Highlights
-
•
Dracocephalum kotschyi Boiss belongs to Lamiaceae and one of the eight species of the genus Dracocephalum, is an endemic species of Iran and is grown in areas such as Isfahan, Yasuj, Mazandaran, and Tabriz. D kotschyi (Zarringiah) using the IUCN grouping criteria is one of the vulnerable species in Iran.
-
•
The lowest concentration of plant sample (15.8489 ± 0.001 μg/ml) was related to flower organs in the second year of cultivation, an increase can be observed in the antioxidant activity of this plant compared to the synthetic antioxidant (19.95 μg/ml).
-
•
The six-year-old plant with the highest antioxidant capacity, phenolic, and flavonoid compounds to produce the best age vegetative antioxidant and is mainly based on flower organs are preferred.
Keywords: Extract, Flavonoids, IC50, Lamiaceae, Phenol
Abstract
Objective
Dracocephalum kotschyi Boiss is one of eight species of Dracocephalum, an endemic species of Iran, and grows in areas such as Isfahan, Yasuj, Mazandaran, and Tabriz. The present study was designed to analyze the antioxidant, phenol and flavonoids contents of this plant under different conditions of cultivation in a completely randomized, factorial design.
Materials and methods
Shrubs of different ages (two, three, and six) in cultivated rangeland collected from three randomly plant at the end of May 2018 simultaneously with the flowering time. Antioxidant activity of methanolic extract of plant samples were evaluated with DPPH method. Total phenolic compounds and the total content of flavonoids were measured using the Folin Sioukhlu and aluminum chloride methods respectively.
Results
The result showed that there was a significant effect of planting time, plant organs and interaction of time and organ on the total antioxidant capacity and total phenol and flavonoids contents. The highest antioxidant activity, total phenol, and total flavonoid have belonged to the flower of six age plants. The highest antioxidant activity, total phenol, and total flavonoid have belonged to the flower of six age. The highest level of antioxidant activity with IC50 15.8489 ± 0.001 μg/ml belonged to the flower of the two-year-old plant, which has a stronger antioxidant activity than the BHT standard with 19.95 μg/ml.
Conclusion
But in general, it can be said that the six-year-old plant with the highest antioxidant capacity, phenolic, and flavonoid compounds is the best age and mainly flower organ is preferred.
1. Introduction
Natural antioxidants increase the strength of the antioxidants in the body and, as a result, reduce the incidence of certain diseases, such as cancers, heart disease, and stroke. Also, Natural antioxidant compounds are significant as important drug compounds and have a high ability to cope with many diseases [1].
Phenols are the largest group of secondary metabolites that are anti-allergic, anti-inflammatory, antimicrobial, antimicrobial, antiviral and anti-oxidant [2]. Phenols have a complete chemical structure to disable free radicals [3]. The most characteristic of all flavonoids is the action of antioxidants. This feature is very important. Because the cells of the body are constantly exposed to damage by free radicals and active oxygen species that are produced during normal oxygen metabolism or external damage (Grout et al., 1994).
Dracocephalum kotschyi Boiss belongs to Lamiaceae and one of the eight species of the genus Dracocephalum, is an endemic species of Iran and is grown in areas such as Isfahan, Yasuj, Mazandaran, and Tabriz [4,5].
Different species of Dracocephalum have flavonoids, deuterenes, tannins and phenolic acids [6]. There is a combination of spinal-z in the leaf of D. kotschyi, which is used to treat cancer. The organs of D. kotschyi are reported to have flavonoids, monoterpenes, terpenoid, phytosterol and beta-caryophyllene Seidenia et al., 2004; [7]. Nine flavonoids have been reported by D. kotschyi [8]. Two new glycosides monoterpene, along with seven terpenoids and phytosterol, have been isolated from the organs of D. kotschyi and tested as an analgesic in mice. [9].
Because of the limited collection of medicinal plants, there is a tendency to cultivate these plants in agricultural systems. The cultivation of medicinal plants under appropriate environmental conditions allows us to produce plants that are rich in the metabolites [46].
Dracocephalum kotschyi Boiss (Zarringiah) using the IUCN grouping criteria is one of the vulnerable species in Iran [10]. The uncontrolled harvesting of this plant at the flowering stage by indigenous people has prevented the seed from planting this plant and, as a result, has reduced the population of this plant. On the other hand, some natural factors such as erosion and abnormalities such exploitation has caused the plant to become extinct. Therefore, the present study was prepared for the first time in Iran to investigate and compare some plant extract compounds from different culture conditions, so that the results of this study are an important step in domestication and planting of this plant to prevent uncontrolled harvesting and extinction.
2. Materials and methods
2.1. Select the habitat
To select the sampling area, firstly, the livestock habitats were identified in Isfahan Province with library and field studies. Then, cultivated rangelands of Meidanak from the Fereydoun Shahr city were selected considering the various factors such as the availability of habitat, the natural conditions of the habitat, suitable planting presence through field visits from different ages, the conditions such as non-fertilization and soil nutrient manipulation, as well as the absence of different irrigation and cultivation treatments. Geographically the sampling site was located along latitude 50˚ 07ʹ 23.7ʺ and latitude 32˚ 47ʹ 13.9ʺ. The average height of the site was 2594 m above sea level.
2.2. The climate situation
The weather in Isfahan province is generally mild. To know the weather conditions of the studied areas, the required data from the synoptic and phytoremedients stations of Fereydoun Shahr with concerning the databases and subregional organizations were extracted for the 30-year statistical period (1966–2018). (e.g. Table 1). All-weather forecast of Isfahan province, Isfahan climate profile (2018)
Table 1.
Some climatic parameters of the sampling site.
| Site | Average rainfall (mm)) | Average temperature (C) | Maximum temperature (° C) | Minimum temperature (° C | Relative mean ghost (%) | Maximum relative humidity(%) | Minimum relative humidity(%) | climate (expanded dormant) |
|---|---|---|---|---|---|---|---|---|
| Fereidon hshr | 567 | 11.5 | 16 | 4.5 | 36 | 50.3 | 26.3 | semi-arid |
2.3. Plant sampling
In the rangeland area, due to the difference in plant growth age, all crops of different growth years (second, third and sixth years) were sampled. To sample this plant, in late May of 2018 and coinciding with the flowering, were collected along a transect and In the same geographical direction from three points randomly of any age of the plant. The plant flowers were collected by garden scissors. The specimens were transferred to the laboratory after harvest and the flowers and leaves were separated and exposed to free air in shade to dry.
