Abstract
Mycoplasma species (spp.) bacteria can infect cell cultures, posing a potential threat to recipients of cell therapy products. Conventional Mycoplasma testing methods are highly sensitive but typically require a minimum of 28 days to produce results. This delay is problematic if rapid results are needed to inform treatment decisions. Nucleic acid amplification technique (NAT) methods have been gaining favor for Mycoplasma testing due to their speed and specificity; however, they must first be qualified as meeting or exceeding the sensitivity of the compendial method. We present herein a NAT method for the detection of Mycoplasma that circumvents the need for live Mycoplasma spp. in the test procedure by instead being qualified using Mycoplasma spp. genomic DNA. We have demonstrated a lower limit of detection that exceeds the regulatory requirements set by Health Canada. This assay is now being used to screen clinical cell therapy products manufactured at our center.
Introduction
Mycoplasma species (spp.) are among the most common contaminants of cell cultures1 and biopharmaceuticals,2 and they pose a potential threat to patients receiving infusions of cell therapy products.3, 4, 5, 6 Consequently, our regulatory body, Health Canada, and regulators in other jurisdictions require the testing of cell therapy products for the absence of Mycoplasma spp. to ensure patient safety. Health Canada adopts the guidelines laid out in the recognized European Pharmacopeia for microbiological testing as stated in the Good Manufacturing Practices guide for drug products7 and Schedule B of the Food and Drugs Act.8 Conventionally, cell therapy products are tested for Mycoplasma contamination by culture and cell indicator methods, as described in the European and United States Pharmacopeias.9,10 Briefly, the culture method involves growth of cultivatable strains in liquid broth and on solid agar medium and is capable of detecting 10 colony forming units (CFU)/mL, while the indicator cell culture method utilizes mammalian cell cultures (such as Vero cells) to support the growth of fastidious strains. Growth is detected by staining cell cultures with a fluorescent DNA binding dye followed by visualization by microscopy. The indicator cell culture method is less sensitive than the culture method, with a sensitivity of 100 CFU/mL.10 These conventional methods provide effective Mycoplasma detection; however, they are time-consuming (a minimum of 28 days to perform). This lengthy turnaround time can be problematic in the field of cell therapy, especially for non-cryopreserved cell products that expire quickly (within 24–48 h) and where Mycoplasma test results are needed immediately to inform treatment decisions.
Nucleic acid amplification technique (NAT)-based assays, such as polymerase chain reaction (PCR) techniques, are a potential solution to this issue. NAT-based tests detect the presence of a nucleic acid sequence unique to potentially contaminating microorganisms of interest, and they are highly sensitive and rapidly executable. To meet regulatory requirements, new assays must be qualified in-house to meet or exceed the sensitivity of the compendial methods which, for Mycoplasma spp., is 10 CFU/mL. Qualified PCR-based Mycoplasma detection assays have previously been reported.11, 12, 13 These assays used live Mycoplasma spp. for qualification. The use of live Mycoplasma spp. is problematic in facilities that generate cell therapy products because it introduces an unnecessary risk of cell product contamination. Herein, we describe a rapid PCR-based assay that we have qualified for use in testing clinical cell therapy products for Mycoplasma spp. contamination. Briefly, our Mycoplasma detection assay utilizes the commercially available MycoTOOL PCR Mycoplasma detection kit (Roche) with a modified protocol in order to obtain the required 10 CFU/mL sensitivity level. The protocol involves DNA extraction from samples of cell therapy products, amplification of Mycoplasma spp. nucleic acid via highly sensitive touchdown PCR, and visualization by gel electrophoresis. The use of live Mycoplasma spp. to demonstrate assay sensitivity is avoided through the addition of defined quantities of Mycoplasma spp. genomic DNA (gDNA) that are converted to CFU/mL values using empirically derived genome copy to CFU (GC/CFU) ratios.14,15
Qualification of an alternative assay must include evaluation of the detection limit, specificity, and robustness. Formally, “detection limit” is defined as the lowest amount of target nucleic acid in a sample that can be detected, and “specificity” is defined as the ability to unequivocally assess target nucleic acid in the presence of components that may be expected to be present. “Robustness” is defined as the capacity to remain unaffected by small but deliberate variations in method parameters, and it provides an indication of reliability during normal usage.10 We qualified our Mycoplasma detection assay using CAR (chimeric antigen receptor)-T cell samples manufactured at our center. Specificity testing verified the ability to detect Mycoplasma spp. in cell therapy samples, and lower limit of detection (LLOD) testing established a level of sensitivity that satisfies the 10 CFU/mL detection requirement. Intermediate precision (measurement of within-laboratory variations)16 was also evaluated as a measure of assay robustness.
