ABSTRACT
In the anterior foregut (AFG) of mouse embryos, the transcription factor SOX2 is expressed in the epithelia of the esophagus and proximal branches of respiratory organs comprising the trachea and bronchi, whereas NKX2.1 is expressed only in the epithelia of respiratory organs. Previous studies using hypomorphic Sox2 alleles have indicated that reduced SOX2 expression causes the esophageal epithelium to display some respiratory organ characteristics. In the present study, we produced mouse embryos with AFG-specific SOX2 deficiency. In the absence of SOX2 expression, a single NKX2.1-expressing epithelial tube connected the pharynx and the stomach, and a pair of bronchi developed in the middle of the tube. Expression patterns of NKX2.1 and SOX9 revealed that the anterior and posterior halves of SOX2-deficient AFG epithelial tubes assumed the characteristics of the trachea and bronchus, respectively. In addition, we found that mesenchymal tissues surrounding the SOX2-deficient NKX2.1-expressing epithelial tube changed to those surrounding the trachea and bronchi in the anterior and posterior halves, as indicated by the arrangement of smooth muscle cells and SOX9-expressing cells and by the expression of Wnt4 (esophagus specific), Tbx4 (respiratory organ specific), and Hoxb6 (distal bronchus specific). The impact of mesenchyme-derived signaling on the early stage of AFG epithelial specification has been indicated. Our study demonstrated an opposite trend where epithelial tissue specification causes concordant changes in mesenchymal tissues, indicating a reciprocity of epithelial-mesenchymal interactions.
KEY WORDS: Anterior foregut, Conditional knockout, Epithelial-mesenchymal interaction, Esophagus, Respiratory organ, SOX2
Summary: In response to the change in the epithelial characteristics from esophagus to trachea/bronchus that happens when SOX2 is inactivated, surrounding mesenchymal tissues also change their characteristics accordingly at all axial levels.
INTRODUCTION
Epithelial components of the vertebrate alimentary tract develop from the endoderm-derived gut tube, which becomes specified into subdivisions along the anteroposterior axis. Early-stage subdivisions of endoderm-derived gut tubes depend on the action of various exogenous signaling molecules (Jacobs et al., 2012; Swarr and Morrisey, 2015; Zhang et al., 2017), and specification to subdivision results in the expression of subdivision-specific transcription factors (TFs). The epithelial tube of the anterior foregut (AFG) and stomach expresses SOX2 after embryonic day (E) 9 (Que et al., 2007), whereas the hindgut, which develops into duodenum and more posterior intestinal regions, expresses CDX2 (Gao et al., 2009). The anterior portion of AFG epithelial tubes is then split into dorsally positioned SOX2-expressing esophagus tissues and ventrally positioned tracheal tissues that express NKX2.1 and SOX2. Bronchi, bronchioles and alveoli that further develop at the distal end of the trachea also express NKX2.1 (Swarr and Morrisey, 2015; Zhang et al., 2017).
TFs that are expressed in endoderm-derived epithelial tubes play determining roles during gut subdivision. For instance, endoderm-specific inactivation of Cdx2 promoted SOX2 expression in hindgut tubes, which assumed forestomach epithelial cell characters (Gao et al., 2009). Loss of Nkx2.1 in embryos resulted in the development of esophagus and tracheal structures that remained joined, and caused defects in lung branching morphogenesis (Minoo et al., 1999).
Contributions of SOX2 to esophageal specification of AFG epithelia have been demonstrated using combinations of hypomorphic Sox2 alleles, leading to degrees of tracheoesophageal fistula (Que et al., 2007), similar to various aspects of human congenital tracheoesophageal fistula cases (Wong et al., 2016; Zhang et al., 2017). Hence, SOX2 function is essential for the separation of trachea and esophagus in the AFG and required for the establishment of esophageal epithelial characteristics. However, complete tissue-specific Sox2 inactivation is required to precisely evaluate the roles of SOX2 in these processes. We achieved endoderm-specific inactivation of Sox2 using a floxed Sox2 allele and a FoxA2-nEGFP-CreERT2 knock-in allele, in which coding sequences were joined via 2A peptide sequences (Imuta et al., 2013). Under these conditions, tamoxifen administration resulted in the development of a single SOX2-deficient AFG tube connecting the pharynx and the stomach, in the middle of which a pair of bronchi branched out.
