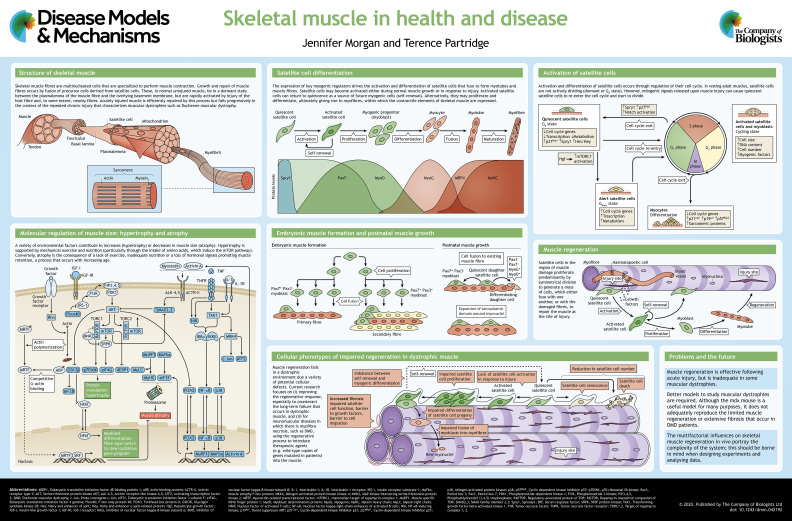
ABSTRACT
Skeletal muscle fibres are multinucleated cells that contain postmitotic nuclei (i.e. they are no longer able to divide) and perform muscle contraction. They are formed by fusion of muscle precursor cells, and grow into elongating myofibres by the addition of further precursor cells, called satellite cells, which are also responsible for regeneration following injury. Skeletal muscle regeneration occurs in most muscular dystrophies in response to necrosis of muscle fibres. However, the complex environment within dystrophic skeletal muscle, which includes inflammatory cells, fibroblasts and fibro-adipogenic cells, together with the genetic background of the in vivo model and the muscle being studied, complicates the interpretation of laboratory studies on muscular dystrophies. Many genes are expressed in satellite cells and in other tissues, which makes it difficult to determine the molecular cause of various types of muscular dystrophies. Here, and in the accompanying poster, we discuss our current knowledge of the cellular mechanisms that govern the growth and regeneration of skeletal muscle, and highlight the defects in satellite cell function that give rise to muscular dystrophies.
KEY WORDS: Muscular dystrophy, Satellite cell, Skeletal muscle regeneration
Summary: The mechanisms of skeletal muscle development, growth and regeneration are described. We discuss whether these processes are dysregulated in inherited muscle diseases and identify pathways that may represent therapeutic targets.
Introduction
Skeletal muscle is composed of linear arrays of multinucleated muscle fibres, each with a complex internal structure dedicated to the conversion of chemical to physical energy. These fibres are ‘end cells’, meaning that they cannot proliferate to expand or restore the population after damage. Instead, they are formed or repaired by fusion of a proliferation-capable population of precursor cells called myoblasts (see Glossary, Box 1). The sequence of transcription factor expression leading to differentiation in the precursor cell population of the main mammalian body musculature is a close reflection of that observed during initial muscle formation in the embryo and the enlargement of muscle fibres in the postnatal and juvenile stages of muscle growth, as well as that observed in muscle repair. However, the behaviour of the myogenic (Box 1) cells differs radically between these situations.
Box 1. Glossary.
Asymmetric division: a cell division that produces daughter cells that have different fates (e.g. one stem cell and one differentiated cell).
CTG expansion: a mutation in which repeats of three nucleotides (trinucleotide repeats) increase in copy number until they cross a threshold above which they become unstable.
DBA2/J, C57Bl/10 and 129/SVemst/J: inbred mouse strains that differ in their genetic backgrounds.
Dy/dy mouse: a model of laminin alpha-2 deficiency (MDC1A) that has a mutation in the LAMA2 gene. This mouse has a moderate fibrotic and dystrophic phenotype (reviewed in Ng et al., 2012).
Dysferlin: a protein that is highly expressed at the sarcolemma of muscle fibres and is involved in repair of the sarcolemma.
Dystroglycanopathy: a muscular dystrophy caused by aberrant glycosylation of dystroglycan.
Gamma-sarcoglycan (Sgcg)-null mouse model: a model of Limb-girdle muscular dystrophy type 2C (LGMD2C).
Genetic modifier: a gene that affects the phenotypic and/or molecular expression of other genes.
Largemyd mouse: a model of congenital muscular dystrophy type 1D (MDC1D). Dystroglycan glycosylation is defective in these mice as a result of a mutation in like-acetylglucosaminyltransferase (LARGE), a glycosyltransferase.
Mdx mouse: X-linked muscular dystrophy mouse model of DMD. Has a mutation in exon 23 of the Dmd gene.
Muscle precursor cell: any cell that is predetermined to differentiate into skeletal muscle.
Myoblast: the progeny of satellite cells.
MyoD: myoblast determination protein 1, a myogenic regulatory factor.
Myogenic: originating in, or produced by, muscle cells.
Niche: a stem cell niche is an interactive structural unit, organized to facilitate cell-fate decisions in a proper spatiotemporal manner (Moore and Lemischka, 2006).
Satellite cell: skeletal muscle stem cell, located between the basal lamina (the internal layer of the basement membrane) and sarcolemma (cell membrane) of a muscle fibre. A satellite cell expresses PAX7 and is quiescent in normal adult muscle.
Symmetric cell division: a cell division that produces daughter cells that have the same fate (e.g. two stem cells, or two differentiated cells).
Utrophin: a cytoskeletal protein that has some structural and functional similarities to dystrophin.
Here, we briefly discuss skeletal muscle formation, growth and repair, with particular reference to muscular dystrophies. Most of the data behind these descriptions are derived from studies in animal models, mainly rodents, or from in vitro models of myogenesis. The relationship between the human condition of interest and the animal models requires careful consideration (Partridge, 2013). Likewise, while in vitro or ex vivo models of myogenesis are the source of much of the molecular biological data on myogenesis, they do not reproduce the interactions with the cellular, matrix and systemic features of the in vivo environment that tune the process of myogenesis to the physiological needs of the animal as a whole. Thus, the applicability of knowledge for disease treatment gained from the above models should be treated with reserve.
Initial muscle fibre formation
Initial muscle fibre formation has predominantly been studied in the limb. During initial myogenesis in the embryonic muscle anlagen, precursor cells proliferate to form compact groups, within which individual cells fuse together in longitudinal arrays to form multinucleated fibres (see poster). This occurs in phases, beginning with a synchronous fusion of cells expressing the paired box transcription factors Pax3 and Pax7 across the whole length of the newly emerging muscle anlagen to form primary muscle fibres (Lee et al., 2013), which act as a scaffolding for subsequent rounds of fibre formation. In mice, a second subset of Pax3+, Pax7− myogenic cells associate and align with the primary fibres. They fuse sequentially with one another, beginning in the middle of the fibre and progressing towards the two ends, to form secondary fibres (Lee et al., 2013) (see poster). In large mammals, a tertiary and even a quaternary phase of myogenesis may occur, although the evidence is uncertain (Edom-Vovard et al., 1999; Bröhl et al., 2012).
Growth of muscle fibres
In mice, neoformation of muscle fibres ceases by birth. Muscle growth occurs by a combination of the progressive addition of myonuclei to each fibre and the expansion of the sarcoplasmic domain around each myonucleus (see poster). In mice, the addition of new myonuclei is largely accomplished by 3-4 weeks of age, and entails both the proliferation and fusion of satellite cells (Box 1). Between 2-3 weeks of age, each mouse extensor digitorum longus (EDL) myofibre increases in myonuclear number from ∼100 to ∼200 myonuclei (Duddy et al., 2015; White et al., 2010). This corresponds to one satellite cell fusion every 2 h and is accomplished by around 5-10 satellite cells per fibre (Duddy et al., 2015; White et al., 2010). Muscle growth beyond 4 weeks of age continues predominantly by an increase in the sarcoplasmic territory around each myonucleus but also involves the addition of myonuclei at about one tenth of the pre-3-week rate, again brought about by the action of a small number of satellite cells per fibre (Duddy et al., 2015).
