Summary
The link between integrin activity regulation and cellular mechanosensing of tissue rigidity, especially on different extracellular matrix ligands, remains poorly understood. Here, we find that primary mouse mammary gland stromal fibroblasts (MSFs) are able to spread efficiently, generate high forces, and display nuclear YAP on soft collagen-coated substrates, resembling the soft mammary gland tissue. We describe that loss of the integrin inhibitor, SHARPIN, impedes MSF spreading specifically on soft type I collagen but not on fibronectin. Through quantitative experiments and computational modeling, we find that SHARPIN-deficient MSFs display faster force-induced unbinding of adhesions from collagen-coated beads. Faster unbinding, in turn, impairs force transmission in these cells, particularly, at the stiffness optimum observed for wild-type cells. Mechanistically, we link the impaired mechanotransduction of SHARPIN-deficient cells on collagen to reduced levels of collagen-binding integrin α11β1. Thus integrin activity regulation and α11β1 play a role in collagen-specific mechanosensing in MSFs.
Subject Areas: Biological Sciences, Cell Biology, Functional Aspects of Cell Biology
Graphical Abstract

Highlights
-
•
Mammary gland stromal fibroblasts are mechanically adapted to a soft environment
-
•
Loss of SHARPIN reduces cell spreading and force generation on soft collagen
-
•
Low integrin α11β1 level in SHARPIN-null cells causes faster unbinding from collagen
-
•
Molecular clutch model predicts the lower forces based on faster integrin unbinding
Biological Sciences; Cell Biology; Functional Aspects of Cell Biology
Introduction
Fibroblasts exert high forces that are implicated in the morphogenetic rearrangement of extracellular matrices (ECMs) (Harris et al., 1981). In the developing mammary gland, stromal cell-mediated organization of the ECM regulates mammary ductal morphogenesis (Brownfield et al., 2013, Ingman et al., 2006). Despite this important function, investigations into mammary stromal components are secondary to that of the mammary epithelium. In addition to ECM organization in normal tissue, mammary gland stromal fibroblasts (MSFs) play a central role in the pro-invasive stiffening of breast tumor stroma (Navab et al., 2016), and therefore, understanding the mechanical aspects of these cells is of clinical interest. Although the role of integrins as cell mechanosensors and transducers is well established, the link between the regulation of integrin activity and the mechanosensing response on different ECM ligands remains poorly understood. SHARPIN is a cytosolic adaptor protein that, among other functions, binds to the intracellular integrin alpha tails and inhibits integrin activity in different cell types in vitro and in vivo (Kasirer-Friede et al., 2019, Peuhu et al., 2017a, Peuhu et al., 2017b, Pouwels et al., 2013, Rantala et al., 2011). We have previously demonstrated that stromal SHARPIN deficiency interferes with normal mouse mammary gland development and collagen fiber assembly in vivo (Peuhu et al., 2017a). However, how SHARPIN mediates integrin-dependent mechanotransduction remains unresolved.
Collagen is abundant in the mammary gland stroma and plays a key role in regulating the physical and biochemical properties of the mammary gland. Alignment of stromal collagen bundles is critical for normal mammary gland development providing migration cues to the outgrowing duct during puberty (Brownfield et al., 2013, Ingman et al., 2006). There are four collagen-binding integrin heterodimers in mammals: the more ubiquitously expressed α1β1, α2β1, and α11β1 and the cartilage-specific α10β1 (Zeltz and Gullberg, 2016). Of these, the fibrillar collagen-binding integrins α2β1 and α11β1 have been strongly linked to collagen remodeling and turnover (Abair et al., 2008, Ivaska et al., 1999, Popova et al., 2007, Riikonen et al., 1995, Tiger et al., 2001) and α11β1 to the induction of cancer stromal stiffness (Navab et al., 2016, Zeltz et al., 2019). Furthermore, “trail blazer” breast cancer cells with high invasive capacity are characterized by high integrin α11β1 expression (Westcott et al., 2015). Nevertheless, integrin α11β1 functions are rather poorly understood, and the role of this receptor in regulating cell-collagen interactions in the mammary gland has not been previously studied.
In order to sense the properties of the surrounding ECM, cells use dynamic molecular bonds, often referred to as molecular clutches, to exert forces within the cell boundary (Elosegui-Artola et al., 2018). A molecular clutch can be defined as a dynamic link between the ECM, integrin adhesion receptors, intracellular adaptor proteins, and the actomyosin cytoskeleton (Elosegui-Artola et al., 2014, Elosegui-Artola et al., 2016). By quantification of the molecular clutch binding dynamics, and using mathematical modeling, one can predict the average force transmission of cells to the ECM as a function of substrate stiffness (Elosegui-Artola et al., 2014, Elosegui-Artola et al., 2016).
Here, we have combined mathematical modeling with cell biology to investigate the biomechanical properties of primary mouse MSFs and to understand how the integrin inhibitor SHARPIN affects integrin-dependent force generation and mechanotransduction. We find that, somewhat counterintuitively, in spite of having higher integrin β1 activity SHARPIN-deficient MSFs were defective in spreading on soft hydrogels with a stiffness similar to the mammary gland tissue in vivo. They also had faster force-induced cell-ECM unbinding rates. Interestingly, both of these defects were specific to collagen and not observed on fibronectin. The molecular clutch model predicted that increased clutch unbinding rates result in the loss of stiffness-dependent traction maximum and increased actin flow rates at low rigidities. Importantly, these predictions were recapitulated experimentally in SHARPIN-deficient primary MSFs on collagen I. SHARPIN-deficient MSFs had significantly downregulated collagen-binding integrin α11β1 levels, explaining mechanistically their unexpected inability to couple to collagen. These data highlight an important divergence in the regulation of collagen I- and fibronectin-binding integrin heterodimers in the mammary gland stroma with implications for the mechanical response of fibroblasts. Moreover, these insights are likely to improve our understanding of fibrotic diseases including cancer where fibroblasts exhibit deregulated integrin activity (Erdogan et al., 2017, Glentis et al., 2017).
