Abstract
Background
Previous studies and case-series showed improvement in left ventricular (LV) function and reverse remodeling after sacubitril/valsartan therapy in real-world studies. We therefore aimed to evaluate whether also right ventricular (RV) function may improve after sacubitril/valsartan therapy.
Methods
Sixty consecutive patients with chronic heart failure and NYHA class II-III were followed up for 12 months after therapy with sacubitril/valsartan. Left and (RV) function was assessed at baseline and after 12 months of therapy.
Results
At 12-month control, therapy with sacubitril/valsartan was associated with a significant improvement in a series of echo parameters: LVEF (p < 0.05), LV end-systolic volume (p < 0.01), left atrium area (p < 0.05).
Right ventricular echo parameters were also improved after sacubitril/valsartan therapy: PAsP (31.0 ± 12.8 vs 34.7 ± 12.5 mmHg, p < 0.05), TAPSE (17.8 ± 3.9 vs 16.5 ± 4.0 mm, p < 0.001); mean PAsP reduction was 3.7 ± 11.4 mmHg (-6.3 ± 37.7%), mean TAPSE increase 1.3 ± 2.5 mm (+9.5 ± 15.7%).
Indexed (%) improvement in PAsP (r 0.33, p < 0.01) and TAPSE (r −0.42, p < 0.01) values were proportional to baseline levels. Improvement in PAsP and TAPSE were independent of left ventricular improvements except for PAsP and end-systolic volumes (r 0.44, p < 0.01).
Conclusions
In a real world scenario, sacubitril/valsartan was associated with an improved RV function.
Keywords: Sacubitril valsartan, Neprilysin inhibitors, ARNI, Right ventricular function, Chronic heart failure
1. Background
Following the results in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) [1], sacubitril/valsartan was endorsed by the latest (2016) recommendations from the European Society of Cardiology (ESC) for the management of chronic heart failure (CHF) patients [2]. Data from real life studies seem to confirm excellent results of randomized studies [3], [4].
Observational studies have also demonstrated that treatment with sacubitril/valsartan may improve left ventricular systolic and diastolic function [5] in subjects with reduced left ventricular ejection fraction (LVEF), reverse remodeling [6], NYHA functional class [7]. Sacubitril/valsartan also reduced costs of hospitalization in real world registries [8].
Subjects with HF and reduced EF (HFrEF), however, are also characterized by an impaired right ventricular (RV) function. The compresence of RV dysfunction indicates HF progression and may carry an additional worse prognostic value, while RV recovery is associated with an improved outcome [9].
On the base of such evidence, we sought to evaluate whether treatment with sacubitril/valsartan may also improve RV function in subjects with HFrEF in an observational study.
2. Methods
Sixty consecutive patients with HFrEF in NYHA functional class II-III, enrolled in the Daunia Heart Failure Registry as reported elsewhere [10], [11], [12], were followed up between September 2016 and January 2019. Enrollment criteria included LVEF ≤ 35%, systolic blood pressure ≥ 100 mmHg, eGFR ≥ 30 mL/min/1.73 m2, potassium levels ≤ 5.4 mmol/l. All patients were treated with stable ACE-inhibitor or angiotensin receptor antagonist doses for at least 6 months and started treatment with sacubitril/valsartan therapy as recommended by the 2016 ESC guidelines on HF diagnosis and treatment 2. Medical history, heart rate, systolic blood pressure, Body Mass Index, NYHA functional class, and medications were recorded and monitored. All patients underwent blood analysis, ECG and conventional and TDI echocardiography in an ambulatory setting under resting conditions at the beginning and after 12 months of therapy with sacubitril/valsartan.
2.1. Echocardiography
Conventional echocardiography was used to assess LV dimensions and LVEF, peak velocities of trans-mitral early (E) and late diastolic (A) LV filling, the ratio of trans-mitral early to late (E/A ratio) LV filling velocity. LV dimensions and LVEF were calculated as recommendations in the joint ASE/ESC guidelines. LVEF was calculated according to the Simpson’s rule.
The TAPSE (tricuspid annular plane systolic excursion) was measured using apical views adjusted to optimize RV structures and to achieve proper orientation for M−mode measures.
Pulsed Doppler mitral inflow velocities were obtained by placing a 1–2 mm sample volume between the tips of the mitral leaflets in the apical four-chamber view. The Doppler beam was aligned parallel to the direction of flow. TDI measurements recorded at the septal mitral annulus in apical four-chamber view included early (E’) diastolic velocities. The trans-mitral to mitral annular early diastolic velocity ratio (E/E') was also calculated.