Models of muscular dystrophies
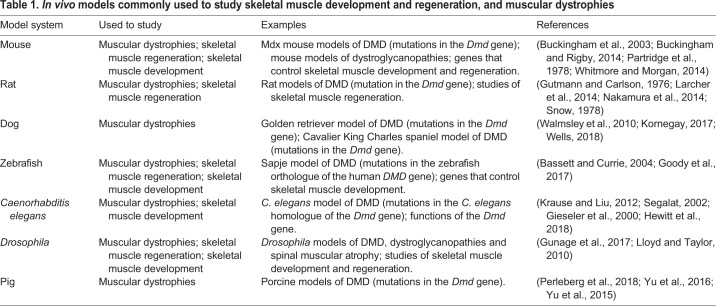
Skeletal muscle development, muscular dystrophies and muscle regeneration have been studied in different in vivo models (Table 1). Of these, the mdx mouse (Box 1) has been the most used, and is thus the source of the most comprehensive set of detailed pathological data; however, its mild clinical course (Bulfield et al., 1984) has raised concerns about its use as a model of Duchenne muscular dystrophy (DMD) in humans. There is a marked difference between the pathology of dystrophin-deficient mice and humans, possibly due to the far greater growth span, larger size and greater loading of muscles in humans than in mice (Grounds and Shavlakadze, 2011). The primary pathology is severe in mdx mice, but is counteracted by robust skeletal muscle regeneration. However, severity is increased in the context of mutations in other genes that affect myoblast proliferation or myofibre stability, such as those that affect telomere length (Sacco et al., 2010) (Yucel et al., 2018), cause a lack of utrophin (Box 1) (Deconinck et al., 1997) or myoblast determination protein 1 (MyoD; also known as Myod1; Box 1) (Megeney et al., 1996), or lead to the deletion of the mouse cytidine monophosphate-N-acetylneuraminic acid hydroxylase (Cmah) gene (Chandrasekharan et al., 2010) (reviewed in Rodrigues et al., 2016).
Table 1.
In vivo models commonly used to study skeletal muscle development and regeneration, and muscular dystrophies
The more severe disease seen in golden retrievers with muscular dystrophy is widely regarded as a closer model of DMD pathology than the mdx mouse and an important intermediate for preclinical research (Kornegay, 2017), but the cost of experiments involving dogs limits their value for fundamental research. Recently developed rat models (Larcher et al., 2014; Nakamura et al., 2014) have yet to be sufficiently described to be fully assessable as experimental models. Fish and invertebrate models offer good access to tools that facilitate the investigation of the genetic and molecular biological aspects of the disease process (Table 1), but the inflammatory responses to damage differ substantially from those in mammals, meaning that these models are of limited value to study the inflammatory aspects of human disease.
Skeletal muscle regeneration
Cellular mechanisms of muscle regeneration
Muscle fibres respond to minor damage with limited immediate repair mechanisms that reseal the muscle fibre's surface membrane (Barthélémy et al., 2018; Horn et al., 2017). Conversely, repair and replacement of irreversibly damaged fibres is achieved by activation of muscle precursor cells (Box 1), which proliferate, move to the area of damage, and fuse with one another and with the surviving segments of damaged muscle fibres (see poster). This latter process does not always perfectly align the surviving fibre stump with the newly forming repair segment, with the result that many fibres become branched after regeneration (Blaveri et al., 1999) (Partridge and Morgan, 2014) and progressively so in the context of a chronic myopathy such as muscular dystrophy (Duddy et al., 2015).
Skeletal muscle regeneration is mediated largely, if not exclusively, by satellite cells (Lepper et al., 2011; Sambasivan et al., 2011; Yamamoto et al., 2018). These cells are normally quiescent in undamaged adult muscle, but become activated in response to injury. They express receptors for growth factors [e.g. fibroblast growth factor receptor 2 (Kästner et al., 2000)], which drive their proliferation upon release of growth factors from damaged muscles or inflammatory cells. This very rapid activation is attributed to the release of RNA coding for the myogenic transcription factor Myf5 from cytoplasmic granules (Crist et al., 2012) and involves a rapid onset of expression of MyoD, with satellite cell proliferation beginning 24-36 h later (Cornelison and Wold, 1997; Zammit et al., 2004). It is hypothesized that some cells contribute to regeneration by fusing with one another and with damaged muscle fibres, while others, distinguished by cessation of MyoD expression, become quiescent and re-enter the satellite cell pool (Zammit et al., 2004). Most of these ideas are based on the study of satellite cells adherent to myofibres, which can be isolated from muscle and subsequently used as an in vitro model of regeneration. But it is becoming increasingly clear that other features of the injury environment play important roles in modulating the sequence of regeneration events. Investigation of the fate of satellite cells (Cousins et al., 2004; Robertson et al., 1990; Tierney et al., 2018a; Webster et al., 2016), myofibre necrosis (Chrzanowski et al., 2017; Filareto et al., 2018) and myofibre regeneration (Baudy et al., 2011) in the context of inflammation (Martinez et al., 2015) in in vivo models are more informative as to the extent of participation of other cells and of the intercellular environment. Recently available markers have shown a complex diversity of myogenetic clones that remain stable during growth and ageing of normal muscle (Tierney et al., 2018b), implying that asymmetric division (Box 1) of a stem cell compartment is a major component of muscle formation and maintenance. During regeneration, however, myogenic clones increase in size and diminish in their complexity, suggesting that, in this process, muscle cells expand predominantly by symmetric cell division (Box 1) of committed cells.
It is increasingly apparent that repair of muscle is a complex collaborative activity, involving several different cell types in addition to satellite cells (Wosczyna and Rando, 2018). Of these, the macrophage (Chazaud et al., 2003; Tidball, 2017) has become prominent, and its effects on the overall repair process are phased. The initial ‘pro-inflammatory’ macrophage population, which is envisaged to act predominantly in the resorption of damaged tissue, is subsequently transformed into, or succeeded by, a more ‘pro-regenerative’ type of macrophage, which secretes cytokines that facilitate the myogenic functions of satellite cells (Kharraz et al., 2013; Saclier et al., 2013b; Tidball et al., 2014; Tidball and Villalta, 2010). The activities of resident fibro-adipogenic cells also influence the balance between fibrosis and myogenesis during the repair process in damaged muscle (Joe et al., 2010; Uezumi et al., 2011) and strongly affect the degenerative mechanisms in dysferlin (Box 1)-deficient muscle (Hogarth et al., 2019). Such discoveries have also revived interest in the changes in structural components of muscle associated with chronic inflammation, which are likely to impact cell mobility and the distribution of cytokines. Fibrosis is an issue in muscular dystrophies, posing a physical barrier to cells and altering muscle stiffness, which can affect satellite cell function (reviewed in Smith and Barton, 2018) (see poster). Furthermore, non-muscle cells, such as fibroblasts (Murphy et al., 2011; Fry et al., 2017; Mackey et al., 2017), and the interactions between satellite cells and cells of the microvasculature (Abou-Khalil et al., 2010; Mounier et al., 2011; Saclier et al., 2013a; Verma et al., 2018), are also involved in mediating the inflammatory response and in promoting satellite cell proliferation and differentiation. The complexity of the cellular interactions in skeletal muscle is too extensive and intricate to be effectively modelled in terms of individual cellular processes. Thus, muscle is perhaps better regarded as an ecosystem within which each of the component parts contributes to a homeostasis that may be disturbed by extreme pathological processes or genetic defects.
There is evidence that cells other than satellite cells contribute to skeletal muscle regeneration, e.g. Twist2-dependent progenitors (Liu et al., 2017), pericytes (Dellavalle et al., 2011; Dellavalle et al., 2007; Meng et al., 2011; Meng et al., 2015; Meng et al., 2016) and CD133+ (also known as PROM1+) cells (Negroni et al., 2009; Torrente et al., 2004; Meng et al., 2014; Meng et al., 2015), but failure of regeneration in the absence of satellite cells questions practical role of these additional cells in this process (Lepper et al., 2011; Sambasivan et al., 2011; Yamamoto et al., 2018).
Effect of gene mutations on satellite cell function
Skeletal muscles regenerate to different extents in different mouse dystrophy models. For example, Largemyd (also known as Large1myd) mice (Box 1) show little regeneration (Bröhl et al., 2012; Almeida et al., 2016) in contrast to the extensive regeneration in the muscles of mdx mice (Almeida et al., 2016). In the context of different genetic defects, it is difficult to determine the extent to which differences in regenerative outcome are influenced by variation in the pattern and extent of fibre degeneration. Interpretation is further complicated by the fact that the perturbation or loss of any of the various genes involved in muscle regeneration may have pleiotropic effects across a number of tissues.
If a defective gene is normally expressed in satellite cells or their progeny, then these cells can be directly affected by the genetic defect. For example, although dystrophin is an important structural protein within skeletal and cardiac muscle and brain, it is also expressed in newly activated satellite cells (Zhang and McLennan, 1994), and its lack in the mdx mouse has been demonstrated to disturb asymmetric division in mdx satellite cells ex vivo (Dumont et al., 2015), with the conjecture that this would deplete the numbers of fusion-competent satellite cells (Dumont et al., 2015) and impair muscle regeneration. However, this prediction conflicts with the fact that dystrophic muscles appear to form normally in all mammalian models of DMD and that mdx limb muscles regenerate very well in response to intrinsic myofibre necrosis [doubling their myonuclear content over the first 3 months of the disease (Duddy et al., 2015)] and in response to experimental injury, even in very old muscles (Boldrin et al., 2015). Such in vivo observations argue against any intrinsic problem with myogenesis of dystrophic myoblasts and satellite cells.
