Abstract
The epothilones are a novel class of antineoplastic agents possessing antitubulin activity. The compounds were originally identified as secondary metabolites produced by the soil-dwelling myxobacterium Sorangium cellulosum. Two major compounds, epothilone A and epothilone B, were purified from the S. cellulosum strain So ce90 and their structures were identified as 16-member macrolides. Initial screening with these compounds revealed a very narrow and selective antifungal activity against the zygomycete, Mucor hiemalis. In addition, strong cytotoxic activity against eukaryotic cells, mouse L929 fibroblasts and human T-24 bladder carcinoma cells was observed. Subsequent studies revealed that epothilones induce tubulin polymerization and enhance microtubule stability. Epothilone-induced stabilisation of microtubules was shown to cause arrest at the G2/M transition of the cell cycle and apoptosis. The compounds are active against cancer cells that have developed resistance to taxanes as a result of acquisition of β-tubulin overexpression or mutations and against multidrug-resistant cells that overexpress P-glycoprotein or multidrug resistance-associated protein. Thus, epothilones represent a new class of antimicrotubule agents with low susceptibility to key tumour resistance mechanisms.
More recently, a range of synthetic and semisynthetic epothilone analogues have been produced to further improve the adverse effect profile (or therapeutic window) and to maximize pharmacokinetic and antitumour properties. Various epothilone analogues have demonstrated activity against many tumour types in preclinical studies and several compounds have been and still are being evaluated in clinical trials. This article reviews the identification and early molecular characterization of the epothilones, which has provided insight into the mode of action of these novel antitumour agents in vivo.
Keywords: Vinca Alkaloid, Ixabepilone, Tubulin Polymerization, Epothilones, Tubulin Isotype
The development of novel antitumour agents has significantly improved the prognosis and survival of patients with various forms of cancer. However, the effectiveness of current treatment modalities is often limited by intrinsic or acquired tumour resistance, which results in disease progression in the majority of cases. Many of the most effective antineoplastic agents currently in use were derived from natural sources. For example, the vinca alkaloid, vinblastine, was obtained from the Madagascar periwinkle plant Catharanthus roseus; anthracyclines are fermentation products of the soil bacterium Streptomyces peucetius var. caesius, and the pacific yew tree is the original source of the taxanes. However, what all of these compounds have in common is that tumours invariably become resistant to their inhibitory activities, frequently because of reduced intracellular concentrations of the antineoplastic agent.[1–6] This limitation drives a continuing search to identify new agents that will overcome mechanisms of tumour resistance and minimize toxicity.
Although rational drug design and screening of synthetic combinatorial libraries have been used with some success, one of the most promising approaches to identify new biologically active agents is to tap the huge reservoir of natural compounds. The significant contributions that microtubule-targeting agents, such as the vinca alkaloids and the taxanes,[7] have made to cancer chemotherapy prompted several pharmaceutical companies to begin the search for new compounds with a similar mechanism of action in extracts of plants and microorganisms. In the 1980s, investigation into the products of a soil-dwelling myxobacterium, Sorangium cellulosum, led to the identification of a new class of compound: the epothilones. These 16-member macrolides were originally selected for their antifungal properties, but were subsequently identified as a new class of highly active microtubule-stabilizing agents. Various synthetic and semisynthetic analogues of the epothilones have shown activity against a wide range of tumour types including multidrug-resistant disease. This review focuses on the early identification and molecular characterization of the epothilones, which has provided an understanding of their mode of action and a rationale for clinical development.
1. Myxobacteria
The myxobacteria are unique microorganisms with unparalleled properties.[8] Myxobacteria are relatively large (0.9–1.0 × 3–6 μm) rod-shaped bacteria (figure 1) that move by gliding or creeping along surfaces. They are strictly aerobic, and are found in soil, decaying organic material, on tree bark and in fresh water. One of their most notable social behaviours is the formation of multicellular fruiting bodies (figure 2), containing dormant myxospores. In times of nutrient deprivation, tens of thousands of cells move toward discrete aggregation sites within the swarm colony (figure 3), where they form a raised mound and from this develop a fruiting body. Within the maturing fruiting body, the rod-shaped cells shorten and fatten. The resultant myxospores are resistant against desiccation, UV radiation, mechanical stress and elevated temperatures, thus helping the organism to survive unfavourable environmental conditions. Myxospores germinate when a nutrient source becomes available.[9] Most relevant to the oncologist is the fact that they frequently produce secondary metabolites with cytotoxic activity.[10–12] It is from one of these organisms that the epothilones were isolated as described below.