Results
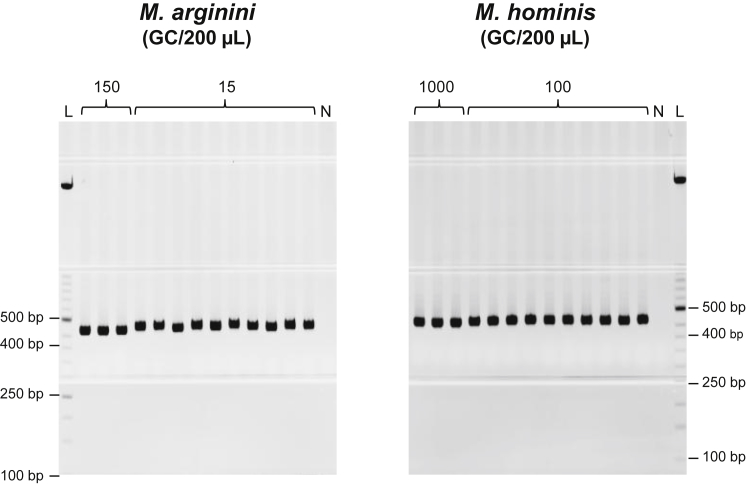
To establish the LLOD for the assay, gDNA samples of each Mycoplasma strain were diluted in Elution Buffer (EB) to the indicated concentrations (Figure 1). Positive 400- to 500-bp bands are present in 3 out of 3 reactions for Mycoplasma arginini gDNA diluted to 150 genome copies (GC)/200 μL, and in 10 out of 10 reactions for 15 GC/200 μL. This establishes the LLOD for M. arginini at 15 GC/200 μL, which is below the 18 GC/200 μL requirement (equivalent to 10 CFU/mL, as described under “Positive Control gDNA” below and Figure 2). Positive bands are present in 3 out of 3 reactions for Mycoplasma hominis diluted to 1,000 GC/200 μL as well as in 10 out of 10 reactions for 100 GC/200 μL. This establishes the LLOD for M. hominis at 100 GC/200 μL, which is also below the 107.2 GC/200 μL requirement.
Figure 1.
Lower Limit of Detection Testing
M. arginini or M. hominis gDNA was diluted to the indicated concentrations in EB and amplified with universal Mycoplasma spp. primers. M. arginini, 150 or 15 GC/200 μL; M. hominis, 1,000 or 100 GC/200 μL. Lane L, ladder; lane N, PCR negative control. Expected Mycoplasma-specific band size was 400–500 bp. Representative gels from two rounds of testing are shown.
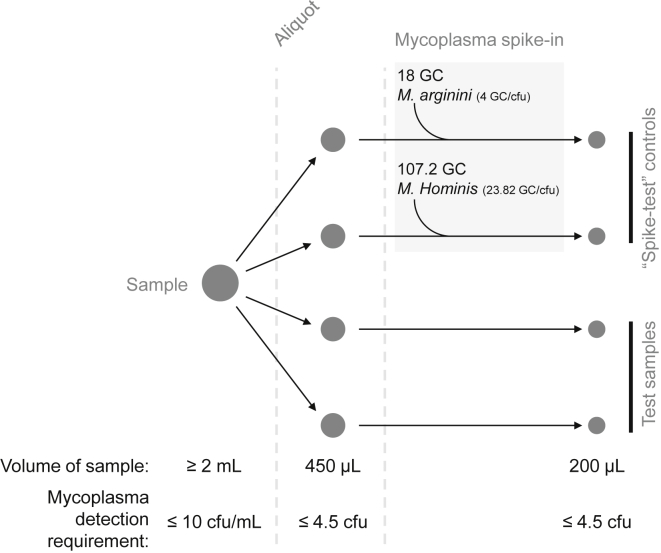
Figure 2.
Sample Processing
This simplified schematic summarizes the standard DNA preparation (≤5 × 106 total cells/mL) sample processing steps and detection requirements. Four 450 μL cell sample aliquots are processed to generate DNA in 190 μL. 10 μL of EB (test samples) or 10 μL of diluted gDNA (spike-test controls) is added to each vial to bring the final volume to 200 μL. M. arginini (18 GC in 10 μL) or M. hominis (107.2 GC in 10 μL) gDNA is added to each of the spike-test control samples to test the 10 CFU/mL detection requirement.
