ABSTRACT
Cellular and molecular mechanisms underlying the switch from self-amplification of cortical stem cells to neuronal and glial generation are incompletely understood, despite their importance for neural development. Here, we have investigated the role of the transcription factor specificity protein 2 (Sp2) in expansive and neurogenic divisions of the developing cerebral cortex by combining conditional genetic deletion with the mosaic analysis with double markers (MADM) system in mice. We find that loss of Sp2 in progenitors undergoing neurogenic divisions results in prolonged mitosis due to extension of early mitotic stages. This disruption is correlated with depletion of the populations of upper layer neurons in the cortex. In contrast, early cortical neural stem cells proliferate and expand normally in the absence of Sp2. These results indicate a stage-specific requirement for Sp2 in neural stem and progenitor cells, and reveal mechanistic differences between the early expansive and later neurogenic periods of cortical development.
This article has an associated ‘The people behind the papers’ interview.
KEY WORDS: Corticogenesis, Mitosis, MADM, Neurogenesis, Sp2, Neural stem cell
Highlighted Article: Specificity protein 2 conditionally deleted mice reveal mechanistic differences between the early expansive and later neurogenic periods of cortical development.
INTRODUCTION
Formation of the cerebral cortex begins in the neuroepithelium of the dorsal telencephalon and culminates in a complex tissue responsible for high-order cognitive processes. The neuroepithelium comprises neural stem cells (NSCs) the cell bodies of which span from the pial (basal) to ventricular (apical) surfaces, and their cell cycle-dependent interkinetic nuclear migration within the ventricular zone (VZ) gives the neuroepithelium a pseudostratified appearance (Bertipaglia et al., 2018). Early during cortical development (in rodents, embryonic days E9.5 to E10.5) symmetric divisions of NSCs amplify the stem cell pool. By E11.5 most NSCs in the VZ transition into neural progenitor cells (NPCs, also called radial glia) that divide asymmetrically to generate another progenitor and a neuronal-fated cell, while maintaining their bipolar morphology (Taverna et al., 2014). The neurogenic daughters migrate basally and can either divide again just outside the VZ in the subventricular zone (SVZ) as intermediate progenitor cells (IPCs) or directly enter the developing cortical plate (CP), migrating past earlier-born neurons to laminate the CP inside out (Farkas and Huttner, 2008). IPCs amplify the number of neurons and contribute to formation of all layers of the cortex, with some evidence for greater contribution to the upper layers (Llorca et al., 2019; Martínez-Cerdeño et al., 2006; Mihalas and Hevner, 2018; Mihalas et al., 2016; Tarabykin et al., 2001). The mechanisms that demarcate the transition from expansion of NSCs to the self-renewing asymmetric divisions of NPCs that directly or indirectly yield neurogenic progeny remain incompletely understood (Uzquiano et al., 2018).
A number of genes linked to human microcephaly and other neurodevelopmental disorders encode proteins that are involved in mitosis (Faheem et al., 2015; Gilmore and Walsh, 2013). Cortical development relies on the programmed output of the appropriate numbers and types of neurons to generate the functional processing units of cortical circuitry (Homem et al., 2015). Disrupted mitosis may cause progenitors to prematurely differentiate or undergo apoptosis, depleting the progenitor population. Because the plasticity of NPCs becomes restricted as development proceeds (Desai and McConnell, 2000), and the number of divisions in individual progenitors may be predefined (Gao et al., 2014; Llorca et al., 2019), the cortex appears to be unable to compensate for premature depletion of the progenitor pool. However, despite mutations in mitotic regulators revealing the sensitivity of the developing cortex to disruptions of cell division, details on the mitotic differences of NSCs and NPCs, or how the timing of specific stages of mitosis are disrupted by microcephaly-inducing mutations is still relatively uncharacterized (Dwyer et al., 2016; Huttner and Kosodo, 2005; Johnson et al., 2017; Mora-Bermúdez et al., 2016, 2014; Vargas-Hurtado et al., 2019). Further delineation of mechanisms that distinguish cell divisions of NSCs and NPCs is crucial to deciphering the underlying mechanisms of neurodevelopmental disorders caused by mutations in cell cycle regulators.
We have previously determined that specificity protein 2 (Sp2) is expressed in mouse cortical NPCs and IPCs at E14.5, and postnatally in progenitors and neuroblasts of the rostral migratory stream (Liang et al., 2013). Deletion of Sp2 during corticogenesis caused mitotic arrest and attenuated progenitor differentiation, resulting in a smaller cortex at birth (Liang et al., 2013). Sp2 is a member of the specificity protein (Sp) family of C2H2 zinc-finger transcription factors characterized by the near similarity of their zinc-finger domains to Sp1 (Bouwman and Philipsen, 2002; Schaeper et al., 2010). Sp1, Sp2, Sp3 and Sp4 tend to be ubiquitously expressed in developing tissues throughout the body, while Sp5, Sp6, Sp7, Sp8 and Sp9 display more-restricted tissue expression (Schaeper et al., 2010; Zhao and Meng, 2005). While the transcriptional activity of Sp2 has been debated (Chen et al., 1998; Moorefield et al., 2004; Terrados et al., 2012; Völkel et al., 2015), Sp2 has repeatedly been demonstrated to be required for early embryonic proliferation and development in multiple cell types and tissues (Baur et al., 2010; Wegener et al., 2017; Xie et al., 2010). Germline deletion of Sp2 in mice is embryonic lethal prior to formation of the cortex (Baur et al., 2010), requiring the use of conditional deletion models to investigate the contribution of Sp2 to tissue formation and cell proliferation. However, the previously characterized tissue-specific bulk deletion models of Sp2 affect cells at multiple differentiation states and may cause cell non-autonomous defects. Thus, the cell-autonomous role of Sp2 in regulation of proliferation and differentiation in NPCs, and whether Sp2 contributes to the functions of symmetrically dividing NSCs remains unknown. Here, we have used multiple approaches to directly and dynamically investigate mitosis in cortical NSCs and NPCs, and have asked how Sp2 deletion alters progenitor expansion and differentiation. Using the mosaic analysis with double markers (MADM) system (Hippenmeyer et al., 2010; Zong et al., 2005), fluorescently labeled clones of cortical progeny were sparsely generated using multiple cre-driver lines to monitor NSC and NPC behavior at high resolution. Further analysis of MADM clones revealed the diminished expansion capacity of Sp2-null (Sp2−/−) NSCs and NPCs in relation to wild-type sibling cells. We show that loss of Sp2 in neurogenic NPCs prolongs mitosis due to the lengthening of early mitotic stages, correlating with a later reduction in the relative population of Sp2−/− progeny, particularly in the upper layers of the cortex. Surprisingly, early Sp2−/− and wild-type NSCs are similar in both the timing of mitosis and the production of early cortical populations prior to neurogenic expansion, indicating a stage-specific requirement for Sp2 in progenitor behavior.
RESULTS
Deletion of Sp2 in NPCs prolongs mitosis
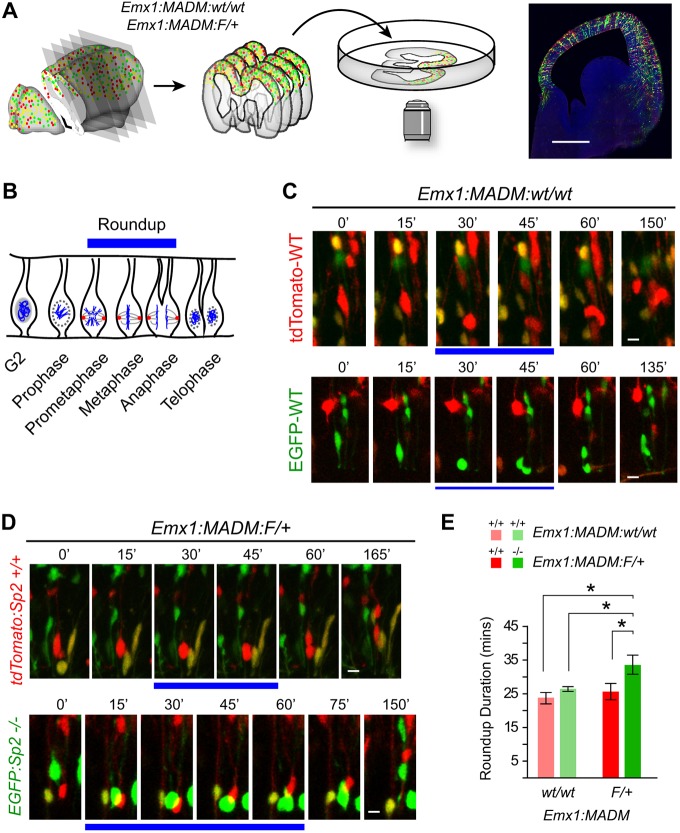
To dynamically analyze progenitor behavior in the presence and absence of Sp2, we used MADM on Chromosome 11 (Hippenmeyer et al., 2010) by crossing mice carrying cre recombinase and each MADM cassette (Fig. S1). Cre-mediated MADM recombination events during G2 phase of the cell cycle in a dividing NSC or NPC results in generation of sibling pairs where one daughter cell expresses EGFP and the other tdTomato. Ubiquitous promoters driving expression of EGFP or tdTomato permanently label the consequent lineages of the two siblings, and the sparse nature of mitotic MADM recombination events allows for high-resolution analysis of each clone independently throughout development (Zong et al., 2005). Moreover, the MADM alleles can be combined with mutant alleles on the same chromosome, allowing for genetic analysis of mutations in the EGFP- and tdTomato-expressing lineages. In this study, we used a floxed allele for Sp2, which is on the mouse chromosome 11, in combination with MADM-11 that we established in a previous study (Liang et al., 2013). To determine baseline MADM recombination events and control for potential off-target effects in NPCs, we used Emx1cre (Gorski et al., 2002) to recombine MADM alleles with the endogenous Sp2 alleles [Emx1:MADM:wt/wt cells; both tdTomato+ (red) and EGFP+ (green) are wild type]. In embryos carrying one floxed Sp2 allele on the MADM background (Emx1:MADM:F/+), the daughter cells generated by interchromosomal recombination will be Sp2+/+ (tdTomato+, red) or Sp2−/− (EGFP+, green) within what is a largely Sp2 heterozygous population due to Emx1cre-driven recombination in the Sp2F/+ background independent of MADM events (Fig. S1).
To dynamically quantitate progression through mitosis in Sp2−/− NPCs, we harvested brains from E14.5 Emx1:MADM:wt/wt and Emx1:MADM:F/+ embryos, prepared organotypic cortical slice cultures and time-lapse imaged apico-basal aspects of the developing somatosensory cortices (Fig. 1A). Dividing cells were imaged and analyzed at the apical surface of the ventricular zone (VZ), the location of the majority of NPC divisions. Analyses were focused on roundup duration, which we defined as when cells at the VZ surface adopted a rounded morphology (start of prometaphase) until a clear cleavage furrow emerged between the separating daughter cells (late anaphase) (Fig. 1B). Quantitative comparison of control Emx1:MADM:wt/wt green and red cells revealed similar roundup duration in the two wild-type siblings (Fig. 1C,E). In contrast, Sp2−/− (green) cells exhibited a significantly longer roundup duration than Sp2+/+ (red) cells in the Emx1:MADM:F/+ cortices (Fig. 1D). Moreover, the increase in roundup duration of Sp2−/− cells was also significant when compared with green and red cells in control cortices (Fig. 1E), supporting the notion that this defect is cell-intrinsic rather than a consequence of unknown background effects of the MADM system. These data indicate a cell-autonomous requirement for Sp2 in the progression through roundup phase of mitosis in NPCs of E14.5 cortex.
