ABSTRACT
Gap junctions are intercellular channels between cells that facilitate cell–cell communication. Connexin 43 (Cx43; also known as GJA1), the predominant gap junction protein in vertebrates, is expressed in premigratory cranial neural crest cells and is maintained throughout the neural crest cell epithelial-to-mesenchymal transition (EMT), but its function in these cells is unknown. To this end, we used a combination of in vivo and ex vivo experiments to assess gap junction formation, and Cx43 function, in chick cranial neural crest cells. Our results demonstrate that gap junctions exist between premigratory and migratory cranial neural crest cells and depend on Cx43 for their function. In the embryo, Cx43 knockdown just prior to EMT delays the emergence of Cx43-depleted neural crest cells from the neural tube, but these cells eventually successfully emigrate and join the migratory stream. This delay can be rescued by introduction of full-length Cx43 into Cx43-depleted cells. Furthermore, Cx43 depletion reduces the size of the premigratory neural crest cell domain through an early effect on neural crest cell specification. Collectively, these data identify new roles for Cx43 in chick cranial neural crest cell development.
KEY WORDS: Connexin 43, Neural crest, Epithelial-to-mesenchymal transition, Gap junction
Summary: Neural crest cells are coupled by Cx43-containing gap junctions. Cx43 knockdown in the chick reduces the premigratory neural crest cell population and delays the emergence of these cells from the neural tube.
INTRODUCTION
Connexins are integral building blocks of gap junctions (Li et al., 2002; Laird, 2014; Sorgen et al., 2018), which are intercellular channels that permit diffusion of small (<1 kDa) molecules and ions between cells (Goodenough et al., 1996; Mese et al., 2007; Abbaci et al., 2008; Defranco et al., 2008; Goodenough and Paul, 2009; Delmar et al., 2018). Connexin proteins have four membrane spanning domains, two extracellular loops, one intracellular loop, and intracellular N- and C-termini (Mese et al., 2007; Abbaci et al., 2008; Defranco et al., 2008; Delmar et al., 2018). Following their synthesis, six connexin proteins are assembled into a hemichannel, also known as a connexon, which can be either homomeric or heteromeric (Goodenough et al., 1996; Mese et al., 2007; Abbaci et al., 2008; Defranco et al., 2008; Goodenough and Paul, 2009; Delmar et al., 2018; Solan and Lampe, 2018). To form a gap junction, hemichannels are trafficked to the cell membrane where they dock head-to-head with a hemichannel or connexon on an adjacent cell, in a homomeric or heteromeric manner, with the newly formed gap junction possessing a half-life of only a few hours (Mese et al., 2007; Dbouk et al., 2009; Goodenough and Paul, 2009). Notably, connexin composition endows the gap junction with distinct properties, including pore size, voltage-dependent gating, open probability and permeability (Vogel and Weingart, 2002; Kanaporis et al., 2011). More recently, connexin proteins have been discovered to have gap junction-independent roles, including binding and organizing cytoskeletal and signaling components as well as regulating junctional conductance and voltage sensitivity (Dbouk et al., 2009; Delmar et al., 2018; Sorgen et al., 2018). Not surprisingly, connexins have been implicated in many human diseases involving a variety of tissues, including cancer metastasis, although cancer-preventative roles have also been ascribed to gap junctions in certain tissue types, highlighting the dynamic nature of gap junction interactions and function (Davy et al., 2006; Laird, 2014; Delmar et al., 2018; Cooreman et al., 2019; Waning et al., 2019; Wu and Wang, 2019).
In humans, 21 connexins have been identified and there are at least 20 in mice (Li et al., 2002; Söhl and Willecke, 2003; Laird, 2014) and, of these, connexin 43 (Cx43; also known as GJA1) is the most abundant and the best studied (Goodenough et al., 1996; Laird, 2014; Delmar et al., 2018; Sorgen et al., 2018; Wu and Wang, 2019). Our recent work revealed that Cx43 is expressed in chick cranial neural crest cells, including both prior to and during their epithelial-to-mesenchymal transition (EMT), and their subsequent migration (Jourdeuil and Taneyhill, 2018). Neural crest cells are a multipotent cell population induced during early embryogenesis within the neural plate border, a region between the neural and non-neural ectoderm (Donoghue et al., 2008; Sauka-Spengler and Bronner-Fraser, 2008; Gammill and Roffers-Agarwal, 2010; Bronner, 2012; Bronner and LeDouarin, 2012; Ivashkin and Adameyko, 2013; Simoes-Costa and Bronner, 2013). During neurulation, premigratory neural crest cells, which lie at the dorsal aspect of the neural folds, undergo EMT, delaminate and migrate into the periphery, where they give rise to a large number of derivatives including intramembranous bones, satellite glial cells, melanocytes, smooth muscle, adipocytes and odontoblasts (Sauka-Spengler and Bronner-Fraser, 2008; Bronner, 2012; Bronner and LeDouarin, 2012; Ishii et al., 2012; Prasad et al., 2012; Stuhlmiller and García-Castro, 2012; Ivashkin and Adameyko, 2013; Simoes-Costa and Bronner, 2013). While Cx43 (and gap junctions) has been identified in human, Xenopus, and mouse migratory neural crest cells (Reaume et al., 1995; Ewart et al., 1997; Lo et al., 1997; Huang et al., 1998; Waldo et al., 1999; Xu et al., 2001, 2006; Li et al., 2002; Rhee et al., 2009; Francis et al., 2011; Kotini et al., 2018), the presence of functional gap junctions in the premigratory cranial neural crest cell population, however, remains unknown. Moreover, nearly 80 distinct mutations in Cx43 gives rise to a multisystem developmental disorder called oculodentodigital dysplasia (ODDD), which is characterized by defects in neural crest-derived craniofacial bones (Paznekas et al., 2003, 2009; Laird, 2014; Delmar et al., 2018). Gap junction-related disorders of the peripheral nervous system have also been identified, which, in the craniofacial region, are derived from neural crest and placode cells (Hamburger, 1961; Delmar et al., 2018).
The robust expression of Cx43 in premigratory cranial neural crest cells suggests that gap junctions may exist within this cell population to facilitate intercellular communication before and during EMT (Sauka-Spengler and Bronner-Fraser, 2008; Bronner, 2012; Schiffmacher et al., 2014, 2016, 2018). To further explore a role for gap junctions and Cx43 within the cranial neural crest cell population of the chick, we performed live imaging and loss-of-function assays. Our data reveal that functional gap junctions are formed between premigratory and migratory neural crest cells. In addition, depletion of Cx43 is sufficient to inhibit gap junction function in both premigratory and migratory neural crest cells, resulting in delayed emigration of Cx43-depleted cells from the neural tube, a delay which can be rescued by the introduction of full-length rat Cx43. Moreover, a reduction in Cx43 led to a concomitant loss of Snail2- (encoded by SNAI2), Pax7- and Sox10-positive premigratory cranial neural crest cells as well as a reduction in the number of Snail2-positive migratory neural crest cells. Altogether, these data suggest that Cx43 plays a role in influencing both the size of the chick premigratory and migratory cranial neural crest cell population, as well as impacting the timing of cranial neural crest cell emigration from the dorsal neural tube.
RESULTS
Multiple isoforms of Cx43 are present in the chick cranial neural crest
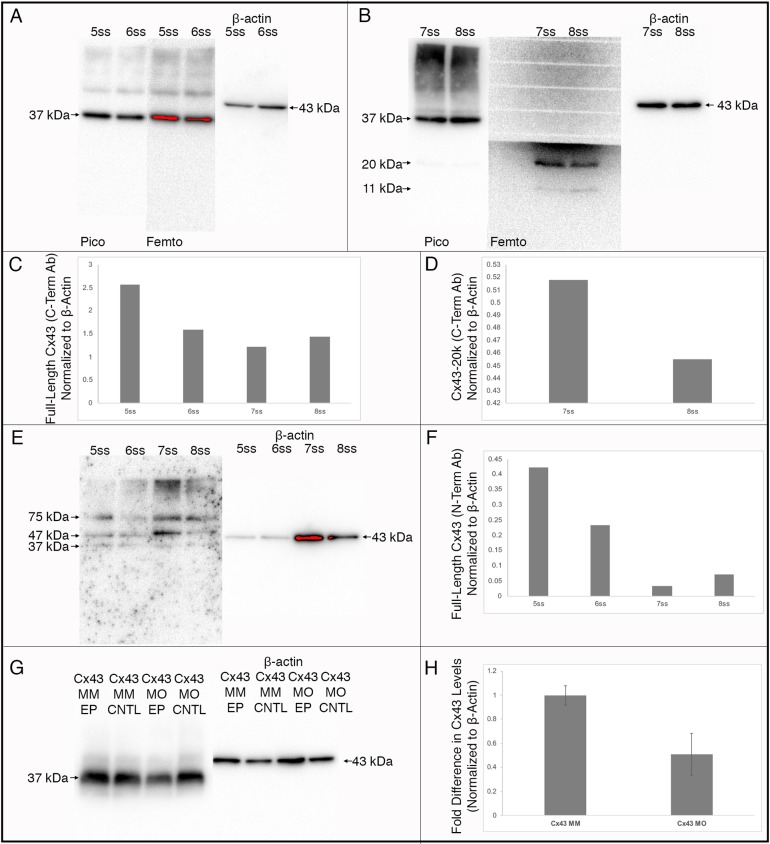
To define the Cx43 isoforms expressed in the chick head prior to and during neural crest cell EMT and early migration {Hamburger–Hamilton (HH)8+ to HH9+ [5 somite stage (ss) to 8ss]} we performed immunoblotting for Cx43 using an antibody that recognizes the Cx43 C-terminus on pooled heads from 12 embryos at each stage. These results revealed that only the full-length Cx43 isoform (37 kDa), and not a short C-terminal Cx43 isoform (Cx43-20k), recently shown to be critical for Xenopus cranial neural crest cell migration via transcriptional activation of CDH2 (Kotini et al., 2018), is present in heads at both the 5ss, in the premigratory neural crest cell population, and at the 6ss, when neural crest cells first begin EMT (Fig. 1A). By the 7ss, when cranial neural crest cells are undergoing EMT, we noted a strong band corresponding to Cx43-20k and a lighter band of ∼11 kDa, the latter of which was also identified previously but does not regulate CDH2 expression (Kotini et al., 2018) (Fig. 1B). The expression of the 20 and 11 kDa isoforms is also maintained at the 8ss (Fig. 1B). The highest level of full-length Cx43 expression was noted before and after the onset of EMT (Fig. 1C, 5ss and 6ss), with lower levels during EMT and early migration (Fig. 1C, 7ss and 8ss). Conversely, levels of the Cx43-20k isoform were most robust upon its onset at the 7ss, with lower levels at the 8ss (Fig. 1D). To confirm the identity of these bands, we performed immunoblotting for Cx43 using an antibody raised against the Cx43 N-terminus on lysate from the same samples, which yielded a band of 37 kDa, corresponding to full-length Cx43 protein, and no C-terminal isoforms (Fig. 1E). Quantification of full-length Cx43 levels obtained with this antibody gave similar results to those noted for the C-terminal antibody above, highest at before and after the onset of EMT (5ss and 6ss) with lower levels during EMT and early migration (7ss and 8ss, Fig. 1F). These data suggest a potential function for full-length Cx43 in premigratory neural crest cells and during the early stages of neural crest cell EMT but do not rule out roles for the Cx43-20k isoform later in development.