Specificity Evaluation
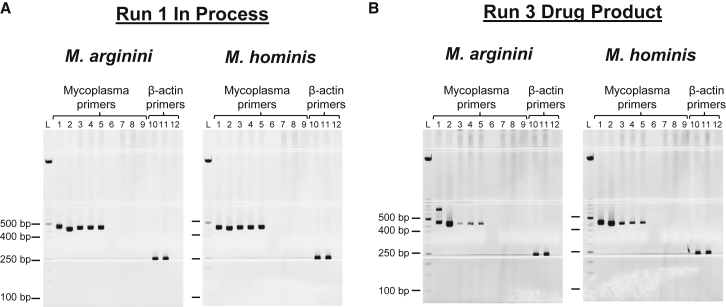
The specificity of the assay was analyzed in 17 rounds of testing with CAR-T cell in-process and drug product sample types. All rounds were successful; gels from two representative assays are shown in Figure 3. Both species of Mycoplasma were detected in at least two out of three “spike-test” sample aliquots (Figures 3A and 3B, lanes 3–5), as evidenced by the presence of positive bands in these lanes between 400 and 500 bp. Additional bands (>500 bp) are sporadically observed in Mycoplasma amplification reactions (Figure 3B, M. arginini gel, lane 1). These bands represent non-specific amplification products, and they are not the result of contamination of the PCR reactions. The absence of contamination is confirmed by the lack of positive 400- to 500-bp bands in the Mycoplasma primer PCR negative control reaction (lane 10). The successful amplification of Mycoplasma-specific bands in spike-test samples from both sample types established the specificity of the assay by demonstrating that the assay was capable of detecting the target in the presence of multiple matrices.
Figure 3.
Specificity Testing
The Mycoplasma Detection Assay was run on in-process and drug product samples to demonstrate that the cell matrix does not interfere with Mycoplasma detection. Two representative assays are shown: run 1, in-process sample (A) and run 3, drug product sample (B). Lane 1, positive control: 18 GC M. arginini or 107.2 GC M. hominis; lane 2, positive control: 1,800 GC M. arginini or 10,720 GC M. hominis; lanes 3–5 and 11, M. arginini or M. hominis spike-test controls; lane 6, blank; lanes 7, 8, and 10: test sample; lanes 9 and 12, negative control; lane L, ladder. Mycoplasma-specific expected band size was 400–500 bp; β-actin was 250 bp.
Robustness Testing
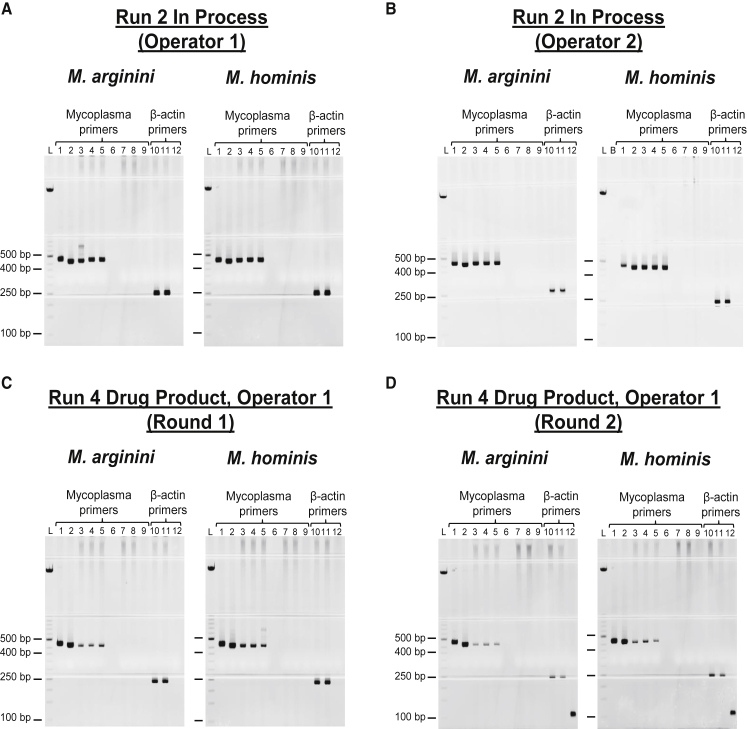
Four CAR-T cell samples were successfully re-tested by additional operators; the results of two representative assays are shown in Figures 4A and 4B. Gels for each round were analyzed following the acceptance criteria (Materials and Methods) and were found to be in agreement: positive spike-test results as well as β-actin control reactions demonstrate that the assay performs as intended, independent of the operator performing the assay. An additional measure of intermediate precision was executed by the repetition of the assay on the run 4 in-process and drug product samples by the same operator on separate days. The results of run 4 drug product testing are shown in Figures 4C and 4D. Both rounds of testing were successful, and the results are in agreement with each other. The replication of results across different days and by different operators demonstrated that the assay was reliable and established the precision of the assay.