Mesenchymal influences on epithelial subdivisions of the AFG have been well documented (Swarr and Morrisey, 2015; Zhang et al., 2017). In this study, we characterized both epithelial and mesenchymal components of the AFG after formation in the absence of SOX2 in the epithelium. Not only the SOX2-deficient AFG epithelia but also the surrounding mesenchymal tissues developed into those of respiratory organs, with clear anteroposterior polarity similar to those of the trachea and bronchi. Thus, once established, the regional identity of the epithelial component of the AFG determined the characters of the surrounding mesenchymal tissues.
RESULTS AND DISCUSSION
Organization of AFG tube development in the absence of endodermal SOX2 expression
To inactivate Sox2 expression in the entire gut tube, we introduced Foxa2nEGFP−CreERT2 (Imuta et al., 2013) into floxed Sox2 homozygous mice (Fig. S1A–C). Tamoxifen was administered to pregnant females on E7 and E8, shortly before the expression of FoxA2 in the foregut at E8.5 (Imuta et al., 2013), and embryos were collected on E11 to E13. In the floor plate-proximal ventral spinal cord, where the expression of Sox2 and FoxA2 normally overlaps, SOX2 expression was abolished (Fig. S1D), confirming that Sox2 was efficiently inactivated by CreERT2.
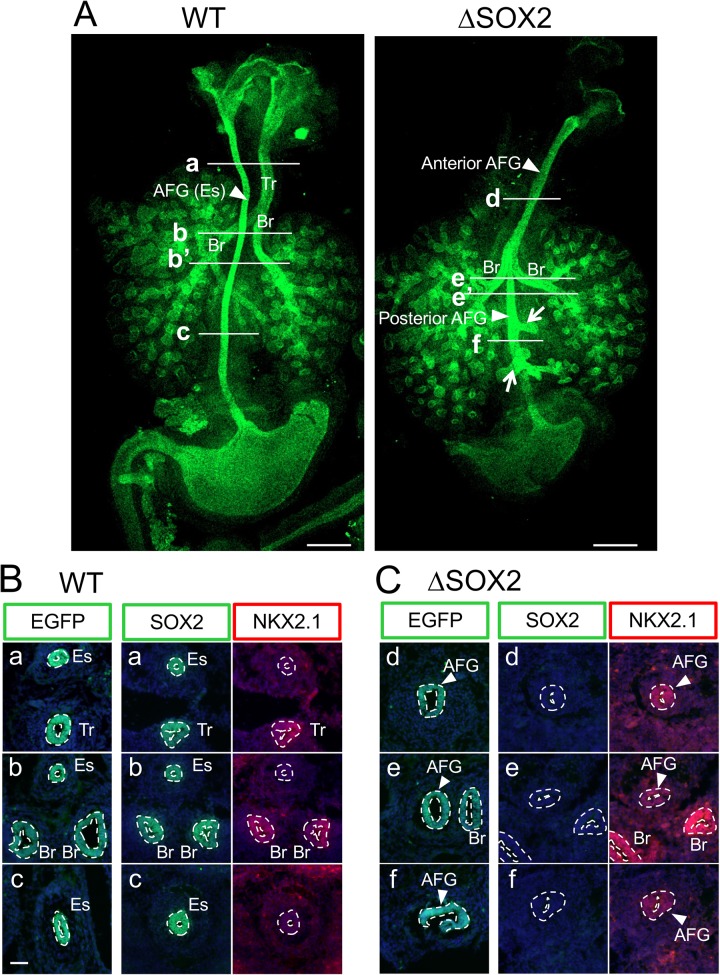
We investigated AFG development in the absence of SOX2 expression (Fig. 1). AFG epithelial tubes were visualized using FoxA2-driven nuclear EGFP fluorescence (Fig. 1A; Fig. S2). In normal E11 to E13.5 embryos, the esophagus joins the pharynx and stomach, and the trachea with a pair of bronchi with lung tissues was already separated (Fig. 1A). In the absence of SOX2 expression, however, a single AFG tube connecting the pharynx and stomach was formed, and a pair of bronchi with lung tissues developed in the middle of these tubes (Fig. 1A). In a significant fraction (12/17) of SOX2-deficient AFG, bronchiole-like tubular tissues developed from the posterior half (Fig. 1A; Fig. S2, arrows). The same AFG tube phenotype was observed using knock-in of CreERT2 into the ROSA26 locus (Cheng et al., 2010), although in this case, brain tissue development was also affected (data not shown). Loss of SOX2 expression in endoderm-derived epithelia expressing EGFP was confirmed by immunostaining of cross sections (Fig. 1B,C).