2.4. Laboratory operation
2.4.1. Plant extraction
Extracts of various samples were produced using a soxhlet device by methanol solvent. For this purpose, at first, 10 g of the dried organ (leaves and flowers separately) was poured into a basket and placed in its place (Timbal). Then, 350 ml of methanol solution were deposited in a 500 ml balloon. َAfter the components were connected and the cold water was drawn and inlet, the extraction was started and lasted for eight hours. Then the rotary evaporator was used to condense the extract solution and transferred to an oven at 45 °C and then transferred to a vacuum oven (35 °C and pressure 20 ml of mercury) to dry the specimens. After 4 days, dried extracts were separated from the plates by spatula..
The extract yield was calculated based on the dry extract weight in 100 g dry matter.
To prevent the degradation of the active ingredients, the extracts were transferred to the impervious dishes and were kept at 4 °C until further tests were performed.
2.4.2. Evaluation of antioxidant properties of the extract (DPPH test)
In this study, the ability of plant extracts to act as an antioxidant for the radical 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was measured. This radical absorbs light at 517 nm and its intensity follows the Beyer-Lambert law. The reduction in absorption by this compound has a linear relationship with the amount of antioxidants. Therefore, if the antioxidant agent is increased, DPPH is more consumed and its color changes from violet to yellow. To investigate the antioxidant property, it seems necessary to use a standard test. In this study, butylated hydroxy toluene (BHT) was used as the standard control. To ensure the accuracy of the test and to reduce the error rate, each of the tests of standard extract and the control was repeated three times, and after averaging the data, the IC50 of each of the samples was determined.
The following solutions were prepared for this test:
AB= Sample absorption
AS= Control absorption
The solution of DPPH: 7.4 mg of solid DPPH was weighed, poured into a 50 mL volumetric flask, and then dissolved in 50 ml of methanol. Since the solution of DPPH degrades and decomposes in light, a dark volumetric flask was used at the end of the work. The color of the resulting solution over time changes from purple to yellow due to its rapid recovery in the environment. DPPH was then stored in a refrigerator at 4 °C. Also, due to DPPH’s corrosivity, it was essential to observe safety precautions when using it. Typically, after preparing the solution of DPPH, a preliminary test was performed to ensure its high standard. One milliliter of DPPH solution was made and 1 mL of methanol was poured into a 5 mL dark balloon and stored at 25 °C for 30 min. After approximately 30 min, using a spectrophotometer (Model UV-2100, Made in the USA), the absorption of the solution was measured at 517 nm. If the absorption read between 1.1 and 1.7, the solution of DPPH was used in the test.
The solution of standard of Butylated hydroxytoluene (BHT): In order to compare the results obtained for the plant samples with the standard BHT specimen, to determine the accuracy of the process, and to assess the antioxidant activity of the standard BHT, 25 mg of standard of BHT was weighed and completely dissolved in 25 ml of methanol in a 25 mL volumetric flask. Then, the solution of BHT was made with a concentration of 1 mg/mL. In the next step, concentrations of 0.8, 0.5, 0.25, 0.1, 5 × 10-2, 5 × 10-3, and 5 × 10−4 mg/mL of the solution of the stock solution were prepared by dilution with methanol in 8 volumetric flasks of 10 mL.
Solutions of extract: To prepare the base solution, 25 mg of each of the methanolic extracts from the plant samples was weighed, poured into a 25 mL volumetric flasks, and mixed with methanol. All components of the extract were completely dissolved in methanol and the resulting solution was homogeneous. The stock solution was prepared at a concentration of 1 mg/mL for each of the samples. Then, solutions with concentrations of 0.8, 0.5, 0.25, 0.1, 5 × 10-2, 5 × 10-3, and 5 × 10−4 mg/mL were prepared. One milliliter of each of the above solutions was poured into the corresponding dark volumetric flask. Then, 1 mL of the DPPH solution was added to each of the dark volumetric flasks and mixed. The dark volumetric flasks were stored at 25 °C for 30 min.
The solution of control: 1 mL of methanol and 1 mL of DPPH solution were added to a 5 mL balloon and mixed.
Finally, after 30 min, the absorption of the solutions at a 517 nm wavelength was read using a UV/vis spectrophotometer, starting with the control solution and then reading the samples in order of increasing concentration. At the end of the calculation, the percentage of antioxidant contained was calculated according to the plot of the negative logarithm of the concentration in EXCEL, and the IC50 was calculated in micrograms per milliliter. The percentage of inhibition was calculated using the following equation:
2.4.3. Measurement of the total amount of phenolic compounds
In this study, the Folin-Ciocalteu method was used to measure the total amount of phenolic compounds. The following solutions were prepared for the Folin–Ciocalteu test:
1-Gallic acid standard solutions: 1.1, 2.2, 3.3, 4.4, 5.5, 6.6, 7.7, 8.8, 9.9, 10.10, and 11 mg of gallic acid were weighed and placed in 11 separate test tubes. One milliliter of ethanol was added to each tube containing gallic acid. Three milliliters of distilled water was added to each of 11 5 mL volumetric flasks, then 0.01 mL (10 μL) of each gallic acid solution was added to one of the 11 volumetric flasks, separately. In the next step, 0.1 mL (100 μL) of Folin-Ciocalteu Merck was added to the volumetric flasks and after 3 min, 0.3 mL (300 μL) of sodium carbonate solution 2 % was added, and then the volumetric flasks with ethanol were reached to the volume and mixed. The volumetric flasks were placed at 25 °C for 2 h.
2-Control sample for the gallic acid samples: 100 mL ethanol instead of the gallic acid solution was used and the same steps as above were repeated.