Figure 4.
Intermediate Precision Testing
The Mycoplasma Detection Assay was run on samples multiple times to demonstrate the reliability of the assay. (A and B) Run 2 in-process samples were tested by operator 1 (A) and operator 2 (B). (C and D) Run 4 drug product samples were tested twice by operator 1 (C [round 1] and D [round 2]). Lane 1, positive control: 18 GC M. arginini or 107.2 GC M. hominis; lane 2, positive control: 1,800 GC M. arginini or 10,720 GC M. hominis; lanes 3–5 and 11, M. arginini or M. hominis spike-test controls; lane 6, blank; lanes 7, 8, and 10, test sample; lanes 9 and 12, negative control; lane L, ladder; lane B, blank. Mycoplasma-specific expected band size was 400–500 bp; β-actin was 250 bp.
Discussion
We describe herein a protocol for the detection of Mycoplasma spp. that we have qualified for Mycoplasma screening of autologous cell therapy products. We have demonstrated that the detection levels for both M. arginini and M. hominis are below the 10 CFU/mL LLOD requirement. We have also demonstrated that the assay detects Mycoplasma spp. in the presence of multiple matrices, while repeated rounds of testing established the reliability and robustness of the assay.
The use of the commercial MycoTOOL PCR Mycoplasma detection kit in this protocol is advantageous because it has been approved by the US Food and Drug Administration (FDA), Health Canada, and the European Medicines Agency (EMA) for use in Mycoplasma spp. testing. Laboratories can leverage data generated by the kit manufacturer in their qualification studies,10 decreasing the cost of implementation as well as the time necessary to validate their procedures. The universal primers included in the kit target the 16S rRNA sequence conserved across multiple Mycoplasma strains,12 including those representing 90%–95% of Mycoplasma cell culture contaminations.1 This allows for the detection of a wide variety of Mycoplasma strains, including fastidious strains that are difficult to detect even by conventional growth-based methods.
The strategy of using gDNA to establish sensitivity enables widespread adoption since the specialized equipment, reagents, and knowledge required for the cultivation of Mycoplasma spp. is not necessary, in addition to avoiding cross-contamination of sensitive cultures. This approach has previously been described;17 however, for qualification of the assay we describe herein, we take the additional step of accounting for the 1/10 sampling at the PCR level in the calculations of LLOD. In our hands, this was necessary for successful qualification of the assay.
This Mycoplasma Detection Assay fulfills an important need in the cell therapy field for a rapid assay that can facilitate the prompt decision-making that is critical for the use of live cell therapies. The use of a PCR-based assay avoids the cultivation time necessary for compendial methods while still performing at the required level of sensitivity. The use of gDNA as a positive control in place of live Mycoplasma spp. allows the assay to be performed in-house, further decreasing the time required to produce results. We share details of this protocol in the hopes that other laboratories generating biologics that require Mycoplasma testing can adopt this approach and expedite Mycoplasma testing of their products to ensure patient safety.
Materials and Methods
Mycoplasma Detection Assay
This assay requires standard precautions for PCR setup such as the use of dedicated workstations, filtered tips, and DNA-free materials and reagents. Additionally, the pre-PCR and post-PCR products were spatially segregated, and extreme care was taken to not cross-contaminate samples with positive control Mycoplasma gDNA during sample processing and PCR setup steps.
Positive Control gDNA
gDNA samples from M. arginini strain G230 (ATCC qCRM-23838D) and M. hominis strain LBD-4 (ATCC qCRM-27545D) were used to establish LLOD for the assay, as well as to assess matrix interference in cell therapy samples. These strains were chosen as representative of the upper (M. hominis, 10 CFU/mL) and lower (M. arginini, 0.1 CFU/mL) detection levels of the MycoTOOL PCR Mycoplasma detection kit.11
Mycoplasma gDNA was used to measure assay sensitivity by adding known numbers of genome copies to PCR reactions and assessing amplification success. The GC/CFU ratio for each species was used to convert the desired CFU number to the corresponding genome copies value. We used previously published GC/CFU ratios of 23.82 for M. hominis strain LBD-415 and 4.0 for M. arginini strain G23014 to calculate the minimum GC that must be detected in the assay in order to satisfy the 10 CFU/mL LLOD requirement. In our assay, a sample of cell product is divided into 450 μL aliquots. DNA is isolated from each aliquot and each sample of isolated DNA has a final volume of 200 μL (Figure 2). The 10 CFU/mL detection requirement means that we need to detect 4.5 CFU in each 450 μL sample aliquot, corresponding to 18 GC of M. arginini or 107.2 GC of M. hominis gDNA. Each 450 μL sample generates DNA in a final volume of 200 μL, and thus the detection requirement for M. arginini is 18 GC/200 μL and 107.2 GC/200 μL for M. hominis.
