Summary
Polymerization and modification play central roles in polymer chemistry and are generally implemented in two steps, which suffer from the time-consuming two-step strategy and present considerable challenge for complete modification. By introducing the radical cascade reaction (RCR) into polymer chemistry, a one-step strategy is demonstrated to achieve synchronized polymerization and complete modification in situ. Attributed to the cascade feature of iron-catalyzed three-component alkene carboazidation RCR exhibiting carbon-carbon bond formation and carbon-azide bond formation with extremely high efficiency and selectivity in one step, radical cascade polymerization therefore enables the in situ synchronized polymerization through continuous carbon-carbon bond formation and complete modification through carbon-azide bond formation simultaneously. This results in a series of α, β, and γ poly(amino acid) precursors. This result not only expands the methodology library of polymerization, but also the possibility for polymer science to achieve functional polymers with tailored chemical functionality from in situ polymerization.
Subject Areas: Polymer Chemistry, Organic Reaction, Polymers
Graphical Abstract
Highlights
-
•
Synchronized polymerization and modification
-
•
Radical cascade polymerization
-
•
Alkene functionalization polymerization
Polymer Chemistry; Organic Reaction; Polymers
Introduction
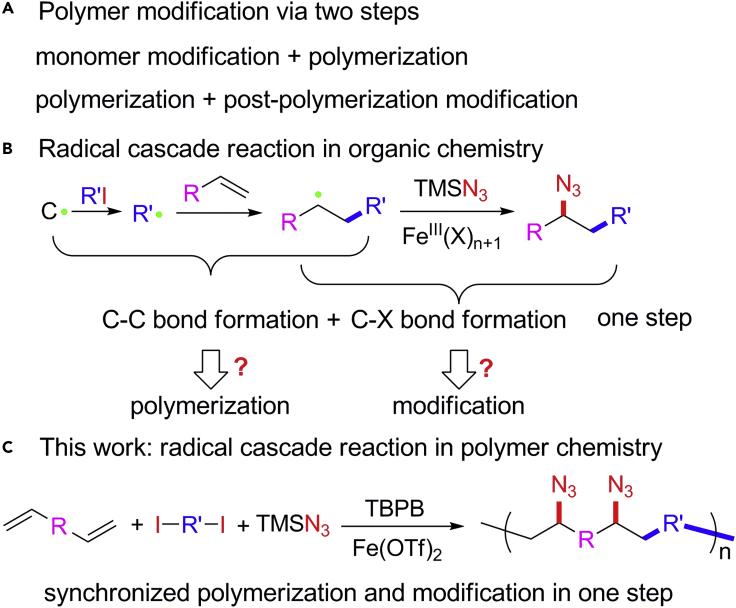
Synthetic polymer materials with desired functionalities in the form of plastics, fibers, rubbers, etc., have played significant roles in human life since last century, which rely mainly on the prosperous development of polymerization and modification methodologies. Polymerization and modification are two fundamental aspects of polymer chemistry and have played central roles in the history of polymer chemistry (Matyjaszewski and Xia, 2001, Kamigaito et al., 2001, Gauthier et al., 2009, Boaen and Hillmyer, 2005, Richards et al., 2012, Britovsek et al., 1999, Moad et al., 2005, Sun et al., 2013). Generally, polymer modification can be implemented either during the monomer synthesis or by post-polymerization modification (Scheme 1A) (Wan et al., 2014, Wan et al., 2017, Lv et al., 2017, Gao et al., 2014, Kakuchi and Theato, 2013, Hedir et al., 2015, Wang et al., 2017b). However, these broadly applied two-step strategies have many disadvantages including time consumption, waste of resources, and solubility issues. And complete post-polymerization modification is a considerable challenge owing to the reduced reactivity of functional groups on the polymer chain and the embedding as well as the shielding effect of the polymer chain on functional groups. We questioned whether a one-step strategy can be developed to overcome the disadvantages by realizing synchronized polymerization and modification in one step, constructing and modifying a polymer simultaneously. To develop a one-step strategy to implement polymerization and modification will enable step-economy and efficient complete modification and is therefore highly desirable.
Scheme 1.
The Introduction of Radical Cascade Reaction into Polymer Chemistry toward Synchronized Polymerization and Modification in One Step
(A) Polymer modification methods. (B) The highly efficient and selective iron-catalyzed three-component alkene carboazidation is used as an example of radical cascade reaction in organic chemistry. (C) Radical cascade polymerization through alkene functionalization polymerization.
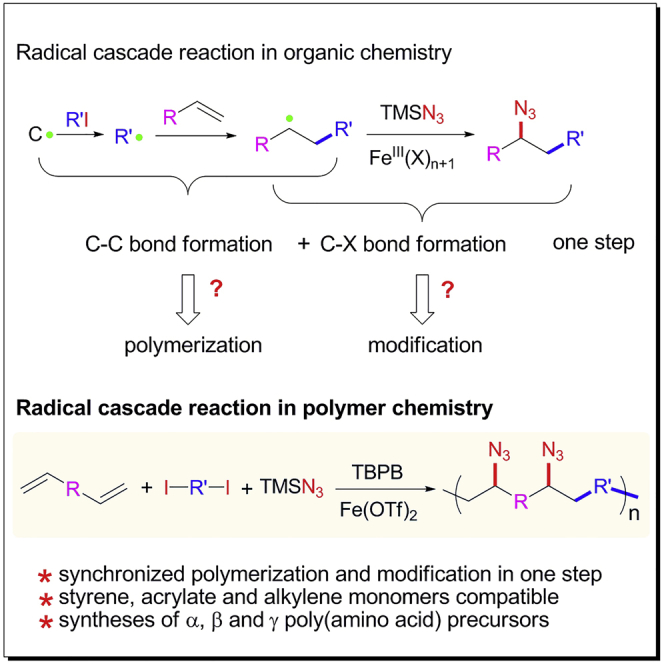
The introduction of organic reactions to polymer chemistry has received considerable research effort for its convenient and versatile effect on the development of new polymerization methodologies. However, not all organic reactions are potential candidates for the design of new polymerization methodology. Only highly efficient and selective organic reactions are suitable to be introduced into polymer chemistry to develop new polymerization methodology. For example, atom transfer radical addition reaction (Wang and Matyjaszewski, 1995, Pintauer and Matyjaszewski, 2008), radical addition-fragmentation reaction (Moad et al., 2008, Chiefari et al., 1998), olefin metathesis reaction (Vougioukalakis and Grubbs, 2010, Bielawski and Grubbs, 2000, Bielawski and Grubbs, 2007), Suzuki coupling reaction (Miyaura et al., 1981, Littke et al., 2000, Kotha et al., 2002, Schluter, 2001, Yokoyama et al., 2007, Baggett et al., 2015), Michael addition reaction (Liu et al., 2003, Wang et al., 2005), Stille coupling reaction (Bao et al., 1995, Littke and Fu, 1999, Yin et al., 2016, Guo et al., 2014), click chemistry reactions (He et al., 2016, He et al., 2017), multiple components reactions (Deng et al., 2012, Deng et al., 2016, Kreye et al., 2011, Wei et al., 2017, Wu et al., 2017, Xue et al., 2016), Barbier reaction (Sun et al., 2017, Jing et al., 2019) etc. have been successfully introduced into polymer chemistry to develop desired polymerization methodologies (Tebben and Studer, 2011, Jiang et al., 2018, Huang et al., 2019, Liu et al., 1999). These polymerization methodologies have expanded the structure and functionality library of polymer chemistry in the past decades. The introduction of highly efficient and selective organic reactions into polymer chemistry is therefore highly desirable for polymer science.
