Abstract
Background
Cue-induced brain reactivity has been suggested to be a fundamental and important mechanism explaining the development, maintenance, and relapse of addiction, including Internet gaming disorder (IGD). Altered activity in addiction-related brain regions has been found during cue-reactivity in IGD using functional magnetic resonance imaging (fMRI), but less is known regarding the alterations of coordinated whole brain activity patterns in IGD.
Methods
To investigate the activity of temporally coherent, large-scale functional brain networks (FNs) during cue-reactivity in IGD, independent component analysis was applied to fMRI data from 29 male subjects with IGD and 23 matched healthy controls (HC) performing a cue-reactivity task involving Internet gaming stimuli (i.e., game cues) and general Internet surfing-related stimuli (i.e., control cues).
Results
Four FNs were identified that were related to the response to game cues relative to control cues and that showed altered engagement/disengagement in IGD compared with HC. These FNs included temporo-occipital and temporo-insula networks associated with sensory processing, a frontoparietal network involved in memory and executive functioning, and a dorsal-limbic network implicated in reward and motivation processing. Within IGD, game versus control engagement of the temporo-occipital and frontoparietal networks were positively correlated with IGD severity. Similarly, disengagement of temporo-insula network was negatively correlated with higher game-craving.
Discussion
These findings are consistent with altered cue-reactivity brain regions reported in substance-related addictions, providing evidence that IGD may represent a type of addiction. The identification of the networks might shed light on the mechanisms of the cue-induced craving and addictive Internet gaming behaviors.
Keywords: behavioral addiction, cue-reactivity, fMRI, functional brain networks, ICA, Internet gaming disorder
Introduction
Internet gaming disorder (IGD), proposed to represent a putative behavioral addiction (Holden, 2001), has been included in the Section III of the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders as a condition deserving further study (American Psychiatric Association, 2013). A common neurobehavioral basis of addictions may be classical and operant conditioning mechanisms that promote the acquisition and persistence of maladaptive behaviors (Heinz, Beck, Grusser, Grace, & Wrase, 2009; Heinz, Beck, Wrase, et al., 2009). That is, addiction-related cue conditioning is implicated in the development and relapse of addictive behaviors (Dickinson, Smith, & Mirenowicz, 2000; Robinson & Berridge, 1993). Thus, the examination of brain responses to Internet gaming cues among IGD may provide insight into one of the most important mechanisms of motivational and compulsive Internet gaming behavior (Tiffany & Conklin, 2000).
Cue-reactivity paradigms have been suggested to be a reliable and ecologically valid way to evaluate craving in substance use disorder and behavioral addiction (Ko et al., 2013; Pelchat, Johnson, Chan, Valdez, & Ragland, 2004; Potenza et al., 2003; Wilson, Sayette, & Fiez, 2004), such as IGD (Ko et al., 2013). These paradigms represent a model of cue-induced responses that provoke memory, positive anticipation, an imbalanced motivational state, withdrawal syndrome, rewarding drive, and lasting craving response (Ko et al., 2013; Sinha & Li, 2007; Skinner & Aubin, 2010).
Multiple studies have examined cue-induced brain reactivity in IGD using functional magnetic resonance imaging (fMRI) and found that IGD showed altered activity in several regions that were implicated in addictive disorders. Specifically, these regions include the orbitofrontal cortex (OFC), related to evaluation and expectancy of reward of stimuli; the nucleus accumbens and caudate, involved in reward processing; the anterior cingulate cortex (ACC), associated with attention and motivation processes; the parahippocampus, implicated in connecting environmental cues to the internal states; the dorsolateral prefrontal cortex (DLPFC), related to processing goal-directed behaviors; the amygdala, associated with initiating conditioned and unconditioned behavior; and the cuneus and precuneus, involved in processing visual memories associated with conditioned stimuli (Ko et al., 2009, 2013; Liu et al., 2017; Sun et al., 2012; Weinstein & Lejoyeux, 2015).
