Abstract
Dispersivity of clay soils is one of the most important issues that should be considered in civil engineering projects. Dispersive soils are clay soils that are easily washed in water with low concentrations of salt; these clay soils usually contain high levels of sodium ions in their adsorption cation sites. Kaolin, sepiolite (fibrous clay), and bentonite soils are among the most important and useful industrial materials. Therefore, in this study, these three clay soils were selected to investigate dispersivity potential by adding 4% of dispersive materials (Sodium hexametaphosphate) and performing shear strength, crumb, double hydrometer, pinhole tests, and chemical experiments. Results indicated a change in the Sodium Adsorption Ratio (SAR) in the following order: kaolin > sepiolite > bentonite. Stabilization practices using chemical methods were done after performing soil divergence with sodium hexametaphosphate. CaCl2, CaSO4, AlCl3, and Al2 (SO4)3 were used for chemical stabilization to assess the effect of ion valence on soil improvement parameters. Results obtained for chemical properties showed that, stabilization potential was in the following order: kaolin > sepiolite > bentonite; meaning that clay soils with lower cation exchange capacity have more remediation potential and are more susceptible to dispersion. The role of calcium and aluminum cations was prominent in improving mechanical and dispersivity properties, respectively. In general, further dispersion potential of clays in the same Na+ concentration was found to be related to a decrease in the cation exchange capacity, specific surface area, and plastic index. Soil dispersion was directly associated with diffuse double layer and electrostatic forces while; soil strength parameters were mainly dependent on cementation and connection of soil particles to each other. Consequently, it was observed that, clay soils with suitable engineering properties (higher strength and compaction or lower Atterberg limits) are more sensitive to dispersion compared to other types of clay with higher CEC and plasticity values.
Keywords: Civil engineering, Geochemistry, Ion, Dispersive soil, Clay mineral, Engineering properties, Chemically stabilization
Civil engineering, Geochemistry, Ion, Dispersive soil, Clay mineral, Engineering properties, Chemically stabilization.
1. Introduction
Dispersive soils, also known as sodic and erodible soils cause a significant challenge for environmental management, agricultural applications, infrastructure construction, and jeopardizing sustainability of many particular sites (Singh et al., 2018). Dispersive soils are the kind of soils having high sodium percentage in their clay content. This clay fraction readily collapses to a suspension form in water (Sherard et al., 1976; Heinzen and Arulanandan, 1977; Holmgren and Flanagan, 1977). Soil degradation resulting from sodicity is a substantial environmental restriction with rigorous negative effects on agricultural sustainability and productivity (Tanji, 1990; Suarez, 2001; Pitman and Lauchli, 2002; Qadir et al., 2006; Rasheed, 2016), Engineering workability, and suitability (Smith and Sullivan, 2014) particularly in arid and semiarid regions of the world.
Laboratory investigations on erosive behavior of consolidated soils utilized a rotating cylinder apparatus indicated that, the amount and type of clay, organic matter, pH, water content, temperature, type and concentration of ions in the pore and eroding fluids, and thixotropy affecting the stress required to initiate erosion (critical shear stress τc) significantly (Alizadeh, 1974; Alizadeh and Arulanandan, 1976).
Dispersive clay soils represent a particular kind of fine-grained soils, which cannot be determined by visual classification or using standard identification – classification tests such as particle size analysis, plasticity, and comparable tests. The crumb and the double hydrometer tests are considered to be straightforward in order to be performed in the field and laboratory, just as preliminary evaluation of dispersive soil. Pinhole test provides evident results in terms of classification of dispersive and non-dispersive soils. Results of the tests have given some uncertainty in classification of the category of medium-dispersive soils, because of lack of defined criteria for the class, particularly with regards to low-value flow rate. In such cases, it is necessary to integrate chemical tests along with existing ones (Ksenija et al., 2018).
The type of ions in the clay soil is one of crucial issues that must always be considered, when they are encountered as natural resources of the earth dams, and so on. Past experiences indicated that, ignoring this factor while implementing the water structures has made many problems and caused damages to the structures by erosion and changes in physical and mechanical soil properties (Asgari and Fakhr, 1994). Marchuk (2013) in a research assessed the behavior of two pure clays (bentonite and illite) and two soil clays in aqueous suspension. As iconicity index increased in the following order Ba2+> Sr2+> Ca2+> Mg2+> K+> Na+> Li+, tendency to covalency increased which in turn, the susceptibility to breakage of the clay-cation bonds in water decreased. Soils with high cation exchange capacity and smectite clay minerals had been dispersed much less than soils dominated through kaolinite and illite clays.