Fig. 1.
Immunoblotting for Cx43 validates the Cx43 antibodies and reveals different Cx43 isoforms during chick development. Immunoblotting results shown for antibodies directed against the C-terminus (A,B,G) or N-terminus (E) of Cx43. (A) The antibody directed against the C-terminus at the 5ss and 6ss revealed the presence of the full-length Cx43 protein (37 kDa) at both low and high exposures. (B) At the 7ss and 8ss, the C-terminal antibody revealed the presence of full-length Cx43 as well as 20 kDa and 11 kDa Cx43 isoforms, which was more evident when the blot was exposed to Femto chemiluminescent substrates. (C) Quantification of the full-length Cx43 protein identified with the C-terminal antibody revealed highest expression before and after the onset of EMT (5ss and 6ss), with lower levels during EMT and early migration (7ss and 8ss). (D) Quantification of the 20 kDa isoform revealed that expression is highest at 7ss. (E) The antibody directed against the Cx43 N-terminus, which is distinct from the C-terminal epitope, does not detect the 20 kDa or 11 kDa isoforms of Cx43. (F) Quantification of full-length Cx43 protein levels with the N-terminal antibody revealed a similar expression profile to that identified with the C-terminal antibody. Immunoblot analysis using the C-terminal antibody (G) reveals a 50% reduction (H) in Cx43 protein (Cx43 MO EP) as compared to the control MM-treated lysate (Cx43 MM EP) and associated contralateral controls (Cx43 MO CNTL and Cx43 MM CNTL). Quantification of full-length C-terminal (C,D) and N-terminal (F) Cx43 were performed on a single blot, while quantification of the effects of the Cx43 MO on Cx43 protein levels (H) was performed in triplicate and are shown as mean±s.d.
Premigratory and migratory cranial neural crest cells possess gap junctions that are dependent upon Cx43 for their function
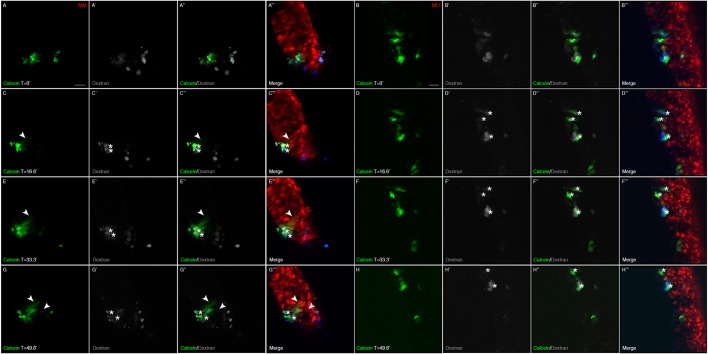
To evaluate whether premigratory cranial neural crest cells form functional gap junctions, we examined the diffusion of Calcein dye, which only passes between cells via gap junctions (Davy et al., 2006; Abbaci et al., 2008), within the premigratory neural crest cell population in vivo containing the Cx43 control morpholino (designated Cx43 electroporated MM; Cx43 MM EP), which we validated by immunoblotting to have levels of Cx43 similar to the unelectroporated, contralateral control side of treated embryos (designated Cx43 MM CNTL) (Fig. 1G). In order to trace the spread of the Calcein between cells that had previously been electroporated with the Cx43 control morpholino, we co-injected a 1:1 solution of Calcein and dextran, the latter being unable to pass through gap junctions due to its large size and is thus maintained in cells receiving the Calcein by injection (Ziambaras et al., 1998). Results of these experiments demonstrated that, in under 3 hours, Calcein diffused to a small number of surrounding premigratory neural crest cells (Fig. 2A–A‴,C–C‴,E–E‴,G–G‴; arrowheads indicate dye spread; Movie 1). To identify approximately how many premigratory neural crest cells received Calcein dye through gap junctions after initial injection, we co-injected the 1:1 solution of Calcein and dextran and immediately examined premigratory neural crest cells in a slice culture assay. In a small population of premigratory neural crest cells, where 25 cells received the initial Calcein and dextran solution, we identified 79 neighboring cells containing Calcein alone, indicating that the dye spread to 54 adjacent cells within a 5-min period (Fig. S1). By contrast, in embryos possessing premigratory cranial neural crest cells electroporated with the Cx43 morpholino (Cx43 MO EP), which leads to a 50% reduction in Cx43 protein levels as determined by immunoblotting compared to the unelectroporated, contralateral control side of treated embryos (Cx43 MO CNTL) (Fig. 1G,H), we noted no passage of Calcein to adjacent cells (Fig. 2B–B‴,D–D‴,F–F‴,H–H‴; Movie 2). Taken together, these results reveal that premigratory cranial neural crest cells form functional gap junctions and that Cx43 knockdown is sufficient to inhibit gap junction-based intercellular communication between them.
Fig. 2.
MO-mediated knockdown of Cx43 abrogates gap junction function in chick premigratory cranial neural crest cells. Images of live neural folds showing diffusion of Calcein (green) from injected cells [asterisks; identified with dextran (gray; blue in A‴–H‴)] to adjacent control MM (red)-electroporated premigratory neural crest cells (A–A‴,C–C‴,E–E‴,G–G‴, arrowheads) over a period of 50 min (time in min denoted with ′). No diffusion is observed with those cells possessing the Cx43 MO (red; B–B‴,D–D‴,F–F‴,H–H‴). All experiments were done at least in triplicate. Scale bars: 20 µm (applicable to their respective image sets).
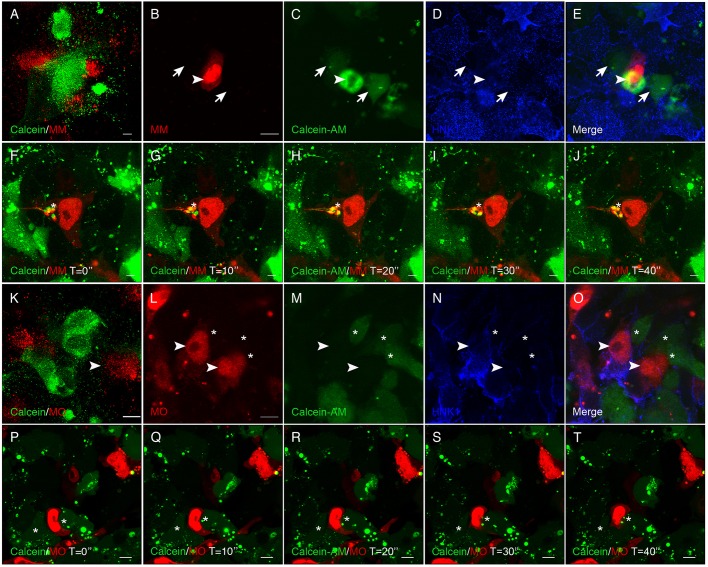
To determine whether migratory cranial neural crest cells also form functional gap junctions, we used an ex vivo culturing system to facilitate live imaging of dye passage, or gap junction function, between neural crest cells. To this end, dorsal neural folds (containing premigratory cranial neural crest cells) from either Cx43 MM EP or Cx43 MO EP embryos were explanted into chamber slides and combined with dorsal neural folds previously treated with Calcein-AM for 30 min (Fig. 3A,K). After overnight incubation, during which time the neural folds attached to the dish and neural crest cells commenced EMT and migrated, we observed that Cx43 MM-treated migratory neural crest cells (Fig. 3B, arrowheads) now possessed Calcein-AM within their cytoplasm due to their contact with adjacent Calcein-treated neural crest cells (Fig. 3C, arrows). We confirmed that these cells were, in fact, migratory neural crest cells by immunostaining fixed cultures with the HNK-1 antibody (Fig. 3B–E). We then repeated the experiment but performed live imaging at high magnification throughout the incubation period to observe dye transfer in real time. Through these assays, we observed that when a control MM-containing migratory neural crest cell extended a filopodia and made contact with an adjacent, Calcein-AM-positive migratory neural crest cell, the dye was taken into the control MM-containing neural crest cell in under 4 min (Fig. 3F–J, asterisks, Calcein contained within an MM-positive cell; Movie 3).
Fig. 3.
MO-mediated knockdown of Cx43 abrogates gap junction function in chick migratory cranial neural crest cells. Images of live (A,F–K,P–T) or fixed and immunostained (B–E,L–O) neural crest cells showing diffusion of Calcein-AM dye (green) from Calcein-AM-treated wild-type (WT) neural crest cells to neural crest cells treated with either the Cx43 mismatch control morpholino (MM) or Cx43 morpholino (MO). When premigratory neural crest cells from MM-treated embryos were combined with Calcein-AM-treated WT premigratory neural crest cells (A–J), passage of Calcein-AM occurred between adjacent MM-positive neural crest cells, noted after fixation (B–E, arrowhead- to arrow-labeled cells) and in live cultures (F–J, asterisks). No dye, however, moved to Cx43 MO-positive neural crest cells (K–O, arrowheads) while adjacent WT neural crest cells freely pass Calcein-AM (asterisks). This is more obvious during live imaging (P–T), where a MO-positive neural crest cell migrates between two adjacent cells and there is no transfer of Calcein-AM (asterisk). During live imaging, photos were acquired every 10 s (time in s denoted with ″; a total of 25 images were captured, but only the first five are shown in stills; see Movies 3,4). Scale bars: 100 µm (A,K), 20 µm (B–E); 5 µm (F–J); 10 µm (L–T). All live imaging experiments were performed, at a minimum, in triplicate.
Next, we conducted the same fixed and live imaging assays described above but this time with Cx43 MO-containing neural crest cells. When Calcein-AM-positive neural folds were incubated with Cx43 MO EP neural folds (Fig. 3K) and cultured to generate migratory neural crest cells from both populations, we did not detect a single cell in which dye transfer had occurred after immunostaining (Fig. 3L–O, arrowheads, MO-positive cells; asterisks, Calcein-positive cells). We confirmed these findings with live imaging, where we observed no dye transfer to Cx43 MO-containing neural crest cells migrating among adjacent, Calcein-AM-positive migratory neural crest cells over a period of 5 min of continuous imaging (Fig. 3P–T, asterisks, Calcein-positive cells; Movie 4). These results demonstrate that migratory neural crest cells also form gap junctions and that a reduction in Cx43 protein levels is sufficient to inhibit gap junction function in this cell population. Intriguingly, as premigratory neural crest cells were electroporated prior to EMT, these data also suggest that Cx43 knockdown is not sufficient to inhibit EMT in an ex vivo culture system.