Fig. 1.
Time-lapse imaging reveals prolonged roundup duration in Sp2−/− NPCs. (A) Schematic for generation and imaging of organotypic slice cultures from MADM cortices. Photomicrograph of an Emx1:MADM:wt/wt forebrain section at E14.5. (B) Schematic of mitotic NPCs with the clearly identifiable stages in our preparations marked by the blue bar. (C) Representative panels from live imaging experiments in control Emx1:MADM:wt/wt cortices. tdTomato-WT (red) and EGFP-WT (green) NPCs exhibited similar roundup durations. (D) Representative panels from Emx1:MADM:F/+ live imaging experiments. Green Sp2−/− progenitors spent more time in roundup than their sibling red Sp2+/+ cells. Elapsed time is indicated in minutes above each frame. Blue bars indicate frames in roundup. (E) Comparison of roundup durations indicates a significant increase in Sp2−/− (green) cells of Emx1:MADM:F/+ cortices compared with all other cell genotypes. Emx1:MADM:wt/wt: red, n=40 cells; green, n=45 cells. Emx1:MADM:F/+: red, n=56; green, n=59 cells; four independent litters analyzed for each genotype. One-way ANOVA, F=7.733; P<0.0001, *P<0.05 after Bonnferroni's multiple comparison correction. Scale bars: 500 µm in A; 10 µm in C,D. Data are mean±s.e.m.
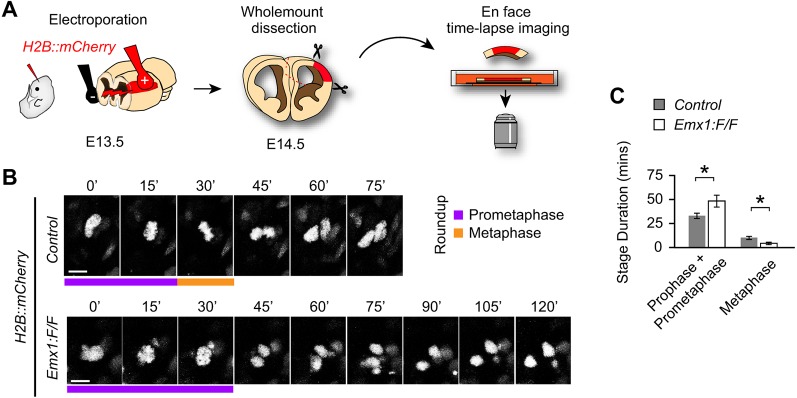
Although the time-lapse imaging data revealed that Sp2−/− cells have prolonged roundup durations, lack of chromatin visualization prevented us from determining the specific mitotic stages affected by loss of Sp2. To remedy this, a plasmid encoding Histone H2B fused to mCherry under a CAG promoter (H2B::mCherry) was delivered into Emx1cre; Sp2F/F (Emx1:F/F; in which Sp2 is bulk-deleted in the cortex) or control (Sp2:F/F) cortices at E13.5 by in utero electroporation. Twenty-four hours later, electroporated cortices were harvested for whole-mount organotypic culture and en face time lapse confocal imaging (Fig. 2A). Duration of distinct mitotic stages was quantified using previously described definitions for cortical NPCs (Pilaz and Silver, 2014) (Fig. 1B); prophase and prometaphase were defined by the initial condensation of H2B::mcherry-labeled chromatin until chromosome alignment at the cell equator forming the metaphase plate. Analysis of these stages revealed that combined prophase and prometaphase length was significantly increased in the Emx1:F/F cortices, while metaphase was absent or significantly shorter in duration compared with controls (Fig. 2B,C). Together, the MADM and bulk time-lapse imaging of mitotic Sp2−/− NPCs revealed increased time spent in prophase and prometaphase with simultaneous decline in metaphase duration compared with Sp2+/+ progenitors in the E14.5 cortex.
Fig. 2.
Sp2−/− NPCs have prolonged early mitosis and decreased metaphase durations. (A) Schematic of in utero electroporation of an H2B::mCherry construct to visualize chromatin in en face time-lapse imaging of organotypic whole-mount preparations. (B) Representative images of cortical NPCs with time noted in minutes above each frame. Prophase+prometaphase (purple) and metaphase (orange) periods are marked as indicated by bars underneath the panels. (C) Quantification of time spent in each stage of mitosis. Sp2F/F (control), n=65 cells from three experiments; Emx1:F/F, n=60 cells from four experiments; prophase+prometaphase, t=2.403, d.f.=123 P=0.02. Metaphase: t=3.75, d.f.=123, P=0.0003. Data are mean±s.e.m. Scale bars: 10 µm. *P<0.05, Student's t-test.
Mosaic deletion of Sp2 reduces NPCs and attenuates neurogenic output
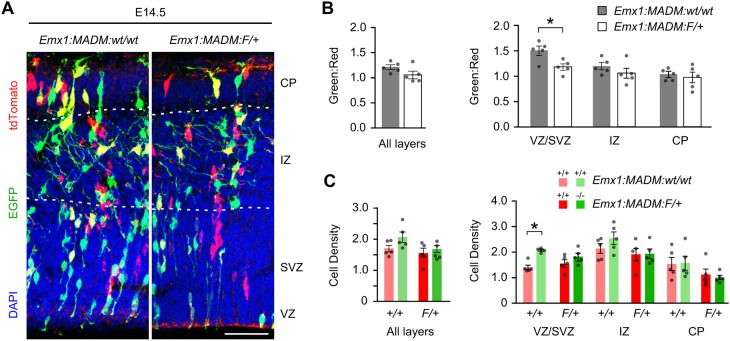
Germline mutations of genes that regulate mitosis often perturb cortical development while sparing most other tissues (Bianchi et al., 2017; Bond et al., 2002; Gai and Di Cunto, 2017). These mutations can cause altered mitotic timing, skewed division angles and/or prevent completion of cytokinesis (Gilmore and Walsh, 2013; Johnson et al., 2017). Mitotic arrest in cortical progenitors can cause apoptosis, thereby depleting the progenitor pool (Chen et al., 2014; Novorol et al., 2013; Pilaz et al., 2016). Less severe disruptions to mitotic timing may alter cell fate, as pharmacological lengthening of mitosis has been reported to inhibit cell proliferation in vitro (Uetake and Sluder, 2010) or to promote symmetric terminal differentiative divisions in the mouse cortex (Pilaz et al., 2016). As we have previously demonstrated loss of Sp2 in NPCs disrupts mitotic arrest without causing cell death (Liang et al., 2013), we sought to determine the role of Sp2 in cell differentiation of NPCs in the cortex. To accomplish this, MADM cell populations were quantified in the E14.5 Emx1:MADM:wt/wt and Emx1:MADM:F/+ developing somatosensory cortices (Fig. 3A). The architecture of the developing cortex enables the tracking of cell differentiation, as cells at different developmental and differentiative stages transition through its distinct compartments (Noctor et al., 2004). MADM recombination will mostly occur in NPCs located in the VZ/SVZ, which will generate neuroblasts either directly or through intermediate progenitors. These fate-restricted cells migrate through the intermediate zone (IZ) towards the cortical plate (CP), where they become terminally differentiated postmitotic neurons. We first compared green-to-red ratios (G:R) of MADM cells in these cortical regions as these cells are initially generated in equal numbers as siblings from single progenitors (Fig. S1), thus any deviation from baseline G:R in control embryos (wt/wt) would reveal if the relative expansion or migration of the green and red populations are altered upon loss of Sp2 in Emx1:MADM:F/+ cortices (Sp2−/−÷Sp2+/+). The G:R for the entire cortical width was similar at E14.5 in Emx1:MADM:F/+ cortices compared with controls, albeit it is noteworthy that the data trends toward a lower value (Fig. 3B). Breaking the Emx1:MADM data into layers revealed that the G:R of Emx1:MADM:F/+ was significantly reduced in the VZ/SVZ when compared with controls (Fig. 3B). As the lower G:R observed in Emx1:MADM:F/+ mice could be due to a reduction in Sp2−/− cells or an expansion of Sp2+/+ cells, we also compared estimated MADM cell densities. The green MADM population in the control background was on average higher in density than the red population, a phenomenon also reported previously (Hippenmeyer et al., 2010). When densities were analyzed by layer, a significant increase in green cell density compared with red cells was found in the VZ/SVZ (Fig. 3C). The G:R discrepancy in MADM cell densities was significantly attenuated in Emx1:MADM:F/+ cortices where the green and red populations were present at similar densities, suggesting the beginning of a decline in Sp2−/− cell expansion in the cortex (Fig. 3C). Altogether, our analysis of mosaic deletion of Sp2 at E14.5 indicates that Sp2−/− cells have prolonged roundup duration relative to their sibling wild-type cells and MADM control mice, resulting in decreased expansion of Sp2−/− cells in the VZ/SVZ.
Fig. 3.
Population analysis of control and Sp2−/− cells in the E14.5 MADM cortices. (A) Representative images of E14.5 Emx1:MADM:wt/wt and Emx1:MADM:F/+ cortices immunoenhanced for EGFP (green), tdTomato (red) and nuclei counterstained with DAPI (blue). Scale bar: 50 µm. (B) Overall green to red ratios (G:R) for total number of cells, and by layer in the developing cortex. VZ/SVZ, ventricular/subventricular zone; IZ, intermediate zone; CP, cortical plate. Total cells, t=1.785, P=0.11; VZ/SVZ, t=3.119, P=0.01; IZ, t=1.196, P=0.27; CP, t=0.4838, P=0.64. Emx1:MADM:wt/wt, n=5; Emx1:MADM:F/+, n=5. (C) Average densities (values ×104/mm3) of MADM cells overall and by layer in the developing cortex. All layers: Emx1:MADM:wt/wt, t=1.883, P=0.10; Emx1:MADM:F/+, t=0.3231, P=0.75. VZ/SVZ: Emx1:MADM:wt/wt, t=6.866, P=0.0001; Emx1:MADM:F/+, t=1.383, P=0.20. IZ: Emx1:MADM:wt/wt, t=1.346, P=0.22; Emx1:MADM:F/+, t=0.1167, P=0.91. CP: Emx1:MADM:wt/wt, t=0.0754, P=0.94; Emx1:MADM:F/+, t=0.4735, P=0.65. Emx1:MADM:wt/wt, n=5; Emx1:MADM:F/+, n=5. Dots represent values from individual embryos. *P<0.05, Student's t-test. Data are mean±s.e.m.