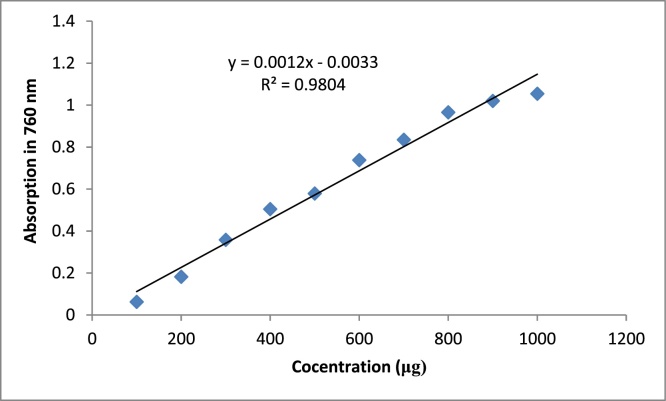
After 2 h, the wavelength of the spectrophotometer device was adjusted on 760 nm. At first, the device was zeroed with the control solution, then the absorbance of each of the solutions was read three times and the average of the three readings taken. From the absorption assays, the absorbance graph of the concentration (μg) was plotted in the EXCEL and the line equation was calculated (Fig. 1).
Fig. 1.
Gallic asid standard curve.
3-Extract solutions: After the total phenol content of the gallic acid standards was analyzed, a total phenol test was performed for the plant samples. For this purpose, 10 mg from each sample of the herbal extract was weighed, placed in a test tube, and 2 mL of DMSO solution was added to completely dissolve the sample. For each extracted sample, three 5 mL the volumetric flasks were used for three replicates. Each of the volumetric flasks contained approximately 3 mL of distilled water and 0.20 mL (20 μL) of each extracted sample. One hundred microlitres of Folin–Ciocalteu reagent was added to the volumetric flasks and after 3 min, 0.3 mL (300 μL) sodium carbonate 2 % was added and then distilled water was added and the volumetric flasks were reached to the volume.
4-Control solution: In addition to the sample volumetric flask, a 5 mL control volumetric flask was used. Instead of 0.20 mL of the extract, 0.20 mL of dimethyl sulfoxide (DMSO) solution was added. The solutions made in the volumetric flasks were uniformly homogeneous and placed at 25 °C for 2 h. After 2 h, the absorbance of each solution was read three times using a spectrophotometer apparatus at a wavelength of 760 nm. For each plant sample, the average of the three readings was calculated and used in the equation of the standard gauge line obtained from the equation of the gallic acid line, and the concentration of the phenolic compounds in the extract of the plant was calculated using the amount of gallic acid, measured in micrograms:
| Absorbance = 0.0012 × Gallic acid (μg) + 0.0033 |
| R2 = 0.98 |
2.4.4. Measurement of the total amount of Flavonoids
1-standard quercetin standard: 2 mg of quercetin samples were weighed in 10 ml volumetric flask and subjected to methanol so that all the components of the extract were completely dissolved in methanol. The required concentrations were prepared in eight volumes of 5 ml cleared volumetric flask from a stock solution: 2.5, 2.5, 1.5, 1, 0.5 and 0.25 ml of stoke solution in 5 ml balloons Brightened with methanol. In each of the volumetric flask, 0.5 ml of each of the prepared concentrations, 1.5 ml of methanol, and then 0.1 ml of the aluminum chloride solution were diluted with distilled water in a volume of 5 ml The blanks were homogeneous and kept at the laboratory for half an hour. After half an hour, the spectrophotometer wavelength was set to 415 nm and read three times. From the adsorption, the absorbance graph of the concentration (μg) was plotted in the Excel program and the line equation was calculated.
2. Extract solution: 10 mg of the extract was weighed to an analytical weight and placed in a 10 ml volumetric flask with methanol so that the extracted sample was completely dissolved. In each of the 0.5 ml bottles of stoke solution, 1.5 ml of methanol, then 0.1 ml of sodium acetate solution and 0.1 ml of aluminum chloride solution were poured into 5 ml distilled water. The spectrophotometer wavelength was adjusted to 415 nm for half an hour and then the device was zeroed with the control solution, and then the absorbance of each of the solutions was read three times and taken from three readings. For each sample, the average of three readings was taken and then in the standard equation of the quercetine standard line, the concentration of flavonoids in the plant extracts was quercetin equivalent in micrograms.
| Absorbance = 0.007 Quercetin (μg)+0.020 |
| R2 =0.999 |
2.5. Statistical analysis
The present study was carried out in a factorial arrangement in a completely randomized design with two factors (elevation and area) in three replications. Statistical analysis was performed using SPSS software. First, the normality of the statistical variables was investigated by the Kolmogorov-Smirnov test, and after ensuring the normality of the data, the analysis of the variance was done by the F-test and the comparison of the means by the Duncan test. The probability level was 5 % error. Pearson correlation test was used to verify the correlation between antioxidant capacity, total phenolic, and flavenoid compounds. Therefore, to ensure the accuracy of the obtained equations, the normalities of the residues, the distortion and the linear data between the data were investigated. Finally, the results were presented in the form of charts or tables.
3. Results
Extract in collected samples in different years elapsed from cultivation in the rangeland showed that in both limbs, the highest extract yield was observed in the second year and the lowest was observed in the third year and at all times the cultivars also had more flower extracts than the leaves (e.g. Table 2)
Table 2.
Effect of extract efficacy and leaves of D. kotschyi on different age of growth time.
| Age of growth | Plant limb | Yield of extracts(%) |
|---|---|---|
| Six years old | flower | 31.8 |
| leaf | 26.9 | |
| Three years old | flower | 31.7 |
| leaf | 24.7 | |
| Two years old | flower | 32.7 |
| leaf | 30.5 |
The results of the analysis of variance of antioxidant capacity in terms of IC50 in D. kotschyi in cultivated pasture showed that the effect of age of growth, plant limb and interaction of growth age on plant limb was significant at the probability level of 1 % error (P < 0.01). Also, the results of the analysis of variance of total phenolic compounds in this plant showed that the effect of the age of growth in the probability level of 5 % and the effect of organ type in the probability level of 1 % error was significant. However, the interaction between age of growth in plant organs on total phenol was not significant. The results of the analysis of variance of total plant flavonoids in cultivated pasture showed that the effect of age of growth, plant organs and interaction of growth age in plant organs at the probability level of 1 % error (P < 0.01) was significant (e.g. Table 3).
Table 3.