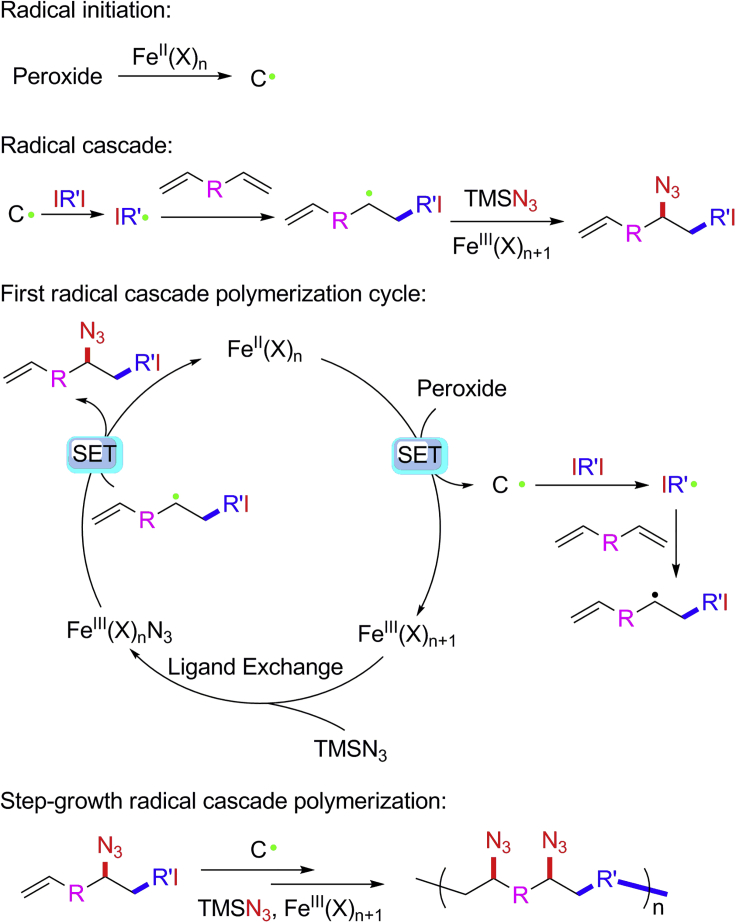
Radical cascade reactions (RCRs) are cascade reactions involving two or more radical procedures and bond formation. They have attracted considerable interest in modern organic synthesis for their versatility in alkene addition and functionalization (Brill et al., 2016, Plesniak et al., 2017, Wang et al., 2017a, Wang et al., 2018, Xiong et al., 2019, Yu et al., 2018, Zhang and Studer, 2015). Attributed to the cascade feature of RCR, they feature the chemical procedure of carbon-carbon bond and complete carbon-X (functional moieties including azide, amine, hydroxide) formation in one step (Scheme 1B). We therefore hypothesize that the exploration of RCR exhibiting extremely high efficiency and selectivity on formation of carbon-carbon and carbon-X bonds and its introduction into polymer chemistry will enable the polymerization through continuous carbon-carbon bond formation and complete modification through carbon-X formation in a single step.
Herein, we demonstrate the introduction of iron-catalyzed three-component alkene carboazidation RCR into polymer chemistry to develop radical cascade polymerization (RCP) through alkene functionalization polymerization, a novel polymerization methodology. This iron-catalyzed three-component alkene carboazidation RCR exhibits extremely high efficiency and selectivity in the formation of carbon-carbon and carbon-azide bonds. Attributed to its efficiency, selectivity, and cascade feature, this RCP enables the polymerization and complete modification in one step. For better understanding the versatility of RCP methodology, RCP polymers are further demonstrated as α, β, and γ amino acid polymer precursors. This work therefore not only expands the methodology library of polymerization, but also opens a window for polymer science to achieve polymer materials with tailored chemical functionality from in situ polymerization.
Results and Discussion
According to our previous work, iron-catalyzed three-component carboazidation of alkenes is an RCR involving alkenes, azidotrimethylsilane (TMSN3). and iodides. It shows high efficiency and selectivity in the formation of carbon-carbon and carbon-azide bonds, resulting in azides containing functional compounds or amino acids precursors when R or R′ contain ester groups (Scheme 1B). (Xiong et al., 2019) In this three-component RCR, cascade radical reactions take place via iron-catalyzed radical generation, iodine atom transfer, radical addition, and iron-catalyzed azido group transfer, producing carbon-carbon bond formation and carbon-azide bond formation. The iodide and TMSN3 serve as carbon and azide sources. In the hypothesis of RCP by introducing RCR into polymer chemistry to realize synchronized polymerization and modification, RCR candidates should show high efficiency and selectivity. This highly efficient and selective carbon-carbon bond formation can avoid other carbon source contamination that may terminate polymerization, whereas that of carbon-azide bond formation can avoid the radical polymerization of alkene by itself.