Dispersion of clay minerals is also related to their composition, structure, and especially nature of exposed external surfaces, as these surfaces interact with surrounding fluids. For instance, concerning evaluating nature of outer surfaces of non-swelling clay minerals (kaolinite and illite) evidently, marked differences may be anticipated. Thus, the exposed basal surfaces of asymmetric 1:1 layer structure of kaolinite has indicated that, it contains of both alumina octahedra and silica tetrahedra, whilst chemical composition of clay mineral has demonstrated that, it has a small Cation Exchange Capacity (CEC) with a minor permanent negative charge and no fixed interlayer cations. In contrast, exposed basal surfaces of symmetric 2:1 layer structure of illite consists only of silica tetrahedra, whilst it has a high permanent negative charge compensated by fixed interlayer cations, as well as a distinctly more increased CEC than that of kaolinite based on its chemical composition (Wilson et al., 2014).
Clay minerals are among some of the most important industrial minerals. Millions of tons of clay minerals are utilized annually in a large variety of applications including geology, process industries, agriculture, environmental remediation, and construction (Murray, 1999). Dispersive clay soils are one of problematic soils in some engineering projects especially in arid regions that would impose high costs to control and stabilize the soils (Abbasi et al., 2017; Norouzian et al., 2018). Most of the studies have only focused on characterization and identification (Sherard et al., 1976; Heinzen and Arulanandan, 1977; Umesh et al., 2011; Ksenija et al., 2018), treatment and remediation (Moravej et al., 2015; Vakili et al., 2017; Abbasi et al., 2017), and problems (Qadir et al., 2006; Abbaslou et al., 2016; Singh et al., 2018) of dispersive soils regardless of clay type properties. However, clay soils are extremely variable in terms of their application and characteristics influencing on applications, properties, and treatment of dispersive soils.
Accordingly, the present research was conducted to evaluate dispersivity of different clay soils (kaolin, sepiolite, and bentonite) and compare them in terms of soil stabilization using conventional methods. Besides, the effect of cation and accompanied anion in soil improvement was also assessed.
2. Materials and methods
2.1. Soil samples
To investigate the effect of types of clay minerals on soil dispersion, three different clay soils were selected: 1) Kaolinite soil obtained from Ore mine in north eastern of Iran, 3) fibrous (Sepiolite soil) clay mineral obtained from north eastern of Iran, and 3) Bentonite soils obtained from south eastern of Iran.
2.2. Soil mineralogy
To determine composition of mineral deposits and mineral purity reservoirs, powder samples were prepared and studied by X-ray diffraction.
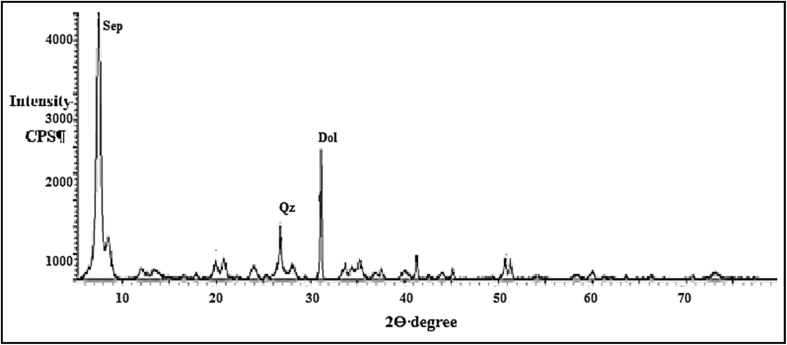
Diffractograms were recorded by using a Philips X-ray diffractometer (Model PW, 1840-Copper target-scanning speed of 0.5 degree per minutes- Voltage 4 kV- Amperage 20 mA) between 4-70° (2 θ). Figures 1, 2, and 3 illustrate main minerals of kaolin, sepiolite, and bentonite soils, respectively.
Figure 1.
X-ray diffraction of kaolin soil containing Kaolinite (Kln), Muscovite (Ms) and Quartz.
Figure 2.
X-ray diffraction of sepiolite (Sep) soil with few quartz (Qz) and dolomite (Dol) impurities.
Figure 3.
X-ray diffraction of bentonite soil containing Montmorillonite (Mnt), Cristobalite (Crs) and Quartz (Qz).
2.3. Soil chemical properties
Calcium Carbonate Equivalent (CCE) (Loppert and Suarez, 1996) was determined for all the samples. Cation Exchange Capacity (CEC) was measured in sodium acetate at a pH of 8.2 (Sumner and Miller, 1996). SAR and ESP of soil samples were measured using laboratory tests as described by the NRCS (1996).