Increasing evidence in cognitive neuroscience suggests that an individual’s behaviors are governed by the interactions of multiple brain regions (Park & Friston, 2013), which make up coherent functional networks (FNs; Smith et al., 2009). A certain brain region may participate in multiple cognitive processes interacting with other structures (Xu et al., 2013), particularly when it involves in multiple FNs. In this case, this brain region may not manifest as significant in general linear model (GLM) analyses, given that it may serve simultaneous but different or even opposite cognitive roles (Xu, Calhoun, & Potenza, 2015). As such, it is critical to identify FNs that integrate several brain regions for a given behavior. Group independent component analysis (ICA) of fMRI has been used to identify large-scale FNs by seeking sources of spatiotemporally coherent components that comprise the BOLD time course (Calhoun, Adali, Pearlson, & Pekar, 2001), and is able to identify alterations in task-related, concurrent, but opposite changes in time courses in the same brain regions that may not be detected by GLM-based analyses (Xu et al., 2015). Previous research using ICA in IGD has identified alterations in frontoparietal executive control networks during cognitive control and risky decision-making (Wang et al., 2016, 2017). However, less is known regarding potential FN alterations associated with cue-induced activity in IGD.
Moreover, most studies on cue-reactivity in IGD examined neural responses to Internet gaming cues relative to non-gaming, non-Internet-related, or neutral object cues (e.g., mosaic pictures; Ko et al., 2009, 2013). However, reported alterations in neural responses to gaming-related cues in IGD might be, at least in part, ascribed to the experience of gaming-related cues as (a) having a positive valence or (b) being associated with Internet use in general. In fact, prior studies found a large overlap in the brain circuitry (e.g., OFC, striatum, and amygdala) that underlay reactivity to cocaine stimuli relative to other evocative, non-drug stimuli (e.g., sex), suggesting the need to distinguish the responses to positive valence from responses to addiction-related cues (Garavan et al., 2000).
In this study, we used a cue-reactivity paradigm that included Internet-gaming stimuli (i.e., game stimuli) and general Internet surfing stimuli (i.e., control stimuli) and sought to identify FNs associated with game-control reactivity (i.e., the direct comparison of responses to Internet gaming vs. general Internet surfing cues) among IGD and age-matched control participants. Internet-related cues instead of neutral cues were used to partition out the potential effects of positive valence and more importantly those of Internet use. Focusing on ICA-identified, game-control-related networks, we aimed to (a) investigate group differences in FN engagement during game-control reactivity and (b) explore the correlations between FN engagement and clinical assessments within IGD (i.e., the severity of IGD and the craving for Internet gaming).
Materials and Methods
Participants and clinical assessments
Given the higher occurrence of IGD among men (Ko et al., 2009), the final sample included 29 male IGD subjects and 23 matched healthy controls (HCs). This final sample of 52 subjects represents a subset of the individuals included in our previous report (Liu et al., 2017), as we used a stricter screen criteria on IGD. Participants represented a total of 432 males (319 potential IGD subjects playing Internet gaming frequently and 113 potential HCs playing Internet gaming occasionally), who were recruited through advertisements posted at local universities and completed initial web-based screening. Similar to our previous study (Zhang et al., 2016), IGD was diagnosed using the following criteria: (a) scores of the Chinese Internet Addiction Scale (CIAS; Chen, Weng, Su, Wu, & Yang, 2003) ≥67 (Ko et al., 2009), (b) spent more than half of the time online on games, and (c) spent ≥14 hr on Internet gaming per week (i.e., with averagely at least 2 hr spent on Internet gaming every day). The criteria of HC included (a) scores of CIAS ≤60 and (b) never spent ≥2 hr per week on Internet gaming. Additional criteria for all the participants included: (a) 18–30 years of age, (b) right-handed, (c) no history of other psychiatric or neurological illness, (d) no current or previous use of illegal substances or gambling, (e) not currently taking any psychotropic medications, and (f) suitable for magnetic resonance imaging (MRI) scanning. All the participants also completed the Beck Anxiety Inventory (BAI; Beck, Brown, Epstein, & Steer, 1988) and Beck Depression Inventory (BDI; Beck, Erbaugh, Ward, Mock, & Mendelsohn, 1961) to assess anxiety and depressive symptoms.
Cue-reactivity task
The cue-reactivity task was the same as that used in our previous study (Liu et al., 2017). Briefly, 24 blocks were presented. Each block contained five stimuli, with each presented in a pseudorandom order for 3.7 s following a 0.3-s fixation cross. These blocks were divided into two separate runs, each of which included three game blocks (i.e., Internet gaming pictures) and three control blocks (i.e., general Internet surfing pictures) alternating with six mosaic baseline blocks (i.e., degraded mosaic stimuli of game and control cues following the corresponding game or control blocks). Following each game or control block, a one-item 6-point Visual Analogue Scale (ranging from 1 = “not craving at all” to 6 = “severe craving”) lasting 4 s was used to assess the craving for Internet gaming or general online surfing, respectively.