Results
Increased Integrin Activity Correlates with Reduced Spreading of Mouse Mammary Gland Fibroblasts on Soft Collagen I, but Not on Soft Fibronectin, -Coated Matrices
Based on previous observations that primary MSFs from SHARPIN-deficient mice (chronic proliferative dermatitis null mutation, cpdm; Sharpincpdm/cpdm; Sharpincpdm) (HogenEsch et al., 1993, Seymour et al., 2007) have increased integrin β1 activity but, counterintuitively, impaired capacity to contract collagen gels (Peuhu et al., 2017a), we sought to investigate the link between integrin activity and force transduction. We first confirmed by flow cytometry the cell-surface levels of total and active integrin β1 with conformation-specific antibodies. As expected, Sharpincpdm MSFs expressed lower total integrin β1 cell-surface levels but equal levels of active integrin β1 compared with SHARPIN-expressing (Sharpin+/+or Sharpincpdm/+; from here on referred to as wild-type) cells, indicating that in Sharpincpdm MSFs a higher proportion of integrin β1 is in the active conformation on the cell surface (Figures 1A and S1A), in line with our previous studies with MSFs (Peuhu et al., 2017a) and other cell types (Peuhu et al., 2017b, Rantala et al., 2011). Next, we studied the ability of wild-type and Sharpincpdm MSFs to spread in response to ECM stiffness and ligand type. MSFs were seeded at equal density on soft (2 kPa) fibronectin or collagen I (a saturating concentration of 20 μg/mL of each ligand was used, Figure S1B) pre-coated polyacrylamide gels, approximating the stiffness of the mammary tissue in vivo (Lopez et al., 2011, Peuhu et al., 2017a, Plodinec et al., 2012). As expected based on the higher integrin β1 activity and faster focal adhesion (FA) turnover compared with wild-type MSFs (Peuhu et al., 2017a, Rantala et al., 2011), Sharpincpdm MSFs spread more compared with wild-type MSFs when seeded on fibronectin-coated hydrogels (Figures 1B and1C). In contrast, on 2 kPa collagen-I-coated hydrogels Sharpincpdm MSFs were less spread than wild-type MSFs (Figures 1B and1C). When cell spreading area was measured on a stiffness range from 0.8 to 13 kPa (Figure 1C), on collagen-I-coated hydrogels, wild-type MSFs displayed a spreading optimum on 2 kPa, whereas Sharpincpdm MSFs were significantly smaller and only fully spread at 13 kPa (Figure 1C). These data present an unexpected conundrum; at lower stiffness [corresponding to the higher end of the rigidity spectrum reported for mammary gland tissue in vivo, 0.1–2 kPa (Peuhu et al., 2017a, Plodinec et al., 2012)], loss of SHARPIN (coinciding with increased integrin β1 activity) correlates with defective MSF spreading on collagen I, whereas on fibronectin the opposite is observed.
Figure 1.
Increased Integrin Activity Correlates with Reduced Spreading of MSFs on Soft Collagen I
(A) Quantification of relative integrin β1 activity [active (clone, 9EG7)/total (clone, HMβ1-1)] cell-surface levels (n = 7 independent experiments) in Sharpincpdm compared with wild-type MSFs by flow cytometry.
(B) Representative images of wild-type and Sharpincpdm MSFs plated for 3–4 h on 2 kPa fibronectin (upper panel) or collagen I (lower panel)-coated polyacrylamide (PAA) hydrogels and labeled for F-actin (white) and nuclei (blue). Cell edges are outlined with yellow dashed lines.
(C) Quantification of cell spreading in wild-type compared with Sharpincpdm MSFs on 0.8, 2, 4, and 13 kPa PAA hydrogels coated with fibronectin (upper panel) or collagen I (lower panel) based on immunofluorescence. Data are pooled from three independent experiments, nwt= 90, 95, 103, 88 and nSharpincpdm= 72, 80, 93, 88 cells (fibronectin, from left to right) and nwt= 64, 105, 91, 109 and nSharpincpdm= 59, 118, 97, 77 cells (collagen I, from left to right).
(D) Representative output images of FA analysis for individual cells plated on 2 kPa collagen-I-coated PAA hydrogels (FA, red; cell borders, black).
(E and F) Quantification of the number of FA per cell (E) and the length of FA (F) in wild-type compared with Sharpincpdm MSFs plated on collagen-I-coated 0.8, 2, 4, and 13 kPa PAA hydrogels. Data are pooled from three independent experiments, nwt= 64, 94, 73, 83, nSharpincpdm= 59, 97, 73, 56 cells (# Adhesion per cell, from left to right), and nwt= 41, 94, 73, 109 and nSharpincpdm= 42, 98, 74, 73 cells (Adhesion length, from left to right).
Mean ± SEM in all graphs. Mann–Whitney U-test; red lines below p values indicate the data points compared. Scale bars: 20 μm. See also Figure S1.
Given that Sharpincpdm MSFs have faster FA dynamics (increased assembly and disassembly rates) on collagen-coated rigid glass substrate (Peuhu et al., 2017a), we next evaluated the effect of SHARPIN deficiency on FA maturation (number and average length) on a range of hydrogel rigidities. Vinculin, a mechanosensitive adaptor molecule recruited to mature FA (Chen et al., 2006, del Rio et al., 2009), was immunolabeled in wild-type and Sharpincpdm MSFs plated on 0.8–13 kPa collagen-I-coated hydrogels (Figures 1D and S1C). Wild-type and Sharpincpdm MSFs (Figure 1D) demonstrated similar FA number and length on collagen-I-coated hydrogels (Figures 1E and 1F). Interestingly, both wild-type and Sharpincpdm primary MSFs formed mature, vinculin-containing adhesions on soft matrices reaching the maturation maxima already on 2–4 kPa (Figure 1F). On fibronectin, Sharpincpdm MSFs demonstrated either longer or more FA on 2–4 kPa hydrogels (Figures S1D–S1F), consistent with increased cell spreading (Figures 1B and1C). Furthermore, MSFs exhibited nuclear localization of the mechanosensitive transcription factor Yes-associated protein (YAP) at 2 kPa stiffness (Figure S1G). This localization was reduced in Sharpincpdm MSFs (Figure S1G, H). The nuclear localization of YAP and the ability of MSFs to generate elongated, stress-fiber-linked adhesions on a low stiffness are counter to the stiffness-induced adhesion reinforcement/maturation and nuclear YAP translocation detected in other cell types (Elosegui-Artola et al., 2016), possibly mirroring the adaption of these primary cells to their soft growth environment in the mammary gland (Lopez et al., 2011, Peuhu et al., 2017a, Plodinec et al., 2012). In conclusion, Sharpincpdm MSFs demonstrate reduced capacity to spread on soft collagen I-coated matrices, whereas the opposite occurs on fibronectin-coated substratum.