Fig. 1.
Absence of the esophagus and expression of NKX2.1 in all epithelial tubes of SOX2-deficient (ΔSOX2) AFG. (A) AFG tube organization was visualized in the dorsal view by FoxA2-driven EGFP in WT AFG and ΔSOX2 AFG on E13.5. WT AFG had separate esophageal (Es) and tracheal (Tr)/bronchial (Br) tubes, whereas ΔSOX2 AFG bronchi developed in the middle of the single tube connecting the pharynx and stomach. Frequently (12/17), bronchiole-like tissues developed from the posterior half of ΔSOX2 AFG, as indicated by arrows. Stomachs of ΔSOX2 were always smaller. Scale bars: 500 µm. For more specimens from earlier developmental stages, see Fig. S2. (B,C) Cross sections of AFG tubes of WT (B) and ΔSOX2 (C) E13 embryos at approximate axial levels as shown in A. The sections were immunostained for EGFP (green), SOX2 (green) and NKX2.1 (red), and the fluorescence images were superimposed on 4′,6-diamidino-2-phenylindole (DAPI) fluorescence to show the cell distribution. SOX2 and NKX2.1 data were from the same embryos, whereas EGFP data were from stage-matched different embryos. Sections of tubular tissues are encircled by broken lines, and those expressing relevant antigens are indicated by the following tissue abbreviations: AFG with an arrowhead indicates the ΔSOX2 AFG epithelium, while Es, Tr, and Br indicate esophagus, trachea, and bronchi, respectively. In ΔSOX2 AFG, the entire epithelial tube lacked SOX2 and expressed NKX2.1. See also Fig. S3. Scale bars: 100 µm.
In normal embryos, proximal branches of the epithelial tubes, i.e. trachea and bronchi, also express SOX2 (Fig. 1B; Fig. S3A) (Gontan et al., 2008; Que et al., 2009; Wong et al., 2016). Airway epithelia that developed in the absence of SOX2 showed tissue growth and branching patterns identical to normal embryos (Fig. 1A; Fig. S2), indicating that SOX2 function is dispensable for lung morphogenesis at least up to the E13 stage. It has been shown that SOX2 is involved in cell type specification at later stages of lung development (Que et al., 2009).
SOX2-deficient stomach tissues were smaller than normal (n>10) (Fig. 1A; Fig. S2), but did not express NKX2.1 (n=3) (Fig. S3B). CDX2 is normally expressed in hindgut epithelia to form SOX2–CDX2 boundaries at the pylorus. In the absence of CDX2 expression, hindgut epithelia expressed SOX2 and assumed a forestomach character (Gao et al., 2009). In contrast, the absence of SOX2 expression did not activate CDX2 expression in the stomach epithelia (n=3) (Fig. S3C).
SOX2-deficient single AFG epithelial tubes have tracheal and bronchial characters
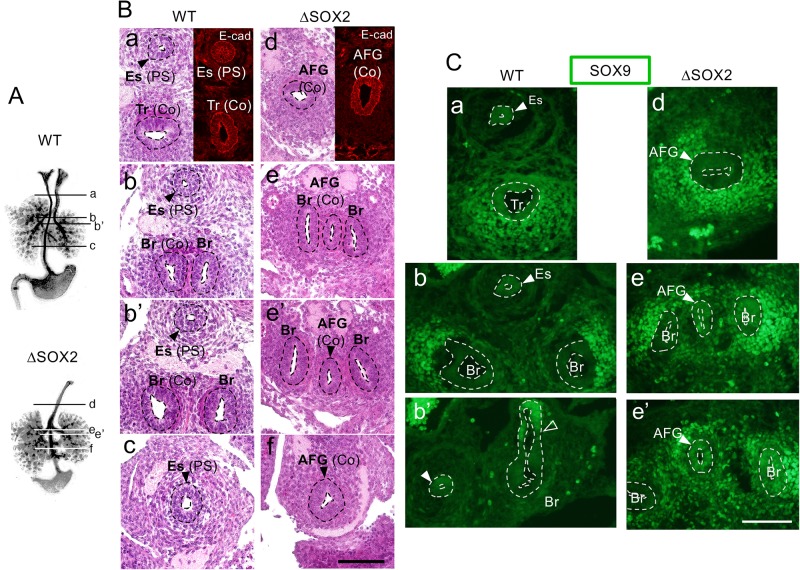
SOX2-deficient AFG epithelial tubes connecting the pharynx and stomach expressed NKX2.1 at all axial levels during the stages E11 to E13.5 (n=4) (Fig. 1C; Fig. S3A,B), indicating tracheal/bronchial characteristics. Accordingly, on E13.5, SOX2-deficient AFG tubes (Fig. 2Bd,e,e′,f) were wider than normal esophagi (Fig. 2Ba,b,b′,c), again showing tracheal and bronchial characteristics. Consistently, SOX2-deficient AFG tubes comprised of columnar (Co) epithelia similar to wild-type (WT) bronchi, as indicated by the distribution of Hematoxylin-stained nuclei (Fig. 2Bd,e,e′,f) and the organization of cell boundaries indicated by E-cadherin immunostaining (Fig. 2Bd), whereas WT esophagi comprised pseudo-stratified (PS) epithelial tissues at this stage (Fig. 2Ba,b,b′,c), a transition state between the earlier columnar and later stratified epithelia of the esophagus (Que, 2015).