Primers
Universal Mycoplasma primer A (forward, 5′-GGCGAATGGGTGAGTAACACG-3′) and primer B (reverse, 5′-CGGATAACGCTTGCGACCTATG-3′) targeting the 16S rRNA gene, originally described by Wong-Lee and Lovett,18 are included in the MycoTOOL kit. This primer set has been validated for the detection of Mycoplasma fermentans, Acholeplasma laidlawii, Mycoplasma hyorhinis, Mycoplasma orale, Mycoplasma pneumoniae, M. arginini, Spiroplasma citri, Mycoplasma salivarium, and M. hominis at 10 CFU/mL and it has limited cross-reactivity to phylogenetically similar Gram-positive Lactobacillus acidophilis, Streptococcus bovis, and Clostridium sporogenes.11
The MycoTOOL kit includes control primers to amplify Gapdh from the Chinese hamster ovary (CHO) cell line. This primer set is unable to amplify GAPDH in human cells. Consequently, primers targeting human β-actin (hACTB393.f, hACTB642.r; PrimerBank ID: 4501885a1)19 were used to confirm cell lysis and DNA recovery in control PCR reactions. Primers were synthesized by Integrated DNA Technologies at a 25-nmol scale with standard desalting. They were resuspended to 100 μM with TE (100 mM Tris-HCl, 1 mM EDTA) (Molecular Probes) and prepared as a 10 μM mixture (5 μM each primer) with EB buffer (QIAGEN) for use in PCR setup.
DNA Extraction
Cell therapy samples were processed using a QC Sample Preparation Kit (Roche). All reagents were included in the kit unless otherwise specified. The DNA extraction procedure is dependent on cell density: samples ≤5 × 106 cells/mL are processed following the standard DNA preparation protocol, while samples >5 × 106 to 1 × 108 cells/mL are processed following the high cell density DNA preparation protocol.
Standard DNA Preparation: ≤5 × 106 Total Cells/mL
Cell samples (≥2 mL) were divided into four aliquots of 450 μL each (Figure 2). 50 μL of proteinase K and 450 μL of lysis buffer were added to each vial followed by vortexing three times for 5-s durations. Samples were incubated for 15 min at 56°C/600 rpm in a Thermomixer R with 2.0 mL block (Eppendorf). 630 μL of precipitation reagent and 2 μL of GlycoBlue coprecipitant (Invitrogen) were added to each vial, followed by 20 inversions and vortexing for 5 s. Samples were then centrifuged for 3 min at 16,000 × g, and supernatants were removed by pipetting. 1 mL of washing buffer was used to wash each pellet. Vials were inverted five times to mix and DNA was pelleted by centrifugation for 3 min at 16,000 × g. Supernatants were completely removed by pipetting. 190 μL of dilution reagent was added to each of the four sample vials, and DNA was resuspended by incubating in the thermomixer at 80°C/900 rpm for 10 min followed by brief vortexing.
High Cell Density DNA Preparation: >5 × 106 to 1 × 108 Total Cells/mL
Two 950 μL aliquots of cell sample were diluted with 950 μL of DNA-free water (Figure S1). Each of the 1,900 μL diluted aliquots was split further into four 450 μL aliquots, for a total of eight 450 μL samples. 50 μL of proteinase K and 700 μL of lysis buffer were added to each vial, followed by vortexing three times for 5-s durations. Samples were incubated for 30 min at 56°C/600 rpm in a Thermomixer R with 2.0 mL block (Eppendorf). 800 μL of precipitation reagent and 2 μL of GlycoBlue coprecipitant (Invitrogen) were added to each vial followed by 20 inversions and vortexing for 5 s. Samples were then centrifuged for 3 min at 16,000 × g, and supernatants were removed by pipetting. 1 mL of washing buffer was used to wash each pellet. Vials were inverted five times to mix and DNA was pelleted by centrifugation for 3 min at 16,000 × g. Supernatants were completely removed by pipetting. 95 μL of dilution reagent was added to each of the eight sample vials and DNA was resuspended by incubating in the thermomixer at 80°C/900 rpm for 15 min, followed by brief vortexing. Pairs of tubes were pooled to generate a total of four vials containing 190 μL of DNA sample in each.