2.4. Soil physical and engineering properties
Engineering properties were determined according to ASTM methods. Atterberg limits (i.e., liquid limit and plastic limit) were determined according to the ASTM D-4318 Standard Test. To determine compaction characteristics, the standard Proctor compaction test was performed according to ASTM D-698 on clay soils. Particle size distribution of the samples was obtained through the hydrometer analysis (ASTM 2006 D-422). Specific gravity of soil solids (Gs) was determined according to ASTM D-854. The effect of dispersivity on shear strength parameters such as friction angle and cohesion of the clay soils (ASTM D3080-04) were conducted by a series of direct shear tests. Samples were tested with three normal stresses of 50 kPa, 100 kPa, and 200 kPa. The experiments were done by using a direct shear box with dimensions of 60 mm × 60 mm × 30 mm. All samples preparation condition included optimum moisture content and maximum dry density. The soil was placed in the mold in three identical layers, and was compacted gently using a small tamper until reaching determined dry unit weight.
Specific Surface Area (SSA) was determined using the BET (theoretical background of adsorption isotherm equation of Brunauer- Emmett- Teller) method described by Fagerlund (1973).
2.5. Dispersive sample preparation and experimental methods
The clay samples were mixed with 4% of Sodium hexametaphosphate (Calgon) with optimum moisture content; then samples were incubated for 30 days at room temperature (22–26 °C), and were blended every day with constant moisture (optimum moisture content). The following tests were conducted to evaluate dispersivity of the soils:
2.6. Soil dispersion test/aggregate stability test/crumb Test-ASTM D6572
Generally, this combination of the tests is very useful to indicate if the soil is sodic and dispersive, done by immersing fragments in fresh water. Cloudy or muddy water is an indicator of dispersive soil.
2.6.1. Pin hole Test-ASTM D4647
In the Pinhole test, distilled water is allowed to flow through a 1.0 mm diameter hole drilled through a compacted specimen. In dispersive clays, the hole is rapidly eroded and the water becomes muddy. For non-dispersive clays, there is no erosion and the water is clear.
2.6.2. Double hydrometer test ASTM D422199
First, particle size distribution was determined using the standard hydrometer test in which the soil specimen is dispersed in distilled water with a chemical dispersant. Then, a parallel hydrometer test is done on a duplicate soil specimen, but without using a chemical dispersant.
2.6.3. Chemical tests
Soil sodicity represents combined effects of (1) salinity as measured by electrical conductivity of the soil, and (2) soluble Na+ concentration relative to soluble divalent cation concentration in soil solution, that is, Sodium Adsorption Ratio (SAR), or Exchangeable Sodium Percentage (ESP). SAR was calculated using Eq. (1):
| (1) |
Where, C represents concentrations in soil solution in terms of mmolc liter−1 (mmolc liter−1 = meq liter−1) of the cations identified as subscripts.
ESP is calculated as proportion of the cation exchange capacity occupied by the sodium ions. ESP was calculated from Eq. (2) by incorporating values of exchangeable Na+ and cation exchange capacity (CEC), both expressed as mmolc kg−1 or cmolc kg−1 (cmolc kg−1 = meq 100 g−1) of the soil.
| (2) |
ESP may also be calculated by replacing CEC in Eq. (2) with the sum of exchangeable cations such as calcium (ECa), magnesium (EMg), potassium (EK), exchangeable sodium (ENa), and aluminum (EAl), with all the cations expressed as mmolc kg−1 or cmolc kg−1 of the soil (Sumner et al., 1998).
An ESP of 15 (~SAR 13) is generally taken to be the threshold, below which soils are classified as nonsodic, and above which soils are dispersive suffering from serious physical problems at the time of applying the water (Marchuk, 2013).
2.7. Soil stabilization and experimental methods
After dispersion process and ensuring of dispersion, soils were chemically stabilized with different salts. To test the effect of anion and cation type on stability of various clay soil samples, CaCl2, AlCl3, Al2 (SO4)3, and CaSO4 were chosen and evaluated with 3 % of cation concentration at optimum moisture content for a period of one month. The clay samples were separately mixed with different solutions, and then soil-electrolyte homogenized mixtures were used in different experiments. For each test, triplicate samples were prepared to confirm reproducibility of results. Average values of results were employed in further computation and plotting of graphs. To analyze flocculation of soils and soil stability, the following experiments were conducted to evaluate the effects of various types and concentrations of solutions on dispersion of different clay soils as described in previous sections:
-
-
Shear Strength
-
-
Pinhole Test
-
-
Chemical Test
2.8. Statistical analysis
To determine significant differences between treatments, mean values were compared based on Duncans̓ Multiple Range Test (DMRT) (p-value<0.05). All statistical analyses were performed using SPSS software (Ver. 17.0) and graphs were drawn in Microsoft Excel 2010.