Fig. 2.
Epithelial tubes in ΔSOX2 AFG exhibit tracheal/bronchial characters. Histological sections of E13 epithelial tubes made at the axial levels shown in Fig. 1A, as schematized in A. (B) H&E staining of embryo cross sections. E-cadherin (E-cad)-stained cryosections at the axial levels (a) and (d) of embryos at similar developmental stages are also shown. ΔSOX2 AFG tubes were wider than WT esophageal tubes, and similar to WT trachea. Whereas WT tracheal (Tr)/bronchial (Br) tubes and ΔSOX2 AFG tubes comprised columnar (Co) epithelium, WT esophageal tubes (Es) comprised pseudo-stratified epithelium (PS), as judged by nuclear arrangements and organization of cell boundaries visualized by E-cadherin immunostaining in panels a and d. (C) Immunostaining of cryosections for SOX9 at approximate axial levels shown in Fig. 1A. SOX9 expression level was high in posterior ΔSOX2 AFG tubes (e′), similar to the distal bronchi in both WT and ΔSOX2 embryos. The open arrowhead indicates the region of transition from low to high SOX9 expression. Scale bars: 100 µm.
On E13, proximal and distal epithelial portions of respiratory organ rudiments differ in SOX9 expression levels (Wong et al., 2016): low in the trachea and proximal bronchi (Fig. 2Ca,b) and high in the distal bronchi (Fig. 2Cb′). In contrast, in WT esophagi, SOX9 expression did not differ significantly between the anterior and posterior halves (n=5), although the expression levels were variable between embryos and sometimes only barely detectable. In SOX2-deficient AFG tubes, epithelial SOX9 expression was low in the anterior part (Fig. 2Cd,e) but higher in a posterior part (n=4) (Fig. 2Ce′), indicating that the anterior and posterior sides of the AFG developed as trachea and bronchi, respectively. Given that ordinary pairs of bronchi branched out from the midpoint of SOX2-deficient AFG tubes, the AFG region between the midpoint and the stomach developed as a third bronchus. Consistently, additional bronchiole-like tissues frequently developed from the posterior half of SOX2-deficient AFG (n=12/17) (Fig. 1A; Fig. S2, arrows).
Surrounding mesenchymal tissues developed concordant with the epithelial tissue identity
We investigated whether mesenchymal tissues surrounding SOX2-deficient AFG tubes developed concordantly with the tracheal and bronchial characteristics of the epithelia.