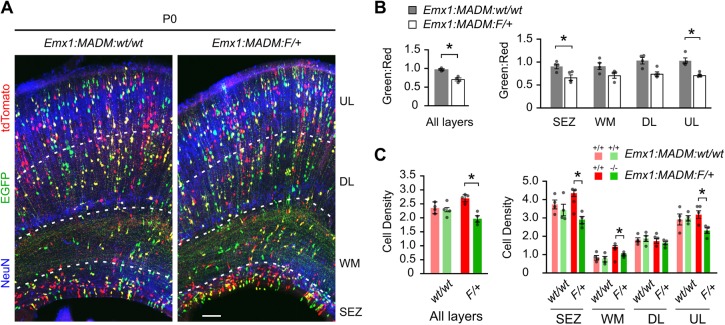
To determine whether loss of Sp2 in NPCs affects the cohort of neurons produced throughout neurogenesis, we examined Emx1:MADM:wt/wt and Emx1:MADM:F/+ cortices on the day of birth (postnatal day 0, P0) when neurogenesis is largely completed and gliogenesis has commenced (Fig. 4A). As such, the cells in the motor and somatosensory regions at P0 should mostly reflect the progeny resulting from neurogenic divisions in the areas we analyzed in previous experiments. The overall G:R throughout the cortical width was significantly decreased in Emx1:MADM:F/+ brains compared with controls. When the cortical width was broken down into the subependymal zone (SEZ), white matter (WM), deep layers (DL) and upper layers (UL), the decreased G:R was most apparent in the SEZ and UL (Fig. 4B). Analysis of average cell densities at P0 in Emx1:MADM:wt/wt revealed similar numbers of red and green wild-type cells, while significantly fewer Sp2−/− cells compared with Sp2+/+ cells were observed in Emx1:MADM:F/+ brains (Fig. 4C). Laminar analysis confirmed the discrepancy is due to absence of Sp2−/− cells in the SEZ and UL. Taken together, our MADM data at E14.5 and P0 indicate that loss of Sp2 in NPCs prolongs prophase and prometaphase, and reduces progenitors in the VZ/SVZ at mid-cortical development. These disruptions have lasting effects on neurogenesis, reducing the densities of progenitors and upper layer cortical cells at the population level in the newborn mouse.
Fig. 4.
Sp2−/− NPCs generate fewer subependymal and upper layer cells in the postnatal cortex. (A) Representative images of P0 Emx1:MADM:wt/wt and Emx1:MADM:F/+ cortices immunoenhanced for EGFP (green), tdTomato (red) and co-stained for NeuN (blue) to delineate the cortical layers. Scale bar: 100 µm. (B) Green to red ratios (G:R) of the P0 cortex for all layers or by layer. SEZ, subpendymal zone; WM, white matter; DL, deep layers; UL, upper layers. All layers: t=6.458, P=0.0007; SEZ: t=3.143, P=0.02; WM: t=2.275, P=0.06; DL: t=1.41, P=0.21; UL: t=4.526, P=0.004; Student's t-test, *P<0.05; m=4 embryos per genotype. (C) Densities (values ×104/mm3) for MADM cells of the P0 cortex for all layers or by layer. All layers: Emx1:MADM:wt/wt, t=0.3883, P=0.71; Emx1:MADM:F/+, t=5.645, P=0.0013. SEZ: Emx1:MADM:wt/wt, t=0.7553, P=0.48; Emx1:MADM:F/+, t=5.753, P=0.0012. WM: Emx1:MADM:wt/wt, t=0.3416, P=0.74; Emx1:MADM:F/+, t=4.619, P=0.0036. DL: Emx1:MADM:wt/wt, t=0.3719, P=0.72; Emx1:MADM:F/+, t=0.8907, P=0.41. UL: Emx1:MADM:wt/wt, t=0.1627, P=0.88; Emx1:MADM:F/+, t=3.393, P=0.014. Student's t-test, *P<0.05. Dots represent values from individual embryos. Data are mean±s.e.m.
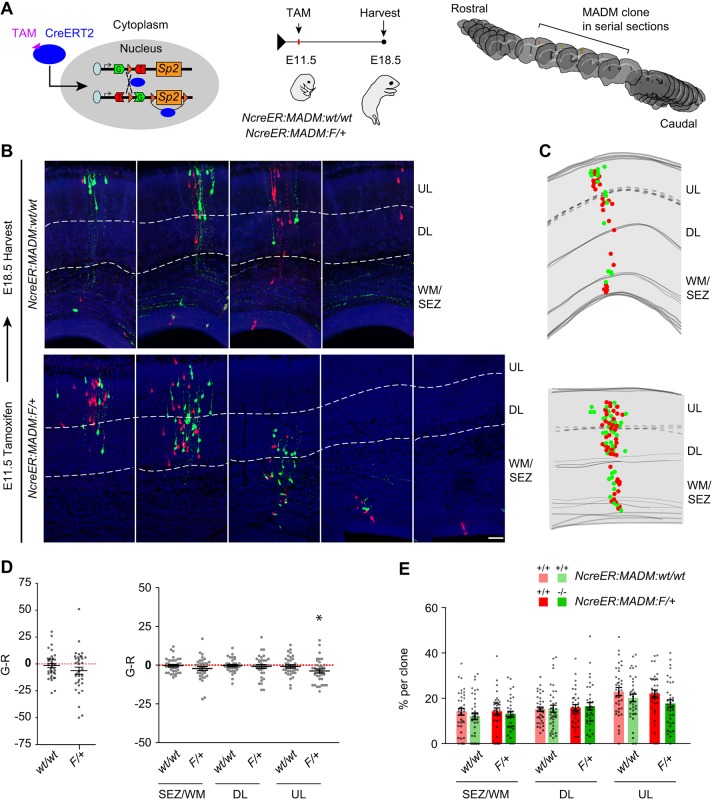
Next, to determine the contribution of individual cortical clones to the observed phenotypes, we used NestincreERT2 (NcreER) (Balordi and Fishell, 2007) on the MADM background to temporally restrict recombination via tamoxifen (TAM) administration, permitting the analysis of progeny from individually labeled progenitors generated from a specific time point (Fig. 5A). To capture the progeny of NPCs throughout the neurogenic period, tamoxifen was administered at E11.5 and embryonic brains were harvested at E18.5 (Fig. 5A). We again used wild-type and floxed Sp2 alleles in combination with the MADM-11 cassettes to generate green and red clones in the cortex. MADM-labeled clones in control mice contain daughter cells with wild-type Sp2 alleles (NcreER:MADM:wt/wt), while in experimental mice one cell will contain the floxed alleles and its sibling will be wild type for Sp2 (NcreER:MADM:F/+; Fig. S1). Analysis was restricted to clones containing both green and red cells and their distribution was quantified within the SEZ/WM, DL and UL (Fig. 5B,C). We computed G minus R (G-R) for individual clones to determine whether there were differences in lineage expansion as G:R masks the subtle differences in clonal composition and cannot be used in instances when cells are absent in a clone. Analysis of G-R revealed that clones in NcreER:MADM:F/+ cortices were biased towards Sp2+/+ cells in the UL (Fig. 5D), confirming the population-level MADM data described earlier. Cell counts across layers for all clones were not significantly different for either genotype, although total clone size was increased in NcreER:MADM:F/+ cortices (Fig. S2A). However, the variability in clone sizes in the mutant genotype was significantly higher than the variability in the control cortices in which both MADM cells are wild type (Fig. S2A). Moreover, we observed that whereas control clones had similar distributions of green and red cells throughout all layers, Sp2−/− cells exhibited a reduction trend in the UL of NcreER:MADM:F/+ cortices (Fig. 5E, Fig. S2A). The broad range of clone sizes (8-127 cells) prompted the reasoning that progenitor contributions from clones that underwent a small number of divisions (small clones) were likely more distinctly affected by loss of Sp2 than were progenitors that were continuously dividing during the E11.5-E18.5 period (large clones). To split our clonal data into large and small groups, we used the median of combined clone sizes enumerated in NcreER:MADM:wt/wt and NcreER:MADM:F/+ cortices (median, 39; n=73 clones from 20 embryos). Clones containing 40 or more cells in NcreER:MADM:F/+ mice had a significantly smaller percentage of Sp2−/− cells in the UL compared with Sp2+/+ siblings (Fig. S2B). In line with this finding, G-R in clones containing ≥40 cells indicated that Sp2+/+ cells were significantly more abundant than Sp2−/− cells in the SEZ/WM and the UL. The smaller clone sizes (≤39) were unaffected by loss of Sp2, possibly owing to their limited time in a progenitor state, as indicated by their small progeny size (Fig. S2C). These results further support our findings that loss of Sp2 in NPCs reduces population expansion with particular impact on cells that laminate the upper layers of the cortex of mice with the conditional genetic backgrounds employed in our study.
Fig. 5.
Clonal analysis of cortical NPCs in the presence and absence of Sp2. (A) Schematic of experimental paradigm to induce individual cortical clones for analysis using the Nestin-creERT2 transgenic mouse that expresses tamoxifen-inducible cre under the control of the Nestin promoter (NcreER) in combination with MADM and floxed Sp2 alleles. Tamoxifen administration at E11.5 permits cre entry into the nucleus to induce highly sparse MADM recombination events in mitotic NPCs. Embryos were sacrificed at E18.5 for fixation, serial sectioning and immunohistochemistry to visualize and quantify clones. (B) Images of serial sections containing individual clones induced at E11.5 in NcreER:MADM:wt/wt and NcreER:MADM:F/+ embryos and harvested at E18.5. Scale bar: 50 µm. (C) Representative mapped clones reconstructed from NcreER:MADM:wt/wt and NcreER:MADM:F/+ embryos. (D) G minus R (G-R) values for total clone composition and by layer in the E18.5 cortex. All layers: NcreER:MADM:wt/wt, t=0.7405, P=0.46; NcreER:MADM:F/+, t=1.894, P=0.067. SEZ/WM: NcreER:MADM:wt/wt, t=0.4133, P=0.68; NcreER:MADM:F/+, t=1.738, P=0.091. DL: NcreER:MADM:wt/wt, t=0.415, P=0.68; NcreER:MADM:F/+, t=0.6746, P=0.50. UL: NcreER:MADM:wt/wt, t=0.8134, P=0.42; NcreER:MADM:F/+, t=2.865, P=0.0071. NcreER:MADM:wt/wt, n=37; NcreER:MADM:F/+, n=35 clones. One-sample t-test. *P<0.05. (E) Laminar distribution of red and green MADM cells in individual clones (dots) by genotype. SEZ/WM: NcreER:MADM:wt/wt, t=0.9489, P=0.35; NcreER:MADM:F/+, t=0.6531, P=0.43. DL: NcreER:MADM:wt/wt, t=0.1238, P=0.9; NcreER:MADM:F/+, t=0.25, P=0.80. UL: NcreER:MADM:wt/wt, t=1.157, P=0.251; NcreER:MADM:F/+, t=1.851, P=0.067. NcreER:MADM:wt/wt, n=37; NcreER:MADM:F/+, n=35 clones. Student's t-test. *P<0.05. Dots represent values from individual embryos. Data are mean±s.e.m.