Fig. 1.
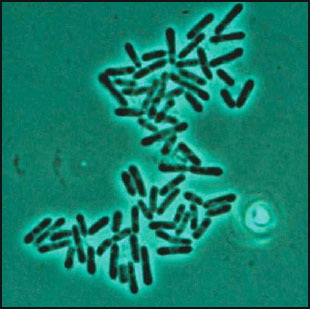
Sorangium cellulosum, vegetative cells. Phase contrast microscopy; 1550×. Individual cells measure 0.9–1.0 × 3–6 μm.
Fig. 2.
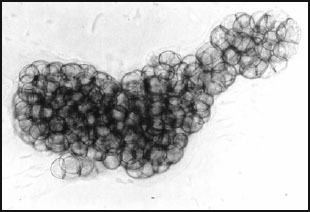
Sorangium cellulosum, fruiting body consisting of tiny sporangioles. Phase contrast microscopy; 460×. The fruiting body measures 275 × 100 μm.
Fig. 3.
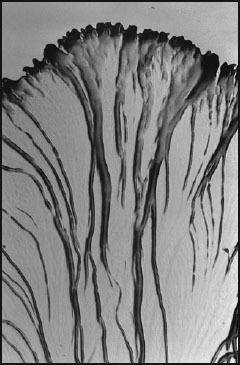
Sorangium cellulosum, section of a swarm colony. The migrating cells pack together into massive radial veins. 25× (width at margin 2.2 mm).
2. Identification of Epothilones
The epothilones were first obtained from cellulose-degrading Sorangium cellulosum, strain So ce90, isolated in 1985 at the Gesellschaft für Bio-technologische Forschung in Braunschweig, Germany. After adaptation of the strain to homogeneous growth in suspension, an antifungal activity was identified from the culture broth of So ce90, with selectivity against the zygomycete, Mucor hiemalis.[13] Following the isolation of the active compounds, it was found that the strain excreted substantial amounts of highly cytotoxic spirangiens; in addition, much lower quantities (around 2 mg/L) of epothilones A and B were produced,[13,14] such that the cytotoxicity observed in the screening was likely a result of the presence of structurally distinct spirangiens.[13–15] The antineoplastic activity of the epothilones became fully apparent when they were purified in 1987. In August of that year, the structures of epothilones A and B (figure 4) were established as 16-membered macrolides[16] and the structures of their biosynthetic precursors, epothilones C and D (figure 4), were determined shortly thereafter.[17,18] So far, no other myxobacterium and indeed no other organism has been found to produce epothilones.
Fig. 4.
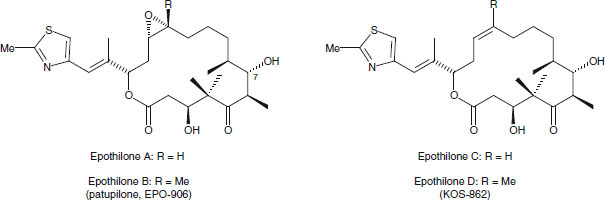
Structures of natural epothilones A–D, derived from Sorangium cellulosum.
Initial screening assays with purified epothilones A and B demonstrated inhibition of the plant pathogenic fungi Pythium infestans, Plasmopara viticola and Phytophtora infestans. Bacteria were not inhibited but strong cytotoxic activity was observed against mouse L929 fibroblasts and human T-24 bladder carcinoma cells.[13] However, because of a lack of interest of pharmaceutical companies in cytotoxic compounds at that time, the mode of action and possible applications in oncology were not pursued.