3. Results and discussion
3.1. Properties of clay soils
Main chemical, physical, and engineering properties of studied clay minerals are presented in Tables 1 and 2.
Table 1.
Chemical Characteristics of clay soils.
| Properties | Kaolin | Sepiolite | Bentonite |
|---|---|---|---|
| CEC (cmol + kg−1) | 3 | 13 | 35 |
| CCE % | 15 | 20 | 25 |
| SAR | 1.5 | 4.3 | 15 |
| ESP % | 2.1 | 4 | 13.9 |
Table 2.
Engineering and geoenvironmental properties of clay soils.
| Characteristics | Quantity measured |
||
|---|---|---|---|
| Kaolin | Sepiolite | Bentonite | |
| Soil classification | CH | CH | CH |
| Liquid Limit % | 60.37 | 164.57 | 131.95 |
| Plastic Limit % | 25.93 | 92.5 | 42.86 |
| Plastic Index | 34.44 | 72.07 | 89.09 |
| Gs | 2.63 | 2.35 | 2.6 |
| SSA (m2 g−1) | 40 | 180 | 220 |
| γdmax KN m−3 | 17.3 | 16.3 | 17.5 |
| ωopt % | 22 | 37.3 | 39 |
Kaolin clay soil has the lowest liquid limit, cation exchangeable capacity, and specific surface area. Sepiolite soil properties are in intermediate level relative to two other clay soils (kaolin and bentonite soils).
3.2. Properties of dispersive soils
Results of chemical experiments (Table 3) showed that, soils were dispersed by adding 4% of Sodium hexametaphosphate.
Table 3.
Dispersion chemical experiments of clay soils.
| Soil Type | Exchangeable Na∗ | Exchangeable Na≠ Meq/100g | Soluble Na∗ ppm | Soluble Na≠ ppm | SAR∗ | SAR≠ | ESP∗ | ESP≠ |
|---|---|---|---|---|---|---|---|---|
| Meq/100g | ||||||||
| Kaolin | 3 | 100 | 1.1 | 75 | 1 | 21 | 1 | 33 |
| Sepiolite | 41.8 | 299.7 | 41 | 115 | 2.3 | 33.9 | 3.25 | 23.1 |
| Bentonite | 503.8 | 616.5 | 448 | 660 | 12 | 40.8 | 14.4 | 17.6 |
Before treatment by dispersing agent, ≠ after treatment by dispersing agent.
Concentration of Na ion in soil pore water layer is one of influential factors in soil dispersivity, because of sodium ion, which is less electronegative due to its mono-valence. Potassium cations are also monovalent, but they enter in interlayer spaces of clay minerals, and are trapped due to their smaller sizes than Na ions. Presence of Na ions in soils results in an increase in the osmosis potential and a decrease in the attractive Van der Waals forces between soil particles. Usually, soils whose concentration of soluble salts in pore water is less than 1 meq/lit (10 meq/100g) are not considered dispersive (Decker and Dunnigan, 1977).
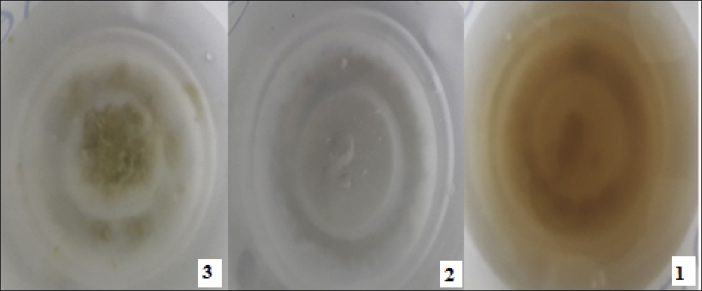
Results obtained from Crump test indicated that, sepiolite reacted more compared to other clay soils in distilled water, while bentonite soil showed the least reaction (Figure 4).
Figure 4.
The clay soils after the crumb test (1: Sepiolite, 2: Kaolin, 3: Bentonite).
Results obtained from double hydrometer test are presented in Table 4. As shown in Table 4, dispersivity is in the following order: sepiolite > bentonite > kaolin; however, differences were not significant between different clay soils.
Table 4.