The peak tricuspid regurgitation velocity was assessed by placing the continuous Doppler through the tricuspid valve and the degree of tricuspid regurgitation was evaluated by the color mode.
Pulmonary artery systolic pressures (PAsP) were estimated using the approach of calculating the systolic pressure gradient between right ventricle and right atrium by the maximum velocity of the tricuspid regurgitant jet using the modified Bernoulli equation and then adding to this value the estimated right atrial pressures based on both the size of the inferior vena cava and the change in caliber of this vessel with respiration, according to international recommendations.
Transthoracic echocardiography was performed using an EPIQ 7C ultrasound system with X5-1 matrix array transducer (Philips Healthcare). All echocardiographic studies were performed and interpreted by experienced physicians. They were blinded of the clinical data.
2.2. Statistical analysis
Continuous variables were expressed as mean ± standard deviation and compared with Student's t-test, categorical variables as percentages and compared with χ2 test. Mean values were compared with Student's t-test for variables with a normal distribution or with the Mann–Whitney non-parametric U test for variables with a non-normal distribution. Linear correlations were determined by measuring the Pearson’s correlation coefficient. Multivariable regression analysis was used to assess possible bias of confounders. A p < 0.05 was considered as statistically significant.
3. Results
Mean age was 66 ± 9 years, LVEF 34 ± 9%, male patients were 88%, NYHA class III represented 29%, hypertension was present in 57%, ischemic heart disease in 43%, diabetes in 31%, COPD in 31%, 50% had an ICD/CRT-D implanted. All patients were treated with beta-blockers, 29% with ivabradine, 57% with mineral-corticoid receptor inhibitors. Population characteristics are given in Table 1.
Table 1.
Population’s characteristics.
| Mean ± SE | (%) | |
|---|---|---|
| Age (years) | 66 ± 9 | |
| Male (%) | 88% | |
| Heart rate (bpm) | 70 ± 14 | |
| Systolic blood pressure (mmHg) | 123 ± 20 | |
| Ischemic etiology (%) | 43% | |
| Hypertension (%) | 57% | |
| COPD (%) | 31% | |
| Diabetes (%.) | 31% | |
| ICD/CRT-D (%) | 50% | |
| LVEF (%) | 34 ± 9 | |
| LVESV | 120 ± 56 | |
| LVEDV | 176 ± 70 | |
| E/E’ ratio | 15.5 ± 5.7 | |
| PAsP | 34 ± 12 | |
| TAPSE | 16.5 ± 4.05 | |
| Creatinine (mg/dl) | 1.16 ± 4.04 | |
| ACE inhibitor (%) | 68% | |
| ARB (%) | 32% | |
| Furosemide (%) | 85% | |
| MRA (%) | 57% | |
| Betablocker (%) | 96% | |
| Ivabradine (%.) | 29% | |
| Digoxin (%) | 13% |
Legend: COPD: Chronic Obstructive Pulmonary Disease; ICD/CRT-D: Implantable Cardioverter-Defibrillator/Cardiac Resynchronization Therapy-Defibrillator; LVEF: Left Ventricular Ejection Fraction; LVEDV: Left Ventricle End-Diastolic Volume; LVESV: Left Ventricular End-Systolic Volume; E/E’ ratio: trans-mitral to mitral annular early diastolic velocity ratio; PAsP: Pulmonary Artery systolic Pressure; TAPSE: Tricuspid Annular Plane Systolic Excursion; ACE: Angiotensin Converting Enzyme; ARB: Angiotensin II Receptor Blocker; MRA: Mineralocorticoid Receptor Antagonist.
At 12-month control, therapy with sacubitril/valsartan was associated with a significant improvement in a series of echo parameters: LVEF (34.0 ± 9.2 vs 39.5 ± 9.8%, p < 0.05), LV end-systolic volume (121.6 ± 55.9 vs 108.2 ± 55.2 mL, p < 0.01), left atrium area (24.9 ± 6.9 vs 23.4 ± 6.3 cm2, p < 0.05) (Table 2).
Table 2.