Cx43 knockdown reduces the size of the premigratory neural crest cell domain in vivo
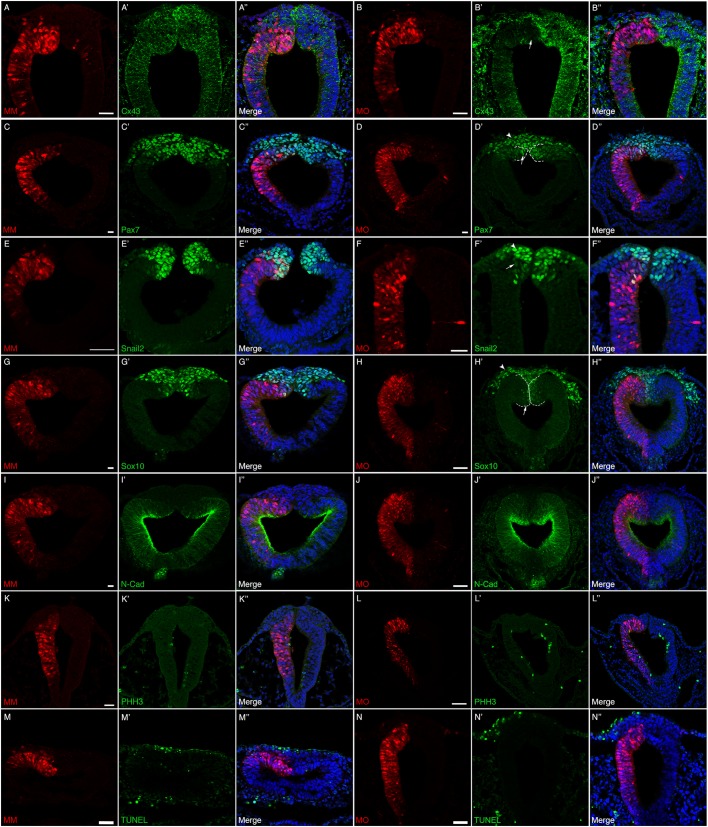
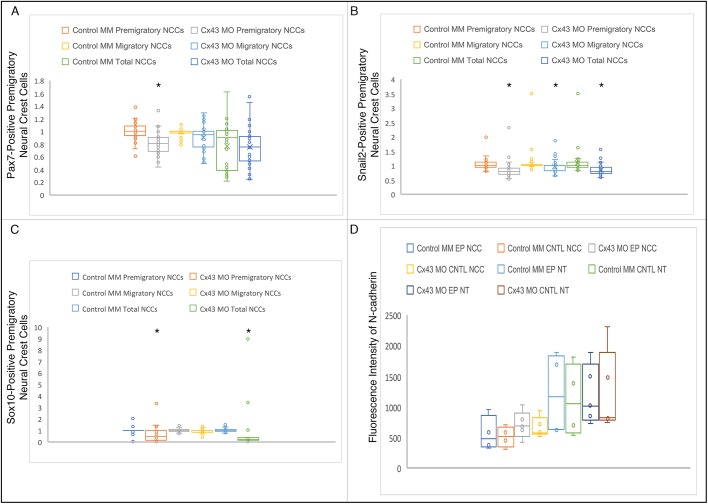
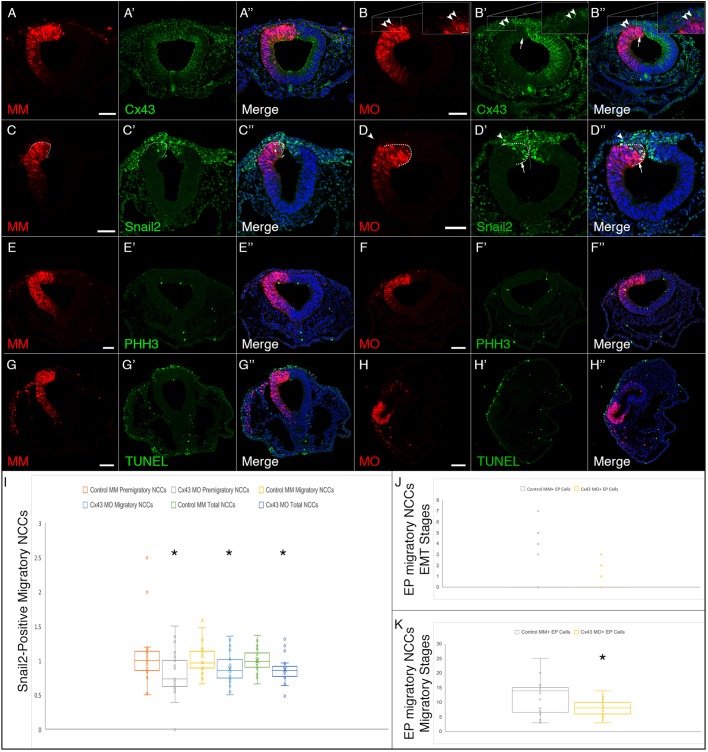
To delineate the function of Cx43, and potentially gap junctions, specifically during neural crest cell EMT, we introduced the Cx43 control MM or Cx43 MO by unilateral electroporation at HH7+ to HH8−, 3 h prior to the onset of EMT and collected the embryos at HH8+ to HH9 (5ss to 7ss). Embryos were fixed and immunostained for a battery of premigratory and migratory neural crest cell markers expressed at these stages (i.e. Cx43, Snail2, Sox10 and Pax7) (Taneyhill et al., 2007; Sauka-Spengler and Bronner-Fraser, 2008; Betancur et al., 2010; Murdoch et al., 2012; Lee et al., 2013; Jourdeuil and Taneyhill, 2018). We also examined N-cadherin given the prior work on its regulation by Cx43-20k (Kotini et al., 2018) and as a way to visualize the neural tube (Dady and Duband, 2017; Jourdeuil and Taneyhill, 2018; Rogers et al., 2018). When premigratory cranial neural crest cells were electroporated with the Cx43 control MM, we noted no change in any of the markers examined (Fig. 4A–A″,C–C″,E–E″,G–G″,I–I″). As expected, electroporation of premigratory cranial neural crest cells with the Cx43 MO reduced the levels of Cx43 in both the dorsal neural folds and the neural tube (Fig. 4B–B″, arrow). Surprisingly, however, Cx43 depletion led to a statistically significant decrease in the number of cells positive for Pax7 (Fig. 4D–D″, arrow, Fig. 5A; P=8.64×10−7), Snail2 (Fig. 4F–F″, arrow, Fig. 5B; P=6.41×10−9) and Sox10 (Fig. 4H–H″, arrow, Fig. 5C; P=0.0001) premigratory neural crest cells on the electroporated side compared to Cx43 control MM-electroporated premigratory neural crest cells (Table S1; all values were normalized to the contralateral control side prior to statistical analysis as per Hutchins and Bronner, 2018). In addition, we saw no appreciable difference in levels and distribution of N-cadherin (compare Fig. 4I–I″ to J–J″), which was validated by fluorescence intensity quantification (Schiffmacher et al., 2016) (Fig. 5D). Importantly, this decreased premigratory neural crest cell domain size was not due to any changes in cell proliferation or cell death. Immunostaining with an antibody to phospho-histone H3 (Fig. 4K–L″) or TUNEL assays (Fig. 4M–N″) showed no appreciable difference in cell proliferation or cell death, respectively, between control MM- and Cx43 MO-treated embryos. These results reveal that the reduction in the size of the premigratory neural crest cell domain may be due to an earlier effect on the cranial neural crest.
Fig. 4.
Cx43 depletion decreases the number of premigratory neural crest cells in the absence of any changes in cell proliferation or cell death. Representative transverse sections taken through the midbrain and hindbrain of HH8+ to HH9 embryos after unilateral electroporation of the neural tube at HH7+ to HH8− (2ss–3ss) with either the control morpholino (MM) or Cx43 MO and immunostaining for various neural crest cell markers. In MM-treated embryos (A–A″,C–C″,E–E″,G–G″,I–I″,K–K″,M–M″), there is no decrease in the level of Cx43 in the neural tube (A′) and no change in any of the premigratory neural crest cells as identified by Pax7 (C′), Snail2 (E′), Sox10 (G′). Similarly, there are no changes in N-cadherin (I–I″), a marker of neural tube cells, cell proliferation [pHH3 (K–K″)]; or cell death [TUNEL (M–M″)]. In Cx43 MO-treated embryos (B–B″,D–D″,F–F″,H–H″,J–J″,L–L″,N–N″), however, there is obvious knockdown of Cx43 in the neural tube (B′, arrow) and also a reduction in the premigratory neural crest cell population observed after Pax7 (D′, arrow), Snail2 (F′, arrow) and Sox10 (H′, arrow) immunostaining. Interestingly, N-cadherin (J–J″) is not affected. Cx43 MO-mediated loss of Cx43 also does not seem to alter levels of either cell proliferation (L–L″) or cell death (N–N″). Additionally, in HH9 embryos, a small number of MO-positive neural crest cells begin to emerge from the neural tube (D′,F′,H′, arrowheads; neural tube indicated by dashed lines). Scale bars: 20 µm (A–K″,M–N″); 50 µm (L–L″).
Fig. 5.
Graphical representation of analyzed premigratory neural crest cell data after control or Cx43 MO treatment. Box and whisker plots showing the third quartile (Q3), median and first quartile (Q1) range of the data as well as data outliers. Whiskers represent minimum and maximum values. (A–C) Graphs depicting the number of premigratory, migratory and total neural crest cells (NCCs) electroporated with either the control MM or Cx43 MO normalized to the un-electroporated contralateral side for the analyzed neural crest cell markers Pax7 (A), Snail2 (B) and Sox10 (C). Asterisks denote statistically significant differences determined with un-paired Mann–Whitney (Wilcoxon) test that employed a Bonferroni correction [A, Cx43 MO premigratory NCCs (P=8.64×10−7); B, Cx43 MO premigratory NCCs (P=6.41×10−9), Cx43 MO migratory NCCs (P=0.0029), Cx43 MO Total NCCs (P=9.64×10−9); C, Cx43 MO Premigratory NCCs (P=0.0001), Cx43 MO Total NCCs (P=2.76×10−6)]. (D) Graph depicting the fluorescence intensity of N-cadherin in premigratory neural crest cells (NCCs) and the neural tube (NT) of control MM- (N=4) or Cx43 MO- (N=5) electroporated cells compared to the contralateral side.