Image acquisition
MRI was conducted using a Siemens Trio 3-Tesla scanner (Siemens, Erlangen, Germany). Forty-one 3.0-mm axial fMRI data were acquired using an echo-planar imaging sequence with the parameters as follows: TR = 2,000 ms; TE = 25 ms; flip angle = 90°; matrix size = 64 × 64; resolution = 3 × 3 mm2. The slices were tilted 30° clockwise from the anterior commissure–posterior commissure (AC-PC) plane to obtain better signals in the OFC. One hundred forty-four high-resolution slices were obtained using T1-weighted sagittal 3-D magnetization prepared rapid gradient-echo sequence: TR = 2,530 ms; TE = 3.39 ms; TI = 1,100 ms; FA = 7°; and FOV = 256 × 256 mm; thickness = 1.33 mm in-plane resolution = 1 × 1 mm2.
Group independent component analysis
Spatial processing of functional data was performed in SPM8 (Welcome Department of Cognitive Neurology, London, UK) and included reorientation to the AC-PC line, realignment, normalization to 3 × 3 × 3 mm3 MNI space, and smoothing with a Gaussian kernel of 5 mm at full width half maximum.
The spatially processed images were then analyzed in a using the Group ICA of fMRI Toolbox (version 2.0e; http://mialab.mrn.org/software/gift). Briefly, data from all the participants were concatenated into a single data set and then were reduced in dimensionality using two stages of principal component analysis (Calhoun et al., 2001). To examine large-scale functional networks, 30 independent components were then extracted from the group aggregate with the Infomax algorithm (Abou-Elseoud et al., 2010; Bell & Sejnowski, 1995). Extraction was repeated 20 times using ICASSO to assess the stability of independent components (Himberg, Hyvärinen, & Esposito, 2004). The time course and spatial map for each component were back-reconstructed for each participant (Calhoun et al., 2001). One-sample t-tests were performed on Z-scaled spatial maps across all participants in SPM8 to determine regions positively significantly integrated into each component at a voxel-level family-wise error (FWE)-corrected pFWE < 0.0001 combined with a cluster extent threshold of 100 voxels.
Each of the 30 components had a cluster quality index greater than 0.8, indicating a highly stable ICA decomposition. We visually inspected the spatial maps, according to previous studies, visualization, and/or prior knowledge (Laird et al., 2011; Smith et al., 2009); 13 of the 30 extracted components were identified as representing artifacts and were excluded from all subsequent analyses.
Model specification and task-relatedness
Modeling of the cue-reactivity task was performed in SPM8, including the onset of task events (i.e., game cues, control cues, and craving rating periods, while mosaic baseline blocks were as reference), convolved with the canonical hemodynamic response function, and the motion parameters from realignment as regressors of no interest. In order to examine the task relevance of each component, a multiple regression was performed to compare component time courses with the modeled event time courses for the game, control, and craving-rating events. Resulting beta weights indicated the extent to which a given network was temporally associated with, or “engaged” during each task condition. Beta weights were averaged across both runs for game and control conditions for each subject.
Statistical analyses of beta weights
To identify which of the 17 non-artifactual components represented cue-reactivity-related FNs, a “game-control” contrast, calculated as the beta weight difference between game cues relative to control cues, was examined with two within-group one-sample t-tests against zero using Bonferroni correction for multiple comparisons (α as .05/34 = .0015). Eight components displayed a significant difference from zero for “game-control” contrast, indicating differential engagement induced by the Internet gaming stimuli versus the general Internet surfing stimuli and were thus considered to represent FNs associated with game-control reactivity.
For the eight FNs associated with cue-reactivity in either IGD or HC, two-sample t-tests were performed between IGD and HC groups to examine the group differences in the “game-control” contrast. Because IGD reported higher anxiety, depression scores, and proportion of smoking than HC, we investigated the group differences in these eight FNs during cue-reactivity regressing BDI, BAI scores, and state of smoking (smoking = 1 and no smoking = 0). Four FNs showed altered activity in IGD (Bonferroni corrected α as .05/8 = .0063). FNs were labeled based on the primary regions of positive spatial integration in each network according to the known intrinsic connectivity networks (Allen et al., 2011; Laird et al., 2011; Smith et al., 2009).