The result of double hydrometer of dispersive clay soils.
| Soil Type | percent of finer 0.005 mm with dispersing agent (A) | The percent of finer 0.005 mm without dispersing agent (B) | Dispertion ratio (B/A) % | Classification |
|---|---|---|---|---|
| Kaolin | 0.0048 | 0.0023 | 47.92 | Dispersive Soil |
| Sepiolite | 0.0048 | 0.0025 | 52.08 | Dispersive Soil |
| Bentonite | 0.0044 | 0.0023 | 50 | Dispersive Soil |
Results obtained from Pinhole test according to standard criterion are shown in Table 5. Besides chemical tests, the Pinhole test is one of the most reliable methods used for assessment of soil dispersion. According to results of Pinhole test, soils were dispersed in the following order: sepiolite ~ kaolin > bentonite.
Table 5.
The Pin-Hole results of clay soils after dispersion.
| Soil Type | Water head (mm) | Time (min) | Final flow rate (ml/s) | Hole diameter (mm) | Classification |
|---|---|---|---|---|---|
| Kaolin | 50 | 5 | 2.48 | 2.8 | D-1 |
| Sepiolite | 50 | 5 | 2.07 | 3 | D-1 |
| Bentonite | 50 | 10 | 2.18 | >1.5 | D-2 |
Results of different tests conducted on all soil samples have indicated different outcomes in relation to dispersivity criteria. Thus, dispersivity of soils needs to be investigated by different methods. In general, potential of dispersivity of soils could be as follows: kaolin > sepiolite > bentonite. It can be concluded that, bentonite soils with high CEC may adsorb high Na concentrations and endure earlier dispersion.
Results of a study on the influence of mineralogy and pore fluid composition on slaking rates of three silty clays containing 20 % kaolin (Hydrite R), montmorillonite, and illite at constant concentration of pore fluid (0.01 N), but at low and high SAR values revealed that, low plasticity and highly flocculated soils have higher slaking rates (Kandiah and Arulanandan, 1974). The soils with low concentrations of Mg2+ and Ca2+ ions relative to Na+ ions, which are generally dispersed and possessing low permeability values showed a lower degree of slaking rate. Considering these observations, it can be concluded that, slaking rates of soils is governed by soil structure, type and amount of the clay, water content, pore fluid composition, and density.
Singh et al. (2018) studied characterization problems and remedies of dispersive soils. They concluded that, chemical analysis of pore water extract and the Double Hydrometer tests are more conservative in indicating the dispersion of soil. The Pinhole test is more reliable, as it simulates field conditions. The Crumb test provides a good indication of the soil regarding potential tendency to erosion.
3.3. Chemical stabilization of dispersive clay soils
Chemical stabilization was done with CaCl2, CaSO4, AlCl3, and Al2 (SO4)3 aimed at evaluating and comparing the effect of different anions (Cl−1, SO42-) and cations (Ca2+, Al3+) on soil stabilization.
Trend related to the effect of chemical stabilizers on soil flocculation using the Pinhole test showed the following order: AlCl3>CaCl2>CaSO4~Al2 (SO4)3. Variations of exchangeable and soluble Sodium after treatment with chemical stabilizers for different dispersive soils are presented in Figures 5 and 6, respectively.
Figure 5.
Variation of Exchangeable Na with different chemical stabilizers after dispersion.
Figure 6.
Variation of soluble Na with different chemical stabilizers after dispersion.
Through chemical stabilization of the soils, sodium was exchanged by Ca2+ and Al3+ cations in the following order: AlCl3>CaCl2>Al2(SO4)3 > Ca(SO4)2, meaning that Al3+ and Ca2+ have exchanged high amounts of Na; in other words, values of exchangeable Na have decreased. Based on amount of sodium exchanged on clay sites, soluble Na increased in the following order: AlCl3>CaCl2>Al2(SO4)3 > Ca(SO4)2. As shown in Figures 5 and 6, type of anion and cation has substantial effect on Na exchange and soil flocculation.
With an increase in the concentration of divalent ions of calcium and trivalent ions of aluminum round clay minerals, these ions are replaced by sodium ion, which is less electronegative due to its mono-valence: thus, thickness of double layer decreases leading to an increase in the attractive forces between minerals.
Goodarzi and Ouhadi (2012) studied the effect of anions on dispersivity ability and engineering characteristics of montmorillonite clay soils. They concluded that, in the same concentration, SO4−2 anion rather than Cl−1 anion caused more diffuse double layer, so dispersivity potential of montmorillonite clay soils containing SO4−2 anions was greater than montmorillonite clay soils containing Cl−1 anions. Anions of Cl−1 and SO42- had same pH variations, thus their interaction with clay surfaces and also difference in cation-anion adsorption power were quite evident in comparison with CO3 (Chorom et al., 1994; Duman and Tunc, 2009).