Increased Integrin Activity Correlates with Faster Integrin-Collagen Binding Dynamics in MSFs
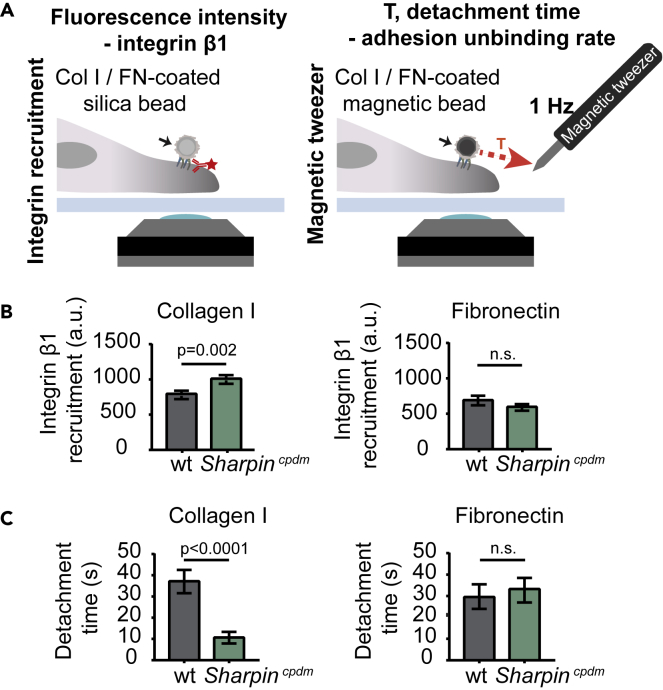
We then assessed how the altered integrin activity in Sharpincpdm MSFs affects the binding and unbinding properties of the cells to matrix ligands (Figure 2A). For this we employed ECM-coated bead recruitment and detachment experiments. Previous studies indicate that adhesions formed by cells on beads of comparable size [3 μm vs. 2.8–4.5 μm; (Guilluy et al., 2011, Jones et al., 2015)] have similar attachment times [30 min vs. 20-35 min; (González-Tarragó et al., 2017, Jones et al., 2015)] and recruit the same core-adhesome proteins as the adhesions that form on the basal side of adherent cells (Guilluy et al., 2011, Jones et al., 2015). Sharpincpdm MSFs displayed slightly increased recruitment of integrin β1 to collagen-I-coated silica beads when compared with wild-type cells, whereas no significant differences in binding to fibronectin-coated beads were observed (Figure 2B). We then employed a magnetic tweezers setup (Elosegui-Artola et al., 2014), a method that allows quantitative measurement of the strength of receptor-ligand bonds, to apply force to collagen I or fibronectin-coated beads attached to cells, and evaluated the time required to detach beads from cells (Figure 2A). Detachment times of collagen-coated beads were significantly lower for Sharpincpdm MSFs compared with wild-type cells (Figure 2C). However, no significant differences were observed with fibronectin-coated beads (Figure 2C). The significant decrease in bead detachment time under force suggests that unbinding rates under force are increased. Thus, overall the results are consistent with an effect of SHARPIN deficiency in increasing both binding and unbinding rates of integrins to collagen, thereby maintaining the overall recruitment constant.
Figure 2.
SHARPIN-Deficient MSFs Show Faster Integrin-Collagen Binding Dynamics
(A) Schematic representation of the set up for integrin recruitment (left panel) and magnetic tweezer (right panel) experiments.
(B) Quantification of integrin β1 recruitment to collagen I (left panel) or fibronectin (right panel)-coated silica beads; nwt = 62 and nSharpincpdm = 51 cells (collagen I) and nwt = 33 and nSharpincpdm = 24 cells (fibronectin) from two independent experiments.
(C) Quantification of detachment time of wild-type and Sharpincpdm MSFs from collagen I (left panel) or fibronectin (right panel)-coated magnetic beads; nwt and nSharpincpdm = 34 cells (collagen) and nwt = 29 and nSharpincpdm = 37 cells (fibronectin) from two independent experiments. Mean ± SEM in all graphs. Unpaired t test. Col I, collagen I; FN, fibronectin.
Molecular Clutch Model Predicts the Absence of Traction Peak in Sharpincpdm Cells at Biologically Relevant Rigidities
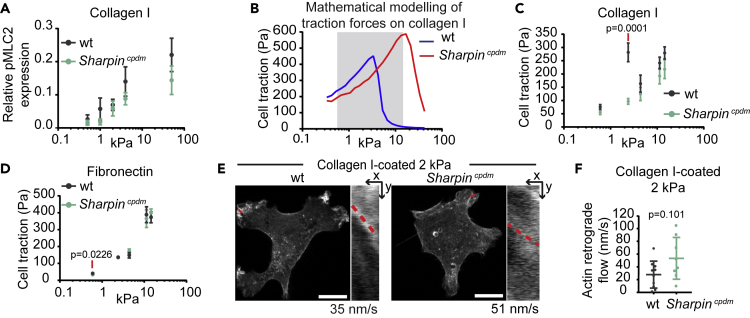
The ability of cells to sense and respond to rigidity, and apply force to the matrix, is regulated by the different components of the adhesive and contractile molecular machinery that functions jointly as a cellular molecular clutch (Case and Waterman, 2015, Chan and Odde, 2008, Elosegui-Artola et al., 2014). Clutch dynamics are a function of several parameters: the number and binding dynamics of integrin-ECM bonds, reinforcement of the integrin-actin link through talin unfolding and vinculin recruitment, actomyosin contractility, and substrate compliance. Unlike previously studied cell types (Elosegui-Artola et al., 2016), the MSFs are able to generate fully mature adhesions at low stiffness. This suggests that MSFs should exhibit the fundamental prediction of the molecular clutch, a biphasic force/rigidity relationship, which is almost always masked by the fact that adhesion growth and reinforcement normally occur only at high rigidities (Chan and Odde, 2008, Elosegui-Artola et al., 2016). This exciting scenario prompted us to measure the remaining necessary parameters required for computational modeling of the molecular clutch in these cells. Actomyosin contractility is an important component of the molecular clutch and is regulated by phosphorylation of myosin light chain 2 (pMLC2). In talin 1−/− knockout mouse embryonic fibroblasts (MEFs) (talin 1−/− MEFs), which display a wild-type phenotype due to compensatory upregulation of endogenous talin 2 and have previously been studied with comparable methods (Elosegui-Artola et al., 2016), pMLC2 levels are largely independent of substrate stiffness (Elosegui-Artola et al., 2016). We compared our MSFs with the talin 1−/− MEFs and observed increasing pMLC2 levels in MSFs in response to increasing stiffness (Figures 3A, S2A, and S2B), similar to the previously observed response of vascular smooth muscle cells (Polte et al., 2004). Furthermore, higher pMLC was observed in MSFs plated on soft substrate as compared with talin 1 −/− MEFs (Elosegui-Artola et al., 2016) (Figure S2A). However, no significant differences in pMLC2 were detected between wild-type and Sharpincpdm MSFs, suggesting that myosin activity remains predominantly unaffected in the absence of SHARPIN.
Figure 3.
Molecular Clutch Model Predicts the Absence of Traction Peak in SHARPIN-Deficient Cells at Biologically Relevant Rigidities
(A) Quantification of relative pMLC2 expression levels in wild-type compared with Sharpincpdm MSFs plated on collagen-I-coated PAA hydrogels with the indicated stiffness; n = 3 independent experiments.
(B) Prediction of the traction forces generated by wild-type and Sharpincpdm MSFs on collagen-I-coated PAA hydrogels based on the molecular clutch model. The stiffness range covered in (C) is highlighted.
(C) Average forces exerted by wild-type compared with Sharpincpdm MSFs on collagen-I-coated PAA hydrogels with the indicated stiffness measured by traction force microscopy, nwt = 20, 20, 21, 20, 19 and nSharpincpdm = 18, 25, 21, 18, 17 cells (from left to right) from two independent experiments.
(D) Average forces exerted by wild-type compared with Sharpincpdm MSFs on fibronectin-coated PAA hydrogels with the indicated stiffness measured by traction force microscopy, nwt= 11, 11, 23, 14, 10 and nSharpincpdm= 10, 13, 23, 11, 10 cells (from left to right) from two independent experiments.