3. Mechanism of Action of Epothilones
Following identification of their cytotoxic activity, the epothilones were shown to bind to β-tubulin subunits with high affinity.[19–23] The tubulin system belongs to one of the best clinically validated anticancer targets. When bound to tubulin, epothilones stimulated its polymerization and stabilized the resulting microtubule structures.[19–21] These effects were also observed under conditions that would normally prevent tubulin polymerization or destabilize microtubules, such as low temperatures (0–25°C), high calcium levels, the absence of guanosine 5′-triphosphate (GTP), the absence of microtubule-associated proteins (MAPs) or dilution of tubulin below the critical concentration required for spontaneous microtubule formation.[19]
The microtubule cytoskeleton is an effective target for antineoplastic agents. The vinca alkaloids inhibit the assembly of tubulin into microtubules and prevent formation of the mitotic spindle.[24] The taxanes stimulate tubulin polymerization, thus enhancing the formation and stability of microtubules.[25–27] Both agents disrupt the dynamic states of microtubule growth and shrinkage that is necessary for proper regulation of cellular functions, including mitosis and meiosis, maintenance of cell shape and intracellular trafficking of macromolecules and organelles.[28–30]
The epothilones were shown to suppress microtubule dynamics. They induce microtubule bundling and formation of multipolar spindles within cells.[19,31–34] The end result of the stimulation of microtubule polymerization is arrest at the G2/M transition of the cell cycle and subsequent cell death via apoptosis.[19,35,36] While this mechanism of tubulin binding by the epothilones appears to be similar to that of paclitaxel, there are some important differences in the properties of these two classes of agents. Firstly, epothilones bind to various β-tubulin isotypes including βIII tubulin, the overexpression of which is associated in vivo and clinically with intrinsic and acquired resistance to the taxanes.[37–40] Secondly, while paclitaxel-induced apoptosis has been reported to occur independently of caspase activation,[41,42] apoptosis induced by epothilones and analogues is associated with activation of caspase 3 and additional caspases in a variety of cell types.[43–46]
4. Biological Effects of Epothilones
In agreement with experiments performed on isolated tubulin, studies on a range of human cancer cell lines have demonstrated that treatment with natural epothilones leads to profound growth inhibition and death of cancer cells. There is a dramatic reduction in the effective concentrations of epothilones required for cellular effects compared with those observed using isolated tubulin. This is consistent with a several hundred-fold accumulation of epothilones within cells.[47] HeLa cells, for example, accumulate 4.2 and 2.6 μmol/L of epothilone A and B, respectively, within 2 hours in the presence of 10 nmol/L concentrations of drugs in the medium; and at a higher drug exposure (above 100 nmol/L), the epothilones reach saturation concentrations of 17 and 26 μmol/L, respectively, which correspond well with the intracellular tubulin concentration of approximately 25 μmol/L.
Consistent with studies using isolated tubulin, epothilone B was found to be more potent than epothilone A in vitro,[48] and both epothilones demonstrated stronger activity than paclitaxel against a panel of tumour cell lines (table I). Although conflicting results were seen when the epothilones were tested in vivo,[49–52] potent antitumour activity has been demonstrated for epothilone B in several drug-sensitive human tumour cell models, including lung, breast, colon and prostate.[52]
Table I.
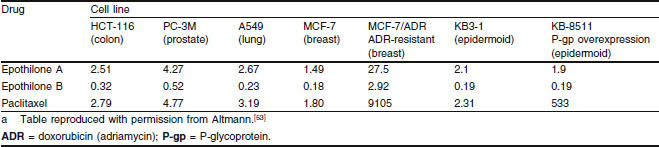
Half maximal inhibitory concentration (IC50) values (nmol/L) of epothilones A and B and paclitaxel in human cancer cell linesa
5. Reduced Susceptibility to Multidrug Resistance
One important feature of the epothilones is that they display reduced susceptibility to multiple mechanisms of tumour resistance. A major cause of intrinsic and acquired tumour resistance is the overexpression of efflux pumps such as P-glycoprotein (P-gp) and multidrug resistance-associated protein, of which many common chemotherapeutic agents are substrates.[1–6] By contrast, many epothilones have low affinity for these efflux pumps; consequently, most multidrug-resistant tumour cell lines, including those that are resistant to paclitaxel, remain sensitive to epothilones.[19,31]
As mentioned above, epothilones are also able to overcome tumour resistance caused by certain mutations in β-tubulin[31] and changes in tubulin isotype composition, as demonstrated by the activity of ixabepilone against Pat-21 breast cancer cells, which are characterized by a loss of βII tubulin isotype and an overexpression of βIII tubulin.[54] A comparison between paclitaxel and epothilone A/B half maximal inhibitory concentration (IC50) values in paclitaxel-resistant cell lines versus their parental cell lines shows that while paclitaxel resistance increased by a factor of 22 to 19 167, the resistance to epothilone B rose only 0.6-[55] to 5.0-fold (table II).[19,32,49,55]
Table II.
