Abstract
Tumor-induced osteomalacia (TIO) is a rare disease in which patients suffer from fractures and progressive disabling bone pain and muscle weakness. TIO is caused by the hypersecretion of Fibroblast Growth Factor 23 (FGF23) from rare neoplasms of mesenchymal origin. This case report describes a 29-year-old male with 2 years of low back/hip pain, gait changes, proximal muscle weakness, and multiple stress fractures. Bone densitometry was remarkable for severe osteoporosis, hypophosphatemia was seen on routine labs, and advanced labs demonstrated an “inappropriately normal” FGF23 level. A 68Ga-DOTATATE scan and MRI showed a 1.3 × 1.1 × 1.0 cm intracranial mass. The patient underwent tumor resection by Neurosurgery. Shortly after, laboratory levels normalized, and the patient's symptoms improved drastically. This case exemplifies the notion that TIO can be caused by FGF23 levels within normal limits, the role of 68-Ga DOTATATE imaging for establishing a diagnosis, and that these tumors can arise anywhere—even intracranially. We also review current surgical and nonsurgical treatment options, as well as emerging novel therapeutics.
Keywords: TIO, Paraneoplastic syndrome, Tumor-induced osteomalacia, Fibroblast growth factor 23, 68Ga-DOTATATE, MRI, DXA, Burosumab
Introduction
Osteomalacia is the softening of bones caused by impaired bone mineralization at sites of bone remodeling and during bone formation primarily due to inadequate levels of available phosphate, calcium, and vitamin D or because of calcium resorption. Only recently, a novel paraneoplastic disease termed tumor-induced osteomalacia (TIO) has been described [1,2]. TIO is caused by an overproduction and secretion of FGF23 from a phosphaturic mesenchymal tumor. Normally, FGF23 is secreted by osteocytes in response to hyperphosphatemia and exogenous calcitriol. FGF23 then acts on the kidneys by decreasing the expression of NPT2, a sodium-phosphate cotransporter in the proximal tubule [3]. Due to its relative overexpression and secretion in this disease, FGF23 in excess decreases phosphate reabsorption while increasing its excretion, causing renal phosphate wasting as well as impairing intestinal phosphate absorption through suppression of renal calcitriol production. Over time, hypophosphatemia will arise and cause osteomalacia due to the inadequate availability of phosphate in the blood for active bone mineralization.
Case report
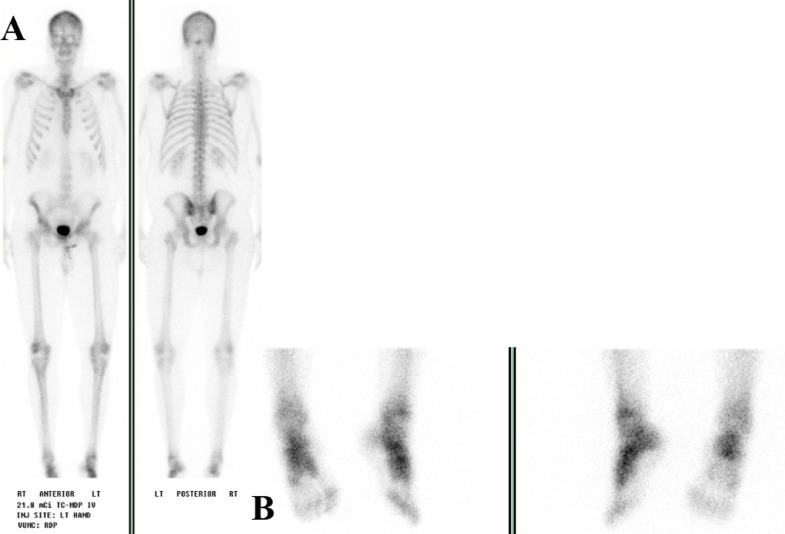
A 29-year-old male presented with 2 years of low back and hip pain, gait changes, proximal muscle weakness, and multiple stress fractures. There was no personal or family history of metabolic bone disorders, rickets, or cancer. He was initially evaluated by rheumatology and diagnosed with psoriatic arthritis, though treatment with steroids and etanercept did not improve his symptoms. An elevated alkaline phosphatase level prompted his referral to endocrinology clinic. In our clinic, a bone scan (Fig. 1) was performed that showed multiple foci of uptake mostly representing fractures. Pertinent laboratory values include a low serum phosphorus (1.3 mg/dL; 2.3-4.7 mg/dL), serum calcium (9.8 mg/dL, 8.4-10.5 mg/dL), low total Vitamin D (15 ng/mL; 25-80 ng/mL), low 1, 25 Dihydroxyvitamin D (10.0 pg/mL; 19.9-79.3 pg/mL), elevated total alkaline phosphatase (354 U/L; 40-150 U/L) elevated bone specific alkaline phosphatase (110.0 mcg/L; 6.5-20.1 mcg/L), parathyroid hormone (79 pg/mL; 16-77 pg/mL). A 24-hour urine phosphorous was performed (1, 160 mg/24hr; 400-1300 mg/24hr). Areal bone density was assessed by dual-energy X-ray absorptiometry (DXA) using a GE Lunar Prodigy Advanced densitometer and revealed a spine BMD of 0.789 g/cm2 (Z score of −4.3) (Fig. 3A), and a total left femur BMD of 0.599 gm/cm2 (Z score of −3.9) (Fig. 3C).