Differences between baseline and follow-up parameters.
| Variables | Baseline values | Follow-up values | P-level |
|---|---|---|---|
| NYHA F. C. | 2.3 ± 0.5 | 2.3 ± 0.5 | n.s. |
| Tricuspid regurgitation | 1.0 ± 0.55 | 1.0 ± 0.52 | n.s. |
| sPAP | 34.7 ± 12.5 | 31.0 ± 12.8 | <0.05 |
| TAPSE | 16.5 ± 4.0 | 17.8 ± 3.9 | <0.001 |
| Systolic Blood Pressure | 123.3 ± 19.8 | 115.8 ± 23.8 | <0.01 |
| HR | 70.3 ± 14.4 | 67.0 ± 9.7 | <0.05 |
| LVEF | 34.0 ± 9.2 | 39.5 ± 9.8 | <0.001 |
| LVEDV | 177.3 ± 71.1 | 174.4 ± 70.1 | n.s. |
| LVESV | 121.6 ± 55.9 | 108.6 ± 55.2 | <0.01 |
| E/E’ ratio | 15.7 ± 5.6 | 15.1 ± 6.2 | n.s. |
| Left atrium area | 24.9 ± 6.9 | 23.4 ± 6.3 | <0.05 |
| NT-pro-BNP | 3049.1 ± 5775.1 | 2305.2 ± 6768.4 | n.s. |
Legend: NYHA F.C.: New York Heart Association Functional Class; PAsP: Pulmonary Artery systolic Pressure; TAPSE: Tricuspid Annular Plane Systolic Excursion; HR: Heart Rate; LVEF: Left Ventricular Ejection Fraction; LVEDV: Left Ventricle End-Diastolic Volume; LVESV: Left Ventricular End-Systolic Volume; E/E’ ratio: trans-mitral to mitral annular early diastolic velocity ratio; NT-pro-BNP: N-terminal fragment of pro-BNP; BNP: B-type natriuretic peptide.
Tricuspid regurgitation is expressed in a semi-quantitative scale from 0 to 3.
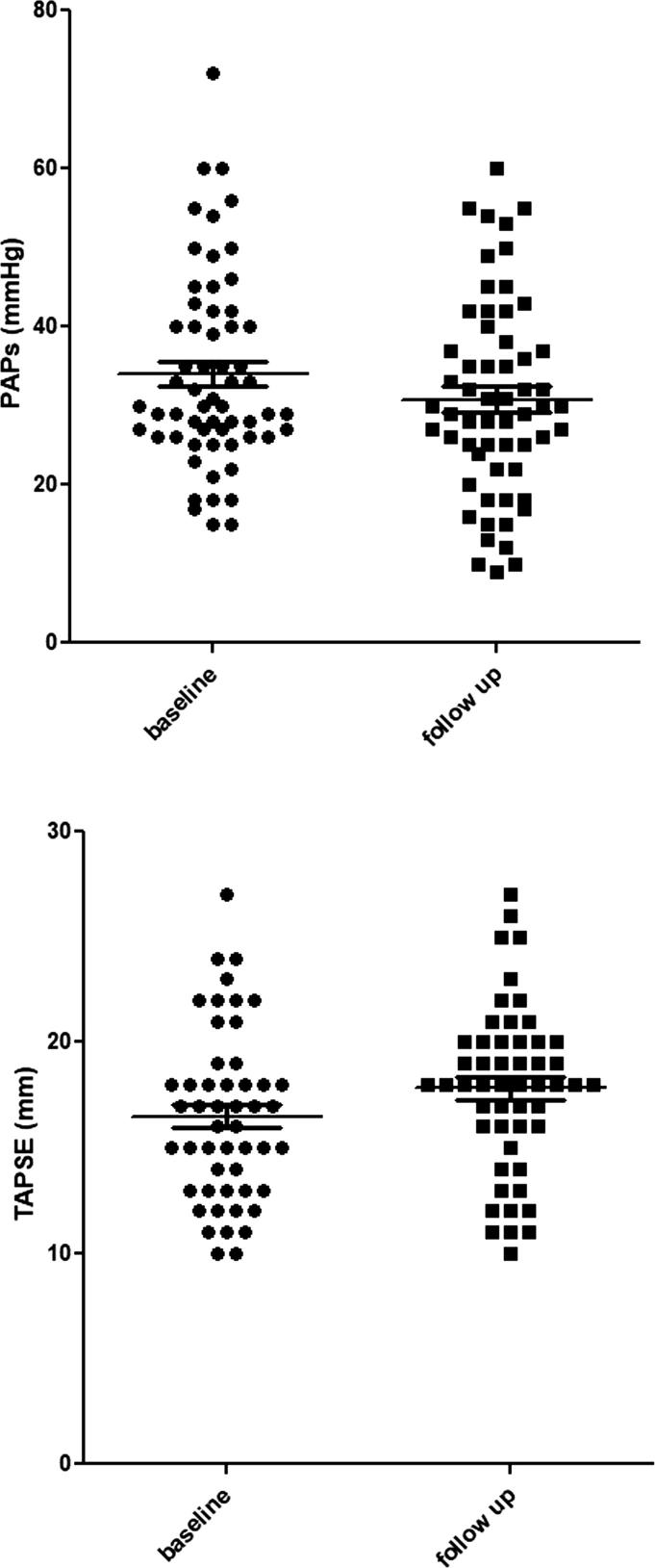
RV echo parameters were also improved after sacubitril/valsartan therapy: PAsP (31.0 ± 12.8 vs 34.7 ± 12.5 mmHg, p < 0.05), TAPSE (17.8 ± 3.9 vs 16.5 ± 4.0 mm, p < 0.001) (Fig. 1); mean PAsP reduction was 3.7 ± 11.4 mmHg (-6.3 ± 37.7%), mean TAPSE increase 1.3 ± 2.5 mm (+9.5 ± 15.7%).