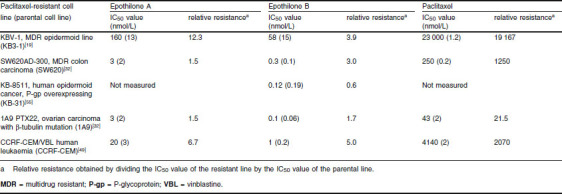
Half maximal inhibitory concentration (IC50) values of epothilones A and B and paclitaxel in paclitaxel-resistant and parental (non-resistant) cell lines
While the in vitro experiments summarized above demonstrated potent antineoplastic properties of the epothilones, translation to in vivo antitumour efficacy was not always satisfactory. This was a result of the poor metabolic stability and unfavourable pharmacokinetic properties of the natural epothilones. Lactone hydrolysis is the main pathway of epothilone B metabolism in mice;[56] epothilones with a lactone are rapidly metabolized in murine plasma, with half-lives of approximately 20 minutes.[57] However, in dogs the half-life is more than 5 hours.[57] In rodents, the degradation rates of the natural epothilones were found to be as follows: epothilone A, 0.50 nmol/min/mg; epothilone B, 1.02 nmol/min/mg; and epothilone D, 1.20 nmol/min/mg serum protein (Bristol-Myers Squibb, data on file). The differences in metabolism between species may be because of differences in the activity of plasma and tissue esterases; however, the data demonstrate the poor metabolic stability of the natural epothilones. This realization led to the development of epothilone analogues with more favourable metabolic and pharmacokinetic profiles.
6. Epothilone Analogues
A vast array of semisynthetic and synthetic epothilone analogues have been synthesized in efforts to improve upon the antitumour activity of the natural epothilones.[53,58] With seven stereogenic centres in a 16-membered macrolide, the total synthesis of epothilones, although challenging, appeared to be far less difficult than that of paclitaxel.[59] Of the synthetic and semisynthetic analogues, the most promising are ixabepilone (BMS-247550, the lactam analogue of epothilone B),[31] BMS-310705 (C21-amine of epothilone B),[60,61] dehydelone (KOS-1584; 9,10-didehydroepothilone D)[62] and sagopilone (ZK-EPO; synthetic epothilone B analogue) [figure 5].[63] It appears that KOS-862 (natural epothilone D) will not undergo further development; however, ixabepilone (BMS-247550), dehydelone (KOS-1584), sago-pilone (ZK-EPO) and patupilone (EPO-906; natural epothilone B) are currently in clinical development. In addition, although not yet in clinical development, the epothilone analogues fludelone (KOS-1591, 26-trifluoro-(E)-9,10-dehydro-12,13-desoxy-epothilone B) and methylthioepothilone B (ABJ879) [figure 5] have shown promise in a range of preclinical xenograft models.[64–67]
Fig. 5.
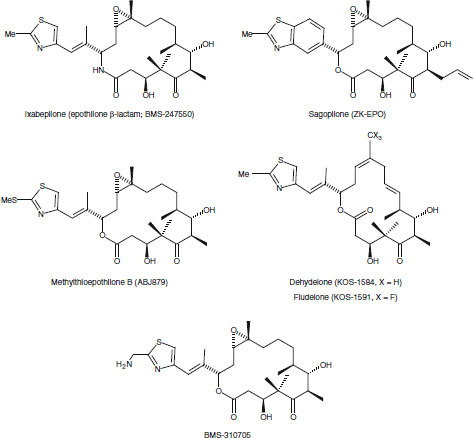
Structures of synthetic and semisynthetic epothilones in development.
The semisynthetic and synthetic analogues benefit from improved pharmacokinetic properties compared with the natural epothilones. For example, the half-life of ixabepilone in mice is 13 hours following intravenous administration of 6 mg/kg and 16 hours following intravenous administration of 10 mg/kg (Bristol-Myers Squibb, data on file). Similarly, the half-life of dehydelone (KOS-1584) is approximately 3-fold that of the natural epothilone D (KOS-862).[62] The degradation rate of ixabepilone is also lower compared with natural epothilone D, i.e. 0.01 nmol/min/mg versus 1.02 nmol/min/mg serum protein (Bristol-Myers Squibb, data on file). Unlike the natural epothilones, data from early clinical trials demonstrated good metabolic stability and availability of epothilone analogues (table III).
Table III.