Cell division in E10.5 NSCs is Sp2 independent
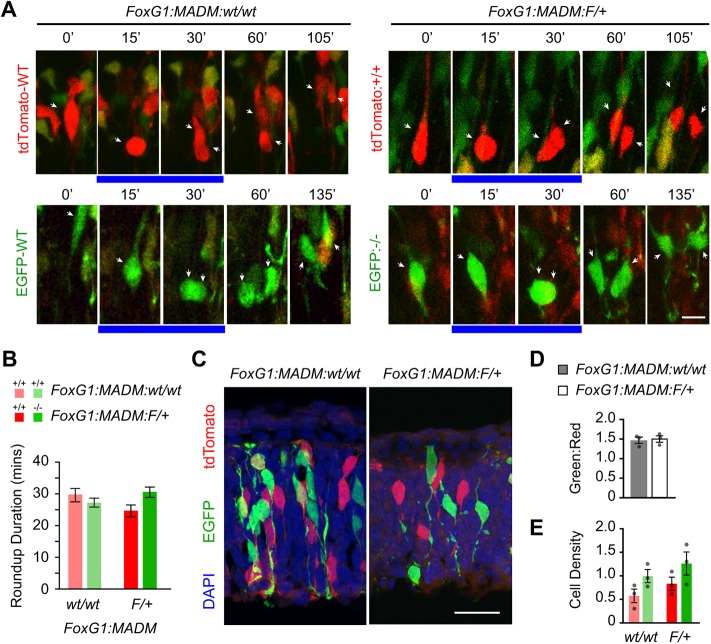
Given our results that deletion of Sp2 delays NPC M-phase progression and reduces progenitor number and neuronal production, we next investigated whether Sp2 is similarly required for these processes in early, symmetrically dividing NSCs of the E10.5 cortex. To directly monitor NSC divisions in the presence and absence of Sp2, we used mice expressing cre from the FoxG1 locus (Hébert and McConnell, 2000), as MADM recombination is inefficiently low in the E10.5 Emx1cre telencephalon in our hands (Fig. S3). Roundup duration of green and red cells was analyzed ex vivo in organotypic whole-mount cultures by time-lapse confocal en face imaging as before (Fig. 2A). We again compared baseline MADM recombination effects using wild-type Sp2 alleles (FoxG1:MADM:wt/wt, both green and red cells are wild type) with embryos with a single floxed Sp2 allele that will generate sibling pairs of Sp2−/− (green) and Sp2+/+ (red) cells (FoxG1:MADM:F/+). In E10.5 FoxG1:MADM:wt/wt cortices, where all cells have the endogenous Sp2 alleles, similar roundup durations were observed between sibling green and red cells (Fig. 6A,B). In FoxG1:MADM:F/+ cortices, Sp2−/− cells had a trend toward longer roundup durations compared with Sp2+/+ siblings; however, comparison of all cell types did not reveal a significant difference between any genotypes at E10.5 (Fig. 6A,B). These results from early NSCs are in contrast to the requirement for Sp2 in NPCs during later neurogenic (asymmetric) divisions and suggest that Sp2 is not required for proliferation in NSCs.
Fig. 6.
Sp2 is dispensable for proliferation and expansion of NSCs. (A) Representative time-lapse en face images of FoxG1:MADM:wt/wt and FoxG1:MADM:F/+ cortical wholemounts (time indicated in minutes above each panel). Arrows indicate cells tracked for each genotype and blue bars indicate frames in roundup. Scale bar: 10 µm. (B) Quantification of the time spent in roundup. FoxG1:MADM:wt/wt: red, n=30 cells; green, n=30 cells. FoxG1:MADM:F/+: red, n=34 cells; green, n=37 cells. One-way ANOVA, F=1.828, P=0.15. (C) Representative images of E10.5 FoxG1:MADM:wt/wt and FoxG1:MADM:F/+ cortices immunoenhanced for EGFP (green) and tdTomato (red). Nuclei are counterstained with DAPI (blue). Scale bar: 25 µm. (D) G:R for cells in E10.5 cortices, t=1.113, P=0.33, n=3 embryos per genotype. (E) Red and green cell density; density values are ×104/mm3. FoxG1:MADM:wt/wt, t=2.082, P=0.11; FoxG1:MADM:F/+, t=1.519, P=0.20. n=3 animals per genotype from three independent litters. Dots represent values from individual embryos. Data are mean±s.e.m.
To determine whether the Sp2−/− cell populations were decreased in FoxG1:MADM:F/+ cortices, MADM populations in FoxG1:MADM:F/+ and FoxG1:MADM:wt/wt at E10.5 were quantified (Fig. 6C). G:R analysis revealed no differences between control and FoxG1:MADM:F/+ embryos (Fig. 6D) and deletion of Sp2 in green cells did not affect MADM cell densities at this stage (Fig. 6E). Analysis of G:R and MADM cell densities in E11.5 FoxG1:MADM:F/+ and control embryos remained similar, further indicating that loss of Sp2 in early NSCs does not affect the earliest stages of corticogenesis (Fig. S4). Taken together, in contrast to NPCs, at E10.5 Sp2−/− NSCs complete roundup with similar timing to controls and generate the appropriate number of progeny by E11.5.
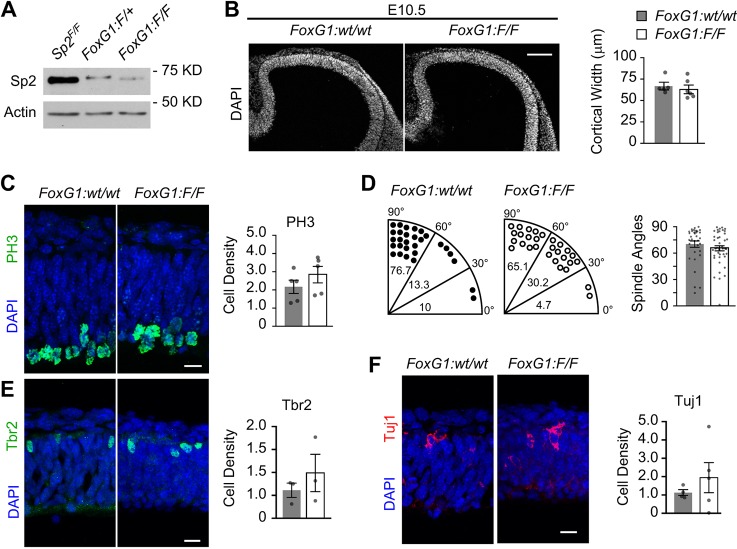
The MADM system generates clones that are Sp2−/− and Sp2+/+ within an Sp2-heterozygous background for the duration of FoxG1-cre expression in mitotic cells. This precluded us from determining whether bulk deletion of Sp2 in early NSCs would impact their ability to proliferate and expand normally, as we had observed in bulk E12.5 and E14.5 Emx1:F/F cortices in our previous study (Liang et al., 2013). To assess bulk effects of Sp2 deletion at E10.5, we generated FoxG1-cre; Sp2F/F (FoxG1:F/F) embryos and confirmed Sp2 deletion in the primordial cortex by comparison with control FoxG1-cre; Sp2+/+ (FoxG1:wt/wt) cortices using western blotting (Fig. 7A). Measurements of cortices in FoxG1:F/F embryos revealed similar widths to controls (Fig. 7B). Additionally, the densities of cells positive for the mitotic marker phospho-histone H3 (PH3) was statistically unchanged compared with controls (Fig. 7C). Comparison of spindle angles for mitotic cells in anaphase or early telophase did not reveal a significant difference in average spindle angle or distribution (Fig. 7D). To determine whether the differentiation paradigm in NSCs was altered upon loss of Sp2, we also analyzed the density of cells expressing the intermediate progenitor cell marker Tbr2 or the post-mitotic neuronal marker β-tubulin III (Tuj1). FoxG1:F/F and control cortices contained similar average densities of Tbr2+ and Tuj1+ cells (Fig. 7E,F). Our combined results in FoxG1:F/F and FoxG1:MADM:F/+ cortices support our findings that Sp2 is dispensable for proliferation and differentiation of E10.5 cortical NSCs.
Fig. 7.
Sp2−/− NSCs proliferate and differentiate in the E10.5 cortex. (A) Sample western blot of E10.5 cortical lysates blotted for Sp2 and actin shows depletion of Sp2 in FoxG1:F/+ and FoxG1:F/F cortices compared with Sp2F/F controls. Weight markers are in kilodaltons (kDa). (B) E10.5 cortices counterstained with DAPI for comparison of average width in FoxG1:wt/wt (n=5) and FoxG1:F/F (n=6) mice; t=0.5565, P=0.59. Scale bar: 100 µm. (C) High-power confocal images of E10.5 cortices immunostained for PH3 and counterstained with DAPI. Quantification of PH3 densities in FoxG1:wt/wt (n=5) and FoxG1:F/F (n=5) cortices; t=1.147, P=0.28. (D) Spindle angle distribution of PH3+ cells in late anaphase or early telophase, as indicated by bins (percent per segment). Angles were measured for the cleavage planes relative to the apical surface. FoxG1:wt/wt, n=33; FoxG1:F/F, n=42; t=0.9107, P=0.37. (E) High-power images of E10.5 cortices immunostained for Tbr2 and counterstained with DAPI. Quantification of Tbr2 densities in FoxG1:wt/wt (n=3) and FoxG1:F/F (n=3) cortices; t=0.8435, P=0.45. (F) High-power images of E10.5 cortices immunostained for Tuj1 and counterstained with DAPI. Quantification of Tuj1 densities in FoxG1:wt/wt (n=4) and FoxG1:F/F (n=5) cortices; t=0.8903, P=0.40. Densities are values ×105 cells/mm3. Dots in charts represent values for individual embryos. Scale bars: 25 µm in C,E,F. Embryos were from at least three independent litters for each genotype. Student's t-test. Dots represent values from individual embryos. Data are mean±s.e.m.
Given the evidence that FoxG1 has a role in proliferation of NPCs and its haploinsufficiency has a neurodevelopmental phenotype (Manuel et al., 2011; Martynoga et al., 2005), we further confirmed that our results were due to loss of Sp2 rather than to FoxG1 haploinsufficiency caused by the knock-in of cre into the FoxG1 locus (Eagleson et al., 2007) by using the recently developed FoxG1IRES-cre mouse. The IRES-cre cassette was inserted into the 3′ UTR of the FoxG1 locus, thereby permitting simultaneous transcription of both FoxG1 and cre without altering endogenous FoxG1 expression or inducing a neurodevelopmental phenotype (Kawaguchi et al., 2016). Western blotting confirmed depletion of Sp2 in FoxG1IRES-cre; Sp2F/F (FoxG1IC:F/F) telencephalons at E10.5 (Fig. S5A,B). Gross measurement of cortical width was similar between FoxG1IC:F/F and littermate Sp2F/F embryos (Fig. S5C) and staining for the same markers of differentiation and proliferation (Tbr2, Tuj1, and PH3) as we did previously for FoxG1:F/F embryos again did not reveal any significant differences in cell densities or spindle angles (Fig. S5D,E,F). Altogether, these results indicate that unlike NPCs, proliferation and differentiation of E10.5 cortical NSCs can occur in the absence of Sp2.