Fig. 1.
Systolic pulmonary arterial pression (p < 0.05) and TAPSE (p < 0.001) improvement after 12-month therapy with sacubitril/valsartan.
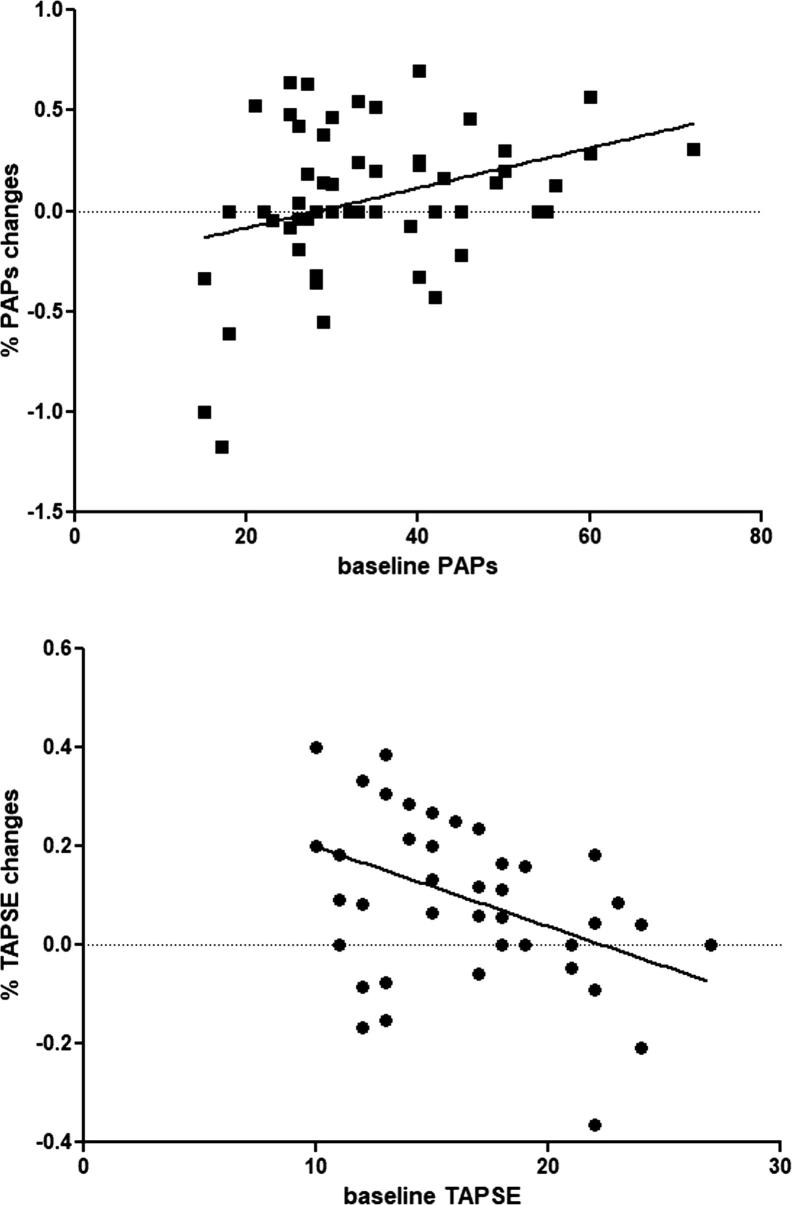
Indexed (%) improvement in PAsP (r 0.33, p < 0.01) and TAPSE (r −0.42, p < 0.01) values were proportional to baseline levels (Fig. 2). Improvement in PAsP and TAPSE were independent of LV improvements except for PAsP and end-systolic volumes (r 0.44, p < 0.01).