Figure 1.
Nuclear medicine scan prior to diagnosis. Whole body bone scan performed prior to diagnosis and 3 months before surgical resection, findings include: (1) Focal uptake at the left femoral neck which may represent a site of insufficiency fracture (black arrow). (2) Multiple foci of uptake involving ribs (dark blue stars) and spinous process of T1 (orange arrow), likely representing fractures. (3) Uptake at the superior aspect of the right acetabulum which is likely related to an insufficiency fracture seen on a prior MRI (yellow arrows). (4) Foci of prominent uptake in the bilateral feet are suggestive of reactive/arthritic changes and/or fractures (light blue double arrow). (5) Focal uptake in the bilateral knees (red arrows) likely representing arthritis changes. (6) Diffuse uptake in the sacroiliac (SI) joints bilaterally (green arrows).
Figure 3.
DXA scans with estimated BMD before and after surgical resection. (A) Preoperative AP Spine DXA Scan calculated an estimated average BMD of 0.789 g/cm2 in regions L1-L4. (B) 9-month postresection AP Spine DXA scan calculated an estimated average BMD of 1.294 g/cm2 in regions L1-L4. (C) Preoperative dual femur DXA scan calculated an estimated average BMD of 0.599 g/cm2 (left total femur) and 0.606 g/cm2 (right total femur). (D) 9-months postresection dual femur DXA scan calculated an estimated average BMD of 1.156 g/cm2 (left total femur) and 1.217 g/cm2 (right total femur).
Due to suspected TIO, a FGF23 level was obtained which was in the upper range of normal (179 RU/mL; ≤ 180 RU/mL). The patient was started on phosphorous and calcitriol. A 68Ga-DOTATATE scan showed an intracranial focal area of uptake near the region of the right temporal lobe of the brain (Fig. 2A). A homogenously enhancing 1.3 × 1.1 × 1.0 cm intracranial lesion, appearing strikingly like a meningioma, arising from the right middle cranial fossa was redemonstrated on MRI (Fig. 2B). The patient was referred to Neurosurgery and underwent a craniotomy to resect the tumor. Pathology showed areas of well-circumscribed proliferation with variable cellularity, prominent hyalinization of blood vessels, and a chondromyxoid matrix [4] (Fig. 2C). These findings are consistent with a final pathologic diagnosis of a benign mesenchymal tumor.
Figure 2.
Characteristics of the intracranial mass. (A) 68Ga-DOTATATE Scan. An intracranial focal area of uptake near the region of the right temporal lobe of the brain (Black Arrow). (B) Fast spoiled gradient-recalled-echo (FSPGR) MRI scan. There is an extra-axial homogenously enhancing mass (White Arrow), arising from the anterior floor of the right middle cranial fossa along the posterior margin of the greater wing of the right sphenoid bone measuring 1.3 × 1.1 × 1.0 cm in AP, transverse, and craniocaudal dimension. (C) Histological Images with Hematoxylin and Eosin (H&E) Staining. Successive H&E histological sections (A–D) show a tumor composed of a mixture of bland spindle cells, adipose tissue, abundant blood vessels, and areas of extracellular chodromyxoid matrix with focal calcifications. The spindle cell component has oval nuclei with eosinophilic cytoplasm and indistinct cell borders. Mitotic figures are not identified. The vessels are ectatic with perivascular hyalinization, with some hemangiopericytoma-like morphology. No giant cells are observed. (A) No magnification. (B–D) Magnified at 20×.