Correlation analyses were used to explore relationships between FN engagement patterns and subjective craving ratings and addiction severity (score of CIAS and the time spent on Internet gaming per week) within the IGD. Moreover, exploratory correlational analyses were performed on the beta weights to examine potential interactions between FNs during cue-reactivity in IGD.
Ethics
This study was approved by the institutional review board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University (study number: ICBIR_A_0075_001), with all participants providing written informed consent.
Results
Demographic and behavioral characteristics
The demographic characteristics of the IGD and HC are shown in Table 1. Compared with HC, IGD reported higher proportion of smoking, higher levels of depression, marginally significant higher anxiety, and higher craving for Internet gaming following game cues versus general Internet surfing cues.
Table 1.
Participants’ characteristics and craving ratings
| IGD (n = 29) | HC (n = 23) | t/χ2/U/F | p | |
|---|---|---|---|---|
| mean ± SD | mean ± SD | |||
| Characteristics | ||||
| Age | 22.59 ± 2.24 | 23.09 ± 2.13 | −0.82 | .418 |
| Years of education | 15.52 ± 1.43 | 15.91 ± 1.47 | −0.98 | .333 |
| CIAS scores | 75.55 ± 7.64 | 45.65 ± 9.80 | 12.37*** | <.001 |
| Time Internet gaming (hours per week) | 21.86 ± 9.82 | 0.92 ± 0.20a | 6.28*** | <.001 |
| Alcohol use (at least once per month) | 25b/0.86c | 17b/0.74c | 1.25 | .264 |
| Cigarette use (at least once per month) | 5b/0.17c | 0b/0.00c | 4.39* | .036 |
| Depression severity (BDI scores) | 7.86 ± 5.67 | 2.91 ± 3.04 | 4.03*** | <.001 |
| Anxiety severity (BAI scores) | 5.48 ± 5.25 | 3.18 ± 3.16 | 1.96 | .056 |
| Craving ratings | ||||
| Background craving | 29.83 ± 7.62 | 13.30 ± 6.93 | 41.35d*** | <.001 |
| Craving for Internet gaming | 4.95 ± 1.16 | 1.52 ± 0.67 | 109.08d (39.15)e*** | <.001 |
| Craving for general Internet surfing | 3.37 ± 1.21 | 2.94 ± 1.10 | 0.78d (0.93)e | .383d (.341)e |
| Craving (game-control) | 1.58 ± 1.09 | −1.42 ± 1.04 | 66.50d (35.93)e*** | <.001 |
Note. SD: standard deviation; IGD: Internet gaming disorder; HC: healthy control; CIAS: Chinese Internet Addiction Scale; FTND: Fagerstrom test for nicotine dependence; BAI: Back Anxiety Inventory; BDI: Beck Depression Inventory; Background craving: assessed by an 8-item Likert scale adapted from the Questionnaire of Smoking Urges; Craving (game-control): scores of Craving for Internet gaming – scores of Craving for general Internet surfing stimuli.
an = 6. bThe number of participants. cThe rate of alcohol and cigarette user. dF test regressing BAI, BDI, and state of smoke. eF test regressing BAI, BDI, state of smoke, and background craving.
*p < .05. ***p < .001.
FNs during reactivity in IGD
A total of four FNs were identified as related to game cues relative to general Internet surfing control cues (i.e, game-control) and differed in game-control engagement between groups. The regional composition of each FN is provided in Table 2. In IGD, all the four FNs were more strongly engaged (or disengaged) during game cues relative to control cues. In comparison, among HCs, the four FNs were either more strongly engaged/disengaged during control cues or did not differ in engagement between cue conditions.
Table 2.