Fig. 2.
Linear correlation between baseline sPAP (r 0.33, p < 0.01) and TAPSE (r −0.42, p < 0.01) levels and indexed (%) changes after 12-month therapy with sacubitril/valsartan.
At multivariable analysis improvement in RV function was independent from age, gender, LV end-systolic and LVEF improvement after sacubitril/valsartan therapy (p < 0.05) (Table 3); PAsP changes were proportional to LV end-systolic changes even at multivariable analysis (p < 0.01).
Table 3.
Multivariable regression analysis.
| b* | Std.Err. | b | Std.Err. | p-value | |
|---|---|---|---|---|---|
| TAPSE | |||||
| age | −0.1197 | 0.1590 | −0.0020 | 0.0027 | 0.4565 |
| male | −0.1879 | 0.1518 | −0.0909 | 0.0734 | 0.2240 |
| variation in end-systolic volume | 0.3064 | 0.1862 | 0.1852 | 0.1126 | 0.1089 |
| variation in LVEF | 0.1973 | 0.1655 | 0.1164 | 0.0976 | 0.2412 |
| variation in PAsP | −0.2693 | 0.1675 | −0.1124 | 0.0699 | 0.1170 |
| baseline TAPSE | −0.3280 | 0.1556 | −0.0127 | 0.0060 | 0.0423 |
| PAsP | |||||
| age | −0.1685 | 0.1288 | −0.0067 | 0.0051 | 0.1975 |
| male | −0.0575 | 0.1270 | −0.0666 | 0.1471 | 0.6531 |
| variation in end-systolic volume | 0.4869 | 0.1393 | 0.7053 | 0.2017 | 0.0011 |
| variation in LVEF | 0.1668 | 0.1406 | 0.2359 | 0.1988 | 0.2417 |
| Baseline PAsP | 0.2630 | 0.1269 | 0.0081 | 0.0039 | 0.0441 |
Legend. TAPSE: Tricuspid Annular Plane Systolic Excursion; LVEF: left ventricular ejection fraction; PAsP: pulmonary arterial systolic pressure.
4. Discussion
To the best of our knowledge, this is the first study showing an improved RV function after therapy with sacubitril/valsartan in a real world registry. Improvements in RV function were proportional to baseline dysfunction and not entirely related to improvement in LV function.
In CHF, development of RV dilation and failure are signs of HF progression carrying an increased risk of cardiac death, irrespective of degree of LV dysfunction [13], [14], [15], [16], [17], [18]. A reduced TAPSE is associated with a higher risk of death or hospitalization during follow-up 13 17 [19]. This parameter has been shown to be an important prognostic marker in patients with HF secondary to ischaemic or non-ischemic dilated cardiomyopathy, either when assessed alone 13 19 [20] or in combination with PAsP [21]. The prognostic role of TAPSE was significantly maintained in multivariable models [22], [23].
In our study we found a positive effect of sacubitril/valsartan therapy on these RV parameters (TAPSE and PAsP) in HFrEF outpatients, in a real-world scenario of patients with CHF. Our findings are in line with previous animal model studies showing that Sacubitril/Valsartan was associated with statistically significant improvement in LVEF [24]. In other animal experimental models, sacubitril/valsartan resulted in superior cardiovascular benefits, as evidenced by sustained improvements in LVEF and end‐diastolic pressure [25]. Ex vivo vascular function, as measured by aortic vasorelaxation responses to acetylcholine and sodium nitroprusside, was significantly improved by valsartan and sacubitril/valsartan, with more sustained improvements achieved by sacubitril/valsartan.
A lack of neprilysin or its reduced expression in hypoxia experimental models led to exacerbation of pulmonary arterial remodelling or pulmonary hypertenion (PH) [26] and to a platelet-derived growth factor levels increase in pulmonary artery smooth muscle cells [27]. The increased platelet-derived growth factor levels result in the proliferation and migration of pulmonary artery smooth muscle cells and endothelial-to-mesenchymal transition [28].