DISCUSSION
Sp2-dependent control of mitosis and cortical neurogenesis
Our current study reveals a stage-specific requirement for Sp2 in proliferation and population size control of neurogenic NPCs but not symmetrically dividing NSCs of the developing cortex. We have previously reported that loss of Sp2 in NPCs results in cell cycle progression defects with prolonged M phase and a smaller forebrain at postnatal stages (Liang et al., 2013). Here, we find that prolonged M phase in Sp2−/− NPCs is due to longer prophase and prometaphase combined with decreased metaphase duration. Such mitotic abnormalities are the predominant phenotype resulting from mutations that underlie microcephaly (Faheem et al., 2015). Many of the microcephaly-associated mutations can result in chromosome segregation errors, chromatin breakage or defects in abscission (last stage of cytokinesis); however, a unifying mechanism underlying microcephaly remains unclear (Gilmore and Walsh, 2013; Li et al., 2016). Nevertheless, a number of sensitive cell biological phenomena have been identified to be susceptible to defects in various stages of mitosis.
For example, the bipolar morphology of NPCs consists of a restricted area of their membrane at the apical surface of the developing cerebral ventricles containing polarity proteins, inheritance of which by daughter cells may be important for determining cell fate (Kosodo et al., 2004; Uzquiano et al., 2018). Mutation of a kinesin motor protein (Kif20b) alters the midbody structure, which typically forms at the apical surface in dividing NPCs and is correlated with widespread apoptosis that results in a smaller brain (Janisch et al., 2013; Little and Dwyer, 2019). Other mutations can affect multiple mitotic stages, in particular prometaphase. Delays in prometaphase and increased apoptosis occur upon knockdown of multiple microcephaly genes in the zebrafish neuroepithelium (Novorol et al., 2013). Moreover, a Wdr62 microcephaly mouse model contains NPCs with delayed prometaphase, resulting in apoptosis (Chen et al., 2014). Pharmacological lengthening of mitosis in the mouse cortex leads to p53-dependent apoptosis, while less severe delays trigger premature terminal symmetric divisions that generate two postmitotic cells (Pilaz et al., 2016). Premature differentiation in the absence of apoptosis likely represents a protective mechanism that ensures blockade of proliferation in unhealthy cells (Uetake and Sluder, 2010). Given that mutations to mitotic regulators will affect multiple mitotic stages, and that errors in mitotic spindle formation may cause defective abscission, it remains unclear whether disruption of any one phase underlies microcephaly or whether a cumulative effect is the culprit. Altogether, these studies suggest that the timing of mitosis, especially prometaphase, may itself be a mechanism of cell fate regulation. Our current findings illustrate that prolonged prophase and prometaphase in Sp2−/− NPCs attenuates population expansion, with lasting effects throughout the period of neurogenesis. How Sp2-dependent control of mitotic length affects the fidelity of the mitotic spindles, midbody structure and inheritance of apically localized fate determinants remains to be studied in the future.
MADM reveals cell-autonomous and non-autonomous roles of Sp2 in control of population size in the cortex
The current study used the MADM system, a powerful tool that permits quantitative lineage analysis of genetically manipulated, clonally derived sibling cells at sparse levels (Zong et al., 2005). Our previous results from bulk-deleted Sp2 cortices using Emx1cre showed alterations in the relative widths of Cux1- and Ctip2-containing upper and deep layers at P0 (Liang et al., 2013). However, that study did not include a quantitative analysis of cell densities within these layers, thus the altered distribution of the cortical cell types could not be attributed to variations in their numbers. Furthermore, as in the bulk model most cells within the cortex should be deleted for Sp2 by ∼E12.5 using Emx1cre (Gorski et al., 2002; Liang et al., 2012), tissue-level phenotypes associated with loss of Sp2 could not be differentiated between cell-autonomous and non-autonomous mechanisms. Comparison of our current MADM results with the phenotypes reported in the past study suggest that aspects of the phenotypes seen in the bulk model are likely due to cell non-autonomous events, particularly in affecting the deep layer neurons, which are specified between E11.5-E14.5 (Dwyer et al., 2016; Molyneaux et al., 2007).
Our clonal lineage MADM experiments reveal that cortical clones arising from Sp2−/− and Sp2+/+ sibling cells at E11.5 have reduced Sp2−/− cells in the upper layers of the cortex, a consistent phenotype at the population level as we observed in the newborn Emx1:MADM:F/+ cortices. We observed a significant increase in clone size in our NcreER:MADM:F/+ experiments, with clones containing 40 or more total cells demonstrating a stronger phenotype in response to Sp2 deletion. These clones likely reflect NPCs that underwent several rounds of symmetric proliferative divisions and/or generated IPCs that continued to divide. Additional rounds of symmetric proliferation would ensure complete depletion of Sp2 protein prior to the neurogenic transition. Another possibility is that IPCs are more sensitive to loss of Sp2 exerting greater impact on upper layer neurons production, which occurs later during cortical development. This is in line with our previous finding that bulk conditional deletion of Sp2 by Emx1cre decreased the percentage of proliferating IPCs (Liang et al., 2013). Moreover, clonal analysis of IPC lineages has revealed IPCs are capable of contributing to all layers in a birthdate-dependent manner, with most of the lineage settling in the upper layers (Mihalas and Hevner, 2018; Vasistha et al., 2015). Reduction of IPCs by conditional deletion of Tbr2 early in neurodevelopment has greatest impact on the development of upper cortical layers (Arnold et al., 2008; Mihalas et al., 2016), likely due to increased IPC production and proliferation as neurodevelopment proceeds (Kowalczyk et al., 2009; Martínez-Cerdeño et al., 2006). IPCs themselves proliferate to directly amplify neuron populations, but they also express delta ligands on filipodia that interact with NPCs to activate Notch signaling and prevent their premature differentiation into IPCs, thereby maintaining the NPC pool (Gaiano et al., 2000; Nelson et al., 2013; Yoon et al., 2008). Therefore, subtle changes to the IPC population could influence the proliferative capacity of NPCs reducing direct and indirect neuronal production, with the greatest cumulative effect on the upper layers.
The smaller NcreER:MADM:wt/wt and NcreER:MADM:F/+ clones could be undergoing a pre-defined program of division and differentiation that is implemented in early stages of cortical development and is not impacted by loss of Sp2, or these clones may not have contained proliferative IPCs. Our FoxG1:MADM:F/+ data at E11.5 indicated that Sp2 depletion in NSCs did not impact the earliest stages of neurogenesis, when IPCs are small in number and deep layer neurons are being generated. In addition, the Emx1:MADM:F/+ analysis indicates that progenitor loss is apparent around E14.5 in the VZ/SVZ, but has not yet manifested in the IZ or CP. Therefore, it appears that later stages of neurogenesis may be more sensitive to loss of Sp2 from NPCs. How Sp2 cell-autonomously regulates IPC production or proliferation is an area of future study. In summary, our results demonstrate that loss of Sp2 most strongly affects NPCs at stages when IPCs and upper layer neurons are generated, resulting in decreased upper layer neurons at P0.
The overall increase in clone sizes observed in NcreER:MADM:F/+ cortices compared with the attenuated populations we observed in the Emx1:MADM:F/+ cortices at P0 could be a consequence of differences between the induction of genetic backgrounds in these experiments. While Emx1:MADM:F/+ cortices will largely be heterozygous for Sp2 by E12.5, NcreER:MADM:F/+ cortices will only sparsely generate Sp2 heterozygous cells in the background due to the low single pulse of tamoxifen. Furthermore, the clonal siblings in these mice are generated from a mother cell that is expressing Sp2 from both the floxed and wild-type alleles, which could alter the initial levels of Sp2 in nascent clones compared with our other models, which may be heterozygous for Sp2 prior to MADM cell generation and expressing Sp2 from a single allele. Another possibility is that the clones generated in NcreER:MADM:F/+ cortices undergo several rounds of symmetric proliferative divisions at the time of induction before transitioning to neurogenic divisions. In comparison, the extended period of MADM recombination in Emx1:MADM:F/+ cortices likely results in more newly born MADM cells generating IPCs or neurons directly. Notwithstanding these potential technical limitations, our data clearly illustrate that Sp2 regulates the expansion of the cellular population generated during neurogenic divisions with a significant effect on production of upper layer neurons, especially in large clones labeled at E11.5.
A curious finding in our analysis of MADM populations is the relative overabundance of green (EGFP+) relative to red (tdTomato+) cells in Emx1:MADM:wt/wt cortices at E14.5, where both populations are wild type in genotype and theoretically expected to be proportional in numbers. This finding matches the previous report of a similar ratio at E14.5 using the same mice (Hippenmeyer et al., 2010) and both studies indicate that this ratio normalizes in cortices of newborn mice. We find that this discrepancy in MADM green:red ratio is greatest in the E14.5 VZ/SVZ, where the majority of MADM cells are newly generated from recent cell divisions. Possible explanations for this discrepancy include potential differences in fluorescent protein stability or visualization. The normalization of cell numbers at P0 in our Emx1:MADM:wt/wt cortices indicates this is not a persistent phenotype and is likely due to differences in fluorescent protein expression onset, and highlights the importance of a wild-type control for these experiments.
Sp2 controls division of NPCs during neurogenesis but not NSCs during early expansive divisions
Using two different FoxG1 knock-in cre lines with clonal and bulk deletion analysis, we found that the size of the progenitor pool and round up duration in NSCs during early cortical development appears to be independent of Sp2 expression. In contrast, G:R in Emx1:MADM:F/+ cortices was reduced in the E14.5 VZ/SVZ, and at P0 Sp2−/− cell density was decreased in the SEZ, WM and UL. We observe significant depletion of Sp2 protein in bulk E10.5 FoxG1:F/F cortices, yet it is possible that the short period from induction of FoxG1-cre expression in NSCs around E8.5 to our observation time at E10.5 is only partially sufficient for Sp2 depletion in MADM clones. Although the majority of cells in the cortex expressing Emx1cre or FoxG1-cre are likely deleted for the Sp2 floxed allele near the onset of cre expression, it is possible that some cells escape the cis-Sp2 deletion or have persistent Sp2 expression over several rounds of division. However, we have previously demonstrated that sorted P0 MADM cells generated using a Nestin-cre transgene genotype correctly (Liang et al., 2013), indicating the cis-deletion of Sp2 occurs sufficiently in mice on the compound MADM background. Furthermore, the presence of a putative PEST domain within Sp2 suggests it is rapidly degraded and therefore unlikely to be present for long after deletion (Bouwman and Philipsen, 2002). Therefore, our analysis of Sp2−/− MADM cells and bulk deletion experiments using two different cre-driver lines support our findings at E10.5 that Sp2 is dispensable for proliferation or differentiation at this stage in NSCs.