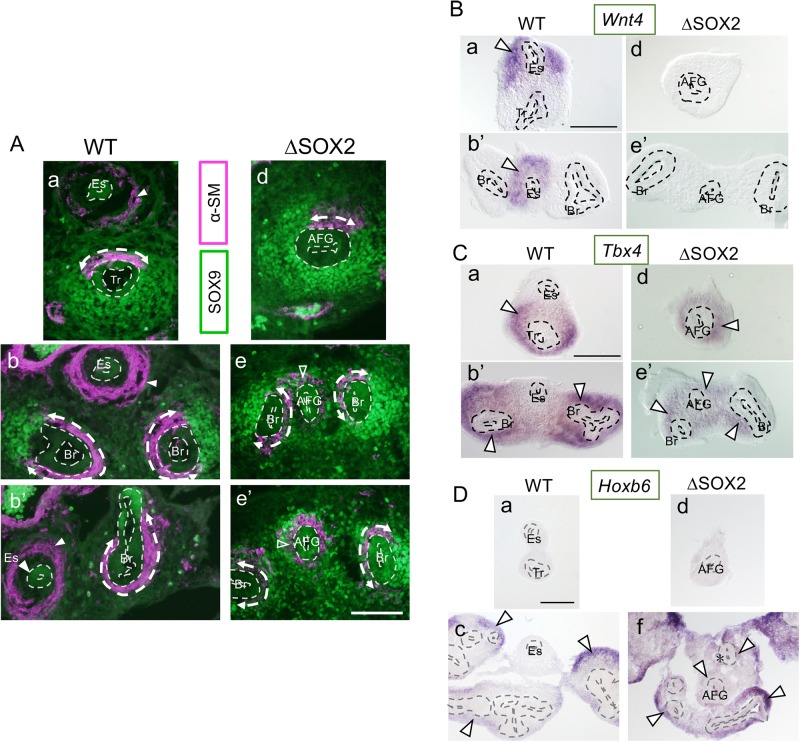
In normal embryos on E13, the epithelial tubes of the esophagus were surrounded by smooth muscle cells expressing smooth muscle α-actin (α-SM) at positions distal from the tube (Fig. 3A, arrowheads) (McHugh, 1995) and were not associated with SOX9-expressing cells (Fig. 3A). In contrast, the epithelial tubes of the trachea and bronchi were tightly wrapped in two patches of mesenchymal cells, representing smooth muscle cells and SOX9-expressing chondrocyte progenitors (Fig. 3A). These smooth muscle patches were positioned at the dorsal face of the trachea and median faces of the bronchi and were broader surrounding the bronchi (Fig. 3A, broken double arrows). The anterior halves of SOX2-deficient AFG tubes were also tightly wrapped in a narrower patch of smooth muscle cells and a broader patch of SOX9-expressing cells, exactly as observed in normal trachea (n=3) (Fig. 3Aa,d). These data indicate that trachea-like development of the anterior epithelial tube elicits mesenchymal developments with characteristics of respiratory organs.
Fig. 3.
Mesenchymal characters are concordant with those of epithelial tubes in the AFG. Histological sections of E13 epithelial tubes are shown at the axial levels indicated in Fig. 1A. (A) Sections shown in Fig. 2B were doubly stained for SOX9 (green) and α-SM (magenta). Mesenchymal tissues surrounding WT and ΔSOX2 trachea/bronchi comprise nearly complementary patches of SOX9-expressing cells and α-SM-expressing cells (broken double arrows). ΔSOX2 AFG tubes were also wrapped in SOX9- and α-SM-expressing mesenchymal tissues, albeit without their separation into patches (open arrowheads). In contrast, WT esophageal tissues lacked these cell types and only had distal α-SM-expressing cells (arrowheads). (B–D) Analogous sections of WT and ΔSOX2 AFG hybridized with mesenchyme-specific gene probes for Wnt4 (esophagus specific), Tbx4 (trachea/bronchus specific), and Hoxb6 (distal bronchus specific). Mesenchymal cells expressing these genes are indicated by open arrowheads. In ΔSOX2 AFG, Wnt4 expression was completely absent (B), epithelial tubes were all surrounded by Tbx4-expressing mesenchyme (C), and the posterior part of the tube was surrounded by Hoxb6-expressing mesenchyme, similar to the distal bronchi (D). Asterisk, an AFG-derived bronchiole-like tube. Scale bars: 100 µm.
In the posterior parts of SOX2-deficient AFG, the epithelial tubes were surrounded by smooth muscle cells and SOX9-expressing cells, resembling bronchi. Yet, smooth muscle cells and SOX9-expressing cells did not segregate (n=3) (Fig. 3Ae,e′, open arrowheads). Presumably, azimuthal specification of the mesenchyme failed to occur in SOX2-deficient AFG.
To investigate the molecular markers of mesenchymal cells that distinguish esophagus and respiratory organs, we performed in situ hybridization using esophagus mesenchyme-specific Wnt4 (Fig. 3Ba,b′) (GSE118641, Gene Expression Omnibus), respiratory organ-specific Tbx4 (Fig. 3Ca,b′) (Arora et al., 2012), and Hoxb6, which is expressed in the distal portion of the bronchus mesenchyme (Fig. 3Dd).