Analysis of variance of growth age and plant limb effects on antioxidant capacity, total phenol and total flavonoid of D. kotschyi.
| Effects | df | MS | ||
|---|---|---|---|---|
| Antioxidant capacity | Total phenolic compounds | Total flav-onoid compounds | ||
| age of growth | 3 | 143.360** | 2240.001* | 8179.078** |
| plant limb | 1 | 9252.670** | 6010.348** | 18355.446** |
| age of growth × plant limb | 3 | 4358.676** | 799.033 ns | 6837.262** |
| Erroe | 16 | 0.741 | 443.316 | 78.190 |
ns: Not meaningful, *: 5 % level of probability in significant **: 1 % level of probability insignificant.
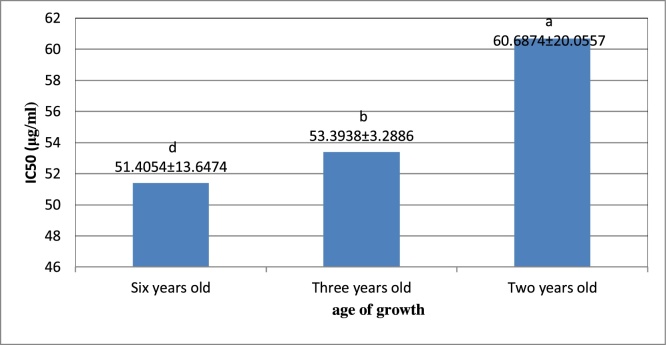
Also, the comparison of the mean IC50 in different age groups indicated that the two-years and six years samples have the highest and lowest IC50 values of 60.6874 and 51.5404 μg/ml, respectively. (e.g. Fig. 2).
Fig. 2.
Comparison of the mean age of growth effect on antioxidant capacity of D. kotschyi using DPPH test according to IC50.
Also, from the results, it can be clearly seen that the collected samples of six years with an average of 117.1157 μg of gallic acid in 1 g of dry matter of extract had the highest phenol content and there was no significant difference between two and three years old plants in terms of total phenol content (e.g. Fig. 3).
Fig. 3.
Comparison of the mean age of growth effect on total phenol of D. kotschyi.
Comparing the mean flavonoids content in different ages of growth in rangelands, the highest amount of flavonoids was obtained from the six-year samples with 147.3095 μg/mg equivalent to total quercetin flavonoid contained in the plant extract. Also, the lowest amount of flavonoids (73.7450 μg/mg equivalent to total quercetin flavonoid contained in the plant extract) was recorded from samples collected from third-year cultivation (e.g. Fig. 4).
Fig. 4.
Comparison of the mean age of growth effect on total flavonoid of D. kotschyi.
On the other hand, based on the results of the comparison of the mean interactions of growth age on plant organs in rangelands, the highest and lowest IC50 values belong to leaf (105.5292 ± 1.4082 μg/ml) and flower (15.8489 ± 0.000 μg/ml) were two years old. Comparing the mean of age of growth and the limbs on the flavonoids of the whole plant, the maximum yield of flowers of the six-year-old plants was 158.952 ± 6.9829 μg/mg and the least amount of this compound was 2.8806 ± 1.3571 μg/mg in the three-year-old leaves. (e.g. Table 4).
Table 4.
Comparison of the mean of region interaction of age of growth × plant limb on antioxidant capacity and total flavonoid of D. kotschy.
| Age of growth | plant limb | Standard deviation ± mean (IC50:μg/ml) | Standard deviation ± mean (Total flavonoid μg/mg) |
|---|---|---|---|
| Six years old | flower | 20.8967 ± 0.4811e | 158.0952 ± 6.9829a |
| leaf | 81.9141 ± 1.0931b | 136.5238 ± 8.6607b | |
| Three years old | flower | 60.7238 ± 0.8103c | 144.6094 ± 114.6094ab |
| leaf | 46.0637 ± 0.0615d | 2.8806 ± 1.3571d | |
| Two years old | flower | 15.8489 ± 0.0000f | 130.2222 ± 15.0033b |
| leaf | 105.5259 ± 1.4082a | 101.9215 ± 3.3557c |
The different letters in each column indicate a significant difference based on the Duncan multi-domain test at a 5 % error rate.
Based on the results of Table 6, we can find that there was a negative correlation between the antioxidant capacity in terms of IC50 and total phenolic compounds in the six-year crop, with a 95 % confidence interval. Also, there was a negative correlation between the antioxidant capacity in terms of IC50 and total flavonoid compounds in the six-year and two-year samples, with a 95 % confidence interval and a strong positive correlation with a 99 % confidence interval for three years. Also, the correlation between the total phenolic and flavonoid compounds of the studied plant was observed only in two-year-old plants with a strong positive correlation with 99 % confidence (e.g. Table 5).
Table 5.
Correlation of antioxidant capacity in terms of IC50, total phenol and flavonoid in different growth ages of D. kotschyi.
| Total flavenoid | Total phenol | Antioxidant capacity according to IC50 | correlation | age of growth |
|---|---|---|---|---|
| −0.854* | −0.912* | 1 | Antioxidant capacity according to IC50 | Six years old |
| 0.621ns | 1 | Total phenol | ||
| 1 | Total flav-onoid | |||
| 0.998** | 0.360 ns | 1 | Antioxidant capacity according to IC50 | Three years old |
| 0.354 ns | 1 | Total phenol | ||
| 1 | Total flav-onoid | |||
| −0.849* | −0.800 ns | 1 | Antioxidant capacity according to IC50 | Two years old |
| 0.938** | 1 | Total phenol | ||
| 1 | Total flav-onoid |
ns: Not meaningful, *: 5 % level of probability in significant **: 1 % level of probability insignificant.
DPPH test on synthetic BHT antioxidant was also performed at the desired concentrations and IC50 was 19.95 μg/ml (Fig. 5) which was compared to the lowest concentration of plant sample (15.8489 ± 0.001 μg/ml) related to flower organs in the second year of cultivation, an increase in the antioxidant activity of this plant compared to the synthetic antioxidant can be observed (less than IC50 showed the highest antioxidant activity). Also, in the flowering organ in the sixth vegetative year, the IC50 content was slightly (20.8967 ± 0.4811 μg/ml) approximately equivalent to the synthetic antioxidant capacity of BHT.
Fig. 5.
Inhibition percentage relative to the negative logarithm of BHT standard sample concentration.