(E) Representative images of Lifeact-GFP-transfected wild-type and Sharpincpdm MSFs plated on 2 kPa collagen-I-coated PAA hydrogels. Insets are kymographs showing actin retrograde flow along the red line (time = 125s, imaged every second). The slope of the line was used to calculate the actin retrograde flow rate.
(F) Quantification of actin retrograde flow in wild-type compared with Sharpincpdm MSFs, nwt = 10 and nSharpincpdm = 8 cells (1 measurement/cell), from three independent experiments.
Mean ± SEM in all graphs. Mann–Whitney U-test, red lines below p values indicate the data points compared. Scale bars: 10 μm. See also Figure S2 and Table S1.
Taking into consideration these experimentally determined MSF features, we employed the computational model of the molecular clutch (Elosegui-Artola et al., 2014, Elosegui-Artola et al., 2016). It predicts that in the absence of changes in integrin recruitment or adhesion growth as a function of stiffness, the cells should display a biphasic force/rigidity response, even if myosin contractility increases with rigidity (Figure 3B). The other prediction arising from our modeling is that increased integrin binding and unbinding rates, as observed in Sharpincpdm MSFs, should displace the traction force peak to higher rigidities. To test the model predictions, we measured cell-matrix force transmission using traction force microscopy. As predicted, wild-type cells on collagen I displayed a biphasic response of force as a function of stiffness, on the lower stiffness range, with a force maximum at 2 kPa (Figure 3C andS2C). Thus, MSFs represent the first mammalian primary cell type that shows this predicted biphasic force response without genetic perturbation. In Sharpincpdm cells, the concomitant increase in binding and unbinding rates should shift the force curve, displacing the force peak to higher rigidities. Accordingly, the maximum force peak at 2 kPa was absent (Figures 3C and S2C), closely replicating the result of the molecular clutch modeling (Figure 3B). In this case, no force peak was observed, potentially because it was displaced to a stiffness above the range where traction forces were measured experimentally. Of note, wild-type cells exhibit a stiffness-dependent additional increase in force transmission above 4 kPa that is not predicted by the model indicating that the modeling corresponds to the experimental data only in the lower stiffness range. The nature of this regime remains unknown and warrants further investigation.
Finally, the molecular clutch model also predicts that changes in force should inversely correlate with actin flow. As predicted, actin flow of actively spreading (plated for 45–105 min) Sharpincpdm cells (average 51 nm/s) on 2 kPa collagen-I-coated hydrogels was also elevated with respect to wild-type cells (average 35 nm/s) (Figures 3E and3F). Because of the limited transfection efficiency in the primary MSFs and the need to isolate them freshly from mouse mammary gland, this single stiffness was selected based on the highest differences in traction force measurements. Interestingly, in stably adhered MSFs (plated for 4 h) on 2 kPa collagen-I-coated hydrogels, very slow actin retrograde flow was observed compared to talin 1−/− MEFs (Figure S2D), and measurement of actin flow in MSFs was beyond the detection limit. This is in stark contrast to the rapid actin flow detected in other cell types on soft substrate (Bangasser et al., 2017, Chan and Odde, 2008, Elosegui-Artola et al., 2016), further demonstrating the adaption of MSFs to a soft environment. This may be attributed, in part, to integrin expression. We compared the cell-surface levels of several integrin subunits in talin 1−/− MEFs and wild-type MSFs and observed that MSFs express nearly two times more integrin β1 and markedly more integrin α1-and α11-subunits compared to talin 1−/− MEFs (Figure S2E) that spread poorly on soft collagen (Figure S2F).
Consistent with the fact that no differences in adhesion behavior under force were observed with fibronectin-coated beads (Figure 2C), wild-type and Sharpincpdm cells exerted the same forces on fibronectin-coated substrates irrespective of stiffness and the cells displayed monotonic increase in force with increasing rigidity (Figure 3D). In contrast to collagen I substrate, MSFs did not show a force maximum at low rigidities on fibronectin. As in Sharpincpdm cells on collagen, this lack of a biphasic force response could result from the limited stiffness range used in these experiments (0.6–14.5 kPa) and suggests that the force maximum for MSF on fibronectin is exerted at a stiffness above 14.5 kPa. Our observations support the view that different integrin heterodimers form integrin-ligand bonds with different strengths depending also on ECM composition, which leads to variations in the force maximum on different ECM ligands.
Together, these data demonstrate that SHARPIN deficiency, and the consequent increase in integrin-collagen unbinding rate, lead to significant effects in mechanotransduction in MSFs, providing a possible explanation to our previous finding that Sharpincpdm MSFs are unable to remodel collagen in vitro and are defective in supporting generation of mammary gland stromal architecture supportive of normal development and ductal outgrowth (Peuhu et al., 2017a).
Integrin α11β1 Protein Levels Regulate the Spreading of MSFs on Soft Matrices
Next, we asked how loss of SHARPIN could affect integrin binding dynamics and what the consequent effects are. One potential explanation is that Sharpincpdm and wild-type MSFs exhibit differences in collagen-binding integrin expression, leading to different ECM binding properties. An RNA sequencing dataset of wild-type and Sharpincpdm MSFs (Peuhu et al., 2017a) was analyzed for all the matrix-binding integrin subtypes (Figure S3A). Of the collagen-binding integrin alpha subunits (Itga1, Itga2, Itga10, Itga11), which all form a heterodimer with the integrin β1 subunit, Itga11 was the predominant α-subunit expressed at mRNA level, Itga1 was detected at low levels and Itga10 or Itga2 were not detected. Importantly, no significant differences in integrin mRNA expression levels were observed between wild-type and Sharpincpdm MSFs (Figure S3A).
Next we analyz Pa collagen-I-coated hydrogels directly after isolation from the mouse mammary gland (Figure S3B), indicating that the observed difference in integrin α11 is not induced by in vitro culture on stiff fibronectin-rich substratum (plastic in the presence of serum fibronectin) and is prominent under conditions when collagen I is the only provided ligand (Figure S3C).
As inactive and active integrins are trafficked and degraded differently (Arjonen et al., 2012, Rainero et al., 2015), we speculated that the increased relative activity of integrin β1 could lead to increased targeting of integrin α11β1 to lysosomal degradation in Sharpincpdm MSFs. This hypothesis was supported by the fact that SHARPIN-deficient MSFs displayed more integrin α11 co-localization with Lamp1 (lysosomal marker) than wild-type MSFs (Figures 4G and 4H). Furthermore, we observed accumulation of integrin α11 to the Lamp1-positive compartment when MSFs were treated with the lysosomal degradation disruptor Bafilomycin A1 (Figures 4G and 4H). These data suggest that a subset of integrin α11 traffics to lysosomal degradation in MSFs and that this is enhanced in SHARPIN-deficient cells.
Figure 4.
SHARPIN Regulates Integrin α11β1 Protein Levels
(A) Analysis of cell-surface expression (median fluorescence) of integrin α1 and α11 in Sharpincpdm relative to wild-type MSFs, nItga1 =6 and nItga11 = 8 from six independent flow cytometry experiments.