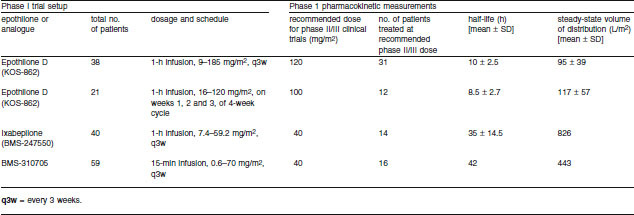
Phase I pharmacokinetic parameters of epothilone analogues in cancer patients[68,69]
7. Conclusions
The epothilones, originally identified as selective antifungal agents, are a family of macrolides specifically produced by the myxobacterium Sorangium cellulosum. Although it is unclear what role the epothilones play in the lifecycle of this organism, their high toxicity toward eukaryotic cells suggests that they may help to protect the ecological niche of the bacterium against competitors and predators, such as fungi, soil protozoa and nematodes. Alternatively, the bacterium may utilize the compounds to secure access to essential nutrients like nitrogen and phosphorus, in its nutrient-poor environment.
Further characterization of the epothilones has demonstrated strong in vitro and in vivo cytotoxic activity toward tumour cells. The biological actions of the epothilones are mediated by induction of tubulin polymerization, microtubule stabilization, cell cycle arrest and apoptosis. Other anti-microtubule agents, such as the taxanes, have been widely and successfully used as chemotherapeutic agents for many years. However, the therapeutic benefit of these drugs has been limited by their susceptibility to tumour cell resistance mechanisms. Cells that overexpress efflux pumps such as P-gp, encoded by the multidrug-resistance gene, resist the cytotoxic effects of taxanes. In addition, cells that lose expression of the tubulin βII isoform (the target of taxanes) and overexpress βIII tubulin have also demonstrated a taxane-resistant phenotype. Unlike the taxanes, the epothilones have demonstrated anti-neoplastic activity in cell lines and in in vivo human xenograft models characterized by P-gp and βIII tubulin overexpression.
The comparatively simple structure of the epothilones is amenable to synthesis, and a multitude of semisynthetic and synthetic analogues have been generated since their initial discovery. The compounds have demonstrated notable antineoplastic activity in a broad range of tumour types, including metastatic tumours. Thus, the epothilones constitute a novel class of antineoplastic agents possessing antitubulin activity and low susceptibility to key tumour resistance mechanisms. Clinical trials are currently ongoing with various natural epothilones and synthetic analogues to examine the efficacy and safety of these compounds in the treatment of cancer.[68,70–73]
Acknowledgements
Research work related to this manuscript was supported by the Helmholtz Centre for Infection Research. The authors gratefully acknowledge the editorial assistance of Roy Garcia, PhD in the preparation of this article and thank Bristol-Myers Squibb for their support in providing access to information on ixabepilone. The authors are consultants for Bristol-Myers Squibb.
Footnotes
Formerly Gesellschaft für Biotechnologische Forschung (GBF) [Department of Natural Products].
References
- 1.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 2.Moscow J, Morrow CS, Cowan KH. Drug resistance and its clinical circumvention. In: Holland JF, Frei E III, editors. Cancer medicine. Toronto: BC Decker; 2003. [Google Scholar]
- 3.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer. 2003;10(1):43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 4.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–71. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 6.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl.1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 7.Lavelle F. What’s new about new tubulin/microtubule-binding agents? Exp Opin Invest Drugs. 1995;4(8):771–5. [Google Scholar]
- 8.Reichenbach H, et al. Order VIII Myxococcales Tchan, Pochon and Prévot. 1948, 398AL. In: Brenner DJ, Krieg NR, Stanley JT, et al., editors. Bergey’s manual of systematic bacteriology. 2nd ed. New York (NY): Springer; 2005. pp. 1059–144. [Google Scholar]
- 9.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60(1):70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Höfle G, Reichenbach H. Biosynthetic potential of the myxobacteria. In: Kuhn W, Fiedler H, editors. Sekundärmetabolismus bei Mikroorganismen. Tübingen: Attempto Verlag; 1995. pp. 61–78. [Google Scholar]