Results obtained from shear strength test for different clay soils (kaolin, sepiolite, and bentonite) under different conditions (non-dispersive, dispersive, and chemically stabilized) are presented in Figure 7. Cohesion (Figure 7A, C, E) and friction angle (Figure 7B, D, F) of clay soils increased after stabilization as soil strength parameters, possibly due to soil flocculation and also reduction in water adsorption. As depicted in Figure 7, the difference in cohesion and friction angle is not very significant between dispersive and non-dispersive soils; but their variation after stabilization was significant at the level of 5%. Among chemical stabilizers, calcium chloride increased strength parameters more than other chemical materials. As a conclusion, trend related to the effect of chemical stabilizers on strength parameters was in the following order: CaCl2>CaSO4~AlCl3>Al2 (SO4)3.
Figure 7.
Variation of shear strength parameters of different clay soils (A) Cohesion of Sepiolite, (B) Inner friction angle of Sepiolite, (C) Cohesion of Bentonite, (D) Inner friction angle of Bentonite, (E) Cohesion of Kaolin, (F) Inner fraction angle of Kaolin Dispersive, Non-dispersive, and chemically stabilized (different letters (a, b, and c) indicate significant difference at p < 0.05 (Duncan's Test)).
Strength of dispersive soil increased with an increase in the lime (0.5–1.0% hydrated lime), alum (0.6–1.0% (25% solution of Aluminium sulfate) to 1.5% of total dry weight of soil) and gypsum (a minimum of 2% by mass of gypsum) content up to specific Limit (Singh et al., 2018).
Spatial arrangement of the clay minerals is amongst the most obvious factors influencing dispersion and migration of clays particularly regarding fabric, their micro-aggregate structure, morphology, surface area, porosity, and particle size distribution. These factors are all highly variable requiring to be reviewed on an individual clay mineral basis later. However, it should be noticed that, generally the clay minerals of most interest with respect to fines migration have formed during diagenesis (Wilson et al., 2014). The ZELIAC (consisting of zeolite, activated carbon, limestone, rice husk ash, Portland cement) was considered as a new stabilizer for treatment of dispersive clay soils according to Vakili et al. (2017). Maximum reduction in dispersivity, compressibility, and plasticity occurred where maximum adsorption capacity of the Ca2+ content by clay particle took place.
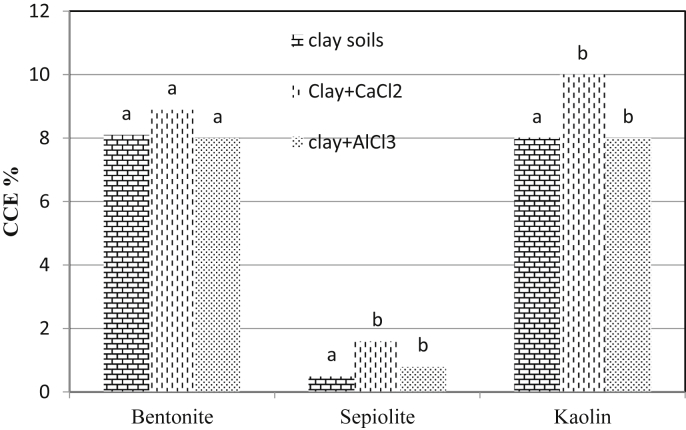
To demonstrate changes in carbonate amount, CCE was measured for different clay soils and also after treatment by CaCl2 and AlCl3 (Figure 8). Results showed that, CaCl2 treatment increased carbonate amounts due to containing Ca2+ ions, so it improved soil strength parameters more than AlCl3. Since, Al3+ is trivalent and also Ca2+ is precipitated by carbonates, therefore Al3+ works as a better treatment for soil dispersivity reduction.
Figure 8.
Amount of CCE percentage for dispersive and chemically stabilized soils by CaCl2 and AlCl3 (different letters (a, b, and c) indicate significant difference at p < 0.05 (Duncan's test)).
4. Conclusion
Dispersion (breakdown of soil into single particles) is controlled by soil texture, type of the clay, soil organic matter, soil salinity, and exchangeable cations. The clay soils (kaolin, sepiolite, and bentonite) demonstrated different engineering characteristics; and they had the following order: bentonite > sepiolite > kaolin clay soils in terms of Atterberg limits, optimum moisture content, and cation exchange capacity. Based on exchangeable amount of Na after dispersion, values of sepiolite and kaolin soils were half and one-third of bentonite soils, respectively. The lower CEC, the more stabilization potential of clay soil will be. This usually means that soils with low CEC are more likely to develop required amount of calcium or aluminum cations (or other divalent and trivalent cations) to overwhelm dispersivity, while soils with high CEC are less susceptible to dispersivity.