Defects in genes that are normally expressed only in the muscle fibre or connective tissue may have indirect effects on satellite cell function. For example, there is no innate defect in the proliferative ability of satellite cells in the dy/dy mouse (Box 1) model of laminin alpha-2 chain/merosin-deficient congenital muscular dystrophy (in which skeletal muscles rapidly degenerate, but regenerate poorly) when they are removed from their niche (Box 1) (Ontell et al., 1992). However, in mouse models of dystroglycanopathy (Box 1) (Ross et al., 2012) and collagen VI deficiency (Urciuolo et al., 2013), the effects of niche defects on satellite cell dysfunction have yet to be fully delineated. Collagen VI deficiency directly affects basement membrane structure, but may also indirectly effect satellite cell behaviour as a consequence of a series of degeneration/regeneration events: in each event, a myofibre undergoes necrosis and it is either repaired by satellite cells, or is replaced by a newly regenerated myofibre. A myofibre may undergo more than one of these degeneration/regeneration events. When each occurs, either a new basal lamina forms within the old one, or the old basal lamina may be removed and replaced by a newly formed basal lamina (Gulati et al., 1983; Vracko and Benditt, 1972). Furthermore, mutations in different components of the dystrophin-associated protein complex (DAPC), which spans the myofibre plasma membrane (reviewed in Gao and McNally, 2015), cause different muscular dystrophies (reviewed in Whitmore and Morgan, 2014) (Table 1). The extent to which members of the DAPC are expressed in satellite cells (Cohn et al., 2002), and whether their expression within satellite cells is an important component of satellite cell function (Dumont et al., 2015), remains unresolved.
Satellite cell defects in muscular dystrophies
Different muscular dystrophies give rise to a variety of defects within satellite cells (Table 2; see poster) (Bigot et al., 2009; Thornell et al., 2009; Beffy et al., 2010; Castets et al., 2011; Logan et al., 2011; Boyden et al., 2012; Di Gioia et al., 2017; Feichtinger et al., 2019). Skeletal muscle in different parts of the body is differentially affected by muscular dystrophies (reviewed in Randolph and Pavlath, 2015), which has been suggested to result from differences in the type and/or the function of satellite cells within different muscles. For example, extraocular muscles are spared in several muscular dystrophies, possibly due to intrinsic developmental differences between extraocular and other muscles (reviewed in McDonald et al., 2015). Furthermore, extraocular muscles contain more satellite cells than hindlimb muscles (Kallestad et al., 2011), and satellite cells from extraocular muscles have a greater proliferative and regenerative capacity than those from limb and diaphragm muscles (Stuelsatz et al., 2015). In contrast, satellite cells of limb and masseter muscle origin contribute similarly to muscle regeneration on transplant into a permissive muscle environment, despite the fact that satellite cells from EDL and masseter muscles have different gene expression profiles, and masseter satellite cells usually proliferate more and differentiate later than those from EDL (Ono et al., 2010).
Table 2.
Defects caused by the different muscular dystrophies
Satellite cell number is also affected in muscular dystrophies, although there is contradictory evidence for their loss in DMD or mdx mouse muscle (Bankolé et al., 2013; Boldrin et al., 2015; Jiang et al., 2014; Kottlors and Kirschner, 2010). The denominator used for their quantification, i.e. loss of satellite cells per myofibre, per myonucleus, per total area or per muscle fibre area, may play some part in this. Satellite cell number is higher in pharyngeal muscles than in limb muscles of oculopharyngeal muscular dystrophy patients (Gidaro et al., 2013), and some of these PAX7+ cells lie outside the satellite cell niche, suggesting a problem with the niche itself, but the implications of these higher satellite cell numbers are unclear. Recessive mutations in the protein O-glycosyltransferase 1 (POGLUT1) gene are associated with decreased Notch signalling and patients have fewer quiescent satellite cells (Servián-Morilla et al., 2016), suggesting a critical role of POGLUT1 in the maintenance of the satellite cell pool. In normal human muscle, satellite cell numbers diminish with age (Sajko et al., 2004). In muscular dystrophies, age-related decreases in satellite cell number can be compounded by the chronic pathology and may occur by different mechanisms (e.g. by proliferative exhaustion). There is a reduction in satellite cell number in a mouse model of recessive selenoprotein 1-related myopathy (Castets et al., 2011), and their numbers are also attenuated in X-linked myotubular myopathy by a combination of apoptosis and reduced proliferation (Lawlor et al., 2012).
Lastly, genetic background also has a profound effect on muscle regeneration. Skeletal muscle pathology of the gamma-sarcoglycan (Sgcg)-null mouse model (Box 1) of limb-girdle muscular dystrophy (LGMD) is worse in a DBA2/J than a 129/SVemst/J genetic background (Fukada et al., 2010; Heydemann et al., 2009). Likewise, the DBA2/J background is associated with a worse mdx pathology than the C57Bl/10 background (Fukada et al., 2010; Coley et al., 2016; van Putten et al., 2019) (Box 1). Furthermore, genetic modifiers (Box 1) in DMD and facioscapulohumeral muscular dystrophy affect membrane-associated proteins that may preserve muscle fibres against degeneration (reviewed in Hightower and Alexander, 2018).
The quality of regeneration of dystrophic skeletal muscle is subject to ongoing discussion. Regenerated muscle fibres in normal, injured and mdx mouse muscles are invariably branched (Bourke and Ontell, 1984; Ontell, 1986; Pichavant and Pavlath, 2014). There is an association between the extent of branching and the vulnerability to contraction-induced injury in older fast-twitch muscles in mdx mice (Chan et al., 2007). Myofibre branching is a major factor in the hypertrophy of mdx muscle (Faber et al., 2014), but its direct effect on muscle strength is difficult to determine.
The main messages from this research are that satellite cells are dysfunctional in many chronic muscle diseases, and that this is compounded by increasing age. There is some debate as to whether myogenic deficit in any particular case is intrinsic to the satellite cells themselves or to the influence of the environment. The concept of epigenetic switching of cell function, by signalling from extracellular sources, now blurs this distinction. Whether ageing is intrinsic to the cells or is a reflection of the cellular response to the ageing environment is under debate.
Hypertrophy of skeletal muscle
Muscle size is greatly influenced by the functional demands made on it, with atrophy being associated with disuse or underuse, while heavy use, particularly under high loads, promotes hypertrophy (Murach et al., 2017). Interestingly, although the molecular mechanisms and pathways associated with atrophy and hypertrophy are well described (reviewed in Egerman and Glass, 2014; Schiaffino et al., 2013) (see poster), the cellular mechanisms involved remain to be fully ascertained.
Conditional Cre ablation of satellite cells has led to mixed results and views on the role of satellite cell participation in the muscle growth response to overload that results from ablation of synergistic muscles (Egner et al., 2016; Lee et al., 2012; Murach et al., 2017). Among the best-described molecular mechanisms behind the control of muscle size are the insulin-like growth factor 1, transforming growth factor beta and myostatin signalling pathways (reviewed in Lee, 2004; Chen et al., 2016; Timmer et al., 2018) (see poster). Inhibition of myostatin has a dramatic effect on muscle size (Lee and McPherron, 2001), although the cellular mechanisms involved are uncertain. A number of investigations have implicated suppression of satellite cell function as a causative mechanism of myostatin action (e.g. McCroskery et al., 2003); however, studies on the myostatin-null mouse have demonstrated no evidence of this (Amthor et al., 2009; Wang and McPherron, 2012).
Potential therapies for muscular dystrophies
Because defects in regeneration play a key role in muscular dystrophies, they have widely been considered as a potential target for improvement of therapy in DMD, either by preventing or reducing myofibre necrosis (Morgan et al., 2018) and/or increasing muscle regeneration (Table 1). This line of thought has been pursued from two different angles. First, it has been proposed to use myoblast fusion during the repair of damaged muscle fibre as vectors for introducing therapeutic genes, e.g. normal copies of the mutant gene, into the repaired muscle fibres (Partridge et al., 1989). However, this approach would only be suitable for a neuromuscular disease in which there is myofibre necrosis (e.g. in DMD) and requires a high success rate of myoblast transplantation, which has not been achieved in any preclinical research projects or in the clinical trials conducted to date (reviewed in Skuk and Tremblay, 2015). The main reason for this poor grafting efficiency is the massive loss of cells within hours of their intramuscular transplantation (Beauchamp et al., 1999; Fan et al., 1996; Guerette et al., 1997; Skuk and Tremblay, 2017), the cause of which remains unexplained and is a clear target for further research. Second, ineffective muscle repair, which is a feature of DMD, at least in the later stages of the disease, is an obvious target for improvement but the causal bases of this phenomenon remain poorly understood. Because the standard mdx mouse does not reproduce this poor regenerative response, a better model of this aspect of the disease – perhaps the DBA/2J mdx mouse (van Putten et al., 2019) – is required.