Endpoint PCR Assay
PCR was performed using the MycoTOOL Mycoplasma detection amplification kit (Roche) with two modifications: (1) the CHO-specific Gapdh primer set included with the kit was replaced with a human β-actin primer set; and (2) the PCR reactions were scaled up from 50 μL to 100 μL total volume. This change was necessary to be able to add more input DNA to the reaction, in order to fulfill the 10 CFU/mL sensitivity requirement. We were unable to establish a LLOD at or below 10 CFU/mL with the original volumes. All other reagents used are included with the kit. Two master mixes were prepared: one to amplify Mycoplasma spp.-specific templates, with the other targeting β-actin. Each 60 μL of master mix contained 1.4 μL of RM1a, 20 μL of RM1b, 14 μL of MgCl2 (25 mM), 2 μL of primer mix Mycoplasma or 10 μL of β-actin primer mix (10 μM, 5 μM each primer), 4 μL of detection dye, and 18.6 μL (for Mycoplasma master mix) or 10.6 μL (for β-actin master mix) of PCR-grade H2O. 60 μL of each master mix was aliquoted to reaction tubes.
Samples were added to reactions in the following order: (1) negative control, (2) test samples, (3) spike-test samples, and (4) PCR-positive controls. 40 μL of EB was added to each of the Mycoplasma and β-actin negative control reactions. 10 μL of EB was added to two of the test samples to bring the final volume to 200 μL. 40 μL of each test sample was run in duplicate in Mycoplasma-specific amplification reactions. To generate the spike-test samples, M. arginini and M. hominis gDNA samples were diluted to the appropriate concentrations and then 10 μL of each diluted sample, respectively, was spiked into the two remaining test sample aliquots (Figure 2). 40 μL of each of these spike-test samples was then added to the Mycoplasma-specific amplification reactions, in triplicate. Each of the test and spike-test control samples (40 μL) was also used as template in β-actin amplification reactions. PCR-positive controls consisted of suitable dilutions of M. arginini and M. hominis gDNA spiked into EB, which were then added to Mycoplasma-specific master mix. All reactions were mixed by pipetting and subjected to PCR cycling.
PCR Cycling and Amplicon Detection
Reactions were cycled on a Bio-Rad Dyad thermal cycler in a touchdown PCR program as described in the MycoTOOL Mycoplasma detection amplification kit instructions: samples were incubated at 40°C for 5 min in a carryover prevention step, followed by initial denaturation at 94°C for 10 min. The touchdown portion of the program includes 20 cycles with a denaturation step at 94°C for 30 s, annealing for 30 s with a decreasing temperature profile (2 cycles each at 70°C, 69°C, 68°C, 67°C, 66°C, 65°C, 64°C, 63°C, 62°C, and 61°C), and an elongation step at 72°C for 45 s. This was followed by 25 cycles with a denaturation step at 94°C for 30 s, an annealing step at 60°C for 30 s, and an elongation step at 72°C for 45 s. The final elongation was at 72°C for 4 min, and samples were then held at 4°C.
Following amplification, 12 μL of PCR product was added to 3 μL of 5× Hi-Density TBE (Tris-borate-EDTA) sample buffer (Invitrogen) and mixed by pipetting up and down. A DNA molecular weight marker (included with kit) was prepared by mixing 16 μL of molecular weight marker with 24 μL of 1× TBE-electrophoresis buffer (Invitrogen), 8 μL of Hi-Density TBE sample buffer, and 1.6 μL of detection dye (included with kit). 10 μL of PCR sample or molecular weight marker was loaded per lane on Novex 6% TBE gels (Invitrogen) and subjected to electrophoresis in XCell Surelock mini-cell (Thermo Fisher Scientific) using a Bio-Rad PowerPac HC at 200 V for 40 min. Gels were visualized on a FLA 9500 (GE Healthcare) using the SYBR Safe (473 nm) settings. Gel results were analyzed based on the acceptance criteria listed below:
-
1.1.1.
The Mycoplasma expected band size is ∼450 bp; any bands between 400 and 500 bp in size are considered a positive result.11 The expected size for the β-actin band is 250 bp.
-
1.1.2.