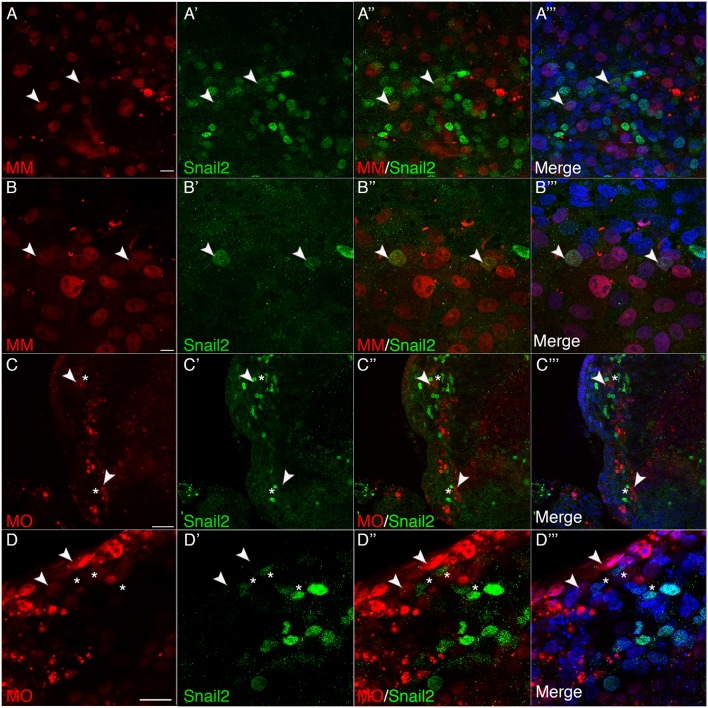
To address this question more rigorously, we evaluated the function of Cx43 earlier in development during cranial neural crest induction. To this end, we performed electroporations at HH4 to target the Cx43 control MM or the Cx43 MO into the epithelium comprising the non-neural ectoderm, neural plate border region (consisting of those cells that will give rise to neural crest and placode cells) and the neural ectoderm. We then explanted the neural ectoderm and neural plate border region of the Cx43 control MM- and Cx43 MO-electroporated embryos and allowed them to develop ex vivo. In Cx43 control MM-treated explants, we observed that a number of the electroporated cells were specified into Snail2-positive premigratory neural crest cells (Fig. 6A–B‴; arrowheads). However, not all electroporated cells would become specified to the neural crest cell fate as we also electroporated tissue fated to become the neural tube, ectoderm and placode cells. Conversely, in Cx43 MO-treated explants, we were unable to identify any MO-positive cells that were also Snail2-positive, even though they were located within a group of MO-negative, Snail2-positive cells (Fig. 6C,D‴; arrowheads, Cx43 MO EP cells; asterisks: adjacent Snail2-positive cells). Altogether, these data point to an important role for Cx43 during the specification and maintenance of the premigratory cranial neural crest cell domain.
Fig. 6.
MO-mediated knockdown of Cx43 prevents specification of neural crest cells. Images of explanted neural plate border and neural ectoderm electroporated at HH4 with control MM (A–B‴) and Cx43 MO (C–D‴) incubated for 40 h to allow for neural crest specification. In A–B‴ control MM explants (N=4 embryos), control MM-positive (red) cells have become specified to a neural crest fate as identified by Snail2 (green) expression (arrowheads). The explant in B–B‴ shows co-labeling (arrowheads) of nuclei with the control MM and Snail2. (C–D‴) In Cx43 MO explants (N=4 embryos), Cx43 MO-positive (red, arrowheads) cells are found within neural crest-positive regions (Snail2, green, asterisks); however, none of these Cx43 MO-positive cells are also Snail2 positive. Scale bars: 10 µm (A–B‴), 50 µm (C–C‴); 20 µm (D–D‴).
Cx43 knockdown results in delayed exit of Cx43-depleted premigratory neural crest cells from the neural tube
While examining the above HH8+ to HH9 embryos, we noted that there were few Cx43 MO-positive migratory neural crest cells, even though EMT was well underway in these samples (Fig. 4H′). This finding was particularly interesting as Cx43 MO-positive neural crest cells completed EMT and migrated in the dorsal neural fold explant experiments (see Fig. 3K–T above). Therefore, in order to evaluate the function of Cx43 during neural crest cell migration in vivo, we unilaterally electroporated embryos with either the Cx43 control MM or Cx43 MO at HH7+ to HH8− (three hours prior to the onset of EMT) and allowed them to develop for seven to 12 h (HH9+ to HH10). Embryos were fixed and immunostained for Cx43, Snail2 and phospho-histone H3 (Taneyhill et al., 2007; Sauka-Spengler and Bronner-Fraser, 2008; Betancur et al., 2010; Murdoch et al., 2012; Lee et al., 2013; Jourdeuil and Taneyhill, 2018). When premigratory neural crest cells were electroporated with the Cx43 control MM and grown to later stages, we noted no change in any of the markers examined (Fig. 7A–A″,C–C″). As expected, electroporation of premigratory cranial neural crest cells with the Cx43 MO reduced the levels of Cx43 in the neural tube as well as the numbers of premigratory cells that had yet to undergo EMT (Fig. 7B′; arrow, arrowheads, and accompanying high magnification inserts). Furthermore, we observed a statistically significant decrease in the number of premigratory (P=0.008), migratory (P=0.02) and total Snail2-positive cells (P=0.002; Fig. 7D′,I; Table S2; all values were normalized to the contralateral control side prior to statistical analysis as per Hutchins and Bronner, 2018). These differences were again independent of any changes in cell death or proliferation, suggesting that this reduction is due to an earlier effect on the size of the premigratory population (Fig. 7E–H″). Interestingly, we noted an increase in the number of Cx43 MO-positive migratory neural crest cells beginning at HH9+ compared to the number of MO-positive migratory neural crest cells seen at the younger stages (compare Figs 4H and 7J to Fig. 7B′,K), suggesting that these cells may experience a delay in their emergence from the neural tube. Furthermore, there was a statistically significant reduction in the number of Cx43 MO-positive migratory neural crest cells when compared to control MM-positive migratory neural crest cells (P=0.001; Fig. 7K). These results are in keeping with our ex vivo explant culture data demonstrating that Cx43 knockdown is not sufficient to inhibit EMT and migration in vivo.
Fig. 7.
Cx43 depletion decreases the number of migratory neural crest cells in the absence of any changes in cell proliferation or cell death. Representative transverse sections taken through the midbrain and hindbrain of HH9+ to HH10 embryos after unilateral electroporation of the neural tube at HH7+ to HH8− with either the control morpholino (MM) or Cx43 MO and immunostaining for Cx43 (A–B″) and Snail2 (C–D″). In control MM-treated embryos (A–A″,C–C″,E–E″,G–G″) there is no decrease in the level of Cx43 in either the neural tube or the migratory neural crest cells (A′) and no change in Snail2 (C′). Cell proliferation (E′) and cell death (G′) were also unaffected. In Cx43 MO-treated embryos (B–B″,D–D″,F–F″,H–H″), by contrast, there is obvious knockdown of Cx43 in the neural tube (B′, arrow) and neural crest cells (B′, arrowheads and high magnification insert) as well as a reduction in the number of Snail2-positive premigratory (compare outlined regions indicated by an arrow in C′ and D′ and migratory neural crest cells [compare number of migratory neural crest cells and the distance they have migrated (arrowhead) between the electroporated and contralateral side, D′]. MO-mediated depletion of Cx43, however, had no effect on cell proliferation (F′) or cell death (H′). Quantification of the number of Snail2-positive premigratory, migratory and total neural crest cells (NCCs) electroporated with either the control MM or Cx43 MO normalized to the un-electroporated contralateral side are presented in a box-and-whisker plot (I) showing the third quartile (Q3), median, and first quartile (Q1) range of the data as well as data outliers. Whiskers represent maximum and minimum values. Asterisks denote statistically significant differences determined with an unpaired Mann–Whitney (Wilcoxon) test that employed a Bonferroni correction. Results demonstrate that there is a significant difference in the number of premigratory (P=0.008), migratory (P=0.02) and total (P=0.002) Snail2-positive cells after MO-mediated depletion of Cx43. Furthermore, this depletion of Cx43 also causes the electroporated cells to delay their exit from the neural tube, as migratory neural crest cells begin to emerge around HH8 (Fig. 4B), and the number of neural crest cells exiting the neural tube is not the same as that in control MM-electroporated embryos (compare A and B). (J,K) To determine the severity of this delay, electroporated cells in embryos ranging from HH8+ to HH9 (EMT stage; J) and HH9+ to HH10 (migration stage; K) were counted and depicted in a box-and-whisker plot. There was a statistically significant difference between the number of electroporated migratory neural crest cells after Cx43 depletion as determined using an unpaired Mann–Whitney (Wilcoxon) text (K; asterisk, P=0.001). Scale bars: 50 µm (A–F″); 10 µm (high magnification inserts in B–B″); 100 µm (G–H″).
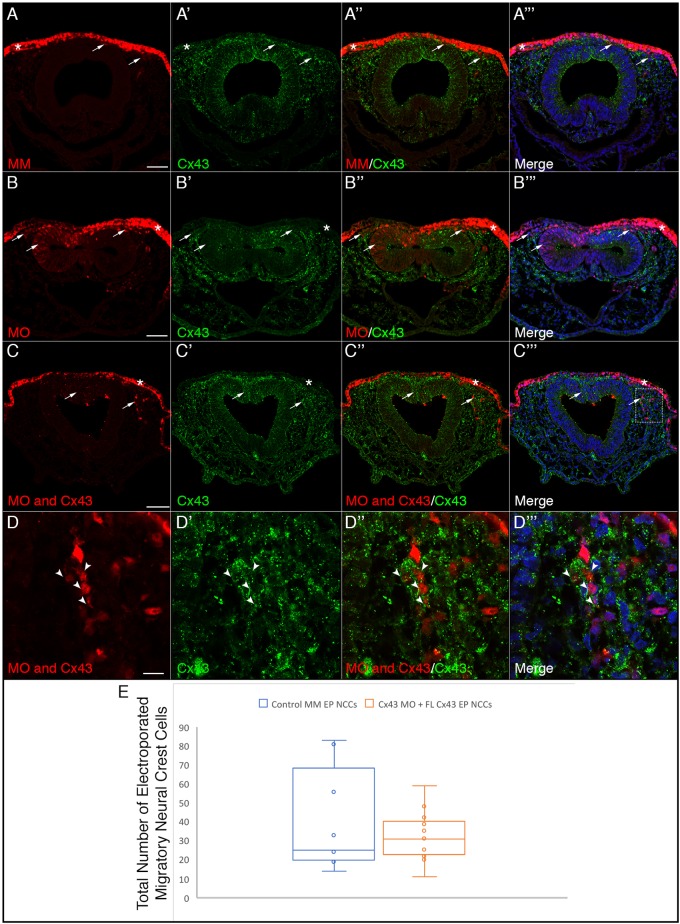
To attempt to rescue the delayed exit from the neural tube exhibited by Cx43 MO-positive cells, as well as further validate our MO, we used a dorsoventral electroporation technique to co-electroporate premigratory neural crest cells with both the Cx43 MO (or control MM) and a full-length rat Cx43 cDNA construct that is refractory to the MO. After electroporation, embryos were re-incubated to HH11 to evaluate rescue of Cx43 expression and cranial neural crest cell migration. To do this, we performed co-immunohistochemistry for Cx43 and HNK-1 to mark neural crest cells (Fig. 8). These data demonstrated that the full-length rat Cx43 rescues Cx43 expression after MO-mediated knockdown of Cx43 [compare Fig. 8A–A‴ (control MM), Fig. 8B–B‴ (Cx43 MO) and Fig. 8C–C‴ (Cx43 MO and full-length Cx43)], with a higher magnification image of the boxed region in Fig. 8C‴ demonstrating that Cx43 MO-positive cells co-electroporated with full-length Cx43 possess Cx43 levels comparable to un-electroporated neighbors (Fig. 8D–D‴, arrowheads). We then counted the number of MO-positive migratory neural crest cells in control MM and Cx43 MO embryos, both co-electroporated with full-length Cx43 (Fig. 8E). These data reveal that there is no significant difference in the number of migratory neural crest cells between these two treatments (P=0.89), indicating that full-length Cx43 is able to rescue the delayed emigration phenotype exhibited by Cx43 MO-positive cranial neural crest cells.