Shortly after surgery, the patient's serum phosphorus normalized. FGF23 was initially undetectable postoperatively and then normalized to the midnormal range where it has remained. Within 3 months postresection, his pain and his ability to stand (Supplementary Video 1) and walk (Supplementary Video 2) improved significantly and he was able to resume swing dancing, which he had not been able to do for nearly a year.
A follow-up 9-month DXA scan showed a remarkable improvement in BMD to the normal range. Total spine BMD at 9 months postresection was 1.294 gm/cm2 (Z score of +0.5), which was an improvement of 64% (Fig. 3A and B), and his left total femur was 1.156 gm/cm2 (Z score of +0.5), which was an improvement of 93% (Fig. 3C and D). A more recent 1-year postresection interval history shows that the patient is doing well—recently joining a gym and working out weekly. All his fractures have healed, and he has some residual arthritis in his knees and ankles but no bone pain, fractures, or gait abnormalities (Fig. 4).
Figure 4.
Nuclear medicine scans 10 months postsurgical resection. (A) Whole body bone scan showing multiple foci of moderately increased radiotracer uptake in the bilateral shoulders, knees, ankles, and feet, albeit much less than preresection. This uptake is consistent with degenerative changes. There is no evidence of acute fracture. (B) Bone scan of bilateral feet shows uptake that is nonspecific and may represent old fractures on a background of degenerative changes. No acute fractures seen.
Discussion
Osteomalacia is the softening of bones caused by impaired bone mineralization at sites of bone remodeling and during bone formation primarily due to inadequate levels of available phosphate, calcium, and vitamin D or because of calcium resorption [5]. Typical signs and symptoms of osteomalacia include diffuse body pains, muscle weakness, fragility/fractures of the bones, and severely low bone mass. Since these symptoms are very generalizable and can range from mild to severe, these patients are often misdiagnosed and/or undiagnosed for many years. Additionally, young men are less likely to be evaluated for osteoporosis and fractures [6].
The most common cause of osteomalacia is vitamin D deficiency, therefore a serum total vitamin D level is a good primary screening test. Less common causes include hereditary disorders of vitamin D or phosphorous [7] which are typically identified in childhood. After a total vitamin D test, a serum phosphorous test should be performed. Hypophosphatemia should prompt 24-hour urine phosphorous and calcium levels to differentiate between malabsorption and phosphate wasting [8].
In our patient, vitamin D was low and additional lab tests showed hypophosphatemia with an increased 24-hour urine phosphorus (100 mg/day or a FEPO4 above 5% is indicative of renal phosphate wasting in patients with hypophosphatemia). Overall, these results suggested a phosphate wasting disorder [9].
Ultimately, differentiating between different phosphate wasting disorders is dependent on FGF23 (Table 1), which was only recently discovered in 2000 [10]. The normal physiological function of FGF23 is to regulate phosphate concentration in the blood. Normally, FGF23 is secreted by osteocytes in response to hyperphosphatemia or exogenous calcitriol. FGF23 then acts on the kidneys by decreasing the expression of NPT2, a sodium-phosphate cotransporter, in the proximal tubule [3]. Due to this, FGF23 decreases the reabsorption of phosphate while increasing its excretion. In addition, recent studies have shown that FGF23 may also suppress 1-alpha-hydroxylase, reducing its ability to activate vitamin D and therefore impairing both intestinal phosphorous and calcium absorption and homeostasis [11].
Table 1.
Phosphate wasting disorder differential diagnosis.