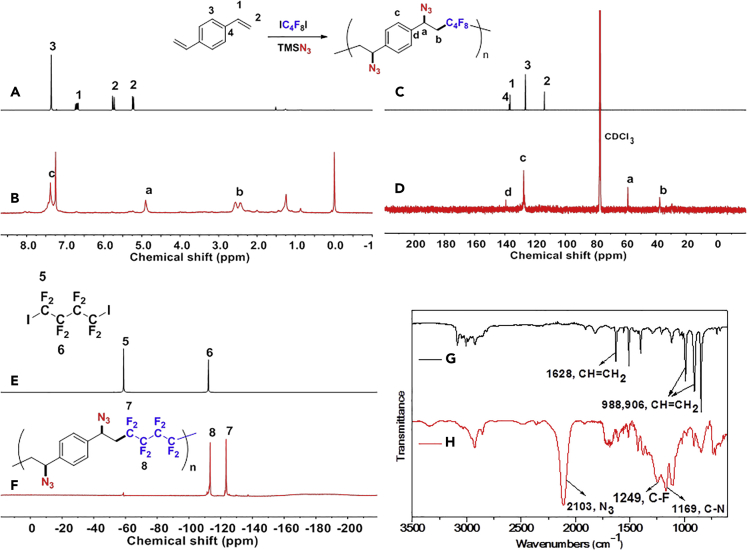
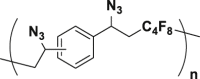
To prove the above hypothesis of introducing iron-catalyzed three-component alkene carboazidation RCR into polymer chemistry to realize synchronized polymerization and modification in one step, the A2+B2 type RCP was therefore designed with dienes, 1,4-diiodoperfluorobutane, and TMSN3 as three-component monomers; iron(II) trifluoromethanesulfonate (Fe(OTf)2) as catalyst; and tert-butyl peroxybenzoate (TBPB) as a radical initiator (Scheme 1C). To verify the above attempt to introduce RCR into polymer chemistry, iron-catalyzed RCPs were carried out in 1,2-dimethoxyethane (DME). A series of azide-containing polymers were successfully synthesized and are shown in Table 1. Taking the RCP of a diene monomer, 1,4-divinylbenzene as an example, its successful RCP was verified by NMR and Fourier transform infrared spectroscopy (FTIR) spectra (Figure 1). From 1H NMR spectra shown in Figures 1A and 1B, the product shows broader peak signals in comparison with the sharp peak signals from the monomer, which is a typical phenomenon associated with polymerization. The successful polymerization can be further verified by the disappearance of signals from the vinyl group at 6.70, 5.72, and 5.24 ppm and the appearance of signals for carbon-carbon bond formation at 2.62–2.36 ppm and carbon-azide bond formation at 4.90 ppm in the 1H NMR spectra (Figures 1A and 1B). From the 13C NMR spectra shown in Figures 1C and 1D, the polymerization is verified by the disappearance of signals from the vinyl group at 136.49 and 113.78 ppm and the appearance of signals for carbon-carbon bond formation at 37.59 ppm and carbon-azide bond formation at 58.62 ppm. From the 19F NMR spectra shown in Figures 1E and 1F, the signal at −58.82 ppm (CF2-I) disappeared and a signal appeared at −123.34 ppm (CF2-CH2) after polymerization. From the FTIR spectra shown in Figures 1G and 1H, the polymerization is verified by the disappearance of absorption bands at 1,628 and 988,906 cm−1, which were assigned to the stretching vibration of C=C and in-plane bending and wagging vibration of = C-H in the alkene, respectively. Meanwhile, new spectral bands due to carbon-carbon bond formation and carbon-azide bond formation appeared at 1,249 and 2,103 cm−1. These are the stretching vibration of C-F and N3, respectively. The tiny amount of residual signal from the vinyl group at 6.70, 5.72, and 5.24 ppm and the signal from the iodide at −58.59 ppm (CF2-I) in 1H NMR and 19F NMR spectra of the polymer verify the presence of the terminal vinyl group and iodide at each end of the polymer chain. The successful preparation of the polymer is further verified by GPC curve with a Mn of 5,100 and PDI of 1.78. These results of vinyl group addition, carbon-carbon bond formation, carbon-azide bond formation, and terminal groups are all confirmed and are evidence for the successful iron-catalyzed RCP of 1,4-divinylbenzene, 1,4-diiodoperfluorobutane, and TMSN3, resulting in an azide-containing polymer with a terminal vinyl group and an iodide on each end of the polymer chain. Other examples of RCP are listed in Table 1 and Figures S1–S9. These results serve to confirm that these RCPs undergo vinyl group addition, carbon-carbon bond formation, and carbon-azide bond formation, resulting in polymerization and complete modification in one step.
Table 1.
The Alkene Structures and Results of Polymers Synthesized by Radical Cascade Polymerization
| Entry | Substrate | Product | Conv. (%)a | Mnb | Mwb | PDIb |
|---|---|---|---|---|---|---|
| 1 | 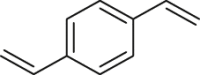 |
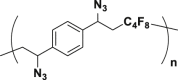 |
99 | 5,100 | 9,100 | 1.78 |
| 2 | 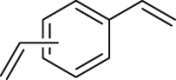 |
 |
99 | 4,300 | 6,600 | 1.53 |
| 3 |  |
 |
98 | 3,000 | 4,400 | 1.47 |
| 4 | 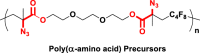 |
98 | 4,200 | 5,700 | 1.36 | |
| 5c | 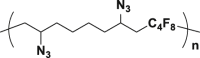 |
97 | 3,400 | 5,400 | 1.59 | |
| 6 | 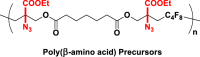 |
99 | 6,800 | 11,700 | 1.72 | |
| 7d | 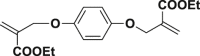 |
 |
99 | --e | ||
| 8d | 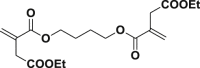 |
 |
99 | 7,800 | 13,700 | 1.76 |
| 9d | 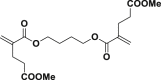 |
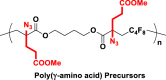 |
98 | 4,600 | 7,200 | 1.57 |
Reaction conditions: alkene (1mmol, 1.0 eq.), IC4F8I (1.0 eq.), TMSN3 (2.5 eq.), TBPB (3.0 eq.), Fe(OTf)2 (5 mol%) and DME (1 mL), r.t, 40 h.