Fig. 8.
Co-electroporation of Cx43 MO and a full-length Cx43 construct refractory to the MO rescues the delayed neural crest cell emergence from the neural tube. Representative transverse sections taken through the midbrain and hindbrain of HH11 embryos after dorso-ventral electroporation at HH8 of the dorsal surface of the neural folds with the control MM (A–A‴), Cx43 MO (B–B‴), and co-electroporation of the dorsal neural folds with Cx43 MO on the dorsal surface and full-length rat Cx43 in the neural tube (C–D‴). Electroporation with the control MO does not alter levels of Cx43 in either the ectoderm (A″, asterisk) or the neural crest cells (A″, arrows) while there is a significant reduction in Cx43 in the ectoderm (B″, asterisk) and neural crest with the Cx43 MO (B″, arrows). Co-electroporation of the Cx43 MO and full-length rat Cx43 does not rescue the knockdown in the ectoderm (C″, asterisk), which does not receive the full-length rat Cx43, but is able to rescue the levels of Cx43 in both the neural tube and the migratory neural crest cells (C″, arrows). The box in C‴ is shown at a higher magnification in D–D″, and shows the rescue of the Cx43 MO-electroporated neural crest cells (arrowheads) to almost normal levels of Cx43. This restoration of Cx43 results in normal numbers of neural crest cells emerging from the neural tube (C″, arrows). (E) Data were quantified with an un-paired Mann–Whitney (Wilcoxon) test, which showed no significant difference in the number of migratory neural crest cells between control MM-treated embryos and Cx43 MO embryos co-electroporated with full-length Cx43 (P=0.89), graphically depicted by a box and whiskers plot as in Fig. 5. Scale bars: 50 µm (A–C‴), 10 µm (D–D‴).
DISCUSSION
Premigratory and migratory neural crest cells form functional gap junctions
Much work over the past two decades has implicated gap junctions, and specifically Cx43, in the formation of neural crest cell-derived organs and tissues. In mouse, Cx43 is expressed in premigratory and migratory neural crest cells as well as those undergoing EMT (Ruangvoravat and Lo, 1992; Lo et al., 1997), with migratory neural crest cells forming functional gap junctions (Lo et al., 1997). Moreover, Cx43-knockout mice die from conotruncal heart malformations and defects associated with the outflow tract, a region of the heart that forms from cardiac neural crest cells (Ruangvoravat and Lo, 1992; Ewart et al., 1997; Lo et al., 1997; Huang et al., 1998; Sullivan et al., 1998; Xu et al., 2001, 2006; Wei et al., 2005; Rhee et al., 2009; Francis et al., 2011). A recent study in Xenopus reveals the importance of a shorter isoform of Cx43 (Cx43-20k) in controlling cranial neural crest cell migration (Kotini et al., 2018). In chick, Cx43 is expressed in cranial neural crest cells from HH8− through to HH17 during trigeminal ganglion assembly (Jourdeuil and Taneyhill, 2018). Through immunoblotting, our new studies have identified full-length Cx43 and multiple C-terminal isoforms of Cx43 (Cx43-20k and Cx43-11k) expressed during chick cranial neural crest cell EMT and early migration. In all of these systems, however, gap junction presence and function in premigratory neural crest cells and those undergoing EMT remained poorly understood, until our studies herein.
Our results now show that gap junctions are present in both chick premigratory and migratory cranial neural crest cells and that their function is dependent on Cx43. In the presence of Cx43, dye readily passes from one cranial neural crest cell to an adjacent cell, but this is not observed upon Cx43 knockdown, which reduces Cx43 levels by ∼50%. The presence of Cx43-containing gap junctions in the neural crest could imply a function for gap junction-based communication within the premigratory and migratory cranial neural crest cell populations, which we investigated in more detail through functional assays in the chick embryo.
Cx43 knockdown reduces the number of premigratory neural crest cells and delays Cx43-depleted neural crest cell exit from the neural tube
Building on our previous studies, we next evaluated the function of Cx43 in the embryo during neural crest cell EMT. Strikingly, unilateral electroporation of the Cx43 MO at HH7+ and HH8− (2ss–3ss) decreased the number of premigratory cranial neural crest cells independently of effects on apoptosis or cell proliferation. In addition, Cx43-depleted premigratory neural crest cells exhibited a delayed emigration from the neural tube, while the EMT and emigration of non-electroporated cells was unaffected. Moreover, this reduction in the premigratory neural crest cell domain is not recovered during later development, as embryos at HH9+ to HH10 have a significantly smaller number of Snail2-positive premigratory, migratory and total neural crest cells on the Cx43 MO-electroporated side. To address this change in domain size, we investigated the impact of Cx43 knockdown at HH4, when neural crest cells are induced. Intriguingly, Cx43 levels MO-positive cells do not express Snail2, suggesting that Cx43 plays a role in specification and/or maintenance of the premigratory neural crest cell population in chick. This contrasts with the phenotype noted in Xenopus, where Cx43 knockdown had no effect on the premigratory neural crest cell population but rather affected neural crest cell migration (Kotini et al., 2018). The presence of this same Cx43-20k isoform identified by Kotini et al. (2018) in chick, however, may imply a potential conserved function in chick neural crest cell migration, which will be tested in future experiments. Collectively, our data suggest that one possible function of Cx43 in the context of gap junctions is to permit the diffusion of an intercellular signal(s) required to maintain the neural crest cell population. A reduction in Cx43 could also affect hemichannel function, which allows small molecule and ion exchange to occur between the cytoplasm and the extracellular environment and has recently been shown to play a role in extracellular signaling and disease function (Nielsen et al., 2012; Delmar et al., 2018; Rovegno and Sáez, 2018; Valdebenito et al., 2018). It will be necessary, in the future, to develop techniques in the chick that will allow us to query hemichannel function in the cranial neural crest.
Interestingly, we also observed that these Cx43 MO-positive neural crest cells exhibit a delayed exit from the neural tube, beginning to leave more broadly by HH9+. It is currently unclear whether this lag in emigration is due to a gap junction-dependent or -independent function of Cx43. In order to confirm that this delayed exit was caused by loss of Cx43, we co-electroporated embryos with the Cx43 MO and full-length rat Cx43 refractory to the MO at HH8, and collected them at HH11. These data both validated that the Cx43 MO is specific to Cx43 and demonstrated that, after rescue, there is no significant difference in the number of MO-positive neural crest cells exiting from the neural tube in control MM- and rescued Cx43 MO-electroporated embryos. It is therefore possible that the observed delay in the exit from the neural tube of Cx43 MO-electroporated neural crest cells is due to the time needed for another connexin protein to become incorporated into neural crest gap junctions to restore gap junction function, given that it is possible that neural crest cells could form heteromeric gap junctions (i.e. composed of two or more different types of connexins). This seems unlikely, however, due to the absence of functional gap junctions in the migratory neural crest cell explants. It is also possible that the 50% reduction in Cx43 protein levels achieved by the Cx43 MO may be sufficient to abrogate gap junction function (i.e. dye passage) but still allow for neural crest cells to emigrate out of the neural tube, albeit with this lag. However, this would likely occur through a gap junction-independent function of Cx43 given the robust loss of intercellular dye passage noted in our ex vivo explant assays. Taken together, these data suggest that connexins, and gap junctions, might be critical for intercellular communication to help establish and/or maintain the appropriate size of the premigratory cranial neural crest cell domain, as well as control the timing at which neural crest cells emerge from the neural tube and migrate to properly pattern the vertebrate embryo.
MATERIALS AND METHODS
Chicken embryo culturing and fixation
Fertilized chicken eggs (Gallus gallus) were obtained from both Moyer's Chicks (Quakertown, PA) as well as the University of Maryland (College Park, MD) and incubated at 38°C in humidified incubators (EggCartons.com, Manchaug, MA). After 30 h of incubation, eggs were removed from the incubator and a window was made in the shell to allow access to the embryo. Samples were staged according to the Hamburger and Hamilton (Hamburger and Hamilton, 1951) staging table. Manipulations were performed between Hamburger and Hamilton (HH) stage 4 through to HH8− (2ss–3ss). Embryos were subsequently collected between HH8− and HH11 (3ss–14ss). Superscript (+) or (−) refer to the specific number of somites indicated in the text.
Cx43 and mismatch control morpholinos
A 3′ lissamine-tagged antisense translation-blocking Cx43 morpholino (Cx43 MO, 5′-CAGGGCACTCCAATCACCCATCTTC-3′), or a 5-base pair mismatch Cx43 control morpholino (Cx43 MM, 5′-CAcGGCAaTCCAATaACaCATaTTC-3′) (start codon underlined and mismatches shown in lower case), was designed to target the Cx43 transcript according to the manufacturer's criteria or serve as a control, respectively (Gene Tools, LLC, Philomath, OR). Both morpholinos were used at a concentration of 500 µM as previously described (Fishwick et al., 2012; Schiffmacher et al., 2014). We confirmed that the morpholinos were specific to Cx43 and did not bind to any other sites by using the NCBI Nucleotide BLAST tool to search the invert complement of the morpholino sequence against the chicken genome, as per Gene Tools' recommendations). Immunoblotting was also performed to demonstrate evidence of Cx43 knockdown (see below).