4. Discussion
The study of extract yield in collected samples in different years elapsed from cultivation in the rangelands showed that in both limbs, the highest extract yield was observed in the second year and the lowest in the third year. Macchia et al. [47] also achieved the yield of T. vulgaris in the first year more than in the following years. However, Garivani et al. [48] observed the opposite in the study of two species of Thymus. Studies have shown that in the first year more aspects of plant deployment are considered and from the second year that plants are located, due to the complete establishment of the plant and access to nutrients and elements of the soil, an increasing trend in growth characteristics and yield. In fact, plant energy in the first year is mainly due to plant deployment and the acceptable and economical performance of the plant since the second year. This is related to the morphological and physiological characteristics of the plant. Koocheki et al. [11] in the study of Crocus sativus L. and Naderi Boroujerdi et al. [12], in the study of Mentha piperita L., achieved similar results. The results of plant extracts yield indicated that the extracts of the flower were more effective in all areas and all cultivars compared to leaf cultivars, which was consistent with the findings of Ghavam et al. [13]. This indicates the importance of reproductive organs in the production of secondary metabolites in the studied plant relative to the vegetative organs
Based on the results of the effect of cultivar and year spent on cultivation on the IC50 value, this was consistent with the results of Asadi-Sanam et al. [16] and Alfaro et al. [14] and with the results of Rezazadeh and Ghasem Nejad [15]. The antioxidant capacity associated with the increase in the year had an increasing trend that reached the highest levels in the six-year-old plants. Various factors, including the age of the plant and season, affect secondary metabolites [49]. Alirezaei Naghandar et al. [50] also showed that raising the age of the plant increases the antioxidant capacity in the study of wild sorrel.
Also, the effect of herbal organs and the interaction between age and herb growth on IC50 was significant, in which Asadi-Sanam et al. [16] achieved similar results. Mostafa et al. [17], in the study of Rumex vesicarius L., the amount of antioxidant activity varies depending on the type of plant organ used. In this study, the highest antioxidant capacity in the second year was observed, which decreased in the third year and increased again in the 6th year. This could be due to environmental stresses in different years and possibly the energy used by the plant in the third year for vegetative growth of the plant and plant sowing and adaptation of the plant in the sixth year. In the limb of the leaf, the reverse process occurred and the third year of growth showed the best antioxidant capacity, which could indicate favorable conditions for the synthesis of the antioxidant compounds of the limb. One of the factors affecting secondary metabolites in plants is plant type so that the amount of secondary metabolites in different organs is different [30]. Borhani et al. [18], in the study of yellow alfalfa, also recorded the highest antioxidant capacity in the limbs. There are plenty of flavonoids, glycosides and synthetic acid derivatives in pollen, and it seems that the antioxidant activity of pollen grains is in phenolic compounds [19].
In the study of the phenolic compounds at different ages in the squat rangelands, the results showed a significant effect of age of growth and organ effects on these compounds at levels of 1 % and 5 %, respectively, and their interaction was insignificant. Alfaro et al. [14] in the study of Mortierella vinacea recognized the effect of culture on phenol. The phenol content of the plant in different years of the second and third was not statistically significant, but in the sixth year, it was the highest. Studies have shown that high concentrations of phenol in the plant are obtained when plants are allowed to produce and store these compounds for more than a year [20]. The aging of the plant also results in the accumulation of phenolic compounds in plants. Elzaawely et al. [21] reported that variations in the amount of phenolic compounds can be due to differences in environmental factors and plant accumulation time. Therefore, the plant can be grown after six years, has been adapted to the environmental conditions and has reached full deployment and has the highest production and storage of phenolic compounds. The ability of Phenols of neutralize free radicals by the presence of their hydroxyl group, which is related to the hydrogen or electron carrier [51].
In evaluating the total flavonoid content at different ages in the rangeland, the results indicated that the effect of time of cultivation on this characteristic was significant. Alfaro et al. [14] in Mortierella vinacea and Rezaei Konty and Gorbani in Frankenia hirsuta Desf. And Climacoptera turcomanica (Litw.) Botsch. Comparison of flavonoid flavonoids at different times of cultivation in the squat rangeland can be obtained from the six and three-year-old samples, respectively, with the highest and lowest flavonoids. Reducing the amount of flavonoids from the second to the third year can be indicative of vegetative growth and plant energy utilization for deployment, which leads to a decrease in the accumulation of secondary metabolites, including flavonoids. The increase of flavonoids over time, up to the sixth year, indicates the cellular strategy to cope with oxidative stress. Flavonoids are one of the broadest and most diverse natural compounds that, like other phenolic compounds, can absorb free radicals. In oxidative stresses, phenol compounds, especially flavonoids, can interact with membrane phospholipids through a hydrogen bond with polar heads of phospholipids, resulting in these compounds being collected at the inside and outside of the membrane and prevented from obtaining damage molecules. The dipole hydrophobic region helps maintain the fluidity and integrity of the membrane [22]. Because the enzymes involved in biosynthesis, flavonoids are affected by puberty. Therefore, reducing the amount of flavonoids during puberty can be due to their synthesis or degradation and conversion to other plant metabolites, which flavonoids are rapidly synthesized in the tissues during cell division, and then synthesized in the cell proliferation phase slow down [23]
The findings indicated the effect of herbal organs and the interaction of growth age on herb on plant flavonoids. Alirezaei Nagandar et al. [50] and Rezazadeh and Ghasem Nejad [15] also achieved similar findings. The six-year-old flower had the highest and third-leaf limb with the least amount of this composition. The specificity of some flavonoids in certain tissues and organs in jujube plants has also been reported [24]. In all the plants of different ages, the limb had more flavonoid content than the leaves. There are plenty of flavonoids, glycosides and cyano acid derivatives in pollen [19]. Plant leaves seem to have little to do with the accumulation of flavonoids. This could be due to the low visibility of ultraviolet radiation in this area.