In preclinical studies on rats with PH, sacubitril/valsartan reduced RV systolic pressure, RV hypertrophy, and dilatation [29]; these effects may be secondary to pulmonary vascular changes, including reduced pulmonary vascular remodeling, as demonstrated by reduction of pulmonary vascular wall thickness in rats with PH in treatment with sacubitril/valsartan [30].
PH due to left heart disease (PH-LHD) frequently complicates HFrEF; sacubitril/valsartan increases levels of natriuretic peptides. The resulting action on natriuresis, diuresis and vasodilation may play an important role in the reduction of pulmonary pressures and so the sacubitril/valsartan may affect RV function.
In our study, patients with lower TAPSE or high PASP showed more benefit with therapy than others maybe because, in such patients, higher levels of neprilysin could be hypothesized alongside with a more relevant effect on pulmonary vascular remodelling.
A meta-analysis of over 69,000 patients by Kramer et al. demonstrated that improvement in LVEF and LV remodeling parameters was associated with lower rates of mortality among patients with HFrEF [31]. Also RV recovery was associated with improved survival in HF patients [9].
How to impact RV impaired function remains, however, an unresolved issue. There are inconsistent data on RV positive remodeling after standard therapy for HFrEF. In a small cohort of patients with HF and RV dysfunction, carvedilol administration was safe and it was associated with positive RV remodelling as well as improved exercise duration [32].
In a multicenter, randomized, double-blind, placebo-controlled, crossover clinical trial in patients with systemic RV dysfunction, losartan did not improve exercise capacity or reduce NT-proBNP levels [33]. The majority of the studies, however, was held in congenital heart disease treated with heart surgery [34].
Also matter of debate is whether the improvement in RV function is mediated by improvement in LV function or not.
Our data are the first showing an improved RV function with a new class of drugs, sacubitril/valsartan. However, these results require confirmation in larger cohorts with longer follow-up periods.
5. Conclusions
In a real world scenario, sacubitril/valsartan was associated with an improved RV function.
6. Limitations
Main limitations of the study are represented by the small number of patients enrolled and the observational nature of the study; these preliminary results need to be confirmed in a properly powered multicentric study and randomized trials.
Conflict of interest
The authors have no conflict of interest to disclose.
CRediT authorship contribution statement
Michele Correale: Conceptualization, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Adriana Mallardi: . Pietro Mazzeo: Investigation, Resources. Lucia Tricarico: Writing - original draft. Claudia Diella: Investigation, Data curation. Valentina Romano: Resources, Data curation. Armando Ferraretti: Conceptualization, Methodology, Formal analysis, Resources, Data curation. Alessandra Leopizzi: Investigation, Data curation. Giuseppina Merolla: Investigation, Resources, Data curation. Matteo Di Biase: Validation, Supervision. Natale Daniele Brunetti: Methodology, Formal analysis, Writing - review & editing, Visualization, Supervision, Project administration.
Contributor Information
Michele Correale, Email: opsfco@tin.it.
Pietro Mazzeo, Email: pietromazzeo91@tiscali.it.
Armando Ferraretti, Email: starmigi@libero.it.
Giuseppina Merolla, Email: merollagiuse71@alice.it.
Natale Daniele Brunetti, Email: natale.brunetti@unifg.it.