The stage-specific sensitivity of NPCs to loss of Sp2 highlights mechanistic differences between early and late cell divisions in the developing cortex, matching previous reports. An important difference between NSCs and NPCs is a change in cell cycle duration, in particular through NPC-specific lengthening of G1 (Miyama et al., 1997). The ability of NSCs to proliferate and generate a sufficient stem cell pool is an important determinant of overall brain size (Gilmore and Walsh, 2013). However, neurogenic divisions by NPCs appear to be more sensitive to subtle disruptions of the cell cycle (Hu et al., 2014). G1 lengthening occurs immediately prior to the onset of neurogenic divisions and manipulating its length promotes or inhibits cell differentiation (Salomoni and Calegari, 2010). Cells from younger cortices can adopt the correct temporal fate of an older environment if transplanted before entering S-phase, indicating that the plasticity of younger progenitors and developmentally defined environments can promote specific cell fates during G1 (Desai and McConnell, 2000; Frantz and McConnell, 1996; McConnell and Kaznowski, 1991). As G1 is a period when cell signaling and differentiative factors are active, a short G1 may prevent the interaction of Sp2 with an activating or inhibitory co-factor. For example, an inhibitory cofactor for Sp2 has been identified in mammalian cell extracts (Moorefield et al., 2004), which may play a role in early NSCs in the E10.5 cortex. Moreover, increased signaling during prolonged G1 may lead to an activating post-translational modification of Sp2. In addition, alternative isoforms of Sp2 could play differential roles in regulation of NPCs and NSCs as there are multiple Sp2 variants expressed from different start sites. At least two distinct cell-type specific promoters for Sp2 in mouse organs outside the brain have been identified, and the 5′ end of Sp2 transcript is subject to alternative splicing (Yin et al., 2010). Although we found that Sp2 is present at both stages by western blotting, the antibody we used fails to distinguish potential alternative isoforms that may be expressed during early and late cortical development. Additionally, there is the possibility that Sp2 is involved with mechanisms in NSCs that are compensated for by other Sp factors both during its experimental deletion, as well as during early NSC cell divisions. Sp factors (Sp1-Sp9) are transcribed from loci on four chromosomes in both mice and humans, where they appear as pairs on three chromosomes and as a triple on one. Sp1 and Sp3 share close structural homologies with Sp2, and are expressed in the cortex at both the early NSC and neurogenic NPC stages (Allen Brain Atlas, developingmouse.brain-map.org/; and Gene Paint, gp3.mpg.de/), suggesting that they may partially compensate for the loss of Sp2. In addition, Sp5 and Sp8 are expressed in the cortex and are downstream effectors of the Wnt signaling pathway (Harrison et al., 2000; Kennedy et al., 2016). Sp5 and Sp8 have reciprocal expression patterns in the developing cortex, where Sp5 expression is downregulated by E13.5 while Sp8 expression is maintained (Sahara et al., 2007). Sp5 has recently been shown to promote NPC proliferation in vivo (Li and Jiao, 2020). The expression of other Sp factors in the cortex is not characterized, but some family members may compensate for loss of Sp2 in NPCs or the regulation of early NSC division individually or cooperatively.
Conclusions
In summary, we have demonstrated a stage-specific requirement for Sp2 in NPCs but not NSCs of the developing cortex. How Sp2 in particular and other Sp factors in general regulate cell division and control of population sizes in various tissues is the subject of future studies. The underlying mechanisms for the temporally restricted requirement for Sp2 is also an area of interest and future research will likely provide further insight into the differences between proliferative and neurogenic divisions in the cortex. Finally, whether tissues that continue to regenerate throughout life (e.g. skin and many internal organs) employ similar mechanisms during expansive and differentiative divisions of stem and progenitor cells during various stages of their development and maintenance is an intriguing issue that requires comparative studies employing similar bulk, population and clonal analytical approaches described in the current study.
MATERIALS AND METHODS
Animal husbandry
Animals were housed at the Laboratory Animal Resources Facility under the guidelines of the Institutional Animal Care and Use Committee and North Carolina State University. Animals were fed ad libitum and housed on a 12 h light/dark cycle. Generation of Sp2 floxed mice has been described previously (Liang et al., 2013). Conditional deletion of the floxed Sp2 allele was achieved through crosses to mice carrying FoxG1-cre [129(Cg)-Foxg1tm1(cre)Skm/J, Jackson Laboratories, 004337], FoxG1IREScre [B6.129T(SJL)-Foxg1tm1.1(cre)Ddmo/J, Jackson Laboratories, #029690] or Emx1IREScre [B6.129S2-Emx1tm1(cre)Krj/J, Jackson Laboratories, 005628]. Cre recombinase activity was reported by crossing to mice with the tdTomato floxed-stop cassette [B6.129S6-Gt (ROSA) 26Sortm14 (CAG-tdTomato) Hze/J; Jackson Laboratories, 007908].
Mice for mosaic analyses were generated using breeding schemes that have been previously described (Hippenmeyer et al., 2010). Briefly, Sp2 floxed mice (Sp2F/F) were crossed with MADM-11TG/TG mice [Igs2tm2(ACTB-TdTomato,-EGFP)Luo/J, Jackson Laboratories, 013751] to generate Sp2F/F; MADM-11TG/TG mice via meiotic recombination. MADM-11GT/GT mice [Igs2tm1(ACTB-EGFP,-tdTomato)Luo/J, Jackson Laboratories, 013749] were crossed with FoxG1-cre, FoxG1IREScre, Emx1IREScre or NestincreERT2 mice [Tg(Nes-cre/ERT2)1Fsh, a generous gift from Gord Fishell, Harvard University, Cambridge, MA, USA)] to generate FoxG1-cre; MADM-11GT/GT, FoxG1IREScre; MADM-11GT/GT, Emx1IREScre; MADM-11GT/GT or NestincreERT2; MADM-11GT/GT mice. For experiments, Sp2F/F; MADM-11TG/TG mice were crossed with MADM-11GT/GT mice with the appropriate cre driver. Control mice were generated by crossing FoxG1-cre; MADM-11GT/GT, FoxG1IREScre; MADM-11GT/GT, Emx1IREScre; MADM-11GT/GT or NestincreERT2; MADM-11GT/GT mice to MADM-11TG/TG mice. For tamoxifen inductions, timed pregnant dams were administered 50 µg/g body weight tamoxifen (Sigma-Aldrich, 10 mg/ml in corn oil) by oral gavage via a plastic feeding tube (Instech).
Genotyping
Sp2 floxed mice were genotyped for the presence of the wild-type and floxed alleles using the primers: TAF-4, 5′-GCAGTGCTGGGGTCGCATGTGTGAGCTGTAGCCTTGCTTC-3′; and TAR-2, 5′-CGCTCAAGCCCCATTGCTGGGCCTGGTGACAA-3′. All other mouse lines were genotyped using protocols provided by Jackson Laboratories.
Tissue processing and immunohistochemistry
Pregnant dams were sacrificed by Avertin overdose (2,2,2 tribromoethanol; 7.5 mg/g body weight) followed by cervical dislocation at the indicated embryonic ages. Embryos were visualized using a dissection microscope (Zeiss steREO Discovery.V12). For embryos younger than E14.5, the entire embryo or head was fixed in 4% PFA in PBS for 6 h at room temperature. Tissue was then submerged in 15% sucrose in PBS overnight followed by 30% sucrose in PBS overnight at 4°C. Embryos were embedded in OCT (Sakura) and snap frozen in a dry ice and ethanol bath. Frozen embryos were sectioned at 25 µm on a cryostat (Leica CM1850). Embryonic brains at E14.5 and older were dissected and submerged into 4% PFA in PBS at 4°C overnight. Brains were embedded in 3% low melting point DNA-grade agarose in 0.01 M PBS and 40-50 µm sections were generated using a vibratome (Leica VT1000S).
Immunohistochemistry
Floating sections were washed with 1× PBS then blocked for 1 h at room temperature in blocking buffer (5% normal donkey or goat serum, 0.15% Triton X-100, 1× PBS). Sections were incubated with primary antibodies diluted in blocking buffer overnight at 4°C, followed by three 5 min washes with 1× PBS at room temperature. Secondary antibodies diluted in blocking buffer were incubated with the sections for 1 h at room temperature, followed by three washes with 1× PBS. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) at 1:5000 during the secondary incubation. Sections were mounted onto glass slides and coverslipped with Faramount aqueous mounting medium (Dako). Images were acquired using an FV1000 confocal microscope (Olympus).
Antibodies
Primary antibodies used were: anti-Actin-HRP (Santa Cruz Biotechnology, sc-1615, 1:5000), anti-GAPDH-HRP (Santa Cruz Biotechnology, sc20357, 1:5000), chicken anti-GFP (Abcam, ab13970, 1:2000), rabbit anti-RFP (Abcam, ab62341, 1:500), mCherry (Abcam, ab167453, 1:1000), rabbit anti-Tbr2 (Abcam, ab23345, 1:300), mouse anti-Tuj1 (Covance, 801201, 1:500), mouse anti-Pax6 (Chemicon, MAB5554, 1:500), rabbit anti-Pax6 (Covance, 901301, 1:500), mouse anti-PH3 (Abcam, ab14955, 1:1000), rabbit anti-Sp2 (Sigma-Aldrich, HPA003357, 1:1000), rabbit anti-Sp2 (Novus, NB100-92358, 1:1000) and mouse anti-NeuN (Millipore, MAB377, 1:1000). Secondary antibodies used were: goat anti-rabbit HRP (Santa Cruz Biotechnology, sc-2004, 1:10,000), Clean-blot IP detection reagent (Thermo Fisher Scientific, 21230, 1:500), AlexaFluor goat anti-rabbit 488 (Thermo Fisher Scientific, A11008, 1:500), AlexaFluor goat anti-mouse 647 (Thermo Fisher Scientific, A21235, 1:500), AlexaFluor goat anti rabbit Cy3 (Thermo Fisher Scientific, A10520, 1:500), AlexaFluor goat anti-chicken 488 (Thermo Fisher Scientific, A11039, 1:500), AlexaFluor goat anti-rabbit 647 (Thermo Fisher Scientific, A21244, 1:500), AlexaFluor donkey anti-rabbit 488 (Thermo Fisher Scientific, A21206, 1:500), AlexaFluor donkey anti-rabbit 647 (Thermo Fisher Scientific, A21244, 1:500), donkey anti-chicken 488 (Jackson ImmunoResearch, 703-545-155, 1:500) and donkey anti-mouse 647 (Jackson ImmunoResearch, 715-605-150, 1:500).