Conclusions
Our knowledge of the molecular mechanisms behind muscle growth and repair has flourished in recent years but our insight into the cellular mechanisms involved in myogenic processes lags greatly behind. The fundamental model of the molecular control of myogenesis in vertebrate limb muscles via a cascade of transcription factors has largely been derived from developmental myogenesis and tissue culture models of adult myogenesis. However, the role of these proteins in the radically different in vivo conditions of neomyogenesis in the embryo and muscle fibre growth, hypertrophy and muscle regeneration in adult muscle has yet to be satisfactorily reconciled. It is possible, for instance, to make a major distinction between those occasions on which the process of satellite cell proliferation is followed by mass cell fusion, e.g. during embryonic neomyogenesis and regeneration of adult muscle, in contrast to the slower continuous proliferation of satellite cells and immediate fusion of committed daughter cells during the growth of fibres in postnatal muscle.
Satellite cells are dysfunctional in many muscular dystrophies. Defective satellite function will have to be addressed, possibly in combination with strategies targeting muscle fibres (e.g. gene therapy or exon skipping to restore dystrophin), to more fully improve muscle performance in patients.
Acknowledgments
This article is part of a special collection ‘A Guide to Using Neuromuscular Disease Models for Basic and Preclinical Studies’, which was launched in a dedicated issue guest edited by Annemieke Aartsma-Rus, Maaike van Putten and James Dowling. See related articles in this collection at http://dmm.biologists.org/collection/neuromuscular.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by Muscular Dystrophy UK (17GRO-PG36-0165 to J.M.).
At a glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dmm.biologists.org/content/13/2/dmm042192/F1.poster.jpg.
References
- Abou-Khalil R., Mounier R. and Chazaud B. (2010). Regulation of myogenic stem cell behavior by vessel cells: the “menage a trois” of satellite cells, periendothelial cells and endothelial cells. Cell Cycle 9, 892-896. 10.4161/cc.9.5.10851 [DOI] [PubMed] [Google Scholar]
- Abu-Baker A., Kharma N., Perreault J., Grant A., Shekarabi M., Maios C., Dona M., Neri C., Dion P. A., Parker A. et al. (2019). RNA-based therapy utilizing oculopharyngeal muscular dystrophy transcript knockdown and replacement. Mol. Ther. Nucleic Acids 15, 12-25. 10.1016/j.omtn.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguti S., Malerba A. and Zhou H. (2018). The progress of AAV-mediated gene therapy in neuromuscular disorders. Expert Opin Biol. Ther. 18, 681-693. 10.1080/14712598.2018.1479739 [DOI] [PubMed] [Google Scholar]
- Almeida C. F., Martins P. C. and Vainzof M. (2016). Comparative transcriptome analysis of muscular dystrophy models Large(myd), Dmd(mdx)/Large(myd) and Dmd(mdx): what makes them different? Eur. J. Hum. Genet. 24, 1301-1309. 10.1038/ejhg.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H., Otto A., Vulin A., Rochat A., Dumonceaux J., Garcia L., Mouisel E., Hourde C., Macharia R., Friedrichs M. et al. (2009). Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc. Natl. Acad. Sci. USA 106, 7479-7484. 10.1073/pnas.0811129106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast S., Beuvin M., Fraysse B., Zhou H., Muntoni F. and Ferreiro A. (2009). Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann. Neurol. 65, 677-686. 10.1002/ana.21644 [DOI] [PubMed] [Google Scholar]
- Azibani F., Brull A., Arandel L., Beuvin M., Nelson I., Jollet A., Ziat E., Prudhon B., Benkhelifa-Ziyyat S., Bitoun M. et al. (2018). Gene therapy via trans-splicing for LMNA-related congenital muscular dystrophy. Mol. Ther. Nucleic Acids 10, 376-386. 10.1016/j.omtn.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankolé L.-C., Feasson L., Ponsot E. and Kadi F. (2013). Fibre type-specific satellite cell content in two models of muscle disease. Histopathology 63, 826-832. 10.1111/his.12231 [DOI] [PubMed] [Google Scholar]
- Barthélémy F., Defour A., Lévy N., Krahn M. and Bartoli M. (2018). Muscle cells fix breaches by orchestrating a membrane repair ballet. J. Neuromuscul. Dis. 5, 21-28. 10.3233/JND-170251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. and Currie P. D. (2004). Identification of a zebrafish model of muscular dystrophy. Clin. Exp. Pharmacol. Physiol. 31, 537-540. 10.1111/j.1440-1681.2004.04030.x [DOI] [PubMed] [Google Scholar]
- Baudy A. R., Sali A., Jordan S., Kesari A., Johnston H. K., Hoffman E. P. and Nagaraju K. (2011). Non-invasive optical imaging of muscle pathology in mdx mice using cathepsin caged near-infrared imaging. Mol. Imaging Biol. 13, 462-470. 10.1007/s11307-010-0376-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J. R., Morgan J. E., Pagel C. N. and Partridge T. A. (1999). Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 144, 1113-1122. 10.1083/jcb.144.6.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffy P., Del Carratore R., Masini M., Furling D., Puymirat J., Masiello P. and Simili M. (2010). Altered signal transduction pathways and induction of autophagy in human myotonic dystrophy type 1 myoblasts. Int. J. Biochem. Cell Biol. 42, 1973-1983. 10.1016/j.biocel.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Bigot A., Klein A. F., Gasnier E., Jacquemin V., Ravassard P., Butler-Browne G., Mouly V. and Furling D. (2009). Large CTG repeats trigger p16-dependent premature senescence in myotonic dystrophy type 1 muscle precursor cells. Am. J. Pathol. 174, 1435-1442. 10.2353/ajpath.2009.080560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaveri K., Heslop L., Yu D. S., Rosenblatt J. D., Gross J. G., Partridge T. A. and Morgan J. E. (1999). Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev. Dyn. 216, 244-256. [DOI] [PubMed] [Google Scholar]
- Boldrin L., Zammit P. S. and Morgan J. E. (2015). Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. 14, 20-29. 10.1016/j.scr.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke D. L. and Ontell M. (1984). Branched myofibers in long-term whole muscle transplants: a quantitative study. Anat. Rec. 209, 281-288. 10.1002/ar.1092090304 [DOI] [PubMed] [Google Scholar]
- Boyden S. E., Mahoney L. J., Kawahara G., Myers J. A., Mitsuhashi S., Estrella E. A., Duncan A. R., Dey F., DeChene E. T., Blasko-Goehringer J. M. et al. (2012). Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics 13, 115-124. 10.1007/s10048-012-0315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröhl D., Vasyutina E., Czajkowski M. T., Griger J., Rassek C., Rahn H.-P., Purfürst B., Wende H. and Birchmeier C. (2012). Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev. Cell 23, 469-481. 10.1016/j.devcel.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Buckingham M. and Rigby P. W. (2014). Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 28, 225-238. 10.1016/j.devcel.2013.12.020 [DOI] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D. and Relaix F. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59-68. 10.1046/j.1469-7580.2003.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A. and Moore K. J. (1984). X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 81, 1189-1192. 10.1073/pnas.81.4.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnaro S., Pellegrini C., Pellegrini M., Chrisam M., Sabatelli P., Toni S., Grumati P., Ripamonti C., Pratelli L., Maraldi N. M. et al. (2016). Autophagy activation in COL6 myopathic patients by a low-protein-diet pilot trial. Autophagy 12, 2484-2495. 10.1080/15548627.2016.1231279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets P., Bertrand A. T., Beuvin M., Ferry A., Le Grand F., Castets M., Chazot G., Rederstorff M., Krol A., Lescure A. et al. (2011). Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum. Mol. Genet. 20, 694-704. 10.1093/hmg/ddq515 [DOI] [PubMed] [Google Scholar]
- Cataldi M. P., Lu P., Blaeser A. and Lu Q. L. (2018). Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat. Commun. 9, 3448 10.1038/s41467-018-05990-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S., Head S. I. and Morley J. W. (2007). Branched fibers in dystrophic mdx muscle are associated with a loss of force following lengthening contractions. Am. J. Physiol. Cell Physiol. 293, C985-C992. 10.1152/ajpcell.00128.2007 [DOI] [PubMed] [Google Scholar]
- Chandrasekharan K., Yoon J. H., Xu Y., deVries S., Camboni M., Janssen P. M., Varki A. and Martin P. T. (2010). A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci. Transl. Med. 2, 42ra54 10.1126/scitranslmed.3000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A.-C., Poron F., Authier F.-J., Dreyfus P. A. and Gherardi R. K. (2003). Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163, 1133-1143. 10.1083/jcb.200212046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Colgan T. D., Walton K. L., Gregorevic P. and Harrison C. A. (2016). The TGF-beta signalling network in muscle development, adaptation and disease. Adv. Exp. Med. Biol. 900, 97-131. 10.1007/978-3-319-27511-6_5 [DOI] [PubMed] [Google Scholar]