The results indicated that there was a direct correlation between the antioxidant capacity and phenol content of the whole plant in the six-year-old plants with a 95 % confidence interval. This is a correlation in findings of many researches such as Javanmardi et al. [25], Cai et al. [35], Pourmorad et al. [26], Unal et al. [27], Kir-ca and Ars-lan [52], Tukun et al., [28], Afrazeh et al. [29], Mehrpour et al. [30],) and Zargoosh et al. [31]. The phenolic compounds are one of the most important antioxidant compounds in plants and the antioxidant activity of plant material is related to the amount of phenolic compounds [21]. Antioxidant activity of phenols is mainly due to its oxidation and reduction properties, which allows it to act as reducing agents, hydrogen, oxygen, and metal hermetic [53]. The key role of phenolic compounds has been reported as free radical-free radicals [32], The riault et al. [45]. In plant cells, phenolic compounds, especially polyphenols, act very effective in reducing the deoxygenation of hydrogen peroxide and act as the backbone of the ascorbate-glutathione cycle to dispose of hydrogen peroxide radicals. When phenols take part in these reactions as antioxidants, they are oxidized to phenoxy radicals. Phenoxyl radicals are initially reacted by ascorbate [33]. Phenylene OH groups are one of the most preferred groups for the loss of a proton from single-electron oxidized forms. The stability of the resulting phenoxyl radicals increases the antioxidant properties and the ability of most compounds containing multiple hydroxyl groups to sweep oxidized free radicals, as well as the formation of free radicals due to lipid peroxidation It prevents (Alavi et al., 2010). Also, in addition to phenol, there was a direct correlation between total flavonoid and plant antioxidant capacity with a 95 % confidence interval. Ferreres et al., [34], Mostafa et al. [17], Alfaro et al. [14], Cai et al. [35], Zlati´c and Stankovi´c, [36] Saadatmad et al., [37], Khalsi Ahvazi et al., [38], Fazelinasab et al. [39], Alireza'i Nandandar et al., Gholizadeh Moghaddam et al. [40] and Borhani et al. [18] also showed that the antioxidant capacity of the plant has a direct correlation with the amount of phenolic and flavonoid compounds. Phenols and flavonoids usually act as sweeteners of free radicals [54,55].
The total flavonoid and the antioxidant capacity of the two-year plant were directly correlated with a 95 % confidence interval. Previous studies on 28 plant products including oily seeds, cereal grains, and medicinal plants have shown that there is a direct correlation between antioxidant activity and total flavonoid compounds [9,26,41]. This phenomenon shows that flavonoids also contribute to the antioxidant properties of these populations. Regarding the correlation between flavonoids and antioxidant capacity, it can be said that the antioxidant effect of many flavonoids, including anthocyanins, flavonoids, and flavonoids, has been reported by researchers [56]. Most herbs contain a significant amount of antioxidants, such as tocopherols (vitamin E), carotenoids, ascorbic acids, flavonoids and tannins [57]. In fact, flavonoid-bearing plants have the strong anti-coagulant properteis [58]. Antioxidant activity of flavonoids increases with the increase of the number of hydroxyl groups subjected to the B ring, especially on the position of carbon 3 or a single hydroxylic acid substrate [59].
Also, there was a strong inverse correlation between total flavonoid and antioxidant capacity of the third-year plant with 99% confidence. The correlations between these two quantities are also confirmed in the findings of Hanaski et al. [42], Rice-Evans et al. [43] and Dastoor et al. [44].
The results indicated that between the total phenolic and flavonoid compounds of the studied plant only two-year-old strong positive correlation with 99 % confidence interval, which was consistent with the findings of Mighani et al. [60] and Hosseini et al. [61], and with The results of Dastoor et al. [44] do not contradict.
Therefore, it is probable that the polyphenolic compounds, and in particular flavonoids, form an important part of the antioxidant compounds of this plant that are formed in very humid climatic conditions and adapted to the newly cultivated environment, which in the year the sixth peaked in the flower organs.
5. Conclusion
Since the two-year-old plant flower organs with IC50 15.8489 ± 0.001 μg/ml, the highest antioxidant than synthetic antioxidant had, optimal conditions and optimal treatment for this property marked. But in general, it can be said that the six-year-old plant with the highest antioxidant capacity, phenolic, and flavonoid compounds to produce the best age vegetative antioxidant and is mainly based on flower organs are preferred.
Author contribution statement
Mansureh Ghavam was the supervisor, designer of the hypotheses, and responsible for all the steps (laboratory, statistical analysis, data analysis, etc.) and wrote the text of the article. Hossein Moradi performed the laboratory work and has created the tables and charts. Ali Tavili is a project consultant at the design and implementation phase of the project. He also contributed to the statistical analyses and data analysis and text editing.
All authors have ethics approval and consent to participate and consent for publication and availability of data and material. The author(s) declare no competing interests. There is no funding
Declaration of Competing Interest
All authors have ethics approval and consent to participate and consent for publication and availability of data and material. The author(s) declare no competing interests. There is no funding
Acknowledgments
Acknowledgments of University of Kashan
References
- 1.Prior R.L., Cao G. Antioxidant phytochemicals in fruits and vegetables. Diet and health implications. Hortic. Sci. 2000;35:588–592. [Google Scholar]
- 2.Soobrattee M.A., Neergheen V.S., Luximon- Ramma A., Aruomab O.I., Bahoruna T. Phenolic as potential antioxidant therapeuticagents: mechanism and actions. Mutation Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Sokol-letowska A., Oszmianski J., Wojdylo A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007;103:853–859. [Google Scholar]