Calculated from 1H NMR spectroscopy.
Measured by GPC.
TMSN3 (4.0 eq.), TBPB (5.0 eq.), 70°C.
50°C.
Not very soluble.
Figure 1.
Radical Cascade Polymerization of an Alkene Through Alkene Functionalization Polymerization
1,4-Diiodoperfluorobutane (IC4F8I) and azidotrimethylsilane (TMSN3) were used as monomers. Reaction conditions: alkene (1 mmol, 1.0 eq.), IC4F8I (1.0 eq.), TMSN3 (2.5 eq.), TBPB (3.0 eq.), Fe(OTf)2 (5 mol%), and DME (1 mL), r.t, 40 h.
(A and B) 1H NMR spectra (A: alkene; B: polymer).
(C and D) 13C NMR spectra (C: alkene; D: polymer).
(E and F) 19F NMR spectra (E: 1,4-diiodoperfluorobutane; F: polymer).
(G and H) FTIR spectra (G: alkene; H: polymer).
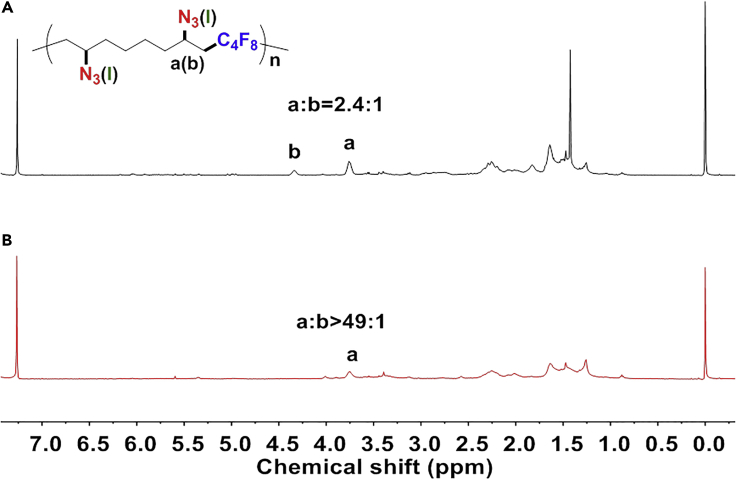
It is also clear that the introduction of RCR into polymer chemistry works smoothly not only for styrene-like monomers, but also for acrylates and alkyl alkenes, giving polymers with moderate molecular weight and high conversion. These results indicate this developed RCP is a versatile method to enable polymerization and modification in one step. For styrene and acrylate monomers, their RCP gives complete conversion of carbon-azide bond formation, resulting in fully functionalized products. When an alkyl alkene was used as monomer, its RCP exhibited controlled azide/iodine modification of the resulting polymers, as shown in Figure 2 and Figure S10. With the increase in the amount of TMSN3, Fe(OTf)2, and temperature, the azide/iodine ratio can be tuned and increased from 2.4:1 to >49:1. Detailed investigations on the influence of catalyst amount, TMSN3 amount, and temperature on controlled modification are shown in Table 2. As Fe(OTf)2, TMSN3, and temperature are increased, the less stable iodine product will form corresponding radical and transfer to azide product, resulting in the decrease of iodine and the increase of azide. So, the azide/iodine ratio can be adjusted from 0.97/1 to >49:1, correspondingly. Traditional cascade or tandem polymerizations are carried out stepwise or in tandem with different polymerization mechanisms, different reaction types, or different monomers in one pot (Grubbs et al., 1997, Qi et al., 2019, Nakatani et al., 2009, Chen et al., 2006, Kang et al., 2017, Wang et al., 2012). This RCP, however, is realized in a one-step radical procedure through RCR. The introduction of RCR into polymer chemistry in the development of the RCP method is therefore novel in that it offers versatile control of polymerization and modification in one step.
Figure 2.
Controlled Azide/Iodine Modification of Resulting Polymers via One-Step RCP
(A) Fe(OTf)2 (5 mol%), TMSN3 (1.5 eq.), TBPB (3.0 eq.), 40°C.
(B) Fe(OTf)2 (15 mol%), TMSN3 (4.0 eq.), TBPB (5.0 eq.), 70°C.
Table 2.