- 11.Reichenbach H, Höfle G. Myxobacteria as producers of secondary metabolites. In: Grabley S, Thierecke R, editors. Drug discovery from nature. Berlin: Springer; 1999. p. 79. [Google Scholar]
- 12.Reichenbach H, Höfle G. Biologically active secondary metabolites from myxobacteria. Biotechnol Adv. 1993;11(2):219–77. doi: 10.1016/0734-9750(93)90042-l. [DOI] [PubMed] [Google Scholar]
- 13.Gerth K, Bedorf N, Höfle G, et al. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (myxobacteria): production, physico-chemical and biological properties. J Antibiot (Tokyo) 1996;49(6):560–3. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 14.Höfle G, Bedorf N. German Patent No. DE413 8042. 1993. [Google Scholar]
- 15.Niggemann J, Bedorf N, Flörke U, et al. Spirangien A and B, highly cytotoxic and antifungal spiroketals from the myxobacterium Sorangium cellulosum: isolation, structure elucidation and chemical modifications. Eur J Org Chem. 2005;23:5013–8. [Google Scholar]
- 16.Höfle G, Bedorf N, Steinmetz H, et al. Epothilone A and B: novel 16-membered macrolides with cytotoxic activity. Isolation, crystal structure, and conformation in solution. Angew Chem Int Ed Engl. 1996;35(13/14):1567–9. [Google Scholar]
- 17.Gerth K, Steinmetz H, Höfle G, et al. Studies on the biosynthesis of epothilones: the PKS and epothilone C/D monooxygenase. J Antibiot (Tokyo) 2001;54(2):144–8. doi: 10.7164/antibiotics.54.144. [DOI] [PubMed] [Google Scholar]
- 18.Gerth K, Steinmetz H, Höfle G, et al. Studies on the biosynthesis of epothilones: the biosynthetic origin of the carbon skeleton. J Antibiot (Tokyo) 2000;53(12):1373–7. doi: 10.7164/antibiotics.53.1373. [DOI] [PubMed] [Google Scholar]
- 19.Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55(11):2325–33. [PubMed] [Google Scholar]
- 20.Buey RM, Diaz JF, Andreu JM, et al. Interaction of epothilone analogs with the paclitaxel binding site: relationship between binding affinity, microtubule stabilization, and cytotoxicity. Chem Biol. 2004;11(2):225–36. doi: 10.1016/j.chembiol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Heinz DW, Schubert WD, Höfle G. Much anticipated: the bioactive conformation of epothilone and its binding to tubulin. Angew Chem Int Ed Engl. 2005;44(9):1298–301. doi: 10.1002/anie.200462241. [DOI] [PubMed] [Google Scholar]
- 22.Bode CJ, Gupta ML, Jr, Reiff EA, et al. Epothilone and paclitaxel: unexpected differences in promoting the assembly and stabilization of yeast microtubules. Biochemistry. 2002;41(12):3870–4. doi: 10.1021/bi0121611. [DOI] [PubMed] [Google Scholar]
- 23.Wartmann M, Altmann KH. The biology and medicinal chemistry of epothilones. Curr Med Chem Anti-Canc Agents. 2002;21:123–48. doi: 10.2174/1568011023354489. [DOI] [PubMed] [Google Scholar]
- 24.Owellen RJ, Hartke CA, Dickerson RM, et al. Inhibition of tubulin-microtubule polymerization by drugs of the vinca alkaloid class. Cancer Res. 1976;36(4):1499–502. [PubMed] [Google Scholar]
- 25.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77(3):1561–5. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnal I, Wade RH. How does taxol stabilize microtubules? Curr Biol. 1995;5(8):900–8. doi: 10.1016/s0960-9822(95)00180-1. [DOI] [PubMed] [Google Scholar]
- 27.Gupta ML, Jr, Bode CJ, Georg GI, et al. Understanding tubulin-Taxol interactions: mutations that impart Taxol binding to yeast tubulin. Proc Natl Acad Sci USA. 2003;100(11):6394–7. doi: 10.1073/pnas.1131967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oakley BR. An abundance of tubulins. Trends Cell Biol. 2000;10(12):537–42. doi: 10.1016/s0962-8924(00)01857-2. [DOI] [PubMed] [Google Scholar]
- 29.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–7. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- 31.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7(5):1429–37. [PubMed] [Google Scholar]
- 32.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol®) J Biol Chem. 1997;272(4):2534–41. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 33.Kamath K, Jordan MA. Suppression of microtubule dynamics by epothilone B is associated with mitotic arrest. Cancer Res. 2003;63(18):6026–31. [PubMed] [Google Scholar]