In utero electroporation
Timed pregnant dams were anesthetized using 5% isoflurane mixed with oxygen then maintained throughout surgery with 1.5% isoflurane. The abdomen was opened and the uterine horns were exposed. Embryos were kept warm and moist with 37°C 0.9% saline. The plasmid pCAG-H2B::mcherry (Addgene) was mixed with 0.5% Fast Green dye to a concentration of 0.5 µg/µl. An air-pressurized injector (Picospritzer III) was used to drive plasmid DNA into the ventricles via micropipette (WPI) at 30 psi. A square wave generator (ECM830) and tweezertrodes (BTX) were set to five electric pulses of 35 mV at 50 ms intervals. The uterus was returned to the abdomen and the abdominal wall and skin were sutured using 4-0 braided silk and a 3/8 reverse cutting needle (Henry Schein). Embryos were harvested after surgery at indicated experimental end points.
Time-lapse imaging
Embryonic brains were prepared for live imaging as wholemounts or slices in culture. For slice cultures, brains were embedded in 3% agarose in complete HBSS [2.5 mM HEPES, 30 mM D-glucose, 1 mM CaCl2, 1 mM MgSO4 and 4 mM NaHCO3 in 1× Hank's Buffered Salt Solution (HBSS)]. Slices of 200 µm thickness were generated using a vibratome (Leica). Slices were placed on a 0.4 µm filter (Millipore) in a 35-mm glass bottom dish (WPI) containing slice culture media [20 mM D-glucose, 1 mM L-glutamine, 25% complete HBSS, 1% penicillin-streptomycin and 5% fetal bovine serum in Basal Medium Eagle (Sigma-Aldrich)]. A stage top incubator (Tokai Hit) was heated to 50°C on its lid and the chamber environment was maintained at 37°C and circulated with 5% CO2. Samples were imaged on an FV1000 confocal microscope (Olympus) every 10-15 min. Mitotic stages were defined using previously established protocols (Pilaz and Silver, 2014). Prophase and prometaphase stages were identified by puncta of chromatin to the fuzzy circular alignment of condensed chromosomes as they begin to attach to the spindle. Metaphase was determined when the plate formed by alignment of chromosomes at the cell equator was visible.
Western blotting
Whole brain or dissected cortices harvested for western blotting were collected into RIPA lysis buffer containing protease and phosphatase inhibitors (Roche, Bio-Rad) and homogenized using a bullet blender followed by sonication. Protein concentration of lysates was determined using a BCA assay (Pierce) and equal amounts of protein were loaded onto 8-10% SDS-Page gels. Separated proteins were transferred to a PVDF membrane (Bio-Rad) in Dunn's Carbonate buffer. Blots were blocked using 5% non-fat milk (LabScientific) in 1× TBS 0.12% Tween-20 (TBS-Tween) for 1 h at room temperature. Primary antibodies were diluted as indicated in 5% milk, TBS-Tween and incubated overnight at 4°C. Blots were washed three times for 5 min in TBS-Tween then incubated in secondary antibodies diluted as indicated in 5% milk, TBS-Tween for 1 h at room temperature. Blots were then washed six times for 5 min with TBS-Tween. All washes were conducted at room temperature. Chemiluminescent signal was generated by incubating blots for 1 min at room temperature with Clarity enhanced chemiluminescence reagent (Bio-Rad) and detected by X-Ray film. Blots were scanned and analyzed using FIJI software.
Quantification and statistical analyses
E14.5 MADM quantifications were performed within a 250 µm wide randomly selected window at least twice within a section and at least three sections were analyzed per animal. Density was determined by measuring the cortical height and multiplying by the window width and tissue thickness (40 µm). For P0 Emx1:MADM quantifications, cell counts were conducted in 300 µm×300 µm grids randomly positioned in motor and somatosensory cortices in tile-scanned sagittal confocal images. A total of 30 grids were counted from three sagittal sections per animal. In the individual clonal lineage experiments, 50 µm serial sections were analyzed for clone presence, with one section containing no cells on either side to demarcate clone boundaries. For E10.5 FoxG1:MADM quantifications, all MADM cells within 210×210 µm grids were counted and cell densities were determined by multiplying cells counted within the grids by tissue thickness (∼25 µm). E10.5 cell density quantifications for bulk deletion experiments were calculated by counting all cells within 150×150 µm grids and densities were calculated by multiplying counted cell numbers within the grids by tissue thickness. Images were analyzed using FIJI software. Statistical analysis was performed in Microsoft Excel and GraphPad Prism. Data are reported as mean±s.e.m. Statistical tests were performed as indicated. Figures were assembled using Adobe Illustrator CS6. For statistical analyses, groups were tested for normality using the D'Agostino-Pearson normality test when sample sizes were sufficient to be tested by this method. Samples violating the assumption of normality were tested by non-parametric analyses as indicated.
Supplementary Material
Acknowledgements
We thank Dr Simon Hippenmeyer (IST Austria) for insightful discussions and Dr Xuying Zhang provided invaluable technical support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.A.J., H.T.G.; Methodology: C.A.J., H.T.G.; Formal analysis: C.A.J., H.T.G.; Investigation: C.A.J.; Writing - original draft: C.A.J., H.T.G.; Writing - review & editing: C.A.J., H.T.G.; Supervision: H.T.G.; Funding acquisition: H.T.G.
Funding
This work was funded by the National Institutes of Health (R01NS089795) and by institutional support to H.T.G. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.186056.supplemental
References
- Arnold S. J., Huang G.-J., Cheung A. F. P., Era T., Nishikawa S.-I., Bikoff E. K., Molnár Z., Robertson E. J. and Groszer M. (2008). The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 22, 2479-2484. 10.1101/gad.475408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balordi F. and Fishell G. (2007). Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 27, 14248-14259. 10.1523/JNEUROSCI.4531-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur F., Nau K., Sadic D., Allweiss L., Elsässer H.-P., Gillemans N., de Wit T., Krüger I., Vollmer M., Philipsen S. et al. (2010). Specificity protein 2 (Sp2) is essential for mouse development and autonomous proliferation of mouse embryonic fibroblasts. PLoS ONE 5, e9587 10.1371/journal.pone.0009587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertipaglia C., Gonçalves J. C. and Vallee R. B. (2018). Nuclear migration in mammalian brain development. Semin. Cell Dev. Biol. 82, 57-66. 10.1016/j.semcdb.2017.11.033 [DOI] [PubMed] [Google Scholar]
- Bianchi F. T., Tocco C., Pallavicini G., Liu Y., Vernì F., Merigliano C., Bonaccorsi S., El-Assawy N., Priano L., Gai M. et al. (2017). Citron kinase deficiency leads to chromosomal instability and TP53-sensitive microcephaly. Cell Rep. 18, 1674-1686. 10.1016/j.celrep.2017.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J., Roberts E., Mochida G. H., Hampshire D. J., Scott S., Askham J. M., Springell K., Mahadevan M., Crow Y. J., Markham A. F. et al. (2002). ASPM is a major determinant of cerebral cortical size. Nat. Genet. 32, 316-320. 10.1038/ng995 [DOI] [PubMed] [Google Scholar]
- Bouwman P. and Philipsen S. (2002). Regulation of the activity of Sp1-related transcription factors. Mol. Cell. Endocrinol. 195, 27-38. 10.1016/S0303-7207(02)00221-6 [DOI] [PubMed] [Google Scholar]
- Chen S.-J., Artlett C. M., Jimenez S. A. and Varga J. (1998). Modulation of human α1(I) procollagen gene activity by interaction with Sp1 and Sp3 transcription factors in vitro. Gene 215, 101-110. 10.1016/S0378-1119(98)00268-6 [DOI] [PubMed] [Google Scholar]
- Chen J.-F., Zhang Y., Wilde J., Hansen K. C., Lai F. and Niswander L. (2014). Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 5, 3885 10.1038/ncomms4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A. R. and McConnell S. K. (2000). Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development 127, 2863-2872. [DOI] [PubMed] [Google Scholar]
- Dwyer N. D., Chen B., Chou S.-J., Hippenmeyer S., Nguyen L. and Ghashghaei H. T. (2016). Neural stem cells to cerebral cortex: emerging mechanisms regulating progenitor behavior and productivity. J. Neurosci. 36, 11394-11401. 10.1523/JNEUROSCI.2359-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson K. L., Schlueter McFadyen-Ketchum L. J., Ahrens E. T., Mills P. H., Does M. D., Nickols J. and Levitt P. (2007). Disruption of Foxg1 expression by knock-in of cre recombinase: effects on the development of the mouse telencephalon. Neuroscience 148, 385-399. 10.1016/j.neuroscience.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faheem M., Naseer M. I., Rasool M., Chaudhary A. G., Kumosani T. A., Ilyas A. M., Pushparaj P., Ahmed F., Algahtani H. A., Al-Qahtani M. H. et al. (2015). Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics 8 Suppl. 1, S4 10.1186/1755-8794-8-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas L. M. and Huttner W. B. (2008). The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr. Opin. Cell Biol. 20, 707-715. 10.1016/j.ceb.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Frantz G. D. and McConnell S. K. (1996). Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron 17, 55-61. 10.1016/S0896-6273(00)80280-9 [DOI] [PubMed] [Google Scholar]
- Gai M. and Di Cunto F. (2017). Citron kinase in spindle orientation and primary microcephaly. Cell Cycle 16, 245-246. 10.1080/15384101.2016.1252584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N., Nye J. S. and Fishell G. (2000). Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26, 395-404. 10.1016/S0896-6273(00)81172-1 [DOI] [PubMed] [Google Scholar]
- Gao P., Postiglione M. P., Krieger T. G., Hernandez L., Wang C., Han Z., Streicher C., Papusheva E., Insolera R., Chugh K. et al. (2014). Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159, 775-788. 10.1016/j.cell.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore E. C. and Walsh C. A. (2013). Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip. Rev. Dev. Biol. 2, 461-478. 10.1002/wdev.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. A., Talley T., Qiu M., Puelles L., Rubenstein J. L. R. and Jones K. R. (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309-6314. 10.1523/JNEUROSCI.22-15-06309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Houzelstein D., Dunwoodie S. L. and Beddington R. S. P. (2000). Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev. Biol. 227, 358-372. 10.1006/dbio.2000.9878 [DOI] [PubMed] [Google Scholar]
- Hébert J. M. and McConnell S. K. (2000). Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol. 222, 296-306. 10.1006/dbio.2000.9732 [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S., Youn Y. H., Moon H. M., Miyamichi K., Zong H., Wynshaw-Boris A. and Luo L. (2010). Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron 68, 695-709. 10.1016/j.neuron.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem C. C. F., Repic M. and Knoblich J. A. (2015). Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 16, 647-659. 10.1038/nrn4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. F., Pomp O., Ben-Omran T., Kodani A., Henke K., Mochida G. H., Yu T. W., Woodworth M. B., Bonnard C., Raj G. S. et al. (2014). Katanin p80 regulates human cortical development by limiting centriole and cilia number. Neuron 84, 1240-1257. 10.1016/j.neuron.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. and Kosodo Y. (2005). Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 17, 648-657. 10.1016/j.ceb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Janisch K. M., Vock V. M., Fleming M. S., Shrestha A., Grimsley-Myers C. M., Rasoul B. A., Neale S. A., Cupp T. D., Kinchen J. M., Liem K. F. et al. (2013). The vertebrate-specific Kinesin-6, Kif20b, is required for normal cytokinesis of polarized cortical stem cells and cerebral cortex size. Development 140, 4672-4682. 10.1242/dev.093286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. A., Wright C. E. and Ghashghaei H. T. (2017). Regulation of cytokinesis during corticogenesis: focus on the midbody. FEBS Lett. 591, 4009-4026. 10.1002/1873-3468.12676 [DOI] [PubMed] [Google Scholar]