A differential diagnosis table for phosphate wasting disorders, including TIO, distinguished by serum FGF23 level [12].
| Phosphate wasting disorders with low FGF23 | Phosphate wasting disorders with high or “inappropriately normal” FGF23 |
|---|---|
| Alcohol consumption | X-linked hypophosphatemia (XLH) |
| Proton pump inhibitor (PPI) Use |
Epidermal nevus syndromes (ENS) |
| Fanconi syndrome | Autosomal dominant/autosomal recessive rickets |
| Myeloma | McCune Albright syndrome |
| Iron use | Cutaneous-skeletal hypophosphatemia syndrome (CSHS) |
| Copper disorders | Tumor-induced osteomalacia (TIO) |
A pertinent distinction between other diagnoses with high or “inappropriately normal” FGF23 (Table 1) and TIO is time of presentation. While most of these diseases present early in life, TIO tends to present later in life with an abrupt time course and a negative family history [1].
In our patient, FGF23 was in the upper range of normal, but not elevated, otherwise known as “inappropriately normal.” The determination of an “inappropriately normal” FGF23 value is dependent on serum phosphorous and calcitriol levels [12]. Compared to previously published cases, a nonelevated FGF23 level was unique to our patient. For example, Chong et al (2013) presents 31 TIO cases; all 31 having elevated FGF23 levels [13]. Therefore, FGF23 levels on the higher end of normal should, in some instances, be pursued [12].
Locating the active tumor in TIO can be difficult since they are usually small and can be located anywhere, but most commonly in the hands, feet, and nasal cavities with intracranial/brain lesions being rare. In recent literature, a full body 68Ga-DOTATATE scan has been shown to be the most specific imaging test [14]. The 68Ga-DOTATATE scan works by utilizing 68Gallium (68Ga)-conjugated somatostatin peptide analogues, such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)TATE, which enables somatostatin receptor imaging with positron emission tomography. This type of scan is most often used for somatostatin receptor positive neuroendocrine tumors and was only recently approved by the US Food and Drug Administration in 2016. Since phosphaturic mesenchymal tumors often express somatostatin receptors, this technique allows for “targeted” imaging of TIO-causing lesions. That being said, false positivity can still be caused by hypersplenism, fractures, sarcoidosis, or even vertebral hemangiomas [15]. If multiple tumors are present, selective FGF23 venous sampling has shown promise in deciphering the FGF23-producing tumor. Complete resection of the tumor is currently the only definitive cure. If the tumor cannot be located, is determined unresectable, or the patient is not a surgical candidate, phosphate and calcitriol should be given. A promising emerging treatment option currently under investigation is Burosumab, an anti-FGF23 IgG1 monoclonal antibody [16].
Finally, osteoporosis has been traditionally considered a postmenopausal disorder of women and DXA scans have been underutilized in men [17]. Phosphate wasting disorders, including TIO, should be considered in the differential diagnosis of unexplained osteoporosis and fractures and a serum phosphorus level should be evaluated as part of a routine metabolic workup. This case demonstrates the utility of DXA to establish severity of disease as well as to monitor our patient's rapid and remarkable response to intervention [18].
Conclusion
TIO is a paraneoplastic syndrome of Fibroblast Growth Factor 23 (FGF23) that causes renal phosphate wasting, ultimately leading to the development of osteomalacia [1]. In patients with TIO, biochemical studies show hypophosphatemia, hyperphosphaturia, and excessive or “inappropriately normal” FGC23 levels. DXA scans should be used to establish disease severity and to monitor recovery post-treatment. A detailed history is important in the final diagnosis of TIO as it is an acquired disorder and thus patients often have an unremarkable childhood history and family history of rickets and/or other bone diseases [10]. Full body imaging should be pursued if there is a high clinical suspicion for TIO [14]. A 68Ga-DOTATAE scan has been shown to be the most specific due to somatostatin-targeting but can have some false positives [15]. With resection, TIO is curable and laboratory values usually normalize shortly after surgery. In this case report, biochemical correction of FGF23 levels after surgical resection led to a remarkable and rapid correction of disability, fractures, and mineralization of bone mass from severely low to normal in a young male in just 9 months. If a mass is not found, is determined to be unresectable, or the patient is not a surgical candidate, phosphorous and calcitriol should be started and taken long-term. Burosumab (an anti-FGF23 monoclonal antibody approved for XLH) is currently under study and may be a potential therapeutic option for these patients [15].