- 4.Fatahi M., Nazeri V., Sefidkon F., Zamani Autecology of Dracocephalum kotschyi Bioss. in Iran. Iranian J. Med. Aromatic Plants. 2019;29(2):325–341. [Google Scholar]
- 5.Rechinger K.H. vol. 150. 1972. (Flora Iranica. Akademische Druck-u. Verlagsanstalt, Graz). [Google Scholar]
- 6.Mehrabani M., Rooh Allahi S., Forumadi A.S. Photochemical investigation Dracocephalum polychaetum Bornm Faculty of Pharmacy. J. Med. Plants. 2005;4(42):38. [Google Scholar]
- 7.Jahanian F., Ebrahimi S., Rahbar-Roshandel N., Mahmoudian M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyi and a potential anti-cancer agent. Phytochem. 2005;66:1581–1592. doi: 10.1016/j.phytochem.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Gohari A., Saeidnia S., Matsuo K., Uchiyama N., Yagura T., Michiho K. Flavonoid constituents of Dracocephalum kotschyi growing in Iran and their trypanocidal activity. Ateneo. Parmense Acta Nat. 2003;57:250–252. [Google Scholar]
- 9.Golshani S., Karamkhani F., Monsef Esfehani H.R., Abdollahi M. Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J. Pharm. Sci. 2004;7:76–79. [PubMed] [Google Scholar]
- 10.Jalili A., Jamzad Z. Research Institute of Forest and Rangelands; Tehran: 1999. Red Data Book of Iran. In Persian. [Google Scholar]
- 11.Koocheki A., Rezvani Moghadam P., Moghvalibi A., Seyedi S.M. Evaluation of the yield of saffron in the first year after cropping in reaction (Crocus sativus L.) to plant density and fertilizer rate. J. Agric. Ecol. 2014;6(72) [Google Scholar]
- 12.Naderi Boroujerdi Gh, Madani H., Khaghani Sh, Chavoshi S., Tahmasbizadeh H. Economic culture, extension, training of medicinal plants of Mentha piperita L. in Central Province. National Conference of Medicinal Plants of Rice and Citrus Research Center; Sari University of Agricultural Sciences and Natural Resources; 2010. [Google Scholar]
- 13.Ghavam M., Azarnivand H., Akhbar M. Identification of phytochemicals, plant compounds smirnovia iranica. J. Herb Drug (An International Journal of Medicinal Herbs) 2015;6:129–135. http://jhd.iaushk.ac.ir/article_643342.html [Google Scholar]
- 14.Alfaro S., Mutis S., Palma R., Quiroz A., Seguel I., Scheuermann E. Influence of genotype and harvest year on polyphenol content and antioxidant activity in murtilla (Ugni molinae Turcz) fruit. Soil Sci. Plant Nutr. 2013;13:67–78. [Google Scholar]
- 15.Rezazadeh A., Ghasem Nejad A. Effect of harvest time on total phenol, flavonoid and antioxidant activity of artichoke leaves (Cynara Scolymus L.). National Conference on Medicinal Plants. Rice and Citrus Research Center; Sari University of Agricultural Sciences and Natural Resources; 2010. [Google Scholar]
- 16.Asadi-Sanam S., Zavareh M., Pirdashti H., Sefidkon F., Nematzadeh G. Evaluation of phytochemical properties of purple coneflower Echinacea purpurea (L.) Moench flowers in intercropping with green beans and different summer time planting dates. Iranian J. Hortic. Sci. 2019;50(1):61–76. [Google Scholar]
- 17.Mostafa H.A.M., Elbakry A.A., Eman A.A. Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius l. (polygonaceae) Int. J. Pharm. Pharm. Sci. 2011;3:109–118. [Google Scholar]
- 18.Borhani G., Mazandaran M., Abbaspour H. Ectophytochemical, Ethnofarmacological, Antioxidant and Antibacterial Study of Different Extract ofMelilotus officinalis L. Medicinal Plants in Chahar Bagh Mountains of Semnan Province. Phytochem. Med. Plants. 2018;6:29–31. [Google Scholar]
- 19.Markham K.R., Campos M.G. 7-α-8-O-Methylherbacetin-3-O-sophorosides from bee pollens and some structure activity observation. Phytochemistry. 1996;43:763–767. [Google Scholar]
- 20.Cech N.B., Eleazer M.S., Shoffner L.T., Crosswhite M.R., Davisand A.C., Mortenson A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromay A. 2006;1103:219–228. doi: 10.1016/j.chroma.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Elzaawely A.A., Xuan T.D., Tawata S. Antioxidant and antibacterial activities of Rumexjaponicus Houtt. aerial parts. Biol. Pharm. Bull. 2005;28:2225–2230. doi: 10.1248/bpb.28.2225. [DOI] [PubMed] [Google Scholar]
- 22.Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J. Environ. Stud. 2006;15(4):523–530. [Google Scholar]
- 23.Ahmad R., Mahbob E.N., Noor Z.M., Ismail N.H., Lajis N.H., Shaari K. Evaluation of antioxidant potential of medicinal plants from Malaysian Rubiaceae (subfamily Rubioideae) Afric. J. Biotech. 2010;9:7948–7954. [Google Scholar]
- 24.Pellotte J.P., Maria A., Martinez D.P. Flavonoid variation with the plant age in zyzyphus mistol leaves. Biocheand Ecol. 1993;2:645–646. [Google Scholar]
- 25.Javanmardi J., Stushnoff C., Locke E. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. [Google Scholar]
- 26.Pourmard F., Hosseinimehr S.J., Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected iranian medicinal plants. Afr. J. Biotechnol. 2006;5:1142–1145. [Google Scholar]
- 27.Unal E., Mavi A., Kara A.A., Cakir A., Şengül M., Yildirim A. Antimicrobial and antioxidant activities of some plants used as remedies in Turkish traditional medicine. Pharma Biol. 2008;46:207–224. [Google Scholar]
- 28.Tukun A.B., Shaheen N., Banu C.P., Mohiduzzaman M., Islam S., Begum M. Antioxidant capacity and total phenolic contents in hydrophilic extracts of selected Bangladeshi medicinal plants. Asian Pac. J. Trop. Med. 2014;7:568–573. doi: 10.1016/S1995-7645(14)60291-1. [DOI] [PubMed] [Google Scholar]
- 29.Afrazeh Z., Bolandi M., Khorshidi M., Mohammadi Nefchi A.S. Evaluation of antioxidant activity of aqueous and alcohol extracts (methanol, ethanol) of saffron petals. J. Agric. Techn Saffron. 2014;2:236. [Google Scholar]
- 30.Mehrpour M., Kashefi B., Moghaddam M. Investigation of phytochemical and antioxidant compounds of different organs of the Angushei medicinal plant in two natural habitats of Semnan and Khorasan provinces. J. Ecophytol. Med. Plants. 2016;4:56–68. [Google Scholar]
- 31.Zargoosh Z., Ghavam M., Bacchetta G., Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019;9:16021. doi: 10.1038/s41598-019-52605-8. https://www.nature.com/articles/s41598-019-52605-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katalinic V., MIlos M., Kulisic T., Jukic M. Screening of 70 medical plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–577. [Google Scholar]
