of psychiatric disorders complicated the interpretation and feasibility of lumbar punctures. Moreover, splenomegaly and palpable adenopathies suggested a systemic progression which was confirmed by the splenic biopsy. Therefore, we decided on a systemic chemotherapy with a well‐established CSF penetration. Although cyclophosphamide and fludarabine are the most frequently used systemic chemotherapies in these cases, as stated in the literature,4 this approach was precluded in our patient as he had experienced major side effects with these drugs in the past. Consequently, high‐dose methotrexate was prescribed as an alternative as it is a well‐accepted treatment for managing CNS involvement in other aggressive B‐cell malignancies notwithstanding the absence of reports of its use in CLL. The management of systemic relapse after ibrutinib treatment was completed with rituximab and venetoclax as described by Seymour et al.11 There is evidence supporting the use of rituximab to treat CNS involvement of B‐cell malignancies such as primary CNS lymphoma.12 But, the potency of venetoclax in treating CNS involvement as described by Reda et al7 was not known at the beginning of our patient's treatment. As a matter of fact, the need to quickly control neurological symptoms was another argument in favor of the administration of high‐dose methotrexate in our case. The contribution of intravenous methotrexate along with corticosteroids to control neurologic deficiencies at the diagnosis of CNS involvement is well‐established in contrast to venetoclax. However, six months after the last methotrexate infusion and under continuous venetoclax treatment neurologic improvements are stable and there are no sequelae nor have any new symptoms arisen. This suggests that venetoclax is effective for treating CNS involvement of CLL. We also highlight the absence of any major side effects. We expect venetoclax to be beneficial as a long‐term control strategy especially in our case in which the patient progressed under ibrutinib treatment.
4. CONCLUSION
Venetoclax in combination with rituximab and high‐dose methotrexate may be an effective and safe therapeutic option to manage CNS involvement of CLL.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
GB: took part in the diagnosis and the initial treatment of CNS involvement of CLL in the reported case and wrote this article. MG, CP, and LO: took part in the diagnosis and the initial treatment. They ensure the patient's hematological follow‐up after hospital discharge. FL and JC: are neurologists and took part in the diagnosis of CNS involvement of CLL. They helped to manage neurologic deficiencies and to implement the anticonvulsivant treatment. They ensure the patient's neurological follow‐up. LY: took part in the management of CNS involvement of CLL and decided to initiate venetoclax in addition to high‐dose methotrexate and rituximab at the diagnosis of CNS involvement. He helped to write this article.
Beziat G, Gauthier M, Protin C, et al. Venetoclax with high‐dose methotrexate and rituximab seem effective and well‐tolerated in the treatment of central nervous system involvement of chronic lymphocytic leukemia: A case report. Clin Case Rep. 2020;8:269–273. 10.1002/ccr3.2580
REFERENCES
- 1. Timmers NKLM, de Maar JS, van Kruijsdijk RCM, Klein SK. Central nervous system localisation of chronic lymphocytic leukaemia, description of two very distinct cases and a review of the literature. Ann Hematol. 2018;97:1627‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strati P, Uhm JH, Kaufmann TJ, et al. Prevalence and characteristics of central nervous system involvement by chronic lymphocytic leukemia. Haematologica. 2016;101:458‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26:v78‐v84. [DOI] [PubMed] [Google Scholar]
- 4. Wanquet A, Birsen R, Bonnet C, et al. Management of central nervous system involvement in chronic lymphocytic leukaemia: a retrospective cohort of 30 patients. Br J Haematol. 2017;176:37‐49. [DOI] [PubMed] [Google Scholar]
- 5. Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18:230‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reda G, Cassin R, Dovrtelova G, et al. Venetoclax penetrates in cerebrospinal fluid and may be effective in chronic lymphocytic leukemia with central nervous system involvement. Haematologica. 2019;104:e222‐e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment: HALLEK. Am J Hematol. 2017;92:946‐965. [DOI] [PubMed] [Google Scholar]
- 9. de Souza S, Santiago F, Ribeiro‐Carvalho M, Arnóbio A, Soares A, Ornellas M. Leptomeningeal involvement in B‐cell chronic lymphocytic leukemia: a case report and review of the literature. BMC Res Notes. 2014;7:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lange CPE, Brouwer RE, Brooimans R, Vecht CHJ. Leptomeningeal disease in chronic lymphocytic leukemia. Clin Neurol Neurosurg. 2007;109:896‐901. [DOI] [PubMed] [Google Scholar]
- 11. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax‐rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107‐1120. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt AM, Herbrand AK, Fox CP, et al. Rituximab in primary central nervous system lymphoma – a systematic review and meta‐analysis. Hematol Oncol. 2019:1‐10. 10.1002/hon.2666 [DOI] [PubMed] [Google Scholar]