Acknowledgments
Acknowledgments
One of the authors (JMC) is supported by NIGMS of the National Institutes of Health under award number T32GM007347. The content in this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Informed consent was obtained from the participant of this case study.
Footnotes
Competing interest: Juan M Colazo, Reid C Thompson, Natalie V Covington, and Kathryn M Dahir declare that they have no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2020.01.039.
Contributor Information
Juan M. Colazo, Email: juan.m.colazo@vanderbilt.edu.
Reid C. Thompson, Email: reid.thompson@vumc.org.
Natalie V. Covington, Email: natalie.covington@vumc.org.
Kathryn M. Dahir, Email: kathryn.dahir@vumc.org.
Appendix. Supplementary materials
References
- 1.McCrance RA. Osteomalacia with Looser's nodes (Milkman's syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q J Med. 1947;16:33–46. [PubMed] [Google Scholar]
- 2.Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005;294:1260–1267. doi: 10.1001/jama.294.10.1260. [DOI] [PubMed] [Google Scholar]
- 3.Phosphate Jüppner H. FGF‐23. Kidney Int Suppl. 2011;79(121):S24–S27. doi: 10.1038/ki.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agaimy A., Michal M., Chiosea S., Petersson F., Hadravsky L., Kristiansen G. Phosphaturic mesenchymal tumors: clinicopathologic, immunohistochemical and molecular analysis of 22 cases expanding their morphologic and Immunophenotypic spectrum. Am J Surg Pathol. 2017;41:1371–1380. doi: 10.1097/PAS.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 5.Francis RM, Selby PL. Osteomalacia. Baillieres Clin Endocrinol Metab. 1997;11:145–163. doi: 10.1016/s0950-351x(97)80569-1. [DOI] [PubMed] [Google Scholar]
- 6.Harper CM, Fitzpatrick SK, Zurakowski D, Rozental TD. Distal radial fractures in older men. A missed opportunity? J. Bone Joint Surg. Am. 2014;96(21):1820–1827. doi: 10.2106/JBJS.M.01497. [DOI] [PubMed] [Google Scholar]
- 7.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Rheum Dis Clin North Am. 2012;38:81–91. doi: 10.1016/j.rdc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(Pt 2):201–206. doi: 10.1177/000456329803500203. Review. [DOI] [PubMed] [Google Scholar]
- 9.Day A.L., Morgan S.L., Saag K.G. Hypophosphatemia in the setting of metabolic bone disease: case reports and diagnostic algorithm. Ther Adv Musculoskelet Dis. 2018;10:151–156. doi: 10.1177/1759720X18779761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Econs MJ. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. doi: 10.1038/81664. PMID 11062477. [DOI] [PubMed] [Google Scholar]
- 11.Perwad F., Zhang M.Y., Tenenhouse H.S., Portale A.A. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol. 2007;293(5):F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Jiang Y, Xia W. FGF23 and phosphate wasting disorders. Bone Res. 2013;1:120–132. doi: 10.4248/BR201302002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res. 2013;28:1386–1398. doi: 10.1002/jbmr.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Zhu Z, Zhong D, Dang Y, Xing H, Du Y. 68Ga DOTATATE PET/CT is an accurate imaging modality in the detection of culprit tumors causing osteomalacia. Clin Nucl Med. 2015;40:642–646. doi: 10.1097/RLU.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 15.Hofman M.S., Eddie Lau W.F., Hicks R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation1. Radiographics. 2015;35:500–516. doi: 10.1148/rg.352140164. [DOI] [PubMed] [Google Scholar]
- 16.Beur Suzanne Jan De, Miller Paul, Weber Thomas, Peacock Munro, Insogna Karl, Kumar Rajiv. OR13-1 Burosumab Improves the Biochemical, Skeletal, and Clinical Symptoms of Tumor-Induced Osteomalacia Syndrome. J Endocr Soc. 2019;3:13–21. Issue Supplement_1, AprilOR. [Google Scholar]
- 17.Management of osteoporosis in postmenopausal women: 2010position statement of The North American Menopause Society Menopause 2010170125–54., quiz 55–56
- 18.Blake G.M., Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med. 2007;83(982):509–517. doi: 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.