Variation of strength parameters and dispersivity was in the following order: kaolin > sepiolite > bentonite. Results revealed that, dispersivity potential of low electrolyte concentration increased because of repulsive forces dominating attractive forces. With a further increase in the specific surface area and cation exchange capacity, dispersivity potential decreased. Since, clays with low plastic index and consequently low CEC will be applied in hydraulic structures. Therefore, potential dispersivity is very important in this regard.
According to treatments of chemical stabilizers (CaCl2, CaSO4, AlCl3, and Al2 (SO4)3), type of cation and anion was found to be different in terms of reduction of soil dispersion and increase in soil flocculation. Further decrease was observed in dispersivity potential by trivalent cations compared to divalent and monovalent cations. However, monovalent anions (Cl−1) further reduced dispersivity potential rather than divalent anions (SO42-). On the other hand, values of strength parameter increased by Ca2+ cations although no distinct trend was observed for anions. It can be concluded that, Ca2+ increased probability of CaCO3 formation and soil cementation, so its role is more important in improvement of soil strength, while sodium cations might be replaced by aluminum ions having higher valences than calcium, causing a deccline in the thickness of double layer. The reduction of repulsive forces will occur due to the reduction in the thickness of the double layer, which in turn, results in decrease of dispersivity potential of soils. Anions with higher valences (SO42-) rather than lower valences (Cl1-) would elevate thickness of diffuse double layer leading to more soil dispersion. Percentage of Na exchanged by cations was around 50–70, 30, and less than 10% for bentonite, sepiolite, and kaolin clay soils, respectively.
The role of di and trivalent cations as an added substance for controlling of soil dispersivity can mainly be ascribed to pH and ion exchange effects, overcoming soil dispersivity, improving cohesion, and friction angle of soil, and also causing an increase in the soil strength due to the change from an arranged structure to a more flocculated structure.
In the current study, the effect of type of the clay, type of cation and anion on soil dispersivity was evaluated using a combination of tests. It is highly recommended to investigate mechanical properties of the soil and amount of ions for soil remediation with respect to pH ranges, Na content, and CEC values of clay soils in future studies.
Declarations
Author contribution statement
Hanie Abbaslou, Ali Reza Ghanizadeh & Hojat Hadifard: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbasi N., Farjad A., Sepehri S. The use of nanoclay particles for stabilization of dispersive clayey soils. Geotech. Geol. Eng. 2017;36(1):327–335. [Google Scholar]
- Abbaslou H., Hadifard H., Poorgohardi A. Characterization of Dispersive problematic soils and engineering improvements: a review. Comput. Mater. Civ. Eng. 2016;1:65–68. [Google Scholar]
- Alizadeh A. University of California; Davis, California: 1974. Amount and Type of clay and Pore Fluid Influences on the Critical Shear Stress and Swelling of Cohesive Soils. PhD Thesis. [Google Scholar]
- Alizadeh A., Arulanandan K. 1976. Physico-chemical Aspects in Cohesive Erosion. Proceedings,” American Society of Civil Engineering, NO. GT. [Google Scholar]
- Asgari F.A., Fakhr A. Jihad Daneshgahi Publications, Tehran University; 1994. Soil Swelling and Dispersion from a Geotechnical Engineering point of View; p. 245. [Google Scholar]
- Chorom M., Rengasamy P., Murray R.S. Lay dispersion as influenced by pH and net particle charge of sodic soils. Soil Res. 1994;32:1243–1252. [Google Scholar]
- Decker R.S., Dunnigan L.P. Development and use of the soil conservation service dispersion test. ASTM STP. 1977;623:94–109. [Google Scholar]
- Duman O., Tunç S. Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous Mesoporous Mater. 2009;117:331–338. [Google Scholar]
- Fagerlund G. Determination of specific surface by the BET method. Mater. Struct. 1973;6(3):239–245. [Google Scholar]
- Goodarzi A.R., Ouhadi V.R. Semnan University; Semnan, Iran: 2012. “Effect of Anions on the Divergence and Engineering Properties of montmorillonite.” 6th National congress of Civil Engineering. (in persion) [Google Scholar]