References
- 1.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K. Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M., Ruilope L.M., Ruschitzka F., Rutten F.H., Van der Meer P. 2016 ESC Guidelines for the diagnosis 535 and treatment of acute and chronic heart failure: The Task Force 536 for the diagnosis and treatment of acute and chronic heart failure 537 of the European Society of Cardiology (ESC). Developed with the 538 special contribution of the Heart Failure Association (HFA) of the 539 ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Valle R., Aspromonte N. Sacubitril/Valsartan in Clinical Practice: The Italian Experience. Cardiology. 2017;138(Suppl 1):1–2. doi: 10.1159/000484865. [DOI] [PubMed] [Google Scholar]
- 4.Vincenzi A., Cesana F., Cirò A., Garatti L., Achilli F. Sacubitril/Valsartan in “Field Practice” Patients with Advanced Heart Failure: A Monocentric Italian Experience. Cardiology. 2017;138:13–16. doi: 10.1159/000484877. [DOI] [PubMed] [Google Scholar]
- 5.Martens P., Beliën H., Dupont M., Vandervoort P., Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 2018;36 doi: 10.1111/1755-5922.12435. [DOI] [PubMed] [Google Scholar]
- 6.Almufleh A., Marbach J., Chih S., Stadnick E., Davies R., Liu P., Mielniczuk L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/Valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017;7:108–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino E.R., Degli Esposti D., Miceli R., Bentivenga C., Landolfo M., Fg Cicero A., Berardi E., Spinardi L., Magri G., Dugato V., Borghi C. Sacubitril/valsartan improves both functional and echocardiographic parameters in patients with chronic heart failure with reduced ejection fraction. Curr. Med. Res. Opin. 2019;35:9–12. doi: 10.1080/03007995.2019.1576481. [DOI] [PubMed] [Google Scholar]
- 8.Correale M., Monaco I., Ferraretti A., Tricarico L., Padovano G., Formica E.S., Tozzi V., Grazioli D., Di Biase M., Brunetti N.D. Hospitalization cost reduction with sacubitril-valsartan implementation in a cohort of patients from the Daunia Heart Failure Registry. Int. J. Cardiol. Heart Vasc. 2019;22:102–104. doi: 10.1016/j.ijcha.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dini F.L., Carluccio E., Simioniuc A., Biagioli P., Reboldi G., Galeotti G.G., Raineri C., Gargani L., Scelsi L., Mandoli G.E., Cannito A., Rossi A., Temporelli P.L., Ghio S. Network Labs Ultrasound (NEBULA) in Heart Failure Study Group. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2016;18:1462–1471. doi: 10.1002/ejhf.639. [DOI] [PubMed] [Google Scholar]
- 10.Correale M., Totaro A., Greco C.A., Musaico F., De Rosa F., Ferraretti A., Ieva R., Di Biase M., Brunetti N.D. Tissue Doppler Time Intervals predict the occurrence of re-hospitalization in chronic heart failure: Data from the Daunia Heart Failure Registry. Echocardiography. 2012;29:906–913. doi: 10.1111/j.1540-8175.2012.01729.x. [DOI] [PubMed] [Google Scholar]
- 11.Correale M., Brunetti N.D., Totaro A., Montrone D., Russo A.R., Fanigliulo A.M., Ieva R., Di Biase M. Statin therapy blunts inflammatory activation and improves prognosis and left ventricular performance assessed by tissue Doppler imaging in subjects with chronic ischemic heart failure: results from the Daunia Heart Failure Registry. Clinics. 2011;66:777–784. doi: 10.1590/S1807-59322011000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correale M., Totaro A., Ferraretti A., Musaico F., Passero T., De Rosa F., Abruzzese S., Ieva R., Di Biase M., Brunetti N.D. Additional Prognostic Value of EAS index in predicting the occurrence of rehospitalizations in chronic heart failure: data from the Daunia Heart Failure Registry. Eur. J. Clin. Invest. 2015;45:1098–1105. doi: 10.1111/eci.12514. [DOI] [PubMed] [Google Scholar]
- 13.Damy T., Kallvikbacka-Bennett A., Goode K., Khaleva O., Lewinter C., Hobkirk J., Nikitin N.P., Dubois-Rande J.L., Hittinger L., Clark A.L., Cleland J.G. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J. Card. Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Damy T., Viallet C., Lairez O., Deswarte G., Paulino A., Maison P., Vermes E., Gueret P., Adnot S., Dubois-Rande J.L., Hittinger L. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur. J. Heart Fail. 2009;11:818–824. doi: 10.1093/eurjhf/hfp111. [DOI] [PubMed] [Google Scholar]
- 15.de Groote P., Fertin M., Goeminne C., Petyt G., Peyrot S., Foucher-Hossein C., Mouquet F., Bauters C., Lamblin N. Right ventricular systolic function for risk stratification in patients with stable left ventricular systolic dysfunction: comparison of radionuclide angiography to echo Doppler parameters. Eur. Heart J. 2012;33:2672–2679. doi: 10.1093/eurheartj/ehs080. [DOI] [PubMed] [Google Scholar]
