Abstract
Background
Phenylketonuria (PKU) is a rare autosomal recessive disorder caused by mutations in the gene encoding phenylalanine hydroxylase, an enzyme that converts phenylalanine to tyrosine. Untreated, PKU is characterized by a range of neuropsychological and neurocognitive impairments. Due to ubiquitous newborn genetic screening programs, treatment for PKU can be commenced shortly after birth and can prevent many of the severe manifestations of the disease. However, lifelong management is critical for patients with PKU as high levels of phenylalanine are neurotoxic. As for all chronic diseases, long-term management can be challenging and most adult patients with PKU become lost to follow-up (LTFU). A survey of PKU clinics across the United States and a multidisciplinary Expert Meeting were conducted to develop best practices to engage LTFU patients with PKU.
Results
We defined LTFU patients with PKU as “patients with no contact with the clinic for at least 2 consecutive years.” Combining the results from our survey and our discussion at the Expert Meeting, we have prepared six best practice recommendations to engage LTFU patients with PKU: 1) Ensure patients are aware of the current treatment guidelines for PKU; 2) Communicate to patients any new treatment and diet options as they become available for PKU; 3) Consider the neuropsychological and neurocognitive aspects of PKU; 4) Prioritize motivated LTFU patients; 5) Explore new approaches of outreach to LTFU patients; and 6) Formalize approaches to track and/or identify PKU patients.
Conclusion
We strongly advocate the importance of engaging LTFU patients with PKU and encourage implementation of our best practice recommendations. Although it takes time and effort to engage LTFU patients, we believe that clinics are capable of supporting this significant patient group.
Keywords: Patient care management, Phenylalanine hydroxylase deficiency, Phenylketonuria
1. Introduction
Phenylketonuria (PKU) is a rare autosomal recessive disorder caused by mutations in the gene encoding phenylalanine hydroxylase (PAH), an enzyme that converts phenylalanine (Phe) to tyrosine. The mutations in the PAH gene result in decreased enzyme activity and subsequent accumulation of Phe in the blood and brain (PKU may be referred to as PAH deficiency). The prevalence of PKU in the United States (US) is about 1:11,400 to 1:15,000 live births [1] and most patients are treated with a low Phe diet supplemented with amino acid-based medicinal foods [2] with or without pharmacotherapy [3]. Untreated, PKU is characterized by: delayed development; intellectual disability; seizures; impairments in the ability to remember, plan, and organize; and a range of psychiatric symptoms, including aberrant behavior, depression, and anxiety, which can lead to a reduced quality of life and create significant social challenges. Due to ubiquitous newborn genetic screening programs [4], treatment for PKU can be commenced shortly after birth and can prevent many of the severe manifestations of the disease. However, lifelong management is critical for patients with PKU as high levels of Phe are neurotoxic and lead to the development of neuropsychiatric symptoms and deficits in executive functioning [5].
As for all chronic diseases, lifelong management of PKU can be challenging and many patients become lost to follow-up. Berry et al. [6] estimated (based on clinic records) that 52% (7,808/14,988) of individuals of any age diagnosed with PKU at birth were not currently in clinic; this was as high as 77% (5,184/6,741) for individuals aged 25 to 45 years. More recently, Jurecki et al. [7] completed an online survey of 44 PKU clinics in the US and found that 32% (1,758/5,530) of patients were not currently actively managed by the respondents’ clinics; this was as high as 55% (944/1,732) for patients aged 30 years or older. Hence, both studies found that clinic attendance was highest for children and decreased with age; by adulthood most patients with PKU did not attend a specialty clinic [6,7].
Adults with PKU become lost to follow-up for a variety of reasons including: discharge from the clinic as a child; personal choice (which may follow from the neuropsychological aspects of the disease); strict dietary and treatment guidelines (often difficult to adhere to because of executive function impairments); treatment costs; lack of insurance coverage for medical formula or foods; lack of adult-specific medical formula or foods; insufficient support systems; unavailability of adult PKU clinics; and factors inherent to managing a chronic medical condition, including the effort to maintain Phe levels over a sustained period of time. In earlier work, we have proposed strategies to keep a previously lost to follow-up patient in clinic after they have re-engaged [8], but there is little information available on how to engage and support the lost to follow-up PKU patient to help them return to clinic. Therefore, our objectives were to clarify the definition of lost to follow-up, to understand challenges faced by clinics, and to develop best practices to engage lost to follow-up patients with PKU. We believe that our findings and recommendations will be a valuable resource for all metabolic clinics that treat adult patients with PKU.