In normal embryos on E11 to 11.5, Wnt4 was expressed in mesenchymal cells surrounding the esophagus (Fig. 3Ba,b′, open arrowheads). In contrast, Wnt4 expression was lost in mesenchymal tissues surrounding SOX2-deficient AFG epithelial tubes (n=3) (Fig. 3Bd,e′), confirming the loss of esophageal phenotypes. In contrast, Tbx4 was expressed exclusively in the mesenchyme of respiratory organs in normal embryos (Fig. 3Ca,b′, open arrowheads) (Arora et al., 2012). Mesenchymal tissues of SOX2-deficient AFG expressed Tbx4 at all axial levels (n=3) (Fig. 3Cd,e′, open arrowheads), concordant with the respiratory organ identity of the epithelial tubes.
In normal embryos, Hoxb6 was expressed in mesenchymal tissues surrounding the distal part of the bronchus (and more strongly in alveoli) (Fig. 3Dc, open arrowheads), but no expression was observed along the trachea (Fig. 3Da). Along the SOX2-deficient AFG, only the mesenchyme surrounding the posterior AFG tubes and posterior AFG-derived bronchiole-like tubes (Fig. 3Df, asterisk) expressed Hoxb6 (n=4) (Fig. 3Df, open arrowheads), as observed in the distal bronchi (Fig. 3Dc,f, open arrowheads). These observations confirmed the bronchial identity of the posterior half of SOX2-deficient AFG, complete with proximodistal polarity in both epithelial and mesenchymal components (Figs 2B and 3D).
Overall impact of the absence of SOX2 during esophageal and tracheal/bronchial development
We investigated the consequences of abolishing SOX2 expression in the anterior endoderm. Under this condition, single AFG epithelial tubes connecting the pharynx and stomach developed, comprising columnar epithelial cells and expressing NKX2.1, indicating a gain of tracheal/bronchial characteristics and loss of esophageal characteristics (Figs 1 and 4). Trisno et al. (2018) similarly ablated Sox2 in the endoderm and reported the development of NKX2.1-expressing AFG epithelia that lacked esophagus-characteristic p63 expression.
Fig. 4.
Summary of this study. (A) The key observations. The loss of SOX2 in the epithelial tissues alters the esophageal characters of the AFG into trachea and bronchi in both epithelial and mesenchymal components. (B) Details of observations. In the diagrams of WT and ΔSOX2 AFG tubes, expression of SOX2, NKX2.1 and SOX9 in the epithelial tissues and expression of α-SM, SOX9, Wnt4, Tbx4 and Hoxb6 in mesenchymal tissues are indicated.
We also observed anteroposterior differentiation of SOX2-deficient AFG epithelial tubes, similar to proximodistal differentiation of respiratory organ epithelia (Fig. 2B). The anterior part of SOX2-deficient AFG epithelial tubes had low SOX9 expression, as in trachea, whereas a posterior part of the tube had high SOX9 expression, as observed in the distal bronchi.
A new important finding of this study is that mesenchymal tissues that surround the epithelial tubes change their characters at all axial levels in concordance with the changes in the epithelial tubes from the esophagus to the respiratory organs (Figs 3 and 4). Studies of alimentary tract development have provided a paradigm of epithelial-mesenchymal interactions during organ development (Swarr and Morrisey, 2015; Zhang et al., 2017). Earlier studies demonstrated the impact of mesenchyme-derived cues on the development of epithelial tubes. For example, dorsoventral differentiation during the early stages of AFG development, leading to esophagus/trachea splitting, is mediated by BMP signaling from the surrounding mesenchymal cells (Domyan et al., 2011; Ishii et al., 1998). Yet, regulatory influences in SOX2-deficient AFG epithelial tubes were in the opposite direction (Fig. 4). All mesenchymal tissues surrounding SOX2-deficient AFG epithelial tubes developed as respiratory organ mesenchyme rather than esophageal mesenchyme. Thus, interactions between epithelial and mesenchymal components during AFG development appear to be bidirectional and dependent on the developmental stage. The underlying mechanisms through which epithelial regional identity determines the characters of the surrounding mesenchyme remain to be elucidated. In a previous study, SOX2-deficient AFG failed to express some Wnt antagonists (Trisno et al., 2018), whereas in other studies, the anteroposterior organization of the gut tube depended on retinoic acid signaling (Bayha et al., 2009; Wang et al., 2006). Hence, the regulation of these signals by epithelial tissues may contribute to the coordinated development of epithelial and mesenchymal components of the AFG.