Results of the Influence of Feed Ratio and Polymerization Condition on Controlled Azide/Iodine Modification via One-Step RCP
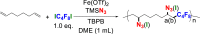 | |||||
|---|---|---|---|---|---|
| Entry | TMSN3(X eq.) | Fe(OTf)2 (X mol%) | TBPB (X eq.) | Temperature (°C) | N3/I (a/b) |
| 1 | 1.0 | 5 | 3.0 | 40 | 0.97:1 |
| 2 | 1.5 | 5 | 3.0 | 40 | 2.4:1 |
| 3 | 2.5 | 5 | 3.0 | r.t | 2:1 |
| 4 | 4.0 | 10 | 5.0 | 40 | 3.0:1 |
| 5 | 4.0 | 10 | 5.0 | 50 | 3.6:1 |
| 6 | 4.0 | 15 | 5.0 | 70 | >49:1 |
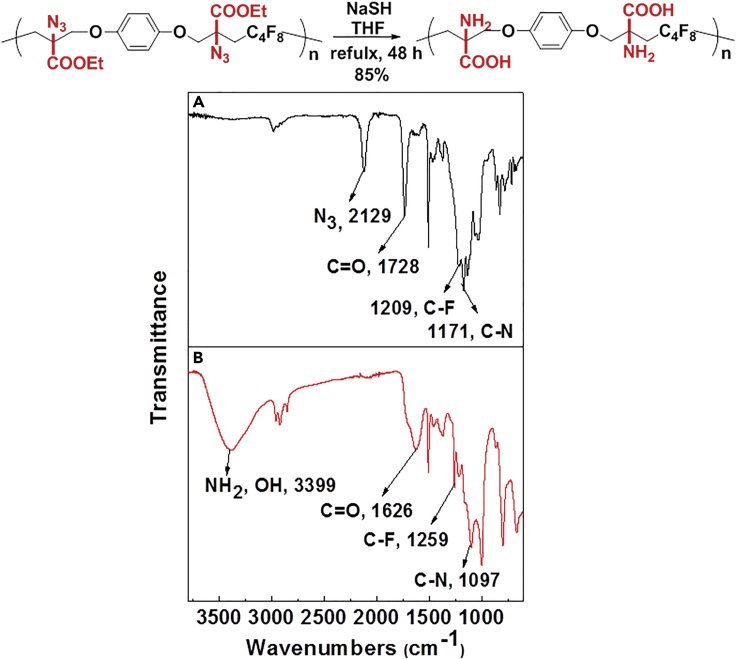
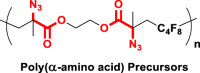
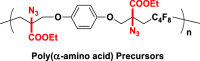
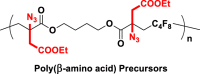
The introduction of RCR into polymer chemistry not only demonstrates versatility in the control of polymerization and modification in one step, but also enables the synthesis of a series of α, β, and γ type poly(amino acid) precursors, as shown in Table 1. Taking the polymer precursor shown in Figure 4 as an example, further reduction of the azide to an amine and deprotection of the ester to a carboxylic acid by NaSH results in polymers containing amino acids. The successful preparation of this α-amino acid containing polymer is confirmed by FTIR spectra, as shown in Figure 3, which shows the complete disappearance of absorption bands at 2,129 cm−1 (stretching vibration of azide group) and the appearance of a new broad signal at 3,399 cm−1 from the stretching vibrations of the amine and hydroxide groups. Meanwhile, the stretching vibration of C=O was shifted from 1,728 to 1,626 cm−1 owing to the conversion of ester groups to carboxylic acid groups. These azide-containing polymers show other potential applications with regards to the versatile azide group. An example of this is the production of bottlebrush polymers.
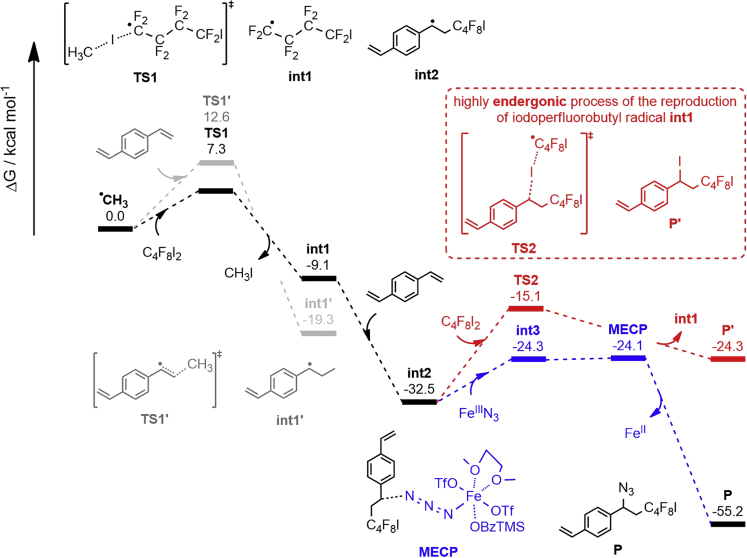
Figure 4.
The Free Energy Profile of RCP Involving 1,4-Divinylbenzene
Figure 3.
Reduction and Deprotection of Precursor in the Preparation of Poly(α-Amino Acid)
(A) FTIR spectrum of poly(α-amino acid) precursor.
(B) FTIR spectrum of poly(α-amino acid).
Mechanistic Study
To further investigate the mechanism of RCP, density functional theory studies were also conducted to exhibit how RCP realizes synchronized polymerization and modification in one cascaded step. The results for RCR of 1,4-divinylbenzene or octa-1,7-diene, 1,4-diiodoperfluorobutane and TMSN3 are shown in Figures 4 and S11, respectively. Once the carbon radical is formed through a single electron transfer from the iron (II) catalyst to TBPB, the iodine abstraction between 1,4-diiodoperfluorobutane and the formed carbon radical in the formation of an iodoperfluorobutyl radical (int1) shows the lowest barrier pathway with only 7.3 kcal/mol of free energy barrier. Then, the perfluorobutyl radical adds onto the 1,4-divinylbenzene without barrier to deliver the benzyl radical int2 with high exergonicity of 32.5 kcal/mol. These free energy-favored processes guarantee the efficiency and selectivity of carbon-carbon bond formation in the formation of int2 to inhibit the radical polymerization of alkene by itself. Further calculations indicate int2 will undergo two competitive pathways through reacting with a C4F8I2 or a FeIIIN3 depending on alkene types, resulting in formation of carbon-azide bond and carbon-iodine bond, respectively. For styrene-type monomer, it shows sufficient efficiency and selectivity on the carbon-azide bond formation, attributed to the unfavorable iodoperfluorobutyl radical reproduction by the stable benzyl radical int2, which is an endergonic pathway with relatively higher barrier of 17.4 kcal/mol (Figure 4). For alkyl alkene-type monomer, the observation of iodine addition product in the reaction of octa-1,7-diene is due to the homenergic iodoperfluorobutyl radical reproduction by alkyl radical int4 with lower barrier (14.5 kcal/mol, Figure S11), resulting in tunable carbon-azide bond and carbon-iodine bond formations by adjusting the amount of TMSN3 and Fe(OTf)2.
Based on the above characterizations of polymer structures and mechanism studies, a plausible mechanism for this iron-catalyzed three-component alkene carboazidation RCP is shown in Scheme 2. This RCP is initiated by a single electron transfer from the iron (II) catalyst to TBPB generating a carbon radical (C⋅) and an iron (III) complex. The iodine atom in the diiodo compound was abstracted by the carbon radical to form a new radical (IR’⋅), which will add to the divinyl alkene giving an intermediate. Meanwhile, the iron (III) complex exchanges the ligand with TMSN3 to generate the iron (III) azide complex, which reacts with a radical intermediate to end one radical cascade polymerization cycle with the formation of an azide-containing iodoalkene compound and iron (II) complex which is used in the next polymerization cycle. With the continuous step-growth RCR, the iron-catalyzed three-component alkene carboazidation type of RCR has been successfully introduced to polymer chemistry in the development of RCP, enabling synchronized polymerization and modification in one pot, where the sufficient efficiency and selectivity on the carbon-carbon bond and carbon-azide bond formations play key roles.