(B and C) (B) Representative Western blot analysis of integrin α11 protein expression in wild-type and Sharpincpdm MSFs and (C) quantification of the relative integrin α11 expression levels, nwt and nSharpincpdm = 7 from five independent experiments. GAPDH was detected for loading control.
(D) Representative images of immunolabelled integrin α11 (green) and total integrin β1 (magenta) in wild-type and Sharpincpdm MSFs plated on 2 kPa collagen-I-coated PAA hydrogels. Nuclei (blue) were co-labeled.
(E and F) (E) Representative Western blot analysis of integrin α11 and SHARPIN protein expression in wild-type MSFs silenced with control or SHARPIN-targeting siRNA and (F) quantification of the relative integrin α11 expression levels; n = 5 independent experiments. GAPDH was detected for loading control.
(G) Representative images of immunolabelled integrin α11 (green) and the lysosomal marker Lamp1 (magenta) in control-treated (ctrl) or Bafilomycin A1-treated (100 nM, 6h) wild-type and Sharpincpdm MSFs plated on collagen. Region of interest (yellow) shows examples of co-localization (white spots, highlighted with red arrow).
(H) Quantification of relative (to wt control) co-localization of integrin α11 and Lamp1 in control-treated or Bafilomycin-A1-treated wild-type and Sharpincpdm MSFs plated on collagen; n = 76, 102, 89, 125 cells (from left to right) pooled from three independent experiments; line under p value indicates which samples are compared with each other.
Mean ± SEM in all graphs. (A) Wilcoxon matched-pairs signed rank test. (C, F, and H) Mann-Whitney U-test. Scale bars: 20 μm. See also Figure S3.
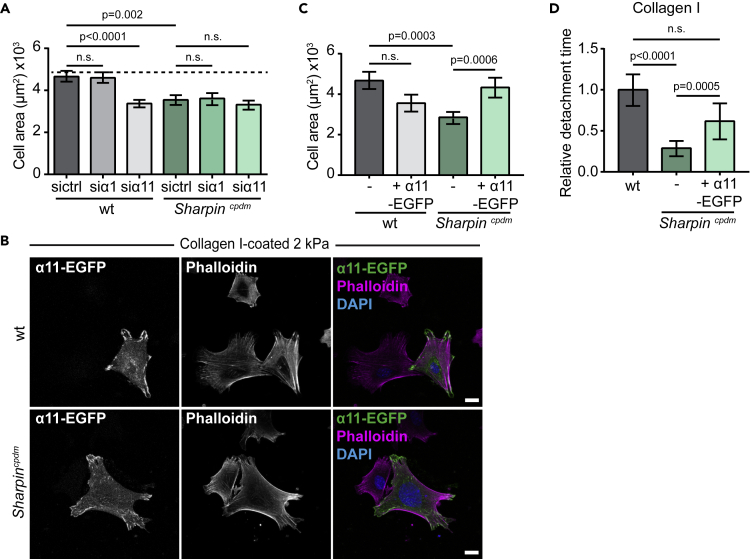
To investigate whether the reduced surface expression of integrin α1 and α11 in Sharpincpdm MSFs could be responsible for the impaired capability to spread on a compliant collagen-I-coated surface, we analyzed cell spreading following siRNA-mediated downregulation of integrin α1 or integrin α11 (Figure 5A), confirmed by qPCR (Figure S3D). In wild-type MSFs downregulation of integrin α11, but not integrin α1, led to significantly reduced cell spreading on 2 kPa collagen-I-coated hydrogels that resembled the phenotype of Sharpincpdm MSFs (Figure 1B). In contrast, no significant differences in cell area were observed when the endogenously lower integrin α1 and integrin α11 levels were silenced in Sharpincpdm MSFs (Figure 5A). These data indicate that appropriate levels of integrin α11, but not integrin α1, are important for regulating the spreading of MSFs on soft collagen-I-coated substrates. This could be linked to the fact that integrin α11 mediates strong binding to and contraction of fibrillar collagen I (Tiger et al., 2001), whereas integrin α1 prefers non-fibrillar collagen IV and has lower binding affinity to fibrillar collagens (types I, II, and III) (Tulla et al., 2001). However, this remains to be investigated.
Figure 5.
Integrin α11β1 Regulates the Spreading of MSFs on Soft Matrices
(A) Quantification of the cell area in wild-type and Sharpincpdm MSFs silenced with control, integrin α1, or integrin α11 targeting siRNA and plated on 2 kPa collagen-I-coated PAA hydrogels; n = 94, 91, 93, 82, 88, 90 cells (from left to right) from three independent experiments.
(B) Representative images of integrin α11-EGFP-transfected wild-type and Sharpincpdm MSFs plated on 2 kPa collagen-I-coated PAA hydrogels and co-labeled for F-actin (magenta) and nuclei (blue).
(C) Quantification of cell area in non-transfected and integrin α11-EGFP-transfected wild-type and Sharpincpdm MSFs plated on 2 kPa collagen-I-coated PAA hydrogels. Data are pooled from two independent experiments; n = 37, 31, 46, 48 cells (from left to right).
(D) Quantification of detachment time of wild-type, Sharpincpdm, and integrin α11-EGFP-transfected Sharpincpdm MSFs from collagen-I-coated magnetic beads. Data are pooled from three independent experiments; n = 91, 101, 46 cells (from left to right).
Mean ± SEM in all graphs. (A) Unpaired t test. (C and D) Mann-Whitney U-test. Line under p value indicates which samples are compared with each other. Scale bars: 20 μm. See also Figure S3.
To verify that integrin α11 levels are critical for collagen I interaction of MSFs, we performed rescue experiments with ectopically expressed EGFP-tagged human integrin α11. Reintroduction of integrin α11 in Sharpincpdm MSFs on collagen-I-coated 2 kPa hydrogels reversed their defective spreading (Figures 5B and 5C). In contrast, overexpression of integrin α11-EGFP in wild-type MSFs modestly, albeit, non-significantly decreased cell spreading (Figures 5B and 5C). In addition, reintroduction of integrin α11 by ectopic expression partially rescued the ligand detachment time in Sharpincpdm MSFs (Figure 5D). Taken together, these results demonstrate that integrin α11β1 is essential in the integrin-collagen I binding dynamics and spreading of MSFs on soft collagen-I-coated substrates and that the impaired mechanotransduction of SHARPIN-deficient MSFs is coupled to reduced integrin α11β1 protein expression level.
Discussion
Here we have investigated the mechanotransduction of mammary gland stromal fibroblasts to gain insight into the biologically essential role of these cells in sculpting the mammary gland stromal architecture in vivo (Peuhu et al., 2017a). Taking this experimentally challenging primary cell model (compared with the immortalized cell lines studied previously) has provided two interesting and unexpected observations related to tissue-specific characteristics of mechanotransduction. First, our data comparing wild-type and SHARPIN-deficient cells provide a striking example of how, even if major mechanical regulators such as myosin contractility are not affected, merely changing integrin properties under force can dramatically affect the cell's mechanoresponse. Second, the fact that wild-type MSFs exhibit mature, vinculin-associated FA irrespective of matrix stiffness allowed us to decouple traction force generation from adhesion maturation, leading to the fundamental clutch model prediction of a biphasic traction-stiffness relationship that is otherwise very elusive to observe (Elosegui-Artola et al., 2018).