2. Methods
2.1. Survey
BioMarin Pharmaceutical Inc. (Novato, CA, USA) designed, in conjunction with the Expert Meeting members, and conducted a survey of clinics (who received fair market payment for their participation) in the US between February and March 2018. The National PKU Alliance (https://npkua.org/) assisted in recruiting clinicians affiliated with a PKU clinic who actively managed at least 5 adult patients with PKU. The survey and survey results are provided as online supplementary information. Participants were asked to answer questions or rank the utility of various approaches.
The objective of the survey was to explore current practices and strategies around locating and re-engaging patients with PKU who are lost to follow-up. The results of the survey were presented at the Expert Meeting to facilitate discussion.
All data were collected and analyzed by Trinity Partners LLC (Waltham, MA, USA). Data were summarized as percentages of respondents and were analyzed using descriptive statistics. Data were presented to a Multidisciplinary Expert Meeting for interpretation and discussion of the results.
2.2. Multidisciplinary expert meeting
A professionally facilitated, external Expert Meeting of clinicians, affiliated with a PKU clinic that actively treated adult patients, and with extensive experience in managing adult PKU patients, was held in April 2018. The Multidisciplinary Expert Meeting consisted of 15 members from PKU clinics across different geographic regions within the US: 6 geneticists, 1 developmental pediatrician, 6 metabolic dietitians, and 2 nurse practitioners. All authors were members of the Expert Meeting; other members are included in the Acknowledgements. Expert Meeting members signed a written contract and received a fair market value payment for their participation in the meeting. The outcomes of the Expert Meeting discussions, and relevant survey findings, are presented in this manuscript.
3. Results and discussion
3.1. Survey
The survey was completed by 67 (of 80 who were sent the survey) respondents across 55 clinics, which is approximately 50% of all PKU clinics in the US, and therefore likely to be representative of the PKU community. The majority (69%) were dietitians; other respondents were metabolic disorder specialists (12%), nurse practitioners (10%), geneticists (7%), and psychiatrists (2%). Clinics saw an average of 45 adult patients with PKU.
3.2. Definition of lost to follow-up
The majority of survey respondents defined an ‘inactive’ PKU patient as a patient who had spent 2 years (55% [34/62] of respondents) out of clinic (1 year: 24% [15/62]; 3 years: 11% [7/62]). Patients were defined as ‘inactive’ because of the number of failed communication/contact attempts (78% [42/54] for 3–5 failed attempts), missed clinic appointments (77% [33/43] for ≥3 missed appointments), or missed laboratory tests/prescription refills (41% [16/39] for 3–5 missed tests/refills and 39% [15/39] for 6–10 missed tests/refills).
Taking into account the survey results, personal experience, and publications, we defined lost to follow-up patients with PKU as “patients with no contact with the clinic for at least 2 consecutive years.” Note that for some clinics, if a patient has had no contact with the clinic for 3 years they are then considered ‘new’ patients if/when they return to clinic.
The findings reported here may also be relevant to additional cohorts of patients: minimally engaged patients, who do not attend clinic but do keep in touch with the clinic, and lost to care patients, who were treated when they were young but for whom contact information is no longer available. We believe that many of the lost to care patients may have been advised by their healthcare provider that lifelong management of PKU was not required. For example, in 1971 Blaskovics and Nelson [9] recommended that dietary restriction to prevent mental retardation was unnecessary beyond 6 years. Each of these cohorts has a different relationship with the clinic and may require modified approaches toward re-engagement.
3.3. Best practice recommendations to engage lost to follow-up patients
Combining the results from our survey and our discussion at the Expert Meeting, we have prepared six best practice recommendations to engage lost to follow-up patients with PKU (Fig. 1) that we believe will assist clinics in helping lost to follow-up patients return to clinic. We provide evidence supporting our recommendations.
Fig. 1.
Best practice recommendations to engage lost to follow-up patients with PKU.