- Chiarini F., Evangelisti C., Cenni V., Fazio A., Paganelli F., Martelli A. M. and Lattanzi G. (2019). The cutting edge: the role of mTOR signaling in laminopathies. Int. J. Mol. Sci. 20, E847 10.3390/ijms20040847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowski S. M., Vohra R. S., Lee-McMullen B. A., Batra A., Spradlin R. A., Morales J., Forbes S., Vandenborne K., Barton E. R. and Walter G. A. (2017). Contrast-enhanced near-infrared optical imaging detects exacerbation and amelioration of murine muscular dystrophy. Mol. Imaging 16, 1536012117732439 10.1177/1536012117732439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirak S., Arechavala-Gomeza V., Guglieri M., Feng L., Torelli S., Anthony K., Abbs S., Garralda M. E., Bourke J., Wells D. J. et al. (2011). Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378, 595-605. 10.1016/S0140-6736(11)60756-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. D., Henry M. D., Michele D. E., Barresi R., Saito F., Moore S. A., Flanagan J. D., Skwarchuk M. W., Robbins M. E., Mendell J. R. et al. (2002). Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110, 639-648. 10.1016/S0092-8674(02)00907-8 [DOI] [PubMed] [Google Scholar]
- Coley W. D., Bogdanik L., Vila M. C., Yu Q., Van Der Meulen J. H., Rayavarapu S., Novak J. S., Nearing M., Quinn J. L., Saunders A. et al. (2016). Effect of genetic background on the dystrophic phenotype in mdx mice. Hum. Mol. Genet. 25, 130-145. 10.1093/hmg/ddv460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison D. D. W. and Wold B. J. (1997). Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191, 270-283. 10.1006/dbio.1997.8721 [DOI] [PubMed] [Google Scholar]
- Cousins J. C., Woodward K. J., Gross J. G., Partridge T. A. and Morgan J. E. (2004). Regeneration of skeletal muscle from transplanted immortalised myoblasts is oligoclonal. J. Cell Sci. 117, 3259-3269. 10.1242/jcs.01161 [DOI] [PubMed] [Google Scholar]
- Crist C. G., Montarras D. and Buckingham M. (2012). Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell 11, 118-126. 10.1016/j.stem.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Deconinck A. E., Rafael J. A., Skinner J. A., Brown S. C., Potter A. C., Metzinger L., Watt D. J., Dickson J. G., Tinsley J. M. and Davies K. E. (1997). Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717-727. 10.1016/S0092-8674(00)80532-2 [DOI] [PubMed] [Google Scholar]
- Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B. G., Messina G., Morosetti R. et al. (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255-267. 10.1038/ncb1542 [DOI] [PubMed] [Google Scholar]
- Dellavalle A., Maroli G., Covarello D., Azzoni E., Innocenzi A., Perani L., Antonini S., Sambasivan R., Brunelli S., Tajbakhsh S. et al. (2011). Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2, 499 10.1038/ncomms1508 [DOI] [PubMed] [Google Scholar]
- Di Gioia S. A., Connors S., Matsunami N., Cannavino J., Rose M. F., Gilette N. M., Artoni P., de Macena Sobreira N. L., Chan W. M., Webb B. D. et al. (2017). A defect in myoblast fusion underlies Carey-Fineman-Ziter syndrome. Nat. Commun. 8, 16077 10.1038/ncomms16077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy W., Duguez S., Johnston H., Cohen T. V., Phadke A., Gordish-Dressman H., Nagaraju K., Gnocchi V., Low S. and Partridge T. (2015). Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet Muscle 5, 16 10.1186/s13395-015-0041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont N. A., Wang Y. X., von Maltzahn J., Pasut A., Bentzinger C. F., Brun C. E. and Rudnicki M. A. (2015). Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21, 1455-1463. 10.1038/nm.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F., Mouly V., Barbet J. P. and Butler-Browne G. S. (1999). The four populations of myoblasts involved in human limb muscle formation are present from the onset of primary myotube formation. J. Cell Sci. 112, 191-199. [DOI] [PubMed] [Google Scholar]
- Egerman M. A. and Glass D. J. (2014). Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 49, 59-68. 10.3109/10409238.2013.857291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner I. M., Bruusgaard J. C. and Gundersen K. (2016). Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 143, 2898-2906. 10.1242/dev.134411 [DOI] [PubMed] [Google Scholar]
- Eren I., Ersen A., Birsel O., Atalar A. C., Oflazer P. and Demirhan M. (2019). Functional outcomes and complications following scapulothoracic arthrodesis in patients with facioscapulohumeral dystrophy. J. Bone Joint Surg. Am. [Epub ahead of print] 10.2106/JBJS.19.00571 [DOI] [PubMed] [Google Scholar]
- Faber R. M., Hall J. K., Chamberlain J. S. and Banks G. B. (2014). Myofiber branching rather than myofiber hyperplasia contributes to muscle hypertrophy in mdx mice. Skelet Muscle 4, 10 10.1186/2044-5040-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Maley M., Beilharz M. and Grounds M. (1996). Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve 19, 853-860. [DOI] [PubMed] [Google Scholar]
- Feichtinger R. G., Mucha B. E., Hengel H., Orfi Z., Makowski C., Dort J., D'Anjou G., Nguyen T. T. M., Buchert R., Juenger H. et al. (2019). Biallelic variants in the transcription factor PAX7 are a new genetic cause of myopathy. Genet. Med. 21, 2521-2531. 10.1038/s41436-019-0532-z [DOI] [PubMed] [Google Scholar]
- Filareto A., Maguire-Nguyen K., Gan Q., Aldanondo G., Machado L., Chamberlain J. S. and Rando T. A. (2018). Monitoring disease activity noninvasively in the mdx model of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 115, 7741-7746. 10.1073/pnas.1802425115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. S., Kirby T. J., Kosmac K., McCarthy J. J. and Peterson C. A. (2017). Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20, 56-69. 10.1016/j.stem.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada S., Morikawa D., Yamamoto Y., Yoshida T., Sumie N., Yamaguchi M., Ito T., Miyagoe-Suzuki Y., Takeda S., Tsujikawa K. et al. (2010). Genetic background affects properties of satellite cells and mdx phenotypes. Am. J. Pathol. 176, 2414-2424. 10.2353/ajpath.2010.090887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q. Q. and McNally E. M. (2015). The dystrophin complex: structure, function, and implications for therapy. Compr. Physiol. 5, 1223-1239. 10.1002/cphy.c140048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidaro T., Negroni E., Perié S., Mirabella M., Laine J., Lacau St Guily J., Butler-Browne G., Mouly V. and Trollet C. (2013). Atrophy, fibrosis, and increased PAX7-positive cells in pharyngeal muscles of oculopharyngeal muscular dystrophy patients. J. Neuropathol. Exp. Neurol. 72, 234-243. 10.1097/NEN.0b013e3182854c07 [DOI] [PubMed] [Google Scholar]
- Gieseler K., Grisoni K. and Segalat L. (2000). Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans. Curr. Biol. 10, 1092-1097. 10.1016/S0960-9822(00)00691-6 [DOI] [PubMed] [Google Scholar]
- Goody M. F., Carter E. V., Kilroy E. A., Maves L. and Henry C. A. (2017). “Muscling” throughout life: integrating studies of muscle development, homeostasis, and disease in zebrafish. Curr. Top. Dev. Biol. 124, 197-234. 10.1016/bs.ctdb.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds M. D. and Shavlakadze T. (2011). Growing muscle has different sarcolemmal properties from adult muscle: a proposal with scientific and clinical implications: reasons to reassess skeletal muscle molecular dynamics, cellular responses and suitability of experimental models of muscle disorders. BioEssays 33, 458-468. 10.1002/bies.201000136 [DOI] [PubMed] [Google Scholar]
- Guerette B., Skuk D., Celestin F., Huard C., Tardif F., Asselin I., Roy B., Goulet M., Roy R., Entman M. et al. (1997). Prevention by anti-LFA-1 of acute myoblast death following transplantation. J. Immunol. 159, 2522-2531. [PubMed] [Google Scholar]
- Gulati A. K., Reddi A. H. and Zalewski A. A. (1983). Changes in the basement membrane zone components during skeletal muscle fiber degeneration and regeneration. J. Cell Biol. 97, 957-962. 10.1083/jcb.97.4.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunage R. D., Dhanyasi N., Reichert H. and VijayRaghavan K. (2017). Drosophila adult muscle development and regeneration. Semin. Cell Dev. Biol. 72, 56-66. 10.1016/j.semcdb.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Gutmann E. and Carlson B. M. (1976). Regeneration and transplantation of muscles in old rats and between young and old rats. Life Sci. 18, 109-114. 10.1016/0024-3205(76)90280-0 [DOI] [PubMed] [Google Scholar]