Rescue experiments
The pSFFV-Cx43 construct, which contains the full-length rat Cx43 open reading frame cloned into the EcoR1 site of the pSFFV-neo plasmid, was kindly provided by Dr Joseph Stains (University of Maryland School of Medicine, Baltimore, MD). This vector, which has modest levels of expression relative to CMV/CAG-based expression vectors, generates more consistent responses than stronger expression vectors and avoids the effects that mimic loss-of-function rather than gain-of-function derived from the use of CMV/CAG-based Cx43 expression vectors (Gupta et al., 2016). In order to confirm that this vector would rescue the expression of chick Cx43 after its MO-mediated knockdown, we used NCBI Blast to compare the sequence of the Cx43 MO (described above) to the full-length rat Cx43 open reading frame. We identified five mismatched nucleotides in similar locations to those found in the Cx43 mismatch control MM, suggesting that the Cx43 MO would not block translation of the rat Cx43 transcript when co-expressed in vivo.
HH4 electroporation and ex ovo culture
HH4 electroporations were performed as described in Sauka-Spengler and Barembaum (2008) with specific modifications for ex ovo culture as described in Basch et al. (2006). In brief, windows were made in embryos as described above and a small volume of thin albumin was set aside. The thick albumin covering the blastoderm was then carefully removed and a circular piece of filter paper with an opening in the middle was placed over the cleared area of the vitelline membrane such that the opening was centered on the blastoderm. The filter paper was then carefully dissected from the yolk and placed in Ringer's solution so that the excess yolk could be removed. The embryo was then transferred, dorsal side up, into the electroporation chamber and submerged into the thin layer of Ringer's solution, 4-mm above the embedded platinum sheet in the petri dish-shaped electroporation chamber. The MO was injected, using a glass needle, under the vitelline membrane and overlying the dorsal side of the embryo. The electrode was then positioned such that it was directly above the embryo, submerged in the Ringer's solution, but so as to not make direct contact with the vitelline membrane in order to prevent burning the membrane. Two square electroporation pulses of 7 V with a duration of 25 ms and a 100 ms intervening rest time were applied. After electroporation, embryos were placed in thin albumin for 1–2 h to recover. The presumptive neural tube and neural plate border region, which contains both the presumptive neural crest cell and placode cell populations, was then dissected out, and the explanted tissue was placed in PB-1 standard medium for 1-2 h before being transferred onto 2-well tissue culture slides that had been coated with poly-L-lysine (Sigma-Aldrich, MO; P5899) and fibronectin (Corning, NY; 356008) containing serum-free DMEM (Corning; 10-013-CV) supplemented with 0.1% penicillin-streptomycin (10,000 U/ml; Gibco, MD; 15140122) and N-2 supplement (Gibco, 17501-048). Embryos were then cultured in DMEM supplemented with 0.1% penicillin-streptomycin and N-2 supplement for 40 h in order to assay for neural crest cells (Basch et al., 2006).
Dorsoventral electroporation of the neural crest
Dorsoventral electroporation of the neural tube was used to target the entire midbrain premigratory cranial neural crest cell population between HH7+ and HH8− for neural fold explants and live imaging experiments. To this end, a drop of Ringer's solution (pH 7.4) containing 0.1% penicillin-streptomycin was applied to the vitelline membrane overlying the embryo. A hole was then made at the edge of the area opaca using a 20-gauge needle. The Cx43 MO or Cx43 control MM was injected between the vitelline membrane and the embryo using fine glass needles to completely flood the area that will give rise to the head. The electrodes were then placed such that the anode was inserted into the hole at the border of the area opaca, underlying the MO-flooded epithelium, with the cathode barely touching the vitelline membrane. Three electric pulses (9V for 50 ms) were applied with a 200 ms refractory period in between (Gemini Systems, BTX, MA). Following electroporation, another drop of Ringers with 0.1% penicillin-streptomycin was applied to the surface of the vitelline membrane, the eggs were sealed with tape and parafilm, and re-incubated for 3 h.
Co-electroporation of the Cx43 MO (or Cx43 control MM) and the full-length rat Cx43 expression construct was similarly achieved using dorsoventral electroporation of the neural tube with the following modifications. All electroporations were performed exclusively on HH8 embryos possessing fused neural tubes. In order to introduce both the positively-charged, lissamine-tagged MO and the negatively-charged plasmid, the lumen of the neural tube was filled with the expression construct, while the surface of the ectoderm was flooded with the MO-containing solution as described above. The electrode was positioned as described above; however, the embryos received two, 25V, 30 ms electric pulses with a 200 ms refractory period in between. Following electroporation, embryos were treated as described above; however, the embryos were allowed to develop to HH11.
All embryos were then screened for the presence of MO using a Zeiss Discovery v8 Stereomicroscope (Carl Zeiss Microscopy, NY), with successfully electroporated embryos fixed with 4% paraformaldehyde (PFA; Thermo Fisher Scientific, MD; 30525-89-4) at 4°C overnight or processed for neural fold explants and live imaging experiments.
In ovo unilateral electroporations
Unilateral electroporation of the early chick neural tube was conducted to target premigratory neural crest cells in the midbrain. The Cx43 MO or Cx43 control MM was injected into the lumen of the neural tube of HH7+ and HH8− (2ss or 3ss) chick embryos using fine glass needles. Electrodes were then placed on either side of the midbrain and two electric pulses (25V for 30 ms) were applied to the midbrain with a 200 ms refractory period in between. Following electroporation, the eggs were sealed with tape and parafilm, and returned to the incubator for 3–12 h. Embryos were then screened as described above and subsequently fixed using 4% PFA or were processed for subsequent experimental analysis as outlined below.
Immunoblotting
To validate both Cx43 antibodies used in these experiments [Sigma-Aldrich, C6219, raised to the C-terminus (CT); Abcam, ab78055, raised to the N-terminus (NT)], chick heads were collected and pooled from 12 HH8 and HH9 (5ss through to 8ss) wild-type embryos. Similarly, the knockdown efficiency of the Cx43 MO was evaluated by collecting and independently pooling the electroporated and contralateral control half neural tubes from the midbrains of Cx43 MO and Cx43 control MO embryos at the 7ss. Samples were rinsed in Ringer's solution, centrifuged at 500 g for 5 min at 4°C, and then snap-frozen in liquid nitrogen. Protein extraction and immunoblotting were then performed as previously described (Schiffmacher et al., 2014, 2018; Shah et al., 2017). Briefly, cell pellets were lysed in lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% IGEPAL CA-630, 1 mM EDTA) supplemented with cOmplete Protease Inhibitor Cocktail (Sigma-Aldrich, 1167498001) and 1 mM PMSF (Sigma-Aldrich, 10837091001) for 30 min at 4°C with periodic mixing. The clarified, solubilized protein fractions were collected following centrifugation at >20,000 g for 15 min at 4°C and protein concentrations were quantified with a Bradford assay (Thermo Fisher Scientific). Equivalent amounts of protein per sample were boiled at 99°C for 5 min in 4× reducing Laemmli sample buffer and then centrifuged at >10,000 g for 5 min at room temperature. The samples were then loaded into a Novex Wedgewell Tris-Glycine gel [either 16% (wild-type; XP00160BOX, Invitrogen, MA) or 10% (Cx43 MO and Cx43 control MO; XP00100BOX, Invitrogen)] and subsequently transferred to a 0.45 µm BioTrace PVDF membrane (Pall, Port Washington, NY). Membranes were incubated in blocking solution [5% dry milk in 1× PBS plus 0.1% Tween-20 (PTW)] for 1 h at room temperature and then incubated overnight at 4°C with the following primary antibodies diluted in blocking solution: CT Cx43 [1:1000 (wild-type); 1:5000 (Cx43 MO and Cx43 control MM)] or NT Cx43 (1:1000). Membranes were washed in PTW and then incubated with species- and isotype-specific horseradish peroxidase-conjugated secondary antibodies (40 ng/ml; Jackson ImmunoResearch) in 5% blocking solution for 1 h at room temperature. Membranes were washed again in PTW and antibody detection was performed using the Supersignal West Pico or Femto chemiluminescent substrates (Thermo Fisher Scientific) and visualized using a ChemiDoc XRS system (Bio-Rad, PA). The immunoblots were then stripped using Restore western blot stripping buffer (Thermo Fisher Scientific) for 2 h at 37°C and re-probed using an anti-β-actin primary antibody (1:1000; Santa Cruz Biotechnology, CA; sc-46668) and its species- and isotype-specific secondary antibody as described above. Immunoblots were analyzed using Image Lab software (Bio-Rad), in order to determine band size and volume. The volume of the Cx43 control MM- and Cx43 MO-electroporated (and their corresponding contralateral controls) were then normalized to the loading control in order to determine the efficacy of MO-mediated knockdown. Levels of Cx43 obtained with the CT and NT antibodies from 5ss to 8ss embryos were similarly normalized to the level of the β-actin loading control in order to quantify stage-specific expression. Additionally, the 20 kDa fragment was always identified on the same blot as the full-length protein using the CT antibody; however, it required a significantly longer exposure and thus the blots are presented as separate images.
Evaluation of gap junction function
Gap junction function was evaluated by introducing either the Cx43 MO or Cx43 control MM via dorsoventral electroporation of the premigratory cranial neural crest cell population as described above. After 3 h of incubation, the embryo, along with the extraembryonic membranes, was dissected off the yolk and carefully washed in Ringer's solution. The vitelline membrane was then removed and a ring of filter paper (VWR, NJ; 28310-128) was placed around the embryo so as to hold the embryo taut. A 1:1 solution of 1 mg/ml Calcein (C481; dissolved in Ringer's solution), which only passes to cells via gap junctions (Davy et al., 2006; Abbaci et al., 2008), and 1 mg/ml dextran, Cascade Blue (D1976; dissolved in ddH2O), to allow for the identification of the injected cells as it does not pass through gap junctions (Ziambaras et al., 1998), was injected into the dorsal neural folds using a fine glass needle. Excess Calcein–dextran solution was washed away using Ringer's solution. Then, the embryo and filter paper were turned over and the tautness of the embryo and extraembryonic tissues were adjusted before a second ring of filter paper was applied to the back of the embryo, creating a sandwich. The embryo was then transferred, dorsal side down, onto a glass bottom culture dish (MatTek, MA, P35G-1.5-20-C) with a 20 mm microwell, and covered with a mixture of 1% low-melting-point agarose (prepared with Ringer's solution; Promega, NC, USA; V2111) and thin albumin in order to immobilize the embryo on the dish.
In order to quantify the number of premigratory neural crest cells that received Calcein dye through gap junctions, a live embryo was embedded in a solution of 5% low-melting point agarose and then live sectioned on a Leica VT1200S vibratome at a thickness of 300 µm. The dorsal neural folds were then injected in a number of places with the Calcein and dextran solution using a fine glass needle. The sample was transferred onto a glass bottom culture dish in a small volume of Ringer's solution and imaged at the 5-min mark as described below. Cells in a small area were then counted to determine the total number of Calcein- and dextran-positive cells as compared to those that were only Calcein positive, which yielded the number of cells that had received Calcein via gap junctions during that 5-min period.