- 16.Gavazzi A., Ghio S., Scelsi L., Campana C., Klersy C., Serio A., Raineri C., Tavazzi L. Response of the right ventricle to acute pulmonary vasodilation predicts the outcome in patients with advanced heart failure and pulmonary hypertension. Am. Heart J. 2003;145:310–316. doi: 10.1067/mhj.2003.146. [DOI] [PubMed] [Google Scholar]
- 17.Ghio S., Gavazzi A., Campana C., Inserra C., Klersy C., Sebastiani R., Arbustini E., Recusani F., Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 18.Meyer P., Filippatos G.S., Ahmed M.I., Iskandrian A.E., Bittner V., Perry G.J., White M., Aban I.B., Mujib M., Dell’Italia L.J., Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghio S., Recusani F., Klersy C., Sebastiani R., Laudisa M.L., Campana C., Gavazzi A., Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am. J. Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 20.Kjaergaard J., Akkan D., Iversen K.K., Køber L., Torp-Pedersen C., Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur. J. Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Ghio S., Temporelli P.L., Klersy C., Simioniuc A., Girardi B., Scelsi L., Rossi A., Cicoira M., Tarro Genta F., Dini F.L. Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur. J. Heart Fail. 2013;15:408–414. doi: 10.1093/eurjhf/hfs208. [DOI] [PubMed] [Google Scholar]
- 22.Dini F.L., Demmer R.T., Simioniuc A., Morrone D., Donati F., Guarini G., Orsini E., Caravelli P., Marzilli M., Colombo P.C. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur. J. Heart Fail. 2012;14:287–294. doi: 10.1093/eurjhf/hfr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carluccio E., Dini F.L., Biagioli P., Lauciello P., Simioniuc A., Zuchi C., Alunni G., Reboldi G., Marzilli M., Ambrosio G. The ‘Echo Heart Failure Score’: an echocardiographic risk prediction score of mortality in systolic heart failure. Eur. J. Heart Fail. 2013;15:868–876. doi: 10.1093/eurjhf/hft038. [DOI] [PubMed] [Google Scholar]
- 24.Suematsu Y., Miura S., Goto M., Matsuo Y., Arimura T., Kuwano T., Imaizumi S., Iwata A., Yahiro E., Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 2016;18:386–393. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi R.K., Polhemus D.J., Li Z., Yoo D., Koiwaya H., Scarborough A., Goodchild T.T., Lefer D.J. Combined Angiotensin Receptor-Neprilysin Inhibitors Improve Cardiac and Vascular Function Via Increased NO Bioavailability in Heart Failure. J. Am. Heart Assoc. 2018;7(5) doi: 10.1161/JAHA.117.008268. pii: e008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey E.C., Wick M.J., Karoor V. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am. J. Pathol. 2009;174:782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahal B.K., Heuchel R., Pullamsetti S.S. Hypoxic pulmonary hypertension in mice with constitutively active platelet-derived growth factor receptor-b. Pulm Circ. 2011;1:259–268. doi: 10.4103/2045-8932.83448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song S., Zhang M., Yi Z. The role of PDGF-B/TGF-b1/neprilysin network in regulating endothelial-to-mesenchymal transition in pulmonary artery remodeling. Cell. Signal. 2016;28:1489–1501. doi: 10.1016/j.cellsig.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Andersen S., Axelsen J.B., Ringgaard S., Nyengaard J.R., Hyldebrandt J.A., Bogaard H.J., de Man F.S., Nielsen-Kudsk J.E., Andersen A. Effects of combined angiotensin II receptor antagonism and neprilysin inhibition in experimental pulmonary hypertension and right ventricular failure. Int. J. Cardiol. 2019;293:203–210. doi: 10.1016/j.ijcard.2019.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Clements R.T., Vang A., Fernandez-Nicolas A., Kue N.R., Mancini T.J., Morrison A.R., Mallem K., McCullough D.J., Choudhary G. Treatment of Pulmonary Hypertension With Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ. Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer D.G., Trikalinos T.A., Kent D.M., Antonopoulos G.V., Konstam M.A., Udelson J.E. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J. Am. Coll. Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giardini A., Lovato L., Donti A., Formigari R., Gargiulo G., Picchio F.M., Fattori R. A pilot study on the effects of carvedilol on right ventricular remodelling and exercise tolerance in patients with systemic right ventricle. Int. J. Cardiol. 2007;114:241–246. doi: 10.1016/j.ijcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Dore A., Houde C., Chan K.L., Ducharme A., Khairy P., Juneau M., Marcotte F., Mercier L.A. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 34.Therrien J., Provost Y., Harrison J., Connelly M., Kaemmerer H., Webb G.D. Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study. Int. J. Cardiol. 2008;129:187–192. doi: 10.1016/j.ijcard.2008.04.056. [DOI] [PubMed] [Google Scholar]