3.3.1. Ensure patients are aware of current treatment guidelines for PKU
The most important guideline recommendation for adult patients with PKU is that treatment should be lifelong. The current American College of Medical Genetics and Genomics (ACMG) practice guidelines [10] recommendations include the following points: “Treatment for PAH deficiency should be lifelong for patients with untreated Phe levels >360 μmol/L” and “Maintaining a treated Phe level of 120–360 μmol/L is recommended for all patients of all ages.” The guidelines note that there is strong evidence to suggest that lifelong management is essential to optimal functioning of patients with PKU. Importantly, the ACMG suggests that patients with PKU may not be aware that lifelong management is recommended, or that new advances in diet or pharmacologic treatment are available, and encourages clinics to engage LFTU patients.
The ACMG recommendations are reinforced by the recent European Guidelines for PKU [11], which state “Treatment for life is recommended for any patient with PKU” and “All adults with PKU should have lifelong, systematic follow-up in specialized metabolic centers due to specific risks which may occur during adulthood.”
3.3.2. Communicate to patients any new treatment and diet options as they become available for PKU
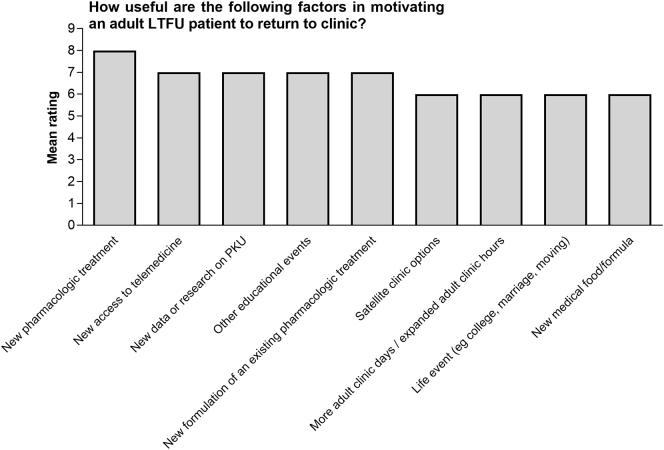
Our survey found that, in the opinion of the respondents, the greatest factor motivating lost to follow-up patients to return to clinic was the availability of a new pharmacologic treatment (mean rating on a scale of 1 to 9 = 8) (Fig. 2). Other factors considered highly motivating were new research on PKU, new types of formula or medical food, or new options for treatment. For example, one of our clinics sent out 50 letters to lost to follow-up patients with PKU informing them that a new treatment for PKU was available and that lifelong treatment is the current clinical recommendation, and this resulted in 10 patients returning to clinic. Another clinic sent out 12 letters to lost to follow-up patients with PKU letting them know that new treatments were available, and this resulted in 4 patients returning to clinic, as well as siblings to whom the information was passed. Patients may consider that the availability of new treatment/information is an incentive for them to return to clinic.
Fig. 2.
Factors motivating lost to follow-up patients with PKU to return to clinic
3.3.3. Consider the neuropsychological and neurocognitive aspects of PKU
The ACMG guidelines recognize that when PKU treatment lapses, as for lost to follow-up patients, a variety of disabling neuropsychological and neurocognitive symptoms can develop, such as deficits in executive functioning, anxiety, depression, and phobias [10]. Indeed, our survey found that the greatest barrier to clinic attendance was “neuropsychological issues affecting cognitive ability” (mean rating on a scale of 1 to 9 = 7). Our survey also found that the “lack of understanding of the burden of disease” was a significant barrier (mean rating = 6.5). This finding is supported by a survey of Italian adult patients with PKU that found that 40% (44/111) of patients did not consider PKU a disease [12]. A combination of not understanding or recognizing the burden of illness and neuropsychological conditions may create a cycle that impedes clinic attendance and adherence to treatment.
The importance of the neuropsychological and neurocognitive aspects of PKU is recognized in the current ACMG practice guidelines [10], which include the following points: “The risk for neurocognitive or psychological symptoms in PAH deficiency is related to age of onset of therapy, lifelong Phe levels, and adherence to treatment” and “Appropriate intellectual and mental health assessments are an important component of care for individuals affected with PKU.” The guidelines note that patients may experience an improvement in neuropsychological and neurocognitive symptoms with the reinstitution of treatment.