- 33.Sakihama Y., Cohen M.F., Grace S.C., Yamasaki H. Plant phenolics antioxidant and pro oxidant activity: Phenolics-induced oxidative damage mediated by metal in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 34.Ferreres F., Ribeiro V., Izquierdo A.G., Rodrigues M.N., Seabra R.M., Andrade P.B., Valento P. Rumex induratus leaves: interesting dietary source of potential bioactive compounds. J. Agric. Food Chem. 2006;54:5782–5789. doi: 10.1021/jf0613233. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plant associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zlati´c N.M., Stankovi´c M.S. Variability of secondary metabolites of the species Cichorium intybus L. From different habitats. Plants. 2017;6:1–9. doi: 10.3390/plants6030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saadatmad L., Gorbani M., Niakan M. Investigation of Changes of the Most Important Substances Secondary Effects and Antioxidant Activity of Different Organs in Different Medicinal Plants of Sanandro, Different Areas of Khorasan Razavi Province. Quarterly J. Med. Plants. 2013;1:58–67. [Google Scholar]
- 38.Khalsi Ahvazi L., Heshmati Gh, Zofan P., Akbarlo M. Phenolic total flavonoid and antioxidant activity of medicinal plant in forage in different stages of growth in four habitats of northeast of Khuzestan province. J. Ecophytol. Med. Plants. 2016;4:33–46. [Google Scholar]
- 39.Fazelinasab B., Sirous Mehr A., Naser Mirzaei N., Soleimani M. Evaluation and comparison of the content of phenol, flavonoids and antioxidant activity of leaf and fruit in 14 different genotypes of medicinal plants in south of Iran. J. Ecophytol. Med. plants. 2016;4:1–14. [Google Scholar]
- 40.Gholizadeh Moghaddam N., Hosseini B., Alirezalou A. Evaluation of variation of some phytochemical indices of leaf extract of genotypes of different species of Barberry. J. Ecophytol. Med. Plants. 2017;3:1–12. [Google Scholar]
- 41.Tawaha K.H., Alali F.Q., Gharaibeh M., Mohammad M., Elimat T.E. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chemis. 2007;104:1372–1378. [Google Scholar]
- 42.Hanasaki Y., Ogawa S., Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radical Biol. Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 43.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 44.Dastoor R., Bakhshi D., Ali Akbar A.R. Evaluation and comparison of total phenol, total flavonoid, resveratrol and antioxidant capacity in fruits of the species Vitis vinifera, (vera Pistacia), (Sambucus nigra) and (Ilex spinigera) J. Ecophyt. Med. Plants. 2017;5:37–48. [Google Scholar]
- 45.The´riault V., Bernatchez L., Dodson J.J. Mating system and individual reproductive success of sympatric anadromous and resident brook charr, Salvelinus fontinalis, under natural conditions. Behav. Ecol. Soc. 2007;62:51–65. [Google Scholar]
- 46.Tabrizi P., Amini Kordkandi K. Study of Production and Biodiversity Systems of Medicinal and Aromatic Plants in Ecosystem of Agricultural Systems of the Province Qazvin. J. Agri Eco. 2014;6:880–890. [Google Scholar]
- 47.Macchia M., Ceccarini L., Andolfil L., Flamini G., Cioni P.L., Morelli I. Agronomicproductive characteristics, yield and essential oil composition of a chemotype of Thymus vulgaris harvested in various phases of its biological cycle. Agric. Mediterranea. 2002;132:44–50. [Google Scholar]
- 48.Garivani G., Sharifi Ashoorabadi E., Safari S., Mirza M. Assessment of domestication and harvesting time on growth and concentrations of active ingredients of two thyme (Thymus L.) species in North Khorasan province of Iran. Iran. J. Med. Aromatic Plants Res. 2014;30(3):445–452. [Google Scholar]
- 49.Harborne J.B., Williams C.A. Advances in flavonoid research since. Phytochemistry. 1992;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 50.Alirezaie Naghandar M., Azizi M., Neamati S., Rezvani Moghaddam P., Rezazadeh S. Variation of some Phytochemical Compound in Shoot and Root of Rumex turcomanicus Czerep. at Different Phenological Stages. J. Med. Plants. 2016;2(58):25–36. [Google Scholar]
- 51.Ghavam M. Plant antioxidants and their evaluation. Health Biotechnol. Biopharma. 2018;2(2):14–18. http://www.healthbiotechpharm.org/Abstract6_files/abs2.htm [Google Scholar]
- 52.Kirca A., Arslan E. Antioxidant capacity and total phenolic content of selected plants from Turkey. Int. J. Food Sci. Technol. 2008;43:2038–2046. [Google Scholar]
- 53.Muscolo A., Sidari M., Panuccio M.R. Tolerance of kikuyu grass to long term salt stress is associated with induction of antioxidant defenses. Plant Growth Regul. 2003;41:57–62. [Google Scholar]
- 54.Pietta P.G. Flavonoids as antioxidants. J. Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 55.Prakash D., Suri S., Upadhyay G., Singh B.N. Total phenol, antioxidant, and free radical scavenging activities of some medicinal plants. Inter. J. Food Sci. Nutr. 2007;58:18–28. doi: 10.1080/09637480601093269. [DOI] [PubMed] [Google Scholar]
- 56.Cao G.H., Alessio H.M., Cutler R.G. Oxygen- radical absorbency capacity assay for antioxidant. Free Radic. Biol. Med. 1993;14(3):303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 57.Larson R.A. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- 58.Badami S., Gupta M.K., Suresh B. Antioxidant activity of the ethanolic extract of Striga orobanchioides. J. Ethnopharmacol. 2003;85:227–230. doi: 10.1016/s0378-8741(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 59.Rajalakshmi D., Narasimhan S. Food antioxidants: sources and methods of evaluation. In: Madhavi D.L., De Shpande S.S., Salunkhe D.K., editors. Food Antioxidants. Marcel Dekker; New York: 1996. pp. 65–83. [Google Scholar]
- 60.Meighani H., Ghasemnezhad M., Hashempour A. Evaluation of physico-chemical properties and antioxidant compounds of different genotypes ber fruit grown in Hormozgan province. J. Crops Improv. 2017;18(4):965–975. [Google Scholar]
- 61.Hosseini B., Sh. Shameh, Alirezaloo A. Evaluation of distribution and phytochemical diversity of roses species (Rosa spp.) in Northwest of Iran. J. Plant Prod. Res. 2018;24(4):31–45. [Google Scholar]