The Mycoplasma and β-actin negative control lanes do not contain a band of the expected sizes, to rule out contamination of the PCR reactions.
-
1.1.3.
The β-actin control reactions all contain the expected 250-bp band to ensure that the cell lysis and DNA isolation were successful.
-
1.1.4.
At least two of the three spike-test reactions have a positive ∼450-bp band to confirm that there was no interference in the PCR reactions by the sample matrix.
-
1.1.5.
If the spike-test controls fail, the results of the positive control reactions can be used to assess whether there was an issue with the PCR reagents/setup/cycling.
-
1.1.6.
If there are 400- to 500-bp bands in any of the four test sample lanes, the sample is considered positive for Mycoplasma spp.
Assay Qualification
The Mycoplasma detection assay qualification process included determination of LLOD, specificity, and intermediate precision testing.
LLOD Determination
Regulators require that NAT-based Mycoplasma detection assays are validated against a panel of species including A. laidlawii, M. fermentans, M. hyorhinis, M. pneumoniae, M. orale, and M. arginini.9,10 The ability of the MycoTOOL PCR Mycoplasma detection kit to detect all of the required species has been previously established11 and was not repeated in our qualification. Instead, we selected two species that were detected with the highest (M. arginini, 0.1 CFU/mL) and lowest (M. hominis, 10 CFU/mL) sensitivity using the MycoTOOL kit11 to evaluate the performance of the kit at our center.
M. arginini and M. hominis gDNA samples were diluted and then spiked into EB at 200 μL total volume to test whether the LLOD was below the 18 GC/200 μL (M. arginini) or 107.2 GC/200 μL (M. hominis) requirements. M. arginini was diluted to 15 and 150 GC/200 μL, and M. hominis was diluted to 100 and 1,000 GC/200 μL. 40 μL of the spiked EB dilutions were added to tubes containing 60 μL of Mycoplasma master mix and mixed by pipetting. PCR cycling and amplicon detection was performed as described in “PCR Cycling and Amplicon Detection” above.
Specificity Testing
Specificity evaluation for NAT-based assays requires confirmation that the test specifically detects a target nucleic acid. As discussed above, the ability of the universal Mycoplasma primer set to detect the required panel of Mycoplasma species was previously established.11 We were able to leverage these data and did not need to perform this aspect of specificity testing in our qualification process.
Specificity is also a measure of the ability to detect a target in the presence of matrix components, which are any substances present in samples in addition to the target of interest. These substances may interfere with template amplification, and thus each sample type to be tested with the Mycoplasma detection assay must be evaluated. We initially developed the Mycoplasma detection assay to screen both in-process and final drug product samples generated during CAR-T cell production runs using the CliniMACS Prodigy system (Miltenyi Biotec) (Figure S3). The in-process sample is taken at day 5 of the CAR-T culture process and consists of cells in TexMACS GMP medium (Miltenyi Biotec) supplemented with gentamicin sulfate (Sandoz) and interleukin-7/-15 (Miltenyi Biotec). The drug product is a subsample of the final infusion product taken at day 12 and contains CAR-T cells in PlasmaLyte (Baxter) with human serum albumin (CSL Behring). In-process and drug product samples from eight CAR-T production runs were used in 17 rounds of testing to evaluate potential matrix interference in the Mycoplasma detection assay (Figure S2).
Intermediate Precision/Robustness Testing
The robustness of the endpoint MycoTOOL kit was previously established by testing the performance of the assay across different kit lots.11 To qualify the assay for use in our center, we evaluated the intermediate precision as an additional measure of the overall robustness of the assay. Intermediate precision measures within-laboratory variation, such as assay performance on different days or by different analysts.16
Two additional operators re-tested four samples in six separate assays to assess any effect of different analysts on the assay outcome. Two samples were also re-tested by the same operator to evaluate assay performance on separate days (Figure S2).
Author Contributions
L.D., M.C., R.A.H., and M.B. designed the qualification study plan. L.D., E.Y., and L.L. performed the experiments. J.R.W. and B.H.N. provided samples. L.D. and R.A.H. wrote the manuscript. R.A.H, K.A.H, B.H.N., and N.K. provided supervision. All authors reviewed and edited the manuscript.
Acknowledgments
We thank Scott D. Brown for assistance with the figures. This work was supported by BioCanRx (Biotherapeutics for Cancer Treatment funded by the Networks of Centres of Excellence), the Canada Foundation for Innovation, and by the BC Cancer Foundation.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.01.009.