- Hewitt J. E., Pollard A. K., Lesanpezeshki L., Deane C. S., Gaffney C. J., Etheridge T., Szewczyk N. J. and Vanapalli S. A. (2018). Muscle strength deficiency and mitochondrial dysfunction in a muscular dystrophy model of Caenorhabditis elegans and its functional response to drugs. Dis. Model. Mech. 11, dmm036137 10.1242/dmm.036137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydemann A., Ceco E., Lim J. E., Hadhazy M., Ryder P., Moran J. L., Beier D. R., Palmer A. A. and McNally E. M. (2009). Latent TGF-β-binding protein 4 modifies muscular dystrophy in mice. J. Clin. Invest. 119, 3703-3712. 10.1172/JCI39845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. M. and Alexander M. S. (2018). Genetic modifiers of Duchenne and facioscapulohumeral muscular dystrophies. Muscle Nerve 57, 6-15. 10.1002/mus.25953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth M. W., Defour A., Lazarski C., Gallardo E., Diaz Manera J., Partridge T. A., Nagaraju K. and Jaiswal J. K. (2019). Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B. Nat. Commun. 10, 2430 10.1038/s41467-019-10438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A., Van der Meulen J. H., Defour A., Hogarth M., Sreetama S. C., Reed A., Scheffer L., Chandel N. S. and Jaiswal J. K. (2017). Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal. 10, eaaj1978 10.1126/scisignal.aaj1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Wen Y., Kuroda K., Hannon K., Rudnicki M. A. and Kuang S. (2014). Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis. Model. Mech. 7, 997-1004. 10.1242/dmm.015917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe A. W. B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A. and Rossi F. M. V. (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153-163. 10.1038/ncb2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallestad K. M., Hebert S. L., McDonald A. A., Daniel M. L., Cu S. R. and McLoon L. K. (2011). Sparing of extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis. Exp. Cell Res. 317, 873-885. 10.1016/j.yexcr.2011.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner S., Elias M. C., Rivera A. J. and Yablonka-Reuveni Z. (2000). Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J. Histochem. Cytochem. 48, 1079-1096. 10.1177/002215540004800805 [DOI] [PubMed] [Google Scholar]
- Kharraz Y., Guerra J., Mann C. J., Serrano A. L. and Muñoz-Cánoves P. (2013). Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013, 491497 10.1155/2013/491497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny P., Selma-Soriano E., Rapisarda A. S., Fernandez-Costa J. M., Perez-Alonso M. and Artero R. (2017). Myotonic dystrophy: candidate small molecule therapeutics. Drug Discov. Today 22, 1740-1748. 10.1016/j.drudis.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Kornegay J. N. (2017). The golden retriever model of Duchenne muscular dystrophy. Skelet Muscle 7, 9 10.1186/s13395-017-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottlors M. and Kirschner J. (2010). Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res. 340, 541-548. 10.1007/s00441-010-0976-6 [DOI] [PubMed] [Google Scholar]
- Krause M. and Liu J. (2012). Somatic muscle specification during embryonic and post-embryonic development in the nematode C. elegans. Wiley Interdiscip Rev. Dev. Biol. 1, 203-214. 10.1002/wdev.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher T., Lafoux A., Tesson L., Remy S., Thepenier V., Francois V., Le Guiner C., Goubin H., Dutilleul M., Guigand L. et al. (2014). Characterization of dystrophin deficient rats: a new model for Duchenne muscular dystrophy. PLoS ONE 9, e110371 10.1371/journal.pone.0110371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor M. W., Alexander M. S., Viola M. G., Meng H., Joubert R., Gupta V., Motohashi N., Manfready R. A., Hsu C. P., Huang P. et al. (2012). Myotubularin-deficient myoblasts display increased apoptosis, delayed proliferation, and poor cell engraftment. Am. J. Pathol. 181, 961-968. 10.1016/j.ajpath.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-J. (2004). Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61-86. 10.1146/annurev.cellbio.20.012103.135836 [DOI] [PubMed] [Google Scholar]
- Lee S.-J. and McPherron A. C. (2001). Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. USA 98, 9306-9311. 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-J., Huynh T. V., Lee Y.-S., Sebald S. M., Wilcox-Adelman S. A., Iwamori N., Lepper C., Matzuk M. M. and Fan C.-M. (2012). Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc. Natl. Acad. Sci. USA 109, E2353-E2360. 10.1073/pnas.1206410109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S. J., Harris J., Bate M., Vijayraghavan K., Fisher L., Tajbakhsh S. and Duxson M. (2013). Initiation of primary myogenesis in amniote limb muscles. Dev. Dyn. 242, 1043-1055. 10.1002/dvdy.23998 [DOI] [PubMed] [Google Scholar]
- Lepper C., Partridge T. A. and Fan C.-M. (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639-3646. 10.1242/dev.067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Garry G. A., Li S., Bezprozvannaya S., Sanchez-Ortiz E., Chen B., Shelton J. M., Jaichander P., Bassel-Duby R. and Olson E. N. (2017). A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 19, 202-213. 10.1038/ncb3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E. and Taylor J. P. (2010). Flightless flies: Drosophila models of neuromuscular disease. Ann. N. Y. Acad. Sci. 1184, e1-e20. 10.1111/j.1749-6632.2010.05432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. V., Lucke B., Pottinger C., Abdelhamed Z. A., Parry D. A., Szymanska K., Diggle C. P., van Riesen A., Morgan J. E., Markham G. et al. (2011). Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD). Nat. Genet. 43, 1189-1192. 10.1038/ng.995 [DOI] [PubMed] [Google Scholar]
- Mackey A. L., Magnan M., Chazaud B. and Kjaer M. (2017). Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J. Physiol. 595, 5115-5127. 10.1113/JP273997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher J., Heidt L., Goyenvalle A., Escobar H., Marg A., Beley C., Benchaouir R., Bader M., Spuler S., Garcia L. et al. (2018). Exon skipping in a Dysf-missense mutant mouse model. Mol. Ther. Nucleic Acids 13, 198-207. 10.1016/j.omtn.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba A., Roth F., Harish P., Dhiab J., Lu-Nguyen N., Cappellari O., Jarmin S., Mahoudeau A., Ythier V., Laine J. et al. (2019). Pharmacological modulation of the ER stress response ameliorates oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 28, 1694-1708. 10.1093/hmg/ddz007 [DOI] [PubMed] [Google Scholar]
- Martinez L., Ermolova N. V., Ishikawa T.-O., Stout D. B., Herschman H. R. and Spencer M. J. (2015). A reporter mouse for optical imaging of inflammation in mdx muscles. Skelet Muscle 5, 15 10.1186/s13395-015-0042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S., Thomas M., Maxwell L., Sharma M. and Kambadur R. (2003). Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135-1147. 10.1083/jcb.200207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A. A., Hebert S. L. and McLoon L. K. (2015). Sparing of the extraocular muscles in mdx mice with absent or reduced utrophin expression: a life span analysis. Neuromuscul. Disord. 25, 873-887. 10.1016/j.nmd.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney L. A., Kablar B., Garrett K., Anderson J. E. and Rudnicki M. A. (1996). MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10, 1173-1183. 10.1101/gad.10.10.1173 [DOI] [PubMed] [Google Scholar]
- Meinen S., Lin S., Thurnherr R., Erb M., Meier T. and Rüegg M. A. (2011). Apoptosis inhibitors and mini-agrin have additive benefits in congenital muscular dystrophy mice. EMBO Mol. Med. 3, 465-479. 10.1002/emmm.201100151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Adkin C. F., Xu S.-W., Muntoni F. and Morgan J. E. (2011). Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS ONE 6, e17454 10.1371/journal.pone.0017454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Chun S., Asfahani R., Lochmuller H., Muntoni F. and Morgan J. (2014). Human skeletal muscle-derived CD133 cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol. Ther. 22, 1008-1017. 10.1038/mt.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Bencze M., Asfahani R., Muntoni F. and Morgan J. E. (2015). The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet Muscle 5, 11 10.1186/s13395-015-0036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Counsell J. R., Reza M., Laval S. H., Danos O., Thrasher A., Lochmüller H., Muntoni F. and Morgan J. E. (2016). Autologous skeletal muscle derived cells expressing a novel functional dystrophin provide a potential therapy for Duchenne muscular dystrophy. Sci. Rep. 6, 19750 10.1038/srep19750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A. and Lemischka I. R. (2006). Stem cells and their niches. Science 311, 1880-1885. 10.1126/science.1110542 [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Prola A., Mariot V., Pini V., Meng J., Hourde C., Dumonceaux J., Conti F., Relaix F., Authier F. J. et al. (2018). Necroptosis mediates myofibre death in dystrophin-deficient mice. Nat. Commun. 9, 3655 10.1038/s41467-018-06057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R., Chrétien F. and Chazaud B. (2011). Blood vessels and the satellite cell niche. Curr. Top. Dev. Biol. 96, 121-138. 10.1016/B978-0-12-385940-2.00005-X [DOI] [PubMed] [Google Scholar]