- 34.Verrills NM, Flemming CL, Liu M, et al. Microtubule alterations and mutations induced by desoxyepothilone B: implications for drug-target interactions. Chem Biol. 2003;10(7):597–607. doi: 10.1016/s1074-5521(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Chen J, Bhalla K, et al. Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem. 2004;279(38):39431–7. doi: 10.1074/jbc.M401530200. [DOI] [PubMed] [Google Scholar]
- 36.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–86. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 37.Verrills NM, Kavallaris M. Improving the targeting of tubulin-binding agents: lessons from drug resistance studies. Curr Pharm Des. 2005;11(13):1719–33. doi: 10.2174/1381612053764706. [DOI] [PubMed] [Google Scholar]
- 38.Kamath K, Wilson L, Cabral F, et al. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280(13):12902–7. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 39.Paradiso A, Mangia A, Chiriatti A, et al. Biomarkers predictive for clinical efficacy of taxol-based chemotherapy in advanced breast cancer. Ann Oncol. 2005;16(4Suppl.):iv14–9. doi: 10.1093/annonc/mdi902. [DOI] [PubMed] [Google Scholar]
- 40.Seve P, Mackey J, Isaac S, et al. Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther. 2005;4(12):2001–7. doi: 10.1158/1535-7163.MCT-05-0244. [DOI] [PubMed] [Google Scholar]
- 41.Ofir R, Seidman R, Rabinski T, et al. Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ. 2002;9:636–42. doi: 10.1038/sj.cdd.4401012. [DOI] [PubMed] [Google Scholar]
- 42.Ahn HJ, Kim YS, Kim JU, et al. Mechanism of taxol-induced apoptosis in human SKOV3 ovarian carcinoma cells. J Cell Biochem. 2004;91:1043–52. doi: 10.1002/jcb.20006. [DOI] [PubMed] [Google Scholar]
- 43.Griffin D, Wittmann S, Guo F, et al. Molecular determinants of epothilone B derivative (BMS 247550) and Apo-2L/TRAIL-induced apoptosis of human ovarian cancer cells. Gynecol Oncol. 2003;89(1):37–47. doi: 10.1016/s0090-8258(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 44.Guo F, Nimmanapalli R, Paranawithana S, et al. Ectopic over-expression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood. 2002;99(9):3419–26. doi: 10.1182/blood.v99.9.3419. [DOI] [PubMed] [Google Scholar]
- 45.Uyar D, Takigawa N, Mekhail T, et al. Apoptotic pathways of epothilone BMS 310705. Gynecol Oncol. 2003;91:173–8. doi: 10.1016/s0090-8258(03)00481-5. [DOI] [PubMed] [Google Scholar]
- 46.Wu KD, Cho YS, Katz J, et al. Investigation of antitumor effects of synthetic epothilone analogs in human myeloma models in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102(30):10640–5. doi: 10.1073/pnas.0504512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wartmann M, Koppler J, Lartigot M. Epothilones A and B accumulate several-hundred fold inside cells [abstract 1362] Proc Am Assoc Cancer Res. 2000;41:213. [Google Scholar]
- 48.Altmann KH. Epothilone B and its analogs: a new family of anticancer agents. Mini Rev Med Chem. 2003;3(2):149–58. doi: 10.2174/1389557033405269. [DOI] [PubMed] [Google Scholar]
- 49.Su DS, Balog A, Meng D, et al. Structure-activity relationship of the epothilones and the first in vivo comparison with paclitaxel. Angew Chem Int Ed Engl. 1997;36(19):2093–6. [Google Scholar]
- 50.Chou TC, Zhang XG, Balog A, et al. Desoxyepothilone B: an efficacious microtubule-targeted antitumor agent with a promising in vivo profile relative to epothilone B. Proc Natl Acad Sci U S A. 1998;95(16):9642–7. doi: 10.1073/pnas.95.16.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothermel J, Wartmann M, Chen T, et al. EPO906 (epothilone B): a promising novel microtubule stabilizer. Semin Oncol. 2003;30(3Suppl.6):51–5. doi: 10.1016/s0093-7754(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 52.Altmann KH, Wartmann M, O’Reilly T. Epothilones and related structures: a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470(3):M79–91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 53.Altmann KH. The chemistry and biology of epothilones: lead structures for the discovery of improved microtubule inhibitors. In: Liang XT, Fang WS, editors. Medicinal chemistry of bioactive natural products. Wiley: Hoboken (NJ); 2006. pp. 1–34. [Google Scholar]
- 54.Jordan MA, Miller H, Ni L, et al. The Pat-21 breast cancer model derived from a patient with primary Taxol® resistance recapitulates the phenotype of its origin, has altered β-tubulin expression and is sensitive to ixabepilone [abstract] Proc Amer Assoc Cancer Res. 2006;47:LB–280. [Google Scholar]