We believe that the neuropsychological and neurocognitive aspects of PKU in adult patients are the principal reason why patients remain lost to follow-up. Not only do patients often deny that they have a disease, they also have little awareness of their symptoms. Patients may not be able to grasp the concept that their Phe levels may cause their symptoms, particularly because the symptoms are not physically obvious. In summary, patients with PKU may not understand the practical impact of poor Phe control on daily life [10]. Furthermore, given the limitations that patients may have for planning and organizing appointments, careful communication with lost to follow-up patients is important in ensuring they attend the clinic appointment. Therefore, helping patients understand their symptoms may be due to elevated Phe, and, despite the challenges involved, offering adult lost to follow-up patients with PKU psychological support may be incentives for them to return to clinic. Importantly, a specific tool for assessing the quality of life of patients with PKU and the effectiveness of treatment and psychotherapy is available [13].
3.3.4. Prioritize motivated lost to follow-up patients
Our survey found that the average wait time for a clinic appointment was up to 3 months, and did not differ between existing patients (29.9% [20/67] respondents note <1 month average wait time for a clinic appointment, 56.7% [38/67] 1–3 months), new patients (40.3% [27/67] <1 month, 47.8% [32/67] 1–3 months), and lost to follow-up patients returning to clinic (32.8% [22/67] <1 month, 52.2% [35/67] 1–3 months). Traditionally, engaged patients, newborns, and pregnant women receive priority care. However, lost to follow-up patients may not return to clinic if there is not immediacy in the response to their enquiry, as there may only be a small window of opportunity to help them re-engage with the clinic. We highly recommend that clinics prioritize appointments for motivated lost to follow-up patients who contact the clinic. We suggest that, even though these approaches may have administrative challenges, clinics consider opening appointments, holding regular group sessions, using telemedicine, and/or initiating home visits to capitalize on the opportunity to begin treatment as soon as possible.
3.3.5. Explore new approaches of outreach to lost to follow-up patients
Our survey found that 11.9% (8/67) of the clinics did not reach out to lost to follow-up patients, although we note that most clinics are limited in their resources. For those clinics who did attempt to contact patients, the communication was usually made by the dietitian (62.7% [42/67] of respondents) or the front office staff (32.8% [22/67]). The first communication attempt was usually by phone (50.7% [34/67] of respondents) and the second attempt by mail (34.3% [23/67]); final attempts at communication were made by reaching out to primary care physicians or family members.
Our survey found that, in the opinion of the respondents, factors motivating lost to follow-up patients to return to clinic included access to telemedicine (mean rating on a scale of 1 to 9 = 7), educational events (mean rating = 7), satellite clinics (mean rating = 6), and adult clinic days/hours (mean rating = 6).
While acknowledging that clinics are limited by funding, time, and staff, we believe there are numerous options available to clinics to engage lost to follow-up patients with PKU. In support of survey findings, our recommendations include:
Tailoring clinic visits to this population. For example, PKU-only clinic days/hours, adult-only clinic days/hours or metabolic centers (to overcome attending a pediatric clinic), and satellite clinics (to overcome transportation issues).
Telemedicine. This may be used to overcome anxiety over clinic visits and transportation issues.
Holding social events for adults. Encourage patients to attend activities, educational seminars, patient dinners, patient panels, or camps that are designed specifically for adults with PKU. Events may also target younger or older adults.
Communication using social media. The use of social media may be limited by the institution, but will target the correct generation and remove the embarrassment associated with personal communication. Clinics should also consider the use of texts and emails rather than phone calls (within workplace guidelines).
Collaboration with local advocacy groups to connect with patients.
Collaboration with companies. This relationship could be used to send out samples of pharmacologic treatments or medical foods/formula to patients, or highlight new clinical trials that patients may like to participate in.
Establishing a PKU registry. This would enable clinics to target communications specifically to patients with PKU.
Preparing publications that target primary care physicians. Primary care physicians can be made aware of the symptoms of PKU and may refer patients to metabolic clinics.