- Murach K. A., White S. H., Wen Y., Ho A., Dupont-Versteegden E. E., McCarthy J. J. and Peterson C. A. (2017). Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle 7, 14 10.1186/s13395-017-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. M., Lawson J. A., Mathew S. J., Hutcheson D. A. and Kardon G. (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625-3637. 10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Fujii W., Tsuboi M., Tanihata J., Teramoto N., Takeuchi S., Naito K., Yamanouchi K. and Nishihara M. (2014). Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci. Rep. 4, 5635 10.1038/srep05635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni E., Riederer I., Chaouch S., Belicchi M., Razini P., Di Santo J., Torrente Y., Butler-Browne G. S. and Mouly V. (2009). In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol. Ther. 17, 1771-1778. 10.1038/mt.2009.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Banks G. B., Hall J. K., Muir L. A., Ramos J. N., Wicki J., Odom G. L., Konieczny P., Seto J., Chamberlain J. R. et al. (2012). Animal models of muscular dystrophy. Prog. Mol. Biol. Transl. Sci. 105, 83-111. 10.1016/B978-0-12-394596-9.00004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C.-T. E. and Campbell C. (2016). Myotonic dystrophy type 1. CMAJ 188, 1033 10.1503/cmaj.151384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Boldrin L., Knopp P., Morgan J. E. and Zammit P. S. (2010). Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 337, 29-41. 10.1016/j.ydbio.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell M. (1986). Morphological aspects of muscle fiber regeneration. Fed. Proc. 45, 1461-1465. [PubMed] [Google Scholar]
- Ontell M. P., Hughes D., Hauschka S. D. and Ontell M. (1992). Transient neonatal denervation alters the proliferative capacity of myosatellite cells in dystrophic (129ReJdy/dy) muscle. J. Neurobiol. 23, 407-419. 10.1002/neu.480230407 [DOI] [PubMed] [Google Scholar]
- Overby S. J., Cerro-Herreros E., Llamusi B. and Artero R. (2018). RNA-mediated therapies in myotonic dystrophy. Drug Discov. Today 23, 2013-2022. 10.1016/j.drudis.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Palma E., Tiepolo T., Angelin A., Sabatelli P., Maraldi N. M., Basso E., Forte M. A., Bernardi P. and Bonaldo P. (2009). Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum. Mol. Genet. 18, 2024-2031. 10.1093/hmg/ddp126 [DOI] [PubMed] [Google Scholar]
- Partridge T. A. (2013). The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 280, 4177-4186. 10.1111/febs.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A. and Morgan J. E. (2014). Multiple insights from myogenic cell transplants. Hum. Gene. Ther. 25, 404-405. 10.1089/hum.2014.035 [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Grounds M. and Sloper J. C. (1978). Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature 273, 306-308. 10.1038/273306a0 [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P. and Kunkel L. M. (1989). Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337, 176-179. 10.1038/337176a0 [DOI] [PubMed] [Google Scholar]
- Perié S., Trollet C., Mouly V., Vanneaux V., Mamchaoui K., Bouazza B., Marolleau J. P., Laforêt P., Chapon F., Eymard B. et al. (2014). Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol. Ther. 22, 219-225. 10.1038/mt.2013.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perleberg C., Kind A. and Schnieke A. (2018). Genetically engineered pigs as models for human disease. Dis. Model. Mech. 11, dmm030783 10.1242/dmm.030783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichavant C. and Pavlath G. K. (2014). Incidence and severity of myofiber branching with regeneration and aging. Skelet Muscle 4, 9 10.1186/2044-5040-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgai E. R., Griffin D. A., Heller K. N., Mendell J. R. and Rodino-Klapac L. R. (2017). Systemic AAV-mediated β-sarcoglycan delivery targeting cardiac and skeletal muscle ameliorates histological and functional deficits in LGMD2E mice. Mol. Ther. 25, 855-869. 10.1016/j.ymthe.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph M. E. and Pavlath G. K. (2015). A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups. Front. Aging Neurosci. 7, 190 10.3389/fnagi.2015.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J. R., Lin S., McKee K. K., Meinen S., Crosson S. C., Sury M., Hobbs S., Maier G., Yurchenco P. D. and Ruegg M. A. (2017). Linker proteins restore basement membrane and correct LAMA2-related muscular dystrophy in mice. Sci. Transl. Med. 9, eaal4649 10.1126/scitranslmed.aal4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T. A., Grounds M. D., Mitchell C. A. and Papadimitriou J. M. (1990). Fusion between myogenic cells in vivo: an ultrastructural study in regenerating murine skeletal muscle. J. Struct. Biol. 105, 170-182. 10.1016/1047-8477(90)90111-O [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Echigoya Y., Fukada S. I. and Yokota T. (2016). Current translational research and murine models for duchenne muscular dystrophy. J. Neuromuscul. Dis. 3, 29-48. 10.3233/JND-150113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Benn A., Jonuschies J., Boldrin L., Muntoni F., Hewitt J. E., Brown S. C. and Morgan J. E. (2012). Defects in glycosylation impair satellite stem cell function and niche composition in the muscles of the dystrophic large(myd) mouse. Stem Cells 30, 2330-2341. 10.1002/stem.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A., Mourkioti F., Tran R., Choi J., Llewellyn M., Kraft P., Shkreli M., Delp S., Pomerantz J. H., Artandi S. E. et al. (2010). Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059-1071. 10.1016/j.cell.2010.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M., Cuvellier S., Magnan M., Mounier R. and Chazaud B. (2013a). Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 280, 4118-4130. 10.1111/febs.12166 [DOI] [PubMed] [Google Scholar]
- Saclier M., Yacoub-Youssef H., Mackey A. L., Arnold L., Ardjoune H., Magnan M., Sailhan F., Chelly J., Pavlath G. K., Mounier R. et al. (2013b). Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31, 384-396. 10.1002/stem.1288 [DOI] [PubMed] [Google Scholar]
- Saha M., Rizzo S. A., Ramanathan M., Hightower R. M., Santostefano K. E., Terada N., Finkel R. S., Berg J. S., Chahin N., Pacak C. A. et al. (2019). Selective serotonin reuptake inhibitors ameliorate MEGF10 myopathy. Hum. Mol. Genet. 28, 2365-2377. 10.1093/hmg/ddz064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajko S., Kubinova L., Cvetko E., Kreft M., Wernig A. and Eržen I. (2004). Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J. Histochem. Cytochem. 52, 179-185. 10.1177/002215540405200205 [DOI] [PubMed] [Google Scholar]
- Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S. and Galy A. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647-3656. 10.1242/dev.067587 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B. and Sandri M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294-4314. 10.1111/febs.12253 [DOI] [PubMed] [Google Scholar]
- Segalat L. (2002). Dystrophin and functionally related proteins in the nematode Caenorhabditis elegans. Neuromuscul. Disord. 12 Suppl. 1, S105-S109. 10.1016/S0960-8966(02)00090-1 [DOI] [PubMed] [Google Scholar]
- Selvaraj S., Dhoke N. R., Kiley J., Mateos-Aierdi A. J., Tungtur S., Mondragon-Gonzalez R., Killeen G., Oliveira V. K. P., Lopez de Munain A. and Perlingeiro R. C. R. (2019). Gene correction of LGMD2A patient-specific iPSCs for the development of targeted autologous cell therapy. Mol. Ther. 27, 2147-2157. 10.1016/j.ymthe.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow Y., Sibley C. R. and Wood M. J. A. (2012). Artificial mirtron-mediated gene knockdown: functional DMPK silencing in mammalian cells. RNA 18, 1328-1337. 10.1261/rna.030601.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servián-Morilla E., Takeuchi H., Lee T. V., Clarimon J., Mavillard F., Area-Gomez E., Rivas E., Nieto-Gonzalez J. L., Rivero M. C., Cabrera-Serrano M. et al. (2016). A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Mol. Med. 8, 1289-1309. 10.15252/emmm.201505815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D. and Tremblay J. P. (2015). Cell therapy in muscular dystrophies: many promises in mice and dogs, few facts in patients. Expert Opin Biol. Ther. 15, 1307-1319. 10.1517/14712598.2015.1057564 [DOI] [PubMed] [Google Scholar]