- 55.Nicolaou KC, Sasmal PK, Rassias G, et al. Design, synthesis, and biological properties of highly potent epothilone B analogues. Angew Chem Int Ed Engl. 2003;42(30):3515–20. doi: 10.1002/anie.200351819. [DOI] [PubMed] [Google Scholar]
- 56.Blum W, Aichholz R, Ramstein P, et al. In vivo metabolism of epothilone B in tumor-bearing nude mice: identification of three new epothilone B metabolites by capillary high-pressure liquid chromatography/mass spectrometry/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001;15(1):41–9. doi: 10.1002/1097-0231(20010115)15:1<41::AID-RCM190>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 57.Chou TC, O’Connor OA, Tong WP, et al. The synthesis, discovery, and development of a highly promising class of microtubule stabilization agents: curative effects of desoxyepothilones B and F against human tumor xenografts in nude mice. Proc Natl Acad Sci U S A. 2001;98(14):8113–8. doi: 10.1073/pnas.131153098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altmann KA, Bold G, Caravatti G, et al. Epothilones and their analogs: potential new weapons in the fight against cancer. Chimia. 2000;54:612–21. [Google Scholar]
- 59.Höfle G, Reichenbach H. Epothilone, a myxobacterial metabolite with promising antitumor activity. In: Cragg G, Kingston D, Newman D, editors. Anticancer agents from natural products. Taylor & Francis Group: Boca Raton (FL); 2005. pp. 413–450. [Google Scholar]
- 60.Vite G, Höfle G, Bifano M. 223rd Am Chem Soc Meeting. 2002. The semisynthesis and preclinical evaluation of BMS-310705, an epothilone analog in clinical development [abstract] [Google Scholar]
- 61.Kolman A. BMS-310705 Bristol Myers Squibb/GBF. Curr Opin Invest Drugs. 2004;5(12):1292–7. [PubMed] [Google Scholar]
- 62.Zhou Y, Zhong Z, Liu F. KOS-1584: a rationally designed epothilone D analog with improved potency and pharmacokinetic (PK) properties [abstract] Proc Amer Assoc Cancer Res. 2005;46:2535. [Google Scholar]
- 63.Klar U, Buchmann B, Schwede W, et al. Total synthesis and antitumor activity of ZK-EPO: the first fully synthetic epothilone in clinical development. Angew Chem Int Ed Engl. 2006;4547:7942–8. doi: 10.1002/anie.200602785. [DOI] [PubMed] [Google Scholar]
- 64.Chou TC, Dong H, Zhang X, et al. Therapeutic cure against human tumor xenografts in nude mice by a microtubule stabilization agent, fludelone, via parenteral or oral route. Cancer Res. 2005;65(20):9445–54. doi: 10.1158/0008-5472.CAN-05-1014. [DOI] [PubMed] [Google Scholar]
- 65.Dilea C, Wartmann M, Maira SM. A PK-PD dose optimization strategy for the microtubule stabilizing agent ABJ879 [abstract] Proc Amer Assoc Cancer Res. 2004;45:5132. [Google Scholar]
- 66.Wartmann M, Loretan J, Reuter R. Preclinical pharmacological profile of ABJ879, a novel epothilone B analog with potent and protracted anti-tumor activity [abstract] Proc Amer Assoc Cancer Res. 2004;45:5440. [Google Scholar]
- 67.Wu KD, Cho YS, Katz J, et al. Investigation of antitumor effects of synthetic epothilone analogs in human myeloma models in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(30):10640–5. doi: 10.1073/pnas.0504512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22(10):2015–25. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Spriggs DR, Dupont J, Pezzulli S, et al. KOS-862 (epothilone D): phase 1 dose escalating and pharmacokinetic (PK) study in patients (pts) with advanced malignancies [abstract] ECCO. 2003;12:547. [Google Scholar]
- 70.Kuppens IELM. Current state of the art of new tubulin inhibitors in the clinic. Curr Clin Pharm. 2006;1:57–70. doi: 10.2174/157488406775268200. [DOI] [PubMed] [Google Scholar]
- 71.Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: the epothilones. Oncologist. 2007;12(3):271–80. doi: 10.1634/theoncologist.12-3-271. [DOI] [PubMed] [Google Scholar]
- 72.Larkin JM, Kaye SB. Epothilones in the treatment of cancer. Expert Opin Investig Drugs. 2006;15(6):691–702. doi: 10.1517/13543784.15.6.691. [DOI] [PubMed] [Google Scholar]
- 73.Lee JJ, Swain SM. Development of novel chemotherapeutic agents to evade the mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32(6Suppl.7):S22–6. doi: 10.1053/j.seminoncol.2005.09.013. [DOI] [PubMed] [Google Scholar]