MATERIALS AND METHODS
Construction of a mouse line carrying floxed Sox2 alleles
The mouse genomic sequence from −6307 bp to +9880 bp relative to the 5′ end of the Sox2 coding sequence, covering the region from N2 to N5 enhancers (Uchikawa et al., 2003), was used as a targeting vector. The STneoB sequence (Katoh et al., 1987) was flanked by a pair of Flp recombinase target (FRT) sequences and inserted into the vector sequence to select recombinant embryonic stem (ES) cells for G418 resistance. The DT-A cassette (Yagi et al., 1993) was added at the end of the vector sequence to select against non-homologous recombinants. After electroporating the vector in R1 ES cells (Nagy et al., 1993) and selecting recombinants as described by Sawai et al. (1991), homologous recombinants were identified by Southern blotting. Homologous recombinants were used to produce mice carrying a floxed Sox2 allele (Fig. S1A).
Handling mice and mouse embryos
Knock-in mice expressing CreERT2 with the specificity of FoxA2 (Foxa2nEGFP−CreERT2) (Imuta et al., 2013) or ROSA26 [Gt(ROSA)26Sortm1(cre/ERT2)Alj] (Cheng et al., 2010) were crossed with mice carrying the floxed Sox2 allele and bred for several generations in C57BL/6;DBA mixed background to achieve homozygosity at both loci. Mouse and embryo genotypes were determined using polymerase chain reaction (PCR) with primer pairs that gave PCR products of sizes indicated in Fig. S1B. Five mg of tamoxifen in peanut oil was orally administered to pregnant females on 7.5 and 8.5 days post coitum, and embryos were collected at the indicated stages. Embryos carrying the homozygous floxed Sox2 allele and the ROSA26 locus-inserted CreERT2 recombinase gene [Gt(ROSA)26Sortm1(cre/ERT2)Alj] (Cheng et al., 2010) showed the ΔSox2 genotype after the administration of tamoxifen to mothers on E7 and E8. Animal experiments were performed at Kyoto Sangyo University, Nagoya University, Osaka University and Tokushima University in accordance with the animal experimentation regulations of the respective institutions.
Histological analyses
Specimens were fixed in 4% paraformaldehyde and processed for histological analyses. Immunofluorescence staining was done using antibodies listed in Table S1. Wholemount in situ hybridization of isolated gut tubes was performed using digoxigenin-labeled probes and conditions described by Takemoto et al. (2011) with Proteinase K treatment at 5 μg/ml, followed by cryosectioning. Paraffin sections of embryos fixed with Bouin's fixative were stained with Hematoxylin and Eosin (H&E). Photo images were taken using Axioplan 2 (Zeiss) or an FV3000 laser microscope (Olympus).
Supplementary Material
Acknowledgements
We thank Hiroshi Sasaki and Alexandra Joyner for granting us the use of their Foxa2nEGFP−CreERT2 and Gt(ROSA)26Sortm1(cre/ERT2)Alj mice, respectively.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.I., H.K.; Methodology: M.T., R.S., K.M., M.U., T.T., A.K., Y.I., H.K.; Validation: M.T., R.S., M.U., T.T., Y.I., H.K.; Formal analysis: M.T., H.K.; Investigation: M.T., R.S., M.U., T.T., Y.I., H.K.; Resources: K.M., M.U., T.T., A.K., H.K.; Data curation: M.T., H.K.; Writing - original draft: M.T., H.K.; Writing - review & editing: M.U., T.T., Y.I., H.K.; Visualization: M.T., R.S., Y.I., H.K.; Supervision: Y.I., H.K.; Project administration: H.K.; Funding acquisition: M.T., H.K.