Of importance is how we communicate with lost to follow-up patients with PKU. We believe that all communications should be motivational because the type and tone of the communication can have a significant influence on the patient’s decision to return to clinic. Some ways to achieve this include focusing communications on:
-
•
recommendations from ACMG guidelines
-
•
the availability of new treatments
-
•
the total wellness of the patient, i.e. is the patient healthy overall and capable
-
•
the idea that low Phe levels can contribute to symptoms and comorbidities
-
•
the quality of life benefits from having Phe levels under control
-
•
achievable goals that are personal and individual
-
•
places to seek further information, e.g. websites
However, communications should NOT focus only on Phe levels as this detracts from a holistic approach to care.
3.3.6. Formalize approaches to track and/or identify PKU patients
At present, there are no formal electronic programs available to track patients that can highlight those individuals who are lost to follow-up. Most current electronic medical record (EMR) systems do not have the capacity for queries or flagging of patients. To overcome this, clinics often go by memory or have monthly meetings to discuss patients who are lost to follow-up. Given that our survey found that some of the difficulties in engaging lost to follow-up patients included the ability to obtain contact information (mean rating on a scale of 1 to 9 = 7), the time needed to contact patients (mean rating = 6), and the accessibility of historical records (mean rating = 5), the development of a patient record system specifically for patients with PKU is warranted. Survey respondents also suggested that more technologically advanced ways of tracking clinic attendance (mean rating on a scale of 1 to 9 = 8), additional staff dedicated to tracking patients (mean rating = 7), and increased cooperation with patient support groups (mean rating = 6) would be useful options for tracking/identifying lost to follow-up patients with PKU.
We suggest that tracking options may also include Department of Health records, state newborn screening records, ICD-10 query of records, regular scheduled formal reviews of patient status with clinic staff, and working with local primary care physicians. However, the development of more appropriate and specific electronic programs is paramount.
3.4. Challenges
As acknowledged in the ACMG guidelines, lost to follow-up patients with PKU present a “major therapeutic challenge” [10]. As expected there are numerous challenges to engage the lost to follow-up patients including, but not limited to:
Neuropsychological and neurocognitive issues associated with PKU. This includes lack of understanding of the burden of illness and the practical implications, such as remembering appointments or anxiety related to clinic visits. As noted previously, this is the major hurdle that clinicians need to overcome to return patients to clinic. In addition to current issues, a patient’s prior clinic and disease experience may have been overwhelmingly negative, and include painful blood draws, physician complaints of noncompliance, negative associations with diet, and social stressors when growing up.
Available resources are limited. This includes time, clinic staff, appointments, distance to clinic, adult clinics, staff training, and non-central scheduling. Ideally, each patient would be assigned a case manager, but in reality, this is rarely a possibility.
Insurance coverage. Patients should be supported in their interactions with insurance companies to ensure coverage or continuing coverage.
Identifying lost to care patients that are currently unknown to clinics. Although this is challenging, this group may be unaware of lifelong management recommendations.
3.5. Limitations
Our best practice recommendations are limited by the survey responses, which only included 50% of PKU clinics in the US, the personal experiences and opinions of the Expert Meeting members, and the relative lack of information available in the literature on the management of patients with PKU. The value of our recommendations is also dependent upon the willingness of clinics to spend time and effort to engage lost to follow-up PKU patients.
4. Conclusions
In conclusion, we strongly advocate the importance of engaging lost to follow-up patients with PKU. In this report, we have presented a framework to assist the clinician with engaging lost to follow-up patients with PKU. We encourage implementation of our best practice recommendations, including ensuring patients are aware of management guidelines, communicating new treatments, considering neuropsychological aspects of PKU, prioritizing lost to follow-up patients, exploring outreach, and formalizing tracking of patients. Although it takes time and effort to engage lost to follow-up patients, we believe that clinics are capable of supporting this significant patient group.
Funding
The survey and Expert Meeting were funded by BioMarin Pharmaceutical Inc. Medical writing assistance was provided by Janelle Keys, PhD, CMPP, and John Bilbruck, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by BioMarin. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). Trinity Partners LLC consulting was contracted, and funded, by BioMarin to conduct the survey.
Author contributions
All authors participated in the Expert Meeting, including the interpretation of survey results and in the drafting, critical revision, and approval of the final version of the manuscript.