The differential regulation of collagen- and fibronectin-binding integrins and the mechanobiological implications of these differences remain poorly understood. Here, we have investigated the consequences of SHARPIN deficiency on cell mechanosensing, integrin ligand-binding dynamics, and traction force generation and conducted computational modeling of these events. Our data demonstrate that in the absence of the integrin activity regulator SHARPIN, the protein levels of the collagen-binding integrin α11β1 are downregulated. This is likely to be the main reason for the significantly increased unbinding of SHARPIN-deficient MSFs from collagen-coated beads under force. As the remaining integrin α11β1 is more likely to be in the primed active conformation, due to the increase in relative integrin β1 activity, this could account for the small increase of integrin recruitment to collagen. These faster cell-collagen binding dynamics are in line with the previously reported rapid adhesion turnover of SHARPIN-deficient MSFs (Peuhu et al., 2017a). Importantly, the molecular clutch model was able to predict the absence of traction peak and increased actin flow rate at low rigidities based on increased clutch binding and unbinding rates to collagen, measured by magnetic tweezers in SHARPIN-deficient cells. Of note, whereas the response to force of integrin-collagen bonds is not known, our model assumed a catch bond behavior. This should, however, not affect our conclusions, because the key aspects of the molecular clutch model found here (biphasic behavior, plus the role of fold changes in binding and unbinding rates) are general to both catch and slip bonds (Bangasser et al., 2013).
Our model failed, however, to predict the final increase in force transmission in wild-type cells with increasing stiffness. Although the details of this regime warrants further investigation, we note that in conditions with very low actin flow rates, mature adhesions, and very stable actin fibers, cytoskeletal reorganization events other than fast actin flow could be determinant of the cellular force transmission (Oakes et al., 2012). In all, we acknowledge that the model prediction corresponds to the experimental data only in the soft stiffness range, possibly because not all cellular parameters have been, and can be, taken into account by the molecular clutch model.
Although SHARPIN regulates the activity of both collagen- and fibronectin-binding integrins (Rantala et al., 2011), the low levels of fibrillar collagen-binding integrin α11 in SHARPIN-deficient MSFs results in different mechanosensitive responses of these cells on collagen and fibronectin. This is in line with the established role of integrin α11β1 (Lehnert et al., 1999), in the regulation of collagen contractility (Popova et al., 2007, Tiger et al., 2001) and in collagen-linked disease conditions, such as fibrosis, cancer invasion, and particularly in cancer-associated fibroblasts (Bansal et al., 2017, Navab et al., 2016). The regulatory pathways modulating the activity of collagen-binding integrins may be distinct from other integrin heterodimers. The vast majority of studies investigating integrin activation are based on the platelet-specific integrin αIIbβ3 and the fibronectin receptors integrin α5β1 and αvβ3, which are primarily regulated by inside-out and outside-in signaling. In turn, only a few studies have addressed activity regulation in the context of collagen-binding integrins. Heterodimerization of α1β1 and α2β1 integrins has been postulated to have a key role in their activity regulation based on the lower affinity of integrin α1 and α2 to integrin β1 (Lu et al., 2016). Thus, regulation of the expression levels of collagen-binding integrins may be particularly important for their ligand-binding dynamics.
SHARPIN is a multifunctional adapter protein that has been implicated in a number of other signaling pathways, including inhibition of integrin activity (Kasirer-Friede et al., 2019, Peuhu et al., 2017a, Peuhu et al., 2017b, Pouwels et al., 2013, Rantala et al., 2011). SHARPIN promotes canonical Nuclear factor (NF)-κB activation (Gerlach et al., 2011, Tokunaga et al., 2009) and other inflammatory signaling cascades as part of the linear ubiquitin assembly complex (LUBAC) (Chattopadhyay et al., 2016, Dubois et al., 2014, Rodgers et al., 2014, Zak et al., 2011). Furthermore, SHARPIN regulates the functions of the Arp2/3 protein complex (Khan et al., 2017), T cell receptor (Park et al., 2016), and caspase 1 (Nastase et al., 2016) in an LUBAC-independent manner and interacts with PTEN (He et al., 2010), SHANK proteins (Lim et al., 2001), and EYA transcription factors (Landgraf et al., 2010). In this study, reduced integrin α11 protein levels were directly linked to mechanobiological phenotypes in SHARPIN-deficient MSFs, whereas integrin expression at the transcriptional level remained comparable to wild-type MSFs. Because NF-κB is a transcription factor that functions predominantly through regulation of gene expression, our data imply that NF-κB might not be the primary mechanism involved in the regulation of integrin α11 in SHARPIN-deficient MSFs. Currently, we lack the detailed mechanisms accounting for the reduced integrin α11 protein levels in SHARPIN-deficient MSFs and in wild-type MSFs upon SHARPIN silencing. However, it is likely that increased lysosomal trafficking of integrin α11, in the absence of SHARPIN, is contributing to elevated integrin degradation in these cells. This would be in line with previous studies linking integrin activation to reduced receptor recycling rates and increased lysosomal degradation (De Franceschi et al., 2015).
Together, our findings demonstrate how altered integrin activity in SHARPIN-deficient primary MSFs correlates with deregulated cell spreading and traction force generation in response to substrate ligand composition and stiffness. The central role for integrin α11, uncovered here, in regulating mechanotransduction on collagen may also be essential to the pathological behavior of fibroblasts in cancerous or fibrotic tissues. As both SHARPIN and integrin α11β1 are significant regulators of cancer tumorigenesis and dissemination, as well as fibroblast contractility and collagen remodeling (He et al., 2010, Navab et al., 2016, Peuhu et al., 2017a, Tamiya et al., 2018, Zhu et al., 2007), increased understanding of their functional interplay is of wide interest. Finally, our finding that the mechanical output of fibroblasts can be strongly influenced by a single parameter of the molecular clutch, the integrin binding dynamics, highlights the tuneability of mechanotransduction and its ability to trigger specific outputs in response to both internal and external parameters.
Limitations of the Study
Our current results indicate that absence of SHARPIN results in downregulation of integrin α11 protein levels. We find that integrin α11 localizes slightly more in lysosomes in SHARPIN-deficient than wild-type cells. This suggests that altered receptor trafficking and increased degradation may contribute partially to the lower integrin α11 expression. However, additional mechanisms, such as altered mRNA translation, may also be involved. Therefore, more follow-on studies are necessary to fully unravel the mechanism of how SHARPIN regulates integrin α11 levels.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank J. Siivonen and P. Laasola for technical assistance; P. Erusappan for integrin α11 antibody protocols; A. Isomursu and the rest of the Ivaska Lab for insightful comments; H. Hamidi for editing of the manuscript; and X. Trepat for thought provoking discussions that inspired this study. Turku Bioscience Cell Imaging Core and Turku Center for Disease Modeling and Biocenter Finland are acknowledged for services, instrumentation, and expertise. This study has been supported by the Academy of Finland (Grant312517), ERC Consolidator Grant (615258), the Sigrid Jusélius Foundation, and the Finnish Cancer Organization. ML has been supported by Turku Doctoral Program of Molecular Medicine, Instrumentarium Science Foundation, Victoriastiftelsen, and The Swedish Cultural Foundation in Finland, and EP by Academy of Finland (259283, 323096) and Finnish Cultural Foundation.