- Heinzen R.T., Arulanandan K. Vol. 623. ASTM Special Technical Publication; 1977. pp. 202–217. (Factors Influencing Dispersive Clays and Methods of Identification). [Google Scholar]
- Holmgren G.G.S., Flanagan C.P. Vol. 623. ASTM Special Technical Publication; 1977. pp. 219–239. (Factors Affecting Spontaneous Dispersion of Soil Materials as Evidenced by the Crumb Test). [Google Scholar]
- Kandiah A., Arulanandan K. Transportation Research Board; 1974. “Hydraulic Erosion of Cohesive soils.” Transportation Research Record 497; pp. 60–68. [Google Scholar]
- Ksenija D., Laslo C., Nenad S., Gordana H.M. XVI Danube - European Conference on Geotechnical Engineering, Skopje, R. Macedonia. 2018. Methods for assessment and identification of dispersive soils; pp. 205–210. [Google Scholar]
- Loppert R.H., Suarez D.L. “Carbonate and gypsum.”. In: Sparks D.L., editor. Method of Soil Analysis. Part III. third ed. Am. Soc. Agron.; Madison, WI: 1996. pp. 437–474. [Google Scholar]
- Marchuk A. The University of Adelaide; 2013. “Effect of Cations on Structural Stability of Salt-Affected soils.” PhD Thesis of Soil Science. School of Agriculture, Food and Wine. [Google Scholar]
- Moravej S., Nikooee E., Habibagahi G., Niazi A. Bio-stabilisation of dispersive soils. Geotech. Eng. Infrastruct. Dev. 2015;1:2835–2840. [Google Scholar]
- Murray H.H. Applied clay mineralogy today and tomorrow. Clay Miner. 1999;34:39–49. [Google Scholar]
- Norouzian K., Abbasi N., Abedi Koupai J. Evaluation of softening of clayey soil stabilized with sewage sludge ash and lime. J. Civ. Eng. 2018;4(4):743–754. [Google Scholar]
- NRCS U. US Department of Agriculture, Natural Resources Conservation Service; Washington, DC: 1996. Soil Survey Laboratory Methods manual.” Soil Survey Investigations Report 2, Version 3.0. [Google Scholar]
- Pitman M.G., Läuchli A. Springer; Netherlands: 2002. “Global Impact of Salinity and Agricultural ecosystems.” Pp. 3-20 in Salinity: Environment-Plants-Molecules, [Google Scholar]
- Qadir M., Noble A.D., Schubert S., Thomas R.J., Arslan A. Sodicityinduced land degradation and its sustainable management: problems and prospects. Land Degrad. Dev. 2006;17:661–676. [Google Scholar]
- Rasheed S.M.K. “The effect of clay content and land use on dispersion Ratio at different locations in sulaimani governorate—kurdistan region—Iraq. Open J. Soil Sci. 2016;6:1–8. [Google Scholar]
- Sherard J.L., Dunnigan L.P., Decker R.S. Identification and nature of dispersive soils. ASCE Geotech. Div. 1976;102:69–87. [Google Scholar]
- Singh B., Gahlot P., Purohit D.G.M. Dispersive soils-characterization, problems and remedies. Int. Res. J. Eng. Technol. 2018;5(6):2478–2484. [Google Scholar]
- Smith J.V., Sullivan L.A. Construction and maintenance of embankments using highly erodible soils in the Pilbara, North-Western Australia. Int. J. Geomate. 2014;6(2):897–902. [Google Scholar]
- Suarez D.L. Sodic soil reclamation: modelling and field study. Aust. J. Soil Res. 2001;39:1225–1246. [Google Scholar]
- Sumner M.E., Miller W.P. Cation exchange capacity and exchange coefficients. In: Sparks D.L., editor. Methods of Soil Analysis, Part III. third ed. Am. Soc. Agron.; Madison, WI: 1996. pp. 1201–1229. [Google Scholar]
- Sumner M.E., Rengasamy P., Naidu R. Sodic soils: a reappraisal. In: Sumner M.E., Naidu R., editors. Sodic Soil: Distribution, Management and Environmental Consequences. Oxford University Press; New York: 1998. pp. 3–17. [Google Scholar]
- Tanji K.K. In: Nature and Extent of Agricultural salinity.” Pp. 1–17 in Agricultural Salinity Assessment and Management, Manuals and Reports on Engineering Practices No. 71. Tanji K.K., editor. American Society of Civil Engineers; New York: 1990. [Google Scholar]
- Umesh T.S., Dinesh S.V., Sivapullaiah P.V. Characterization of dispersive soils. Mater. Sci. Appl. 2011;2:629–633. [Google Scholar]
- Vakili A.H., Selamat M.R., Aziz H.B.A., Mojiri A., Ahmad Z., Safarzadeh M. Treatment dispersive clay soil by ZELIAC. Geoderma. 2017;285:270–279. [Google Scholar]
- Wilson M.J., Wilson L., Patey I. The influence of individual clay minerals on formation damage of reservoir sandstones: a critical review with some new insights. Clay Miner. 2014;49:147–164. [Google Scholar]