To assess the effects of Cx43 knockdown on migratory neural crest cells, embryos were first unilaterally electroporated at HH7+ or HH8− with either the Cx43 MO or Cx43 mismatch MM and then cultured for an additional 3 h at 38°C. The electroporated embryos were dissected off the yolk and washed with Ringer's solution. The midbrain dorsal neural folds (containing the premigratory neural crest cells) were then dissected from both the electroporated and the contralateral control side in PB-1 standard medium and placed into either a glass bottom culture dish with a 10 mm Microwell [MatTek, P35G-1.5-10-C (N=4)] or an 8-well Lab-Tek II Chamber Slide [Naige Nunc International, 154534; if the samples were to be immunostained (N=4)] that had been coated with poly-L-lysine and fibronectin-containing serum-free DMEM supplemented with 0.1% penicillin-streptomycin and N-2 supplement. To each of these treatments (Cx43 MO or Cx43 control MM), dissected wild-type neural folds that had been incubated for 30 min at 37°C in a 1:500 Calcein-AM (Invitrogen, C3099) in DMEM (supplemented with penicillin-streptomycin and N-2) solution in an uncoated chamber slide, and subsequently washed three times with DMEM, were added. The neural folds were then cultured overnight in order to allow the neural crest cells to undergo EMT and migrate out onto the underlying fibronectin. Cultures were then checked hourly until the Cx43 MO- or Cx43 control MM-treated neural crest cells made contact with the Calcein-AM-treated neural crest cells.
All cultures were imaged live using a Zeiss LSM800 confocal microscope with an AiryScan detection system held within a temperature-controlled unit which was maintained at 37°C and 5% CO2 for the duration of imaging. Images were acquired using Zen 2.0 (Blue Edition) and processed into movies using Adobe Photoshop CC 2018 and Adobe Premiere Pro CC 2018. After live imaging, samples that had been incubated in an eight-well chamber slide were fixed in 4% PFA for 1 h at room temperature and prepared for immunostaining.
Immunohistochemistry
After fixation, whole embryos were washed in 1× phosphate-buffered saline (PBS), gelatin-embedded and serially sectioned at 16 µm. Samples were then de-gelatinized and blocked in 1× PBS containing 0.2% Triton X-100 (1× PBST; EMD Millipore Corporation, MA; TX1561-1) and 10% sheep serum (Lampire, PA; 733900). Cultured cells, on the other hand, were just washed in 1× PBST. Samples were then incubated with various primary antibodies [Cx43 (1:500; Sigma-Aldrich, C6219), Pax7 (1:10; Developmental Studies Hybridoma Bank, DSHB, IA), Sox10 (1:500; GeneTex, CA; GTX128374), Snail2 (1:100; Cell Signaling Technology, MA; C1967), phospho-histone H3 (pHH3, 1:200; Millipore), N-cadherin (1:50; DSHB clone MNCD2), E-cadherin (1:500; BD Transduction Laboratories, CA, USA; 610181) and HNK-1 (1:50); DSHB]. The following day, after washing four times for 30 min with 1× PBST, sections were incubated with appropriate secondary antibodies and incubated for 2–3 h at room temperature or overnight at 4°C [goat anti-rabbit IgG 488 or 647; Invitrogen, A11034 and Jackson ImmunoResearch, PA, USA; 111-605-003, respectively (for Cx43, Sox10, pHH3 and Snail2); goat anti-mouse IgG1 647; Invitrogen, A21240 (for Pax7 and E-cadherin); goat anti-rat IgG; Life Technologies, MD; A21247 (for N-cadherin)]. All primary and secondary antibodies were diluted in 1× PBST and 5% sheep serum. After washing four times for 30 min, coverslips were mounted using DAPI Fluoromount-G (Southern Biotech, AL; 0100-20) to mark cell nuclei. For early EMT stage embryos, a minimum of three serial sections from at least five embryos were examined for all of the markers described. At older stages, a minimum of three serial sections from at least five embryos were examined for Snail2 and pHH3 (Sox10, Pax7 and N-cadherin were not examined at these stages). For rescue experiments, at least four serial sections were examined from at least two embryos. Number of embryos and sections examined for statistical analysis are presented in Tables S1–S3. Images of serial, transverse sections were acquired with the LSM Zeiss LSM800 confocal microscope with AiryScan detection and processed using Zen 2.0 (Blue Edition) software and Adobe Photoshop CC 2018 (Adobe Systems, Inc, CA).
TUNEL
A TUNEL assay (in situ cell death detection kit, Fluorescein; Roche, Mannheim, Germany; 1164795910) was performed on 4% PFA-fixed, cryopreserved sections to detect apoptotic cells as described previously (Wu et al., 2014; Shah et al., 2017). Following TUNEL, coverslips were mounted with DAPI Fluoromount-G and imaged as described above. For all TUNEL assays, at least five embryos were examined.
Fluorescence intensity measurements
In order to determine whether MO-mediated knockdown of Cx43 altered the levels of N-cadherin in either the neural tube or premigratory neural crest cell population, fluorescence intensity was determined on original Zeiss CZI files using the profile line intensity tool in the Zen Blue software (Zeiss; Schiffmacher et al., 2016). To this end, an area of the neural tube and premigratory neural crest cell population containing at least 10 cells was selected using a rectangular tool, which was then paired with a rectangle of the exact same dimensions on the contralateral side. These measurements were performed for both control MM (N=4) and Cx43 MO (N=5) embryos.
Statistical analyses
In order to quantify the effects of Cx43 depletion on EMT and migration, embryos were divided into two categories: early EMT stages (HH8 to HH9; 4ss to 7ss) and later EMT/migratory stages (HH9+ to HH11+; 8ss to 14ss). In each of these groups, the number of premigratory, migratory and total neural crest cells were counted in at least three transverse sections from Cx43 MO- or Cx43 control MM-electroporated embryos and normalized to the number of premigratory, migratory and total neural crest cells, respectively, on the contralateral side, as in Hutchins and Bronner (2018) (see Tables S1 and S2 for N values). Neural crest cells in early EMT stage embryos were identified with either Snail2, Pax7 or Sox10 immunohistochemistry, as described above. As the Pax7 antibody also labels a population of neural tube cells, we only counted those Pax7-positive cells that were also Snail2- or Sox10-positive. Snail2 immunohistochemistry was conducted on embryos at the late EMT/migratory stages. In order to further quantify the effects of Cx43 depletion on exclusively the delamination of MO-positive cells, the number of Cx43- and control MM-positive cells were quantified in all embryos with migratory neural crest cells in each group. Embryos co-electroporated with the full-length rat Cx43 construct and Cx43 MO (or control MM) were analyzed by counting the number of MO-positive neural crest cells to evaluate the rescue. All samples were tested for normality by histogram analysis. For normally distributed data, an unpaired two-tailed Student's t-test was performed to assess statistical significance, while for non-normally distributed data, an unpaired Mann–Whitney (Wilcoxon) test was performed to assess statistical significance for embryos at all time points. In order to account for multiple comparisons, a Bonferroni correction was used and the α-level for each analysis was set to 0.016; therefore, the corrected P values, which are presented in the manuscript, were considered significant at below 0.05.
Supplementary Material
Acknowledgements
The authors would like to thank Ms Irina Kolesnik, Ms Sophia Liu, Ms Linda Farley, and Ms Caroline Halmi for excellent technical assistance, and Dr Joseph Stains (University of Maryland School of Medicine, Maryland, USA) for the kind gift of the full-length rat Cx43 cDNA plasmid.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.J., L.A.T.; Methodology: K.J., L.A.T.; Formal analysis: K.J.; Investigation: L.A.T.; Resources: L.A.T.; Data curation: K.J.; Writing - original draft: K.J.; Writing - review & editing: K.J., L.A.T.; Supervision: L.A.T.; Project administration: L.A.T.; Funding acquisition: K.J., L.A.T.