Author Contributions
PRC, EP, and JI contributed to the conception and design of the study. ML, AEA, MG, JZK, and EP designed, conducted, and analyzed in vitro experiments. AEA and JZK performed traction force microscopy. ML and AEA conducted the magnetic tweezer experiments. CG, AEA, JZK, and AI analyzed the TFM data and confirmed gel stiffness with AFM. ML, EP, and JI wrote the manuscript, and AEA, PRC, and DG edited the manuscript. DG, PRC, EP, and JI supervised the research. Funding acquisition: PRC and JI.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100907.
Contributor Information
Emilia Peuhu, Email: emilia.peuhu@utu.fi.
Johanna Ivaska, Email: johanna.ivaska@utu.fi.
Supplemental Information
References
- Abair T.D., Sundaramoorthy M., Chen D., Heino J., Ivaska J., Hudson B.G., Sanders C.R., Pozzi A., Zent R. Cross-talk between integrins alpha1beta1 and alpha2beta1 in renal epithelial cells. Exp. CellRes. 2008;314:3593–3604. doi: 10.1016/j.yexcr.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjonen A., Alanko J., Veltel S., Ivaska J. Distinct recycling of active and inactive β1 integrins. Traffic. 2012;13:610–625. doi: 10.1111/j.1600-0854.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser B.L., Shamsan G.A., Chan C.E., Opoku K.N., Tüzel E., Schlichtmann B.W., Kasim J.A., Fuller B.J., McCullough B.R., Rosenfeld S.S., Odde D.J. Shifting the optimal stiffness for cell migration. Nat. Commun. 2017;8:15313. doi: 10.1038/ncomms15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser B.L., Rosenfeld S.S., Odde D.J. Determinants of maximal force transmission in a motor-clutch model of cell traction in a compliant microenvironment. Biophys. J. 2013;105:581–592. doi: 10.1016/j.bpj.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Nakagawa S., Yazdani S., van Baarlen J., Venkatesh A., Koh A.P., Song W., Goossens N., Watanabe H., Beasley M.B. Integrin alpha 11 in the regulation of the myofibroblast phenotype: implications for fibrotic diseases. Exp. Mol. Med. 2017;49:e396. doi: 10.1038/emm.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield D.G., Venugopalan G., Lo A., Mori H., Tanner K., Fletcher D.A., Bissell M.J. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr.Biol. 2013;23:703–709. doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L.B., Waterman C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. CellBiol. 2015;17:955–963. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.E., Odde D.J. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S., Kuzmanovic T., Zhang Y., Wetzel J., Sen G. Ubiquitination of the transcription factor IRF-3 activates RIPA, the apoptotic pathway that protects mice from viral pathogenesis. Immunity. 2016;44:1151–1161. doi: 10.1016/j.immuni.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Choudhury D.M., Craig S.W. Coincidence of actin filaments and talin is required to activate vinculin. J. Biol. Chem. 2006;281:40389–40398. doi: 10.1074/jbc.M607324200. [DOI] [PubMed] [Google Scholar]
- De Franceschi N., Hamidi H., Alanko J., Sahgal P., Ivaska J. Integrin traffic - the update. J. Cell. Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J.M., Sheetz M.P. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S.M., Alexia C., Wu Y., Leclair H.M., Leveau C., Schol E., Fest T., Tarte K., Chen Z.J., Gavard J., Bidère N. A catalytic-independent role for the LUBAC in NF-κB activation upon antigen receptor engagement and in lymphoma cells. Blood. 2014;123:2199–2203. doi: 10.1182/blood-2013-05-504019. [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A., Bazellières E., Allen M.D., Andreu I., Oria R., Sunyer R., Gomm J.J., Marshall J.F., Jones J.L., Trepat X., Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 2014;13:631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui-Artola A., Oria R., Chen Y., Kosmalska A., Pérez-González C., Castro N., Zhu C., Trepat X., Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. CellBiol. 2016;18:540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A., Trepat X., Roca-Cusachs P. Control of mechanotransduction by molecular clutch dynamics. Trends CellBiol. 2018;28:356–367. doi: 10.1016/j.tcb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Erdogan B., Ao M., White L.M., Means A.L., Brewer B.M., Yang L., Washington M.K., Shi C., Franco O.E., Weaver A.M. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. CellBiol. 2017;216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach B., Cordier S.M., Schmukle A.C., Emmerich C.H., Rieser E., Haas T.L., Webb A.I., Rickard J.A., Anderton H., Wong W.W. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Glentis A., Oertle P., Mariani P., Chikina A., El Marjou F., Attieh Y., Zaccarini F., Lae M., Loew D., Dingli F. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017;8:924. doi: 10.1038/s41467-017-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Tarragó V., Elosegui-Artola A., Bazellières E., Oria R., Pérez-González C., Roca-Cusachs P. Binding of ZO-1 to α5β1 integrins regulates the mechanical properties of α5β1-fibronectin links. Mol. Biol. Cell. 2017;28:1847–1852. doi: 10.1091/mbc.E17-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia-Mata R., O’Brien E.T., Superfine R., Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- He L., Ingram A., Rybak A.P., Tang D. Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J. Clin. Invest. 2010;120:2094–2108. doi: 10.1172/JCI40778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HogenEsch H., Gijbels M.J., Offerman E., van Hooft J., van Bekkum D.W., Zurcher C. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am. J. Pathol. 1993;143:972–982. [PMC free article] [PubMed] [Google Scholar]
- Ingman W.V., Wyckoff J., Gouon-Evans V., Condeelis J., Pollard J.W. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Ivaska J., Reunanen H., Westermarck J., Koivisto L., Kähäri V.M., Heino J. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J. CellBiol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.C., Humphries J.D., Byron A., Millon-Frémillon A., Robertson J., Paul N.R., Ng D.H.J., Askari J.A., Humphries M.J. Isolation of integrin-based adhesion complexes. Curr.Protoc.Cell Biol. 2015;66:9.8.1–9.8.15. doi: 10.1002/0471143030.cb0908s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasirer-Friede A., Tjahjono W., Eto K., Shattil S.J. SHARPIN at the nexus of integrin, immune, and inflammatory signaling in human platelets. Pnas. 2019;116:4983–4988. doi: 10.1073/pnas.1819156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.H., Salomaa S.I., Jacquemet G., Butt U., Miihkinen M., Deguchi T., Kremneva E., Lappalainen P., Humphries M.J., Pouwels J. The Sharpin interactome reveals a role for Sharpin in lamellipodium formation via the Arp2/3 complex. J. CellSci. 2017;130:3094–3107. doi: 10.1242/jcs.200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf K., Bollig F., Trowe M., Besenbeck B., Ebert C., Kruspe D., Kispert A., Hänel F., Englert C. Sipl1 and Rbck1 are novel Eya1-binding proteins with a role in craniofacial development. Mol. Cell.Biol. 2010;30:5764–5775. doi: 10.1128/MCB.01645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert K., Ni J., Leung E., Gough S.M., Weaver A., Yao W.P., Liu D., Wang S.X., Morris C.M., Krissansen G.W. Cloning, sequence analysis, and chromosomal localization of the novel human integrin alpha11 subunit (ITGA11) Genomics. 1999;60:179–187. doi: 10.1006/geno.1999.5909. [DOI] [PubMed] [Google Scholar]