Supplemental Information
References
- 1.Drexler H.G., Uphoff C.C. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002;39:75–90. doi: 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong S.E., Mariano J.A., Lundin D.J. The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals. 2010;38:211–213. doi: 10.1016/j.biologicals.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Yacoub A.T., Khwaja S.I., Taiwo T., Kim J.H., Nanjappa S., Greene J.N. Mycoplasma pneumoniae in the immunocompromised host: a case series and review of literature. Infect. Dis. Clin. Pract. 2016;24:310–313. [Google Scholar]
- 4.MacKenzie C.R., Nischik N., Kram R., Krauspe R., Jäger M., Henrich B. Fatal outcome of a disseminated dual infection with drug-resistant Mycoplasma hominis and Ureaplasma parvum originating from a septic arthritis in an immunocompromised patient. Int. J. Infect. Dis. 2010;14(Suppl 3):e307–e309. doi: 10.1016/j.ijid.2010.02.2253. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M., Hitomi S., Goto M., Hasegawa Y. Bloodstream infection due to Mycoplasma arginini in an immunocompromised patient. J. Clin. Microbiol. 2012;50:3133–3135. doi: 10.1128/JCM.00736-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yechouron A., Lefebvre J., Robson H.G., Rose D.L., Tully J.G. Fatal septicemia due to Mycoplasma arginini: a new human zoonosis. Clin. Infect. Dis. 1992;15:434–438. doi: 10.1093/clind/15.3.434. [DOI] [PubMed] [Google Scholar]
- 7.Government of Canada . 2009. Good manufacturing practices guide for drug products (GUI-0001)https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/gmp-guidelines-0001/document.html#a4.2 [Google Scholar]
- 8.Government of Canada . 2019. Food and Drugs Act (R.S., c. F-27, s.1)https://laws-lois.justice.gc.ca/eng/acts/F-27/page-14.html#docCont [Google Scholar]
- 9.United States Pharmacopeia . US Pharmacopeial Convention; 2017. USP <63> Mycoplasma Tests; pp. 5978–5984. [Google Scholar]
- 10.European Pharmacopoeia (2011). Mycoplasmas. In European Pharmacopoeia (EDQM), Chapter 2.6.7.
- 11.Deutschmann S.M., Kavermann H., Knack Y. Validation of a NAT-based Mycoplasma assay according European Pharmacopoiea. Biologicals. 2010;38:238–248. doi: 10.1016/j.biologicals.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Eldering J.A., Felten C., Veilleux C.A., Potts B.J. Development of a PCR method for mycoplasma testing of Chinese hamster ovary cell cultures used in the manufacture of recombinant therapeutic proteins. Biologicals. 2004;32:183–193. doi: 10.1016/j.biologicals.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Zhi Y., Mayhew A., Seng N., Takle G.B. Validation of a PCR method for the detection of mycoplasmas according to European Pharmacopoeia section 2.6.7. Biologicals. 2010;38:232–237. doi: 10.1016/j.biologicals.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Dabrazhynetskaya A., Furtak V., Volokhov D., Beck B., Chizhikov V. Preparation of reference stocks suitable for evaluation of alternative NAT-based mycoplasma detection methods. J. Appl. Microbiol. 2014;116:100–108. doi: 10.1111/jam.12352. [DOI] [PubMed] [Google Scholar]
- 15.Dabrazhynetskaya A., Volokhov D.V., Lin T.L., Beck B., Gupta R.K., Chizhikov V. Collaborative study report: evaluation of the ATCC experimental mycoplasma reference strains panel prepared for comparison of NAT-based and conventional mycoplasma detection methods. Biologicals. 2013;41:377–383. doi: 10.1016/j.biologicals.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Working Group. (2005) Validation of Analytical Procedures: Text and Methodology, Q2(R1) (IFPMA). http://academy.gmp-compliance.org/guidemgr/files/Q2(R1).PDF.
- 17.Chisholm J., Bhatt S., Chaboureau A., Viswanathan S. Strategy for an abbreviated in-house qualification of a commercially available rapid microbiology method (RMM) for Canadian regulatory approval. Cytotherapy. 2017;19:1529–1536. doi: 10.1016/j.jcyt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Wong-Lee J.G., Lovett M. Rapid and sensitive PCR method for identification of Mycoplasma species in tissue culture. In: Persing P.H., Smith T.F., Tenover F.C., White T.J., editors. Diagnostic Molecular Microbiology. Principles and Applications. American Society for Microbiology; 1993. pp. 257–260. [Google Scholar]
- 19.Spandidos A., Wang X., Wang H., Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.