Funding
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research [JP19K16184] to M.T. and [JP26251024 and JP17H03680] to H.K.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.048728.supplemental
References
- Arora R., Metzger R. J. and Papaioannou V. E. (2012). Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 8, e1002866 10.1371/journal.pgen.1002866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayha E., Jørgensen M. C., Serup P. and Grapin-Botton A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE 4, e5845 10.1371/journal.pone.0005845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Sudarov A., Szulc K. U., Sgaier S. K., Stephen D., Turnbull D. H. and Joyner A. L. (2010). The engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development 137, 519-529. 10.1242/dev.027045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan E. T., Ferretti E., Throckmorton K., Mishina Y., Nicolis S. K. and Sun X. (2011). Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138, 971-981. 10.1242/dev.053694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., White P. and Kaestner K. H. (2009). Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev. Cell 16, 588-599. 10.1016/j.devcel.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C., de Munck A., Vermeij M., Grosveld F., Tibboel D. and Rottier R. (2008). Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev. Biol. 317, 296-309. 10.1016/j.ydbio.2008.02.035 [DOI] [PubMed] [Google Scholar]
- Imuta Y., Kiyonari H., Jang C.-W., Behringer R. R. and Sasaki H. (2013). Generation of knock-in mice that express nuclear enhanced green fluorescent protein and tamoxifen-inducible cre recombinase in the notochord from Foxa2 and T loci. Genesis 51, 210-218. 10.1002/dvg.22376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Rex M., Scotting P. J. and Yasugi S. (1998). Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial-mesenchymal interactions. Dev. Dyn. 213, 464-475. [DOI] [PubMed] [Google Scholar]
- Jacobs I. J., Ku W.-Y. and Que J. (2012). Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev. Biol. 369, 54-64. 10.1016/j.ydbio.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Takahashi Y., Hayashi S. and Kondoh H. (1987). Improved mammalian vectors for high expression of G418 resistance. Cell Struct. Funct. 12, 575-580. 10.1247/csf.12.575 [DOI] [PubMed] [Google Scholar]
- McHugh K. M. (1995). Molecular analysis of smooth muscle development in the mouse. Dev. Dyn. 204, 278-290. 10.1002/aja.1002040306 [DOI] [PubMed] [Google Scholar]
- Minoo P., Su G., Drum H., Bringas P. and Kimura S. (1999). Defects in tracheoesophageal and lung morphogenesis inNkx2.1(−/−) mouse embryos. Dev. Biol. 209, 60-71. 10.1006/dbio.1999.9234 [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W. and Roder J. C. (1993). Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90, 8424-8428. 10.1073/pnas.90.18.8424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J. (2015). The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus. Wiley Interdiscip. Rev. Dev. Biol. 4, 419-430. 10.1002/wdev.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K.-T., Kurotani R., Morrisey E. E., Taranova O., Pevny L. H. and Hogan B. L. M. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521-2531. 10.1242/dev.003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Luo X., Schwartz R. J. and Hogan B. L. (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136, 1899-1907. 10.1242/dev.034629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai S., Shimono A., Hanaoka K. and Kondoh H. (1991). Embryonic lethality resulting from disruption of both N-myc alleles in mouse zygotes. New Biol. 3, 861-869. [PubMed] [Google Scholar]
- Swarr D. T. and Morrisey E. E. (2015). Lung endoderm morphogenesis: gasping for form and function. Annu. Rev. Cell Dev. Biol. 31, 553-573. 10.1146/annurev-cellbio-100814-125249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto T., Uchikawa M., Yoshida M., Bell D. M., Lovell-Badge R., Papaioannou V. E. and Kondoh H. (2011). Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470, 394-398. 10.1038/nature09729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisno S. L., Philo K. E. D., McCracken K. W., Catá E. M., Ruiz-Torres S., Rankin S. A., Han L., Nasr T., Chaturvedi P., Rothenberg M. E. et al. (2018). Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell 23, 501-515.e7. 10.1016/j.stem.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa M., Ishida Y., Takemoto T., Kamachi Y. and Kondoh H. (2003). Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell 4, 509-519. 10.1016/S1534-5807(03)00088-1 [DOI] [PubMed] [Google Scholar]
- Wang Z., Dollé P., Cardoso W. V. and Niederreither K. (2006). Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 297, 433-445. 10.1016/j.ydbio.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Wong N. C., Jiang A. P., Ku M., Jacobs W.-Y. and Que I. J. (2016). SOX2 in the development and maintenance of the trachea, lung and esophagus. In Sox2: Biology and Role in Development and Disease (ed. Hisato K. and Robin L.-B.). London, San Diego, Waltham, Oxford: Academic Press/Elsevier. [Google Scholar]
- Yagi T., Nada S., Watanabe N., Tamemoto H., Kohmura N., Ikawa Y. and Aizawa S. (1993). A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal. Biochem. 214, 77-86. 10.1006/abio.1993.1459 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jiang M., Kim E., Lin S., Liu K., Lan X. and Que J. (2017). Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 66, 25-35. 10.1016/j.semcdb.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.