Scheme 2.
Mechanism of Iron-Catalyzed Three-Component Alkene Carboazidation RCP Through Alkene Functionalization Polymerization
Conclusion
In summary, we have successfully introduced the radical cascade reaction into polymer chemistry. This has resulted in the development of radical cascade polymerization toward synchronized polymerization and modification in one pot. In this process, the high efficiency and selectivity of iron-catalyzed three-component alkene carboazidation on the carbon-carbon and carbon-azide bond formation play significant and critical roles in alkene functionalization polymerization. Through iron-catalyzed three-component alkene carboazidation radical cascade polymerization, a series of azide-containing functional polymers have been prepared, including a series of α, β, and γ poly(amino acid) precursors. This work therefore expands the methodology library of polymerization and provides a one-step strategy for synchronized polymerization and modification. In view of the rapid development in radical cascade reactions of alkene difunctionalization in the last 10 years and the ever-increasing research interests on this area in organic chemistry, this work also opens up a window for polymer science to achieve functional polymers with tailored chemical functionality not limited to azide during in situ polymerization.
Limitations of the Study
This study focuses on the demonstration of synchronized polymerization and modification in one step, by introducing radical cascade reaction into polymer chemistry to develop a new polymerization methodology, i.e., radical cascade polymerization. The substrates used in this study contain perfluorobutanes, which may cause difficulty in investigating the functionalities of resulting poly(amino acid)s in aqueous solution. Fortunately, the solubility deriving from the substrates will form hydrophobic domain of poly(amino acid)s and endow poly(amino acid)s with amphiphilicity, which may exhibit special functionality as self-assemblies in aqueous solution.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the NSFC (21922112, 21971236, 21672213, and 21871258), the National Key R&D Program of China (2017YFA0700103), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the Haixi Institute of CAS (CXZX-2017-P01) for financial support.
Author Contributions
H.B. and W.-M.W. directed the investigations and prepared the manuscript. N.Z., M.-F.C., and Y.L. contributed to the discussion and preparation of the manuscript. N.Z., H.X., Min Su, and Muqiao Su performed the experiments and analyzed the experimental data. M.-F.C. worked on the theoretical calculations.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100902.
Contributor Information
Wen-Ming Wan, Email: wanwenming@fjirsm.ac.cn.
Hongli Bao, Email: hlbao@fjirsm.ac.cn.
Supplemental Information
References
- Baggett A.W., Guo F., Li B., Liu S.Y., Jäkle F. Regioregular synthesis of azaborine oligomers and a polymer with a syn conformation stabilized by NH⋅⋅⋅π interactions. Angew. Chem. Int. Ed. 2015;54:11191–11195. doi: 10.1002/anie.201504822. [DOI] [PubMed] [Google Scholar]
- Bao Z., Chan W.K., Yu L. Exploration of the Stille coupling reaction for the synthesis of functional polymers. J. Am. Chem. Soc. 1995;117:12426–12435. [Google Scholar]
- Bielawski C.W., Grubbs R.H. Highly efficient ring-opening metathesis polymerization (ROMP) using new ruthenium catalysts containing N-Heterocyclic carbene ligands. Angew. Chem. Int. Ed. 2000;39:2903–2906. doi: 10.1002/1521-3773(20000818)39:16<2903::aid-anie2903>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Bielawski C.W., Grubbs R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007;32:1–29. [Google Scholar]
- Boaen N.K., Hillmyer M.A. Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 2005;34:267–275. doi: 10.1039/b311405h. [DOI] [PubMed] [Google Scholar]
- Brill Z.G., Grover H.K., Maimone T.J. Enantioselective synthesis of an ophiobolin sesterterpene via a programmed radical cascade. Science. 2016;352:1078–1082. doi: 10.1126/science.aaf6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britovsek G.J.P., Gibson V.C., Wass D.F. The search for new-generation olefin polymerization catalysts: life beyond metallocenes. Angew. Chem. Int. Ed. 1999;38:428–447. doi: 10.1002/(SICI)1521-3773(19990215)38:4<428::AID-ANIE428>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Chen G.H., Huynh D., Felgner P.L., Guan Z.B. Tandem chain walking polymerization and atom transfer radical polymerization for efficient synthesis of dendritic nanoparticles for bioconjugation. J. Am. Chem. Soc. 2006;128:4298–4302. doi: 10.1021/ja0573864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiefari J., Chong Y.K., Ercole F., Krstina J., Jeffery J., Le T.P.T., Mayadunne R.T.A., Meijs G.F., Moad C.L., Moad G. Living free-radical polymerization by reversible Addition−Fragmentation chain Transfer: the RAFT process. Macromolecules. 1998;31:5559–5562. [Google Scholar]
- Deng H.Q., Han T., Zhao E.G., Kwok R.T.K., Lam J.W.Y., Tang B.Z. Multicomponent click polymerization: a facile strategy toward fused heterocyclic polymers. Macromolecules. 2016;49:5475–5483. [Google Scholar]
- Deng X.X., Li L., Li Z.L., Lv A., Du F.S., Li Z.C. Sequence regulated poly(ester-amide)s based on passerini reaction. ACS Macro Lett. 2012;1:1300–1303. doi: 10.1021/mz300456p. [DOI] [PubMed] [Google Scholar]