- Lim S., Sala C., Yoon J., Park S., Kuroda S., Sheng M., Kim E. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol. Cell Neurosci. 2001;17:385–397. doi: 10.1006/mcne.2000.0940. [DOI] [PubMed] [Google Scholar]
- Lopez J.I., Kang I., You W., McDonald D.M., Weaver V.M. In situ force mapping of mammary gland transformation. Integr. Biol. (Camb) 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Mathew S., Chen J., Hadziselimovic A., Palamuttam R., Hudson B.G., Fässler R., Pozzi A., Sanders C.R., Zent R. Implications of the differing roles of the β1 and β3 transmembrane and cytoplasmic domains for integrin function. Elife. 2016;5:e18633. doi: 10.7554/eLife.18633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase M., Zeng-Brouwers J., Frey H., Hsieh L.T., Poluzzi C., Beckmann J., Schroeder N., Pfeilschifter J., Lopez-Mosqueda J., Mersmann J. An essential role for SHARPIN in the regulation of caspase 1 activity in sepsis. Am. J. Pathol. 2016;186:1206–1220. doi: 10.1016/j.ajpath.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Navab R., Strumpf D., To C., Pasko E., Kim K.S., Park C.J., Hai J., Liu J., Jonkman J., Barczyk M. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35:1899–1908. doi: 10.1038/onc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes P.W., Beckham Y., Stricker J., Gardel M.L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. CellBiol. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Jin H., Lopez J., Lee J., Liao L., Elly C., Liu Y. SHARPIN controls regulatory T cells by negatively modulating the T cell antigen receptor complex. Nat. Immunol. 2016;17:286–296. doi: 10.1038/ni.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuhu E., Kaukonen R., Lerche M., Saari M., Guzmán C., Rantakari P., De Franceschi N., Wärri A., Georgiadou M., Jacquemet G. SHARPIN regulates collagen architecture and ductal outgrowth in the developing mouse mammary gland. Embo J. 2017;36:165–182. doi: 10.15252/embj.201694387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuhu E., Salomaa S.I., De Franceschi N., Potter C.S., Sundberg J.P., Pouwels J. Integrin beta 1 inhibition alleviates the chronic hyperproliferative dermatitis phenotype of SHARPIN-deficient mice. PLoS One. 2017;12:e0186628. doi: 10.1371/journal.pone.0186628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plodinec M., Loparic M., Monnier C.A., Obermann E.C., Zanetti-Dallenbach R., Oertle P., Hyotyla J.T., Aebi U., Bentires-Alj M., Lim R.Y.H., Schoenenberger C. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012;7:757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- Polte T.R., Eichler G.S., Wang N., Ingber D.E. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 2004;286:518. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- Popova S.N., Barczyk M., Tiger C., Beertsen W., Zigrino P., Aszodi A., Miosge N., Forsberg E., Gullberg D. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol. Cell.Biol. 2007;27:4306–4316. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels J., De Franceschi N., Rantakari P., Auvinen K., Karikoski M., Mattila E., Potter C., Sundberg J.P., Hogg N., Gahmberg C.G. SHARPIN regulates uropod detachment in migrating lymphocytes. Cell Rep. 2013;5:619–628. doi: 10.1016/j.celrep.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero E., Howe J., Caswell P., Jamieson N., Anderson K., Critchley D., Machesky L., Norman J. Ligand-occupied integrin internalization links nutrient signaling to invasive migration. Cell Rep. 2015;10:398–413. doi: 10.1016/j.celrep.2014.12.037. [DOI] [PubMed] [Google Scholar]
- Rantala J.K., Pouwels J., Pellinen T., Veltel S., Laasola P., Mattila E., Potter C.S., Duffy T., Sundberg J.P., Kallioniemi O. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. CellBiol. 2011;13:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen T., Westermarck J., Koivisto L., Broberg A., Kähäri V.M., Heino J. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J. Biol. Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Rodgers M.A., Bowman J.W., Fujita H., Orazio N., Shi M., Liang Q., Amatya R., Kelly T.J., Iwai K., Ting J., Jung J.U. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J. Exp. Med. 2014;211:1333–1347. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R.E., Hasham M.G., Cox G.A., Shultz L.D., Hogenesch H., Roopenian D.C., Sundberg J.P. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416–421. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- Tamiya H., Kim H., Klymenko O., Kim H., Feng Y., Zhang T., Han J.Y., Murao A., Snipas S.J., Jilaveanu L. SHARPIN-mediated regulation of protein arginine methyltransferase 5 controls melanoma growth. J. Clin. Invest. 2018;128:517–530. doi: 10.1172/JCI95410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger C.F., Fougerousse F., Grundström G., Velling T., Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Sakata S., Saeki Y., Satomi Y., Kirisako T., Kamei K., Nakagawa T., Kato M., Murata S., Yamaoka S. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat. Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Tulla M., Pentikäinen O.T., Viitasalo T., Käpylä J., Impola U., Nykvist P., Nissinen L., Johnson M.S., Heino J. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J. Biol. Chem. 2001;276:48206–48212. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- Westcott J.M., Prechtl A.M., Maine E.A., Dang T.T., Esparza M.A., Sun H., Zhou Y., Xie Y., Pearson G.W. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J. Clin. Invest. 2015;125:1927–1943. doi: 10.1172/JCI77767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak D.E., Schmitz F., Gold E.S., Diercks A.H., Peschon J.J., Valvo J.S., Niemistö A., Podolsky I., Fallen S.G., Suen R. Systems analysis identifies an essential role for SHANK-associated RH domain-interacting protein (SHARPIN) in macrophage Toll-like receptor 2 (TLR2) responses. Pnas. 2011;108:11536–11541. doi: 10.1073/pnas.1107577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltz C., Alam J., Liu H., Erusappan P.M., Hoschuetzky H., Molven A., Parajuli H., Cukierman E., Costea D., Lu N., Gullberg D. α11β1 integrin is induced in a subset of cancer-associated fibroblasts in desmoplastic tumor stroma and mediates in vitro cell migration. Cancers (Basel) 2019;11:653–664. doi: 10.3390/cancers11060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltz C., Gullberg D. The integrin-collagen connection - a glue for tissue repair? J. Cell.Sci. 2016;129:1284. doi: 10.1242/jcs.188672. [DOI] [PubMed] [Google Scholar]
- Zhu C., Popova S.N., Brown E.R.S., Barsyte-Lovejoy D., Navab R., Shih W., Li M., Lu M., Jurisica I., Penn L.Z. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.