Funding
This research was supported by grants from the National Institutes of Health (NIH; R01DE024217, L.A.T.), the American Cancer Society (RSG-15-023-01-CSM, L.A.T.), and a Postdoctoral Fellowship (American Association for Anatomy, K.J.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.235440.supplemental
References
- Abbaci M., Barberi-Heyob M., Blondel W., Guillemin F. and Didelon J. (2008). Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. BioTechniques 45, 33-52, 56-62. 10.2144/000112810 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Bronner-Fraser M. and García-Castro M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature, 441, 218-222. 10.1038/nature04684 [DOI] [PubMed] [Google Scholar]
- Betancur P., Bronner-Fraser M. and Sauka-Spengler T. (2010). Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl. Acad. Sci. USA, 107, 3570-3575. 10.1073/pnas.0906596107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner M. E. (2012). Formation and migration of neural crest cells in the vertebrate embryo. Histochem. Cell Biol. 138, 179-186. 10.1007/s00418-012-0999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner M. E. and LeDouarin N. M. (2012). Development and evolution of the neural crest: an overview. Dev. Biol. 366, 2-9. 10.1016/j.ydbio.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooreman A., Van Campenhout R., Ballet S., Annaert P., Van Den Bossche B., Colle I., Cogliati B. and Vinken M. (2019). Connexin and pannexin (Hemi)channels: emerging targets in the treatment of liver disease. Hepatology 69, 1317-1323. 10.1002/hep.30306 [DOI] [PubMed] [Google Scholar]
- Dady A. and Duband J.-L. (2017). Cadherin interplay during neural crest segregation from the non-neural ectoderm and neural tube in the early chick embryo. Dev. Dyn. 246, 550-565. 10.1002/dvdy.24517 [DOI] [PubMed] [Google Scholar]
- Davy A., Bush J. O. and Soriano P. (2006). Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 4, e315 10.1371/journal.pbio.0040315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk H. A., Mroue R. M., El-Sabban M. E. and Talhouk R. S. (2009). Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun. Signal 7, 4 10.1186/1478-811X-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranco B. H., Nickel B. M., Baty C. J., Martinez J. S., Gay V. L., Sandulache V. C., Hackam D. J. and Murray S. A. (2008). Migrating cells retain gap junction plaque structure and function. Cell Commun. Adhes. 15, 273-288. 10.1080/15419060802198298 [DOI] [PubMed] [Google Scholar]
- Delmar M., Laird D. W., Naus C. C., Nielsen M. S., Verselis V. K. and White T. W. (2018). Connexins and disease. Cold Spring Harb. Perspect Biol. 10, a029348 10.1101/cshperspect.a029348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue P. C. J., Graham A. and Kelsh R. N. (2008). The origin and evolution of the neural crest. BioEssays 30, 530-541. 10.1002/bies.20767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart J. L., Cohen M. F., Meyer R. A., Huang G. Y., Wessels A., Gourdie R. G., Chin A. J., Park S. M., Lazatin B. O. and Villabon S. (1997). Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development 124, 1281-1292. [DOI] [PubMed] [Google Scholar]
- Fishwick K. J., Neiderer T. E., Jhingory S., Bronner M. E. and Taneyhill L. A. (2012). The tight junction protein claudin-1 influences cranial neural crest cell emigration. Mech. Dev. 129, 275-283. 10.1016/j.mod.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Xu X., Park H., Wei C.-J., Chang S., Chatterjee B. and Lo C. (2011). Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS ONE 6, e26379 10.1371/journal.pone.0026379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill L. S. and Roffers-Agarwal J. (2010). Division of labor during trunk neural crest development. Dev. Biol. 344, 555-565. 10.1016/j.ydbio.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. and Paul D. L. (2009). Gap junctions. Cold Spring Harb. Perspect Biol. 1, a002576 10.1101/cshperspect.a002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Goliger J. A. and Paul D. L. (1996). Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 65, 475-502. 10.1146/annurev.bi.65.070196.002355 [DOI] [PubMed] [Google Scholar]
- Gupta A., Anderson H., Buo A. M., Moorer M. C., Ren M. and Stains J. P. (2016). Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell. Signal. 28, 1048-1057. 10.1016/j.cellsig.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. (1961). Experimental analysis of the dual origin of the trigeminal ganglion in the chick embryo. J. Exp. Zool. 148, 91-123. 10.1002/jez.1401480202 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92. 10.1002/jmor.1050880104 [DOI] [PubMed] [Google Scholar]
- Huang G. Y., Cooper E. S., Waldo K. L., Kirby M. L., Gilula N. B. and Lo C. W. (1998). Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J. Cell Biol. 143, 1725-1734. 10.1083/jcb.143.6.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins E. J. and Bronner M. E. (2018). Draxin acts as a molecular rheostat of canonical Wnt signaling to control cranial neural crest EMT. J. Cell Biol. 217, 3683-3697. 10.1083/jcb.201709149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M., Arias A. C., Liu L., Chen Y.-B., Bronner M. E. and Maxson R. E. (2012). A stable cranial neural crest cell line from mouse. Stem Cells Dev. 21, 3069-3080. 10.1089/scd.2012.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkin E. and Adameyko I. (2013). Progenitors of the protochordate ocellus as an evolutionary origin of the neural crest. EvoDevo 4, 12 10.1186/2041-9139-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdeuil K. and Taneyhill L. A. (2018). Spatiotemporal expression pattern of Connexin 43 during early chick embryogenesis. Gene Expr. Patterns 27, 67-75. 10.1016/j.gep.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaporis G., Brink P. R. and Valiunas V. (2011). Gap junction permeability: selectivity for anionic and cationic probes. Am. J. Physiol. Cell Physiol. 300, C600-C609. 10.1152/ajpcell.00316.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotini M., Barriga E. H., Leslie J., Gentzel M., Rauschenberger V., Schambony A. and Mayor R. (2018). Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat. Commun. 9, 3846 10.1038/s41467-018-06368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird D. W. (2014). Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 588, 1339-1348. 10.1016/j.febslet.2013.12.022 [DOI] [PubMed] [Google Scholar]
- Lee R. T. H., Nagai H., Nakaya Y., Sheng G., Trainor P. A., Weston J. A. and Thiery J. P. (2013). Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme. Development 140, 4890-4902. 10.1242/dev.094680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. E. I., Waldo K. L., Linask K. L., Chen T., Wessels A., Parmacek M. S., Kirby M. L. and Lo C. W. (2002). An essential role for connexin 43 gap junctions in mouse coronary artery development. Development 129, 2031-2042. [DOI] [PubMed] [Google Scholar]
- Lo C. W., Cohen M. F., Huang G.-Y., Lazatin B. O., Patel N., Sullivan R., Pauken C. and Park S. M. J. (1997). Cx43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev. Genet. 20, 119-132. [DOI] [PubMed] [Google Scholar]
- Mese G., Richard G. and White T. W. (2007). Gap junctions: basic structure and function. J. Invest. Dermatol. 127, 2516-2524. 10.1038/sj.jid.5700770 [DOI] [PubMed] [Google Scholar]
- Murdoch B., DelConte C. and García-Castro M. I. (2012). Pax7 lineage contributions to the mammalian neural crest. PLoS ONE 7, e41089 10.1371/journal.pone.0041089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. S., Axelsen L. N., Sorgen P. L., Verma V., Delmar M. and Holstein-Rathlou N. H. (2012). Gap junctions. Compr. Physiol. 2, 1981-2035. 10.1002/cphy.c110051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas W. A., Boyadjiev S. A., Shapiro R. E., Daniels O., Wollnik B., Keegan C. E., Innis J. W., Dinulos M. B., Christian C., Hannibal M. et al. (2003). Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408-418. 10.1086/346090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas W. A., Karczeski B., Vermeer S., Lowry R. B., Delatycki M., Laurence F., Koivisto P. A., Van Maldergem L., Boyadjiev S. A., Bodurtha J. N. et al. (2009). GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 30, 724-733. 10.1002/humu.20958 [DOI] [PubMed] [Google Scholar]
- Prasad M. S., Sauka-Spengler T. and LaBonne C. (2012). Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Dev. Biol. 366, 10-21. 10.1016/j.ydbio.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S., Kidder G. and Rossant J. (1995). Cardiac malformation in neonatal mice lacking connexin 43. Science 267, 1831-1834. 10.1126/science.7892609 [DOI] [PubMed] [Google Scholar]
- Rhee D. Y., Zhao X.-Q., Francis R. J., Huang G. Y., Mably J. D. and Lo C. W. (2009). Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 136, 3185-3193. 10.1242/dev.032334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. D., Sorrells L. K. and Bronner M. E. (2018). A catenin-dependent balance between N-cadherin and E-cadherin controls neuroectodermal cell fate choices. Mech. Dev. 152, 44-56. 10.1016/j.mod.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovegno M. and Sáez J. C. (2018). Role of astrocyte connexin hemichannels in cortical spreading depression. Biochim. Biophys. Acta Biomembr. 1860, 216-223. 10.1016/j.bbamem.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Ruangvoravat C. P. and Lo C. W. (1992). Connexin 43 expression in the mouse embryo: localization of transcripts within developmentally significant domains. Dev. Dyn. 194, 261-281. 10.1002/aja.1001940403 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T. and Barembaum M. (2008). Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol. 87, 237-256. 10.1016/S0091-679X(08)00212-4 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T. and Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557-568. 10.1038/nrm2428 [DOI] [PubMed] [Google Scholar]
- Schiffmacher A. T., Padmanabhan R., Jhingory S. and Taneyhill L. A. (2014). Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol. Biol. Cell 25, 41-54. 10.1091/mbc.e13-08-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmacher A. T., Xie V. and Taneyhill L. A. (2016). Cadherin-6B proteolysis promotes the neural crest cell epithelial-to-mesenchymal transition through transcriptional regulation. J. Cell Biol. 215, 735-747. 10.1083/jcb.201604006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmacher A. T., Adomako-Ankomah A., Xie V. and Taneyhill L. A. (2018). Cadherin-6B proteolytic N-terminal fragments promote chick cranial neural crest cell delamination by regulating extracellular matrix degradation. Dev. Biol. 444 Suppl 1, S237-S251. 10.1016/j.ydbio.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Schiffmacher A. T. and Taneyhill L. A. (2017). Annexin A6 controls neuronal membrane dynamics throughout chick cranial sensory gangliogenesis. Dev. Biol. 425, 85-99. 10.1016/j.ydbio.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M. and Bronner M. E. (2013). Insights into neural crest development and evolution from genomic analysis. Genome Res. 23, 1069-1080. 10.1101/gr.157586.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G. and Willecke K. (2003). An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes., 10, 173-180. 10.1080/cac.10.4-6.173.180 [DOI] [PubMed] [Google Scholar]
- Solan J. L. and Lampe P. D. (2018). Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 1860, 83-90. 10.1016/j.bbamem.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgen P. L., Trease A. J., Spagnol G., Delmar M. and Nielsen M. S. (2018). Protein−protein interactions with connexin 43: regulation and function. Int. J. Mol. Sci. 19, E1428 10.3390/ijms19051428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller T. J. and García-Castro M. I. (2012). Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 69, 3715-3737. 10.1007/s00018-012-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Huang G. Y., Meyer R. A., Wessels A., Linask K. K. and Lo C. W. (1998). Heart malformations in transgenic mice exhibiting dominant negative inhibition of gap junctional communication in neural crest cells. Dev. Biol., 204, 224-234. 10.1006/dbio.1998.9089 [DOI] [PubMed] [Google Scholar]
- Taneyhill L. A., Coles E. G. and Bronner-Fraser M. (2007). Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134, 1481-1490. 10.1242/dev.02834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito S., Barreto A. and Eugenin E. A. (2018). The role of connexin and pannexin containing channels in the innate and acquired immune response. Biochim. Biophys. Acta Biomembr. 1860, 154-165. 10.1016/j.bbamem.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. and Weingart R. (2002). The electrophysiology of gap junctions and gap junction channels and their mathematical modelling. Biol. Cell 94, 501-510. 10.1016/S0248-4900(02)00022-9 [DOI] [PubMed] [Google Scholar]
- Waldo K. L., Lo C. W. and Kirby M. L. (1999). Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev. Biol. 208, 307-323. 10.1006/dbio.1999.9219 [DOI] [PubMed] [Google Scholar]
- Waning D. L., Guise T. A. and Mohammad K. S. (2019). A “Connexin” responsible for the fatal attraction of cancer to bone. Cell Metab., 29, 6-8. 10.1016/j.cmet.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.-J., Francis R., Xu X. and Lo C. W. (2005). Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J. Biol. Chem. 280, 19925-19936. 10.1074/jbc.M412921200 [DOI] [PubMed] [Google Scholar]
- Wu J. I. and Wang L. H. (2019). Emerging roles of gap junction proteins connexins in cancer metastasis, chemoresistance and clinical application. J. Biomed. Sci. 26, 8 10.1186/s12929-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-Y., Hooper R. M., Han K. and Taneyhill L. A. (2014). Migratory neural crest cell alphaN-catenin impacts chick trigeminal ganglia formation. Dev. Biol. 392, 295-307. 10.1016/j.ydbio.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li W. E. I., Huang G. Y., Meyer R., Chen T., Luo Y., Thomas M. P., Radice G. L. and Lo C. W. (2001). Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J. Cell Biol. 154, 217-230. 10.1083/jcb.200105047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Francis R., Wei C. J., Linask K. L. and Lo C. W. (2006). Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development 133, 3629-3639. 10.1242/dev.02543 [DOI] [PubMed] [Google Scholar]
- Ziambaras K., Lecanda F., Steinberg T. H. and Civitelli R. (1998). Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Miner. Res. 13, 218-228. 10.1359/jbmr.1998.13.2.218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.