- Gao Y., Wang X., Tong L., Qin A.J., Sun J.Z., Tang B.Z. A new strategy of post-polymerization modification to prepare functionalized poly(disubstituted acetylenes) Polym. Chem. 2014;5:2309–2319. [Google Scholar]
- Gauthier M.A., Gibson M.I., Klok H.A. Synthesis of functional polymers by post-polymerization modification. Angew. Chem. Int. Ed. 2009;48:48–58. doi: 10.1002/anie.200801951. [DOI] [PubMed] [Google Scholar]
- Grubbs R.B., Hawker C.J., Dao J., Fréchet J.M.J. A tandem approach to graft and dendritic graft copolymers based on 'living' free radical polymerizations. Angew. Chem. Int. Ed. 1997;36:270–272. [Google Scholar]
- Guo F., Yin X., Pammer F., Cheng F., Fernandez D., Lalancette R.A., Jäkle F. Regioregular organoborane-functionalized poly(3-alkynylthiophene)s. Macromolecules. 2014;47:7831–7841. [Google Scholar]
- He B.Z., Su H.F., Bai T.W., Wu Y.W., Li S.W., Gao M., Hu R.R., Zhao Z.J., Qin A.J., Ling J., Tang B.Z. Spontaneous amino-yne click polymerization: a powerful tool toward regio- and stereospecific poly(beta-aminoacrylate)s. J. Am. Chem. Soc. 2017;139:5437–5443. doi: 10.1021/jacs.7b00929. [DOI] [PubMed] [Google Scholar]
- He B.Z., Zhen S.J., Wu Y.W., Hu R.R., Zhao Z.J., Qin A.J., Tang B.Z. Cu(I)-Catalyzed amino-yne click polymerization. Polym. Chem. 2016;7:7375–7382. [Google Scholar]
- Hedir G.G., Bell C.A., O'reilly R.K., Dove A.P. Functional degradable polymers by radical ring-opening copolymerization of MDO and vinyl bromobutanoate: synthesis, degradability and post-polymerization modification. Biomacromolecules. 2015;16:2049–2058. doi: 10.1021/acs.biomac.5b00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Wang W., Zhou Z., Sun B., An M., Haeffner F., Niu J. Radical ring-closing/ring-opening cascade polymerization. J. Am. Chem. Soc. 2019;141:12493–12497. doi: 10.1021/jacs.9b05568. [DOI] [PubMed] [Google Scholar]
- Jiang K.M., Zhang L., Zhao Y.C., Lin J., Chen M. Palladium-catalyzed cross-coupling polymerization: a new access to cross-conjugated polymers with modifiable structure and tunable optical/conductive properties. Macromolecules. 2018;51:9662–9668. [Google Scholar]
- Jing Y.-N., Li S.-S., Su M., Bao H., Wan W.-M. Barbier Hyperbranching polymerization-induced emission toward facile fabrication of white light-emitting diode and light-harvesting film. J. Am. Chem. Soc. 2019;141:16839–16848. doi: 10.1021/jacs.9b08065. [DOI] [PubMed] [Google Scholar]
- Kakuchi R., Theato P. Three-component reactions for post-polymerization modifications. ACS Macro Lett. 2013;2:419–422. doi: 10.1021/mz400144q. [DOI] [PubMed] [Google Scholar]
- Kamigaito M., Ando T., Sawamoto M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001;101:3689–3745. doi: 10.1021/cr9901182. [DOI] [PubMed] [Google Scholar]
- Kang C., Park H., Lee J.K., Choi T.L. Cascade polymerization via controlled tandem olefin metathesis/metallotropic 1,3-shift reactions for the synthesis of fully conjugated polyenynes. J. Am. Chem. Soc. 2017;139:11309–11312. doi: 10.1021/jacs.7b04913. [DOI] [PubMed] [Google Scholar]
- Kotha S., Lahiri K., Kashinath D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron. 2002;58:9633–9695. [Google Scholar]
- Kreye O., Toth T., Meier M.A.R. Introducing multicomponent reactions to polymer science: passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011;133:1790–1792. doi: 10.1021/ja1113003. [DOI] [PubMed] [Google Scholar]
- Littke A.F., Dai C., Fu G.C. Versatile catalysts for the Suzuki cross-coupling of arylboronic acids with aryl and vinyl halides and triflates under mild conditions. J. Am. Chem. Soc. 2000;122:4020–4028. [Google Scholar]
- Littke A.F., Fu G.C. The first general method for Stille cross-couplings of aryl chlorides. Angew. Chem. Int. Ed. 1999;38:2411–2413. doi: 10.1002/(sici)1521-3773(19990816)38:16<2411::aid-anie2411>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Liu M., Vladimirov N., Fréchet J.M.J. A new approach to Hyperbranched polymers by ring-opening polymerization of an AB monomer: 4-(2-Hydroxyethyl)-ϵ-caprolactone. Macromolecules. 1999;32:6881–6884. [Google Scholar]
- Liu Y., Wu D.C., Ma Y.X., Tang G.P., Wang S., He C.B., Chung T.S., Goh S. Novel poly(amino ester)s obtained from Michael addition polymerizations of trifunctional amine monomers with diacrylates: safe and efficient DNA carriers. Chem. Commun. (Camb.) 2003:2630–2631. doi: 10.1039/b309487a. [DOI] [PubMed] [Google Scholar]
- Lv X.H., Li S.S., Tian C.Y., Yang M.M., Li C., Zhou Y., Sun X.L., Zhang J., Wan W.M. Borinic acid polymer: simplified synthesis and enzymatic biofuel cell application. Macromol. Rapid Commun. 2017;38:1600687. doi: 10.1002/marc.201600687. [DOI] [PubMed] [Google Scholar]
- Matyjaszewski K., Xia J.H. Atom transfer radical polymerization. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- Miyaura N., Yanagi T., Suzuki A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synth. Commun. 1981;11:513–519. [Google Scholar]
- Moad G., Rizzardo E., Thang S.H. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005;58:379–410. [Google Scholar]
- Moad G., Rizzardo E., Thang S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer. 2008;49:1079–1131. [Google Scholar]
- Nakatani K., Terashima T., Sawamoto M. Concurrent tandem living radical polymerization: gradient copolymers via in situ monomer transformation with alcohols. J. Am. Chem. Soc. 2009;131:13600–13601. doi: 10.1021/ja9058348. [DOI] [PubMed] [Google Scholar]