Regional integration of altered game-control-related FNs
| Network/region | Hemisphere | κ | x | y | z | Z |
|---|---|---|---|---|---|---|
| Temporo-occipital network | ||||||
| Middle occipital gyrus/fusiform | R/L | 4,922 | 33 | −66 | −12 | 30.15 |
| Temporo-insular network | ||||||
| Superior temporal gyrus/insula | R | 2,344 | 42 | −18 | 15 | 24.24 |
| Superior temporal gyrus/insula | L | 2,229 | −36 | −24 | 3 | 21.63 |
| Supplementary motor area | R/L | 364 | 6 | −3 | 63 | 15.48 |
| Frontoparietal network | ||||||
| Middle frontal gyrus/inferior parietal lobe | L | 4,347 | −45 | −42 | 45 | 23.08 |
| Inferior frontal gyrus/middle frontal gyrus | R | 774 | 45 | 9 | 33 | 18.06 |
| Cerebellum posterior lobe | R | 654 | 24 | −75 | −45 | 19.63 |
| Middle temporal gyrus | L | 506 | −54 | −54 | −9 | 20.27 |
| Inferior parietal lobule | R | 403 | 33 | −66 | 36 | 18.15 |
| Inferior temporal gyrus | R | 251 | 57 | −48 | −12 | 15.86 |
| Middle frontal gyrus | R | 205 | 30 | 6 | 54 | 14.24 |
| Anterior cingulum | R/L | 108 | 0 | 6 | 27 | 20.94 |
| Dorsal-limbic network | ||||||
| Midbrain/thalamus/putamen | R/L | 2027 | 6 | −21 | −3 | 22.87 |
| Cingulate gyrus/supplementary motor area | R/L | 623 | 0 | 6 | 54 | 15.37 |
Note. Voxel-level family-wise error (FWE) corrected pFWE < .0001, cluster extent threshold (κ) > 100 voxels. Peak coordinates are reported in MNI space. FNs: functional networks.
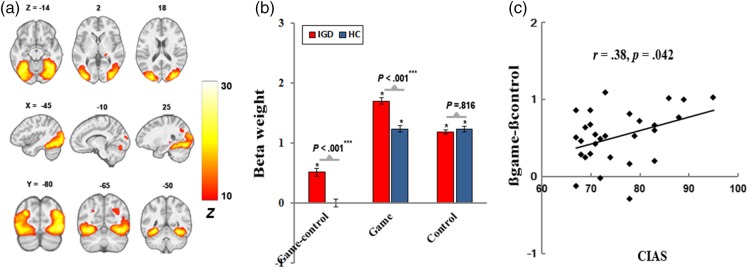
A temporo-occipital network (Figure 1), consistent with IC48 of Allen et al. (2011) and IC10 of Laird et al. (2011), consisted of coordinated signals in the bilateral middle occipital and temporal cortices. Activation in this network was greater for the “game-control” contrast and positively correlated with the CIAS scores among IGD (r = .38, p = .042).
Figure 1.
The temporo-occipital network. (a) Spatial map displayed at a threshold of pFWE < .0001 and cluster size (κ) > 100; (b) greater engagement for “game-control” contrast in IGD; and (c) positive correlation of cue-induced FN engagement with CIAS scores
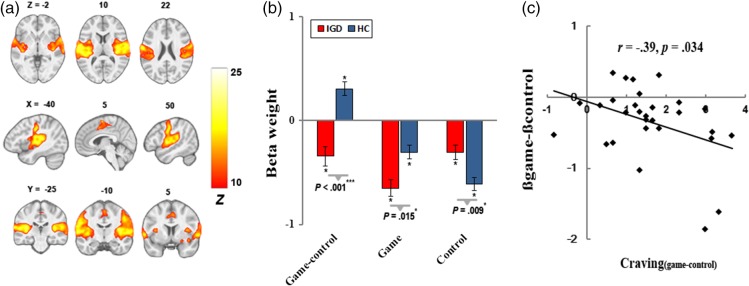
A temporo-insular network (Figure 2), corresponding to IC17 of Allen et al. (2011) and IC16 of Laird et al. (2011), included coordinated signals in the bilateral primary auditory cortices, superior temporal gyrus (STG), and insular. IGD showed greater disengagement (negatively engaged) for the “game-control” contrast, and there was a negative association between activity of this network and craving for Internet gaming (following game cues vs. general Internet surfing cues) within IGD (r = −.39, p = .034).
Figure 2.
The temporo-insular network. (a) Spatial map displayed at a threshold of pFWE < .0001 and cluster size (κ) > 100; (b) greater disengagement for “game-control” contrast in IGD; and (c) negative correlation of cue-induced FN activity with craving for Internet gaming