- Kawaguchi D., Sahara S., Zembrzycki A. and O'Leary D. D. M. (2016). Generation and analysis of an improved Foxg1-IRES-Cre driver mouse line. Dev. Biol. 412, 139-147. 10.1016/j.ydbio.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Chalamalasetty R. B., Thomas S., Garriock R. J., Jailwala P. and Yamaguchi T. P. (2016). Sp5 and Sp8 recruit β-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription. Proc. Natl Acad. Sci. USA 113, 3545-3550. 10.1073/pnas.1519994113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y., Röper K., Haubensak W., Marzesco A.-M., Corbeil D. and Huttner W. B. (2004). Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 23, 2314-2324. 10.1038/sj.emboj.7600223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T., Pontious A., Englund C., Daza R. A. M., Bedogni F., Hodge R., Attardo A., Bell C., Huttner W. B. and Hevner R. F. (2009). Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb. Cortex 19, 2439-2450. 10.1093/cercor/bhn260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. and Jiao J. (2020). Deficiency of TRPM2 leads to embryonic neurogenesis defects in hyperthermia. Sci. Adv. 6, 1-15. 10.1126/sciadv.aay6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Bielas S. L., Zaki M. S., Ismail S., Farfara D., Um K., Rosti R. O., Scott E. C., Tu S., Chi N. C. et al. (2016). Biallelic mutations in citron kinase link mitotic cytokinesis to human primary microcephaly. Am. J. Hum. Genet. 99, 501-510. 10.1016/j.ajhg.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Hippenmeyer S. and Ghashghaei H. T. (2012). A Nestin-cre transgenic mouse is insufficient for recombination in early embryonic neural progenitors. Biol. Open 1, 1200-1203. 10.1242/bio.20122287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Xiao G., Yin H., Hippenmeyer S., Horowitz J. M. and Ghashghaei H. T. (2013). Neural development is dependent on the function of specificity protein 2 in cell cycle progression. Development 140, 552-561. 10.1242/dev.085621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. N. and Dwyer N. D. (2019). p53 deletion rescues lethal microcephaly in a mouse model with neural stem cell abscission defects. Hum. Mol. Genet. 28, 434-447. 10.1093/hmg/ddy350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca A., Ciceri G., Beattie R., Wong F. K., Diana G., Serafeimidou-Pouliou E., Fernández-Otero M., Streicher C., Arnold S. J., Meyer M. et al. (2019). A stochastic framework of neurogenesis underlies the assembly of neocortical cytoarchitecture. eLife 8, e51381 10.7554/eLife.51381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M. N., Martynoga B., Molinek M. D., Quinn J. C., Kroemmer C., Mason J. O. and Price D. J. (2011). The transcription factor Foxg1 regulates telencephalic progenitor proliferation cell autonomously, in part by controlling Pax6 expression levels. Neural Dev. 6, 9 10.1186/1749-8104-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V., Noctor S. C. and Kriegstein A. R. (2006). The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb. Cortex 16 Suppl. 1, i152-i161. 10.1093/cercor/bhk017 [DOI] [PubMed] [Google Scholar]
- Martynoga B., Morrison H., Price D. J. and Mason J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 283, 113-127. 10.1016/j.ydbio.2005.04.005 [DOI] [PubMed] [Google Scholar]
- McConnell S. K. and Kaznowski C. E. (1991). Cell cycle dependence of laminar determination in developing neocortex. Science 254, 282-285. 10.1126/science.1925583 [DOI] [PubMed] [Google Scholar]
- Mihalas A. B. and Hevner R. F. (2018). Clonal analysis reveals laminar fate multipotency and daughter cell apoptosis of mouse cortical intermediate progenitors. Development 145, dev164335 10.1242/dev.164335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalas A. B., Elsen G. E., Bedogni F., Daza R. A. M., Ramos-Laguna K. A., Arnold S. J. and Hevner R. F. (2016). Intermediate progenitor cohorts differentially generate cortical layers and require Tbr2 for timely acquisition of neuronal subtype identity. Cell Rep. 16, 92-105. 10.1016/j.celrep.2016.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyama S., Takahashi T., Nowakowski R. S. and Caviness V. S. (1997). A gradient in the duration of the G1 phase in the murine neocortical proliferative epithelium. Cereb. Cortex 7, 678-689. 10.1093/cercor/7.7.678 [DOI] [PubMed] [Google Scholar]
- Molyneaux B. J., Arlotta P., Menezes J. R. L. and Macklis J. D. (2007). Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427-437. 10.1038/nrn2151 [DOI] [PubMed] [Google Scholar]
- Moorefield K. S., Fry S. J. and Horowitz J. M. (2004). Sp2 DNA binding activity and trans-activation are negatively regulated in mammalian cells. J. Biol. Chem. 279, 13911-13924. 10.1074/jbc.M313589200 [DOI] [PubMed] [Google Scholar]
- Mora-Bermúdez F., Matsuzaki F. and Huttner W. B. (2014). Specific polar subpopulations of astral microtubules control spindle orientation and symmetric neural stem cell division. eLife 3, 7610 10.7554/eLife.02875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Bermúdez F., Badsha F., Kanton S., Camp J. G., Vernot B., Köhler K., Voigt B., Okita K., Maricic T., He Z. et al. (2016). Differences and similarities between human and chimpanzee neural progenitors during cerebral cortex development. eLife 5, e18683 10.7554/eLife.18683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. R., Hodge R. D., Bedogni F. and Hevner R. F. (2013). Dynamic interactions between intermediate neurogenic progenitors and radial glia in embryonic mouse neocortex: potential role in Dll1-Notch signaling. J. Neurosci. 33, 9122-9139. 10.1523/JNEUROSCI.0791-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeño V., Ivic L. and Kriegstein A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Novorol C., Burkhardt J., Wood K. J., Iqbal A., Roque C., Coutts N., Almeida A. D., He J., Wilkinson C. J. and Harris W. A. (2013). Microcephaly models in the developing zebrafish retinal neuroepithelium point to an underlying defect in metaphase progression. Open Biol. 3, 130065 10.1098/rsob.130065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz L.-J. and Silver D. L. (2014). Live imaging of mitosis in the developing mouse embryonic cortex. J. Vis. Exp., e51298 10.3791/51298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz L.-J., McMahon J. J., Miller E. E., Lennox A. L., Suzuki A., Salmon E. and Silver D. L. (2016). Prolonged mitosis of neural progenitors alters cell fate in the developing brain. Neuron 89, 83-99. 10.1016/j.neuron.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara S., Kawakami Y., Izpisua Belmonte J. and O'Leary D. D. M. (2007). Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2, 10 10.1186/1749-8104-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P. and Calegari F. (2010). Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 20, 233-243. 10.1016/j.tcb.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Schaeper N. D., Prpic N.-M. and Wimmer E. A. (2010). A clustered set of three Sp-family genes is ancestral in the Metazoa: evidence from sequence analysis, protein domain structure, developmental expression patterns and chromosomal location. BMC Evol. Biol. 10, 88 10.1186/1471-2148-10-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabykin V., Stoykova A., Usman N. and Gruss P. (2001). Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development 128, 1983-1993. [DOI] [PubMed] [Google Scholar]
- Taverna E., Götz M. and Huttner W. B. (2014). The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465-502. 10.1146/annurev-cellbio-101011-155801 [DOI] [PubMed] [Google Scholar]
- Terrados G., Finkernagel F., Stielow B., Sadic D., Neubert J., Herdt O., Krause M., Scharfe M., Jarek M. and Suske G. (2012). Genome-wide localization and expression profiling establish Sp2 as a sequence-specific transcription factor regulating vitally important genes. Nucleic Acids Res. 40, 7844-7857. 10.1093/nar/gks544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y. and Sluder G. (2010). Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr. Biol. 20, 1666-1671. 10.1016/j.cub.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzquiano A., Gladwyn-Ng I., Nguyen L., Reiner O., Götz M., Matsuzaki F. and Francis F. (2018). Cortical progenitor biology: key features mediating proliferation versus differentiation. J. Neurochem. 146, 500-525. 10.1111/jnc.14338 [DOI] [PubMed] [Google Scholar]
- Vargas-Hurtado D., Brault J.-B., Piolot T., Leconte L., Da Silva N., Pennetier C., Baffet A., Marthiens V. and Basto R. (2019). Differences in mitotic spindle architecture in mammalian neural stem cells influence mitotic accuracy during brain development. Curr. Biol. 29, 2993-3005.e9. 10.1016/j.cub.2019.07.061 [DOI] [PubMed] [Google Scholar]
- Vasistha N. A., García-Moreno F., Arora S., Cheung A. F. P., Arnold S. J., Robertson E. J. and Molnár Z. (2015). Cortical and clonal contribution of Tbr2 expressing progenitors in the developing mouse brain. Cereb. Cortex 25, 3290-3302. 10.1093/cercor/bhu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkel S., Stielow B., Finkernagel F., Stiewe T., Nist A. and Suske G. (2015). Zinc finger independent genome-wide binding of Sp2 potentiates recruitment of histone-fold protein Nf-y distinguishing it from Sp1 and Sp3. PLoS Genet. 11, e1005102 10.1371/journal.pgen.1005102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A., Küspert M., Sock E., Philipsen S., Suske G. and Wegner M. (2017). Sp2 is the only glutamine-rich specificity protein with minor impact on development and differentiation in myelinating glia. J. Neurochem. 140, 245-256. 10.1111/jnc.13908 [DOI] [PubMed] [Google Scholar]
- Xie J., Yin H., Nichols T. D., Yoder J. A. and Horowitz J. M. (2010). Sp2 is a maternally inherited transcription factor required for embryonic development. J. Biol. Chem. 285, 4153-4164. 10.1074/jbc.M109.078881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Nichols T. D. and Horowitz J. M. (2010). Transcription of mouse Sp2 yields alternatively spliced and sub-genomic mRNAs in a tissue- and cell-type-specific fashion. Biochim. Biophys. Acta 1799, 520-531. 10.1016/j.bbagrm.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K.-J., Koo B.-K., Im S.-K., Jeong H.-W., Ghim J., Kwon M.-C., Moon J.-S., Miyata T. and Kong Y.-Y. (2008). Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 58, 519-531. 10.1016/j.neuron.2008.03.018 [DOI] [PubMed] [Google Scholar]
- Zhao C. and Meng A. (2005). Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev. Growth Differ. 47, 201-211. 10.1111/j.1440-169X.2005.00797.x [DOI] [PubMed] [Google Scholar]
- Zong H., Espinosa J. S., Su H. H., Muzumdar M. D. and Luo L. (2005). Mosaic analysis with double markers in mice. Cell 121, 479-492. 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.