- Pintauer T., Matyjaszewski K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008;37:1087–1097. doi: 10.1039/b714578k. [DOI] [PubMed] [Google Scholar]
- Plesniak M.P., Huang H.M., Procter D.J. Radical cascade reactions triggered by single electron transfer. Nat. Rev. Chem. 2017;1 [Google Scholar]
- Qi C.X., Zheng C., Hu R.R., Tang B.Z. Direct construction of acid-responsive poly(indolone)s through multicomponent tandem polymerizations. ACS Macro Lett. 2019;8:569–575. doi: 10.1021/acsmacrolett.9b00297. [DOI] [PubMed] [Google Scholar]
- Richards S.J., Jones M.W., Hunaban M., Haddleton D.M., Gibson M.I. Probing bacterial-toxin inhibition with synthetic glycopolymers prepared by tandem post-polymerization modification: role of linker length and carbohydrate density. Angew. Chem. Int. Ed. 2012;51:7812–7816. doi: 10.1002/anie.201202945. [DOI] [PubMed] [Google Scholar]
- Schluter A.D. The tenth anniversary of Suzuki polycondensation (SPC) J. Polym. Sci. A Polym. Chem. 2001;39:1533–1556. [Google Scholar]
- Sun J.T., Hong C.Y., Pan C.Y. Recent advances in RAFT dispersion polymerization for preparation of block copolymer aggregates. Polym. Chem. 2013;4:873–881. [Google Scholar]
- Sun X.L., Liu D.M., Tian D., Zhang X.Y., Wu W., Wan W.M. The introduction of the Barbier reaction into polymer chemistry. Nat. Commun. 2017;8:1210. doi: 10.1038/s41467-017-01472-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebben L., Studer A. Nitroxides: applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. 2011;50:5034–5068. doi: 10.1002/anie.201002547. [DOI] [PubMed] [Google Scholar]
- Vougioukalakis G.C., Grubbs R.H. Ruthenium-Based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010;110:1746–1787. doi: 10.1021/cr9002424. [DOI] [PubMed] [Google Scholar]
- Wan W.M., Cheng F., Jäkle F. A borinic acid polymer with fluoride ion- and thermo-responsive properties that are tunable over a wide temperature range. Angew. Chem. Int. Ed. 2014;53:8934–8938. doi: 10.1002/anie.201403703. [DOI] [PubMed] [Google Scholar]
- Wan W.M., Li S.S., Liu D.M., Lv X.H., Sun X.L. Synthesis of electron-deficient borinic acid polymers with multiresponsive properties and their application in the fluorescence detection of alizarin red S and electron-rich 8-Hydroxyquinoline and fluoride ion: substituent effects. Macromolecules. 2017;50:6872–6879. [Google Scholar]
- Wang D., Liu Y., Hu Z.C., Hong C.Y., Pan C.Y. Michael addition polymerizations of trifunctional amines with diacrylamides. Polymer. 2005;46:3507–3514. [Google Scholar]
- Wang F., Chen P.H., Liu G.S. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 2018;51:2036–2046. doi: 10.1021/acs.accounts.8b00265. [DOI] [PubMed] [Google Scholar]
- Wang J.S., Matyjaszewski K. Controlled/"living" radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995;117:5614–5615. [Google Scholar]
- Wang S.Q., Fu C.K., Zhang Y., Tao L., Li S.X., Wei Y. One-pot cascade synthetic strategy: a smart combination of chemoenzymatic transesterification and raft polymerization. ACS Macro Lett. 2012;1:1224–1227. doi: 10.1021/mz300444w. [DOI] [PubMed] [Google Scholar]
- Wang X., Xia D., Qin W., Zhou R., Zhou X., Zhou Q., Liu W., Dai X., Wang H., Wang S. A radical cascade enabling collective syntheses of natural products. Chem. 2017;2:803–816. [Google Scholar]
- Wang Z.L., Yu Y., Li Y.S., Yang L., Zhao Y., Liu G.Q., Wei Y., Wang X., Tao L. Post-polymerization modification via the Biginelli reaction to prepare water-soluble polymer adhesives. Polym. Chem. 2017;8:5490–5495. [Google Scholar]
- Wei B., Li W.Z., Zhao Z.J., Qin A.J., Hu R.R., Tang B.Z. Metal-free multicomponent tandem polymerizations of alkynes, amines, and formaldehyde toward structure- and sequence-controlled luminescent polyheterocycles. J. Am. Chem. Soc. 2017;139:5075–5084. doi: 10.1021/jacs.6b12767. [DOI] [PubMed] [Google Scholar]
- Wu H.B., Wang Z.M., Tao L. The Hantzsch reaction in polymer chemistry: synthesis and tentative application. Polym. Chem. 2017;8:7290–7296. [Google Scholar]
- Xiong H.G., Ramkumar N., Chiou M.F., Jian W.J., Li Y.J., Su J.H., Zhang X.H., Bao H.L. Iron-catalyzed carboazidation of alkenes and alkynes. Nat. Commun. 2019;10:122. doi: 10.1038/s41467-018-07985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H.D., Zhao Y., Wu H.B., Wang Z.L., Yang B., Wei Y., Wang Z.M., Tao L. Multicomponent combinatorial polymerization via the biginelli reaction. J. Am. Chem. Soc. 2016;138:8690–8693. doi: 10.1021/jacs.6b04425. [DOI] [PubMed] [Google Scholar]
- Yin X., Guo F., Lalancette R.A., Jäkle F. Luminescent main-chain organoborane polymers: highly robust, electron-deficient poly(oligothiophene borane)s via Stille coupling polymerization. Macromolecules. 2016;49:537–546. [Google Scholar]
- Yokoyama A., Suzuki H., Kubota Y., Ohuchi K., Higashimura H., Yokozawa T. Chain-growth polymerization for the synthesis of polyfluorene via Suzuki-Miyaura coupling reaction from an externally added initiator unit. J. Am. Chem. Soc. 2007;129:7236–7237. doi: 10.1021/ja070313v. [DOI] [PubMed] [Google Scholar]
- Yu X.Y., Chen J.R., Wang P.Z., Yang M.N., Liang D., Xiao W.J. A visible-light-driven iminyl radical-mediated C-C single bond cleavage/radical addition cascade of oxime esters. Angew. Chem.Int. Ed. 2018;57:738–743. doi: 10.1002/anie.201710618. [DOI] [PubMed] [Google Scholar]
- Zhang B., Studer A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015;44:3505–3521. doi: 10.1039/c5cs00083a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.