- Skuk D. and Tremblay J. P. (2017). Cell therapy in myology: dynamics of muscle precursor cell death after intramuscular administration in non-human primates. Mol. Ther. Methods Clin. Dev. 5, 232-240. 10.1016/j.omtm.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. R. and Barton E. R. (2018). Regulation of fibrosis in muscular dystrophy. Matrix Biol. 68-69, 602-615. 10.1016/j.matbio.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow M. H. (1978). An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 186, 535-540. 10.1007/BF00224941 [DOI] [PubMed] [Google Scholar]
- Soheili T., Gicquel E., Poupiot J., N'Guyen L., Le Roy F., Bartoli M. and Richard I. (2012). Rescue of sarcoglycan mutations by inhibition of endoplasmic reticulum quality control is associated with minimal structural modifications. Hum. Mutat. 33, 429-439. 10.1002/humu.21659 [DOI] [PubMed] [Google Scholar]
- Sreetama S. C., Chandra G., Van der Meulen J. H., Ahmad M. M., Suzuki P., Bhuvanendran S., Nagaraju K., Hoffman E. P. and Jaiswal J. K. (2018). Membrane stabilization by modified steroid offers a potential therapy for muscular dystrophy due to dysferlin deficit. Mol. Ther. 26, 2231-2242. 10.1016/j.ymthe.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelsatz P., Shearer A., Li Y., Muir L. A., Ieronimakis N., Shen Q. W., Kirillova I. and Yablonka-Reuveni Z. (2015). Extraocular muscle satellite cells are high performance myo-engines retaining efficient regenerative capacity in dystrophin deficiency. Dev. Biol. 397, 31-44. 10.1016/j.ydbio.2014.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasfaout H., Cowling B. S. and Laporte J. (2018). Centronuclear myopathies under attack: a plethora of therapeutic targets. J. Neuromuscul. Dis. 5, 387-406. 10.3233/JND-180309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell L. E., Lindstom M., Renault V., Klein A., Mouly V., Ansved T., Butler-Browne G. and Furling D. (2009). Satellite cell dysfunction contributes to the progressive muscle atrophy in myotonic dystrophy type 1. Neuropathol. Appl. Neurobiol. 35, 603-613. 10.1111/j.1365-2990.2009.01014.x [DOI] [PubMed] [Google Scholar]
- Tidball J. G. (2017). Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17, 165-178. 10.1038/nri.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G. and Villalta S. A. (2010). Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173-R1187. 10.1152/ajpregu.00735.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G., Dorshkind K. and Wehling-Henricks M. (2014). Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 141, 1184-1196. 10.1242/dev.098285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M. T., Stec M. J., Rulands S., Simons B. D. and Sacco A. (2018a). Muscle stem cells exhibit distinct clonal dynamics in response to tissue repair and homeostatic aging. Cell Stem Cell 22, 119-127.e3. 10.1016/j.stem.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M. T., Stec M. J. and Sacco A. (2018b). Assessing muscle stem cell clonal complexity during aging. Methods Mol. Biol.. 2045, 1-11. 10.1007/7651_2018_139 [DOI] [PubMed] [Google Scholar]
- Timmer L. T., Hoogaars W. M. H. and Jaspers R. T. (2018). The role of IGF-1 signaling in skeletal muscle atrophy. Adv. Exp. Med. Biol. 1088, 109-137. 10.1007/978-981-13-1435-3_6 [DOI] [PubMed] [Google Scholar]
- Torrente Y., Belicchi M., Sampaolesi M., Pisati F., Meregalli M., D'Antona G., Tonlorenzi R., Porretti L., Gavina M., Mamchaoui K. et al. (2004). Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 114, 182-195. 10.1172/JCI20325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M. M., Miyagoe-Suzuki Y. et al. (2011). Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 124, 3654-3664. 10.1242/jcs.086629 [DOI] [PubMed] [Google Scholar]
- Urciuolo A., Quarta M., Morbidoni V., Gattazzo F., Molon S., Grumati P., Montemurro F., Tedesco F. S., Blaauw B., Cossu G. et al. (2013). Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 4, 1964 10.1038/ncomms2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten M., Putker K., Overzier M., Adamzek W. A., Pasteuning-Vuhman S., Plomp J. J. and Aartsma-Rus A. (2019). Natural disease history of the D2-mdx mouse model for Duchenne muscular dystrophy. FASEB J. 33, 8110-8124. 10.1096/fj.201802488R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannoy C. H., Leroy V. and Lu Q. L. (2018). Dose-dependent effects of FKRP gene-replacement therapy on functional rescue and longevity in dystrophic mice. Mol. Ther. Methods Clin. Dev. 11, 106-120. 10.1016/j.omtm.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M., Asakura Y., Murakonda B. S. R., Pengo T., Latroche C., Chazaud B., McLoon L. K. and Asakura A. (2018). Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell 23, 530-543.e9. 10.1016/j.stem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R. and Benditt E. P. (1972). Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J. Cell Biol. 55, 406-419. 10.1083/jcb.55.2.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L. M., Saad N. Y., Pyne N. K., Fowler A. M., Eidahl J. O., Domire J. S., Griffin D. A., Herman A. C., Sahenk Z., Rodino-Klapac L. R. et al. (2018). Pre-clinical safety and off-target studies to support translation of AAV-mediated RNAi therapy for FSHD. Mol. Ther. Methods Clin. Dev. 8, 121-130. 10.1016/j.omtm.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley G. L., Arechavala-Gomeza V., Fernandez-Fuente M., Burke M. M., Nagel N., Holder A., Stanley R., Chandler K., Marks S. L., Muntoni F. et al. (2010). A duchenne muscular dystrophy gene hot spot mutation in dystrophin-deficient cavalier king charles spaniels is amenable to exon 51 skipping. PLoS ONE 5, e8647 10.1371/journal.pone.0008647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. and McPherron A. C. (2012). Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J. Physiol. 590, 2151-2165. 10.1113/jphysiol.2011.226001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. T., Manor U., Lippincott-Schwartz J. and Fan C. M. (2016). Intravital imaging reveals ghost fibers as architectural units guiding myogenic progenitors during regeneration. Cell Stem Cell 18, 243-252. 10.1016/j.stem.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. J. (2018). Tracking progress: an update on animal models for Duchenne muscular dystrophy. Dis. Model. Mech. 11, dmm035774 10.1242/dmm.035774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. B., Bierinx A. S., Gnocchi V. F. and Zammit P. S. (2010). Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev. Biol. 10, 21 10.1186/1471-213X-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore C. and Morgan J. (2014). What do mouse models of muscular dystrophy tell us about the DAPC and its components? Int. J. Exp. Pathol. 95, 365-377. 10.1111/iep.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M. N. and Rando T. A. (2018). A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev. Cell 46, 135-143. 10.1016/j.devcel.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Legendre N. P., Biswas A. A., Lawton A., Yamamoto S., Tajbakhsh S., Kardon G. and Goldhamer D. J. (2018). Loss of MyoD and Myf5 in skeletal muscle stem cells results in altered myogenic programming and failed regeneration. Stem Cell Reports 10, 956-969. 10.1016/j.stemcr.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Bao B., Echigoya Y. and Yokota T. (2015). Dystrophin-deficient large animal models: translational research and exon skipping. Am. J. Transl. Res. 7, 1314-1331. [PMC free article] [PubMed] [Google Scholar]
- Yu H. H., Zhao H., Qing Y. B., Pan W. R., Jia B. Y., Zhao H. Y., Huang X. X. and Wei H. J. (2016). Porcine zygote injection with Cas9/sgRNA results in DMD-modified pig with muscle dystrophy. Int. J. Mol. Sci. 17, E1668 10.3390/ijms17101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel N., Chang A. C., Day J. W., Rosenthal N. and Blau H. M. (2018). Humanizing the mdx mouse model of DMD: the long and the short of it. NPJ Regen. Med. 3, 4 10.1038/s41536-018-0045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P. S., Golding J. P., Nagata Y., Hudon V., Partridge T. A. and Beauchamp J. R. (2004). Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166, 347-357. 10.1083/jcb.200312007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. and McLennan I. S. (1994). Use of antibodies to identify satellite cells with a light microscope. Muscle Nerve. 17, 987-994. 10.1002/mus.880170905 [DOI] [PubMed] [Google Scholar]