Role of the sponsor
BioMarin was involved in the survey design (in conjunction with members of the Expert Meeting), organized the Expert Meeting, and reviewed the final version of the manuscript. Data collection and analysis for the survey results were performed by Trinity Partners LLC and funded by BioMarin.
Declaration of Competing Interest
All authors were members of the Expert Meeting, organized by BioMarin. Expert Meeting members signed a written contract and received a fair market value payment for their participation in the meeting. AT was on the speaker’s bureau for BioMarin and is a recent employee of BioMarin; all other authors have no additional conflicts of interest to declare.
Acknowledgments
The authors would like to thank other members of Expert Meeting, the PKU Lost to Follow-Up Recommendations Group, for their participation and insights: Zineb Ammous (Indiana Children’s Hospital), Gerald Berry (Boston Children’s Hospital), Melanie Colville (Phoenix Children’s Hospital), Brittany Holmes (Yale University School of Medicine), Jenny Hudson Antony (University of Texas Health Science Center at Houston), Stephanie Offord (Children’s Hospital of Wisconsin), Carlos Prada (Cincinnati Children’s Hospital), and Bridget Tomic (Washington University in St Louis).
Contributor Information
Jennifer Beazer, Email: jenn@pkunews.org.
Jane Breck, Email: breck.jane999@gmail.com.
Caroline Eggerding, Email: eggerding-caroline@cooperhealth.edu.
Patricia Gordon, Email: pgordon@pennstatehealth.psu.edu.
Stephanie Hacker, Email: shacker@med.miami.edu.
Amie Thompson, Email: amotmetrd@gmail.com.
References
- 1.Camp K.M., Parisi M.A., Acosta P.B., Berry G.T., Bilder D.A., Blau N. Phenylketonuria scientific review conference: state of the science and future research needs. Mol. Genet. Metab. 2014;112:87–122. doi: 10.1016/j.ymgme.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Singh R.H., Rohr F., Frazier D., Cunningham A., Mofidi S., Ogata B. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet. Med. 2014;16:121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strisciuglio P., Concolino D. New strategies for the treatment of phenylketonuria (PKU) Metabolites. 2014;4:1007–1017. doi: 10.3390/metabo4041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Therrell B.L., Padilla C.D., Loeber J.G., Kneisser I., Saadallah A., Borrajo G.J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015;39:171–187. doi: 10.1053/j.semperi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Bilder D.A., Noel J.K., Baker E.R., Irish W., Chen Y., Merilainen M.J. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev. Neuropsychol. 2016;41:245–260. doi: 10.1080/87565641.2016.1243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry S.A., Brown C., Grant M., Greene C.L., Jurecki E., Koch J. Newborn screening 50 years later: access issues faced by adults with PKU. Genet. Med. 2013;15:591–599. doi: 10.1038/gim.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurecki E.R., Cederbaum S., Kopesky J., Perry K., Rohr F., Sanchez-Valle A. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017;120:190–197. doi: 10.1016/j.ymgme.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Thomas J., Nguyen-Driver M., Bausell H., Breck J., Zambrano J., Birardi V. Strategies for successful long-term engagement of adults with phenylalanine hydroxylase deficiency returning to the clinic. J. Inborn Errors Metab. Screenings. 2017;5:1–9. [Google Scholar]
- 9.Blaskovics M.E., Nelson T.L. Phenylketonuria and its variations. A review of recent developments. Calif. Med. 1971;115:42–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Vockley J., Andersson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 2014;16:188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 11.van Wegberg A.M.J., MacDonald A., Ahring K., Bélanger-Quintana A., Blau N., Bosch A.M. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 2017;12 doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazzorla C., Bensi G., Biasucci G., Leuzzi V., Manti F., Musumeci A. Living with phenylketonuria in adulthood: the PKU ATTITUDE study. Mol. Genet. Metab. Rep. 2018;16:39–45. doi: 10.1016/j.ymgmr.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnault A., Burlina A., Cunningham A., Bettiol E., Moreau-Stucker F., Benmedjahed K. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: the phenylketonuria - quality of life (PKU-QOL) questionnaires. Orphanet J. Rare Dis. 2015;10 doi: 10.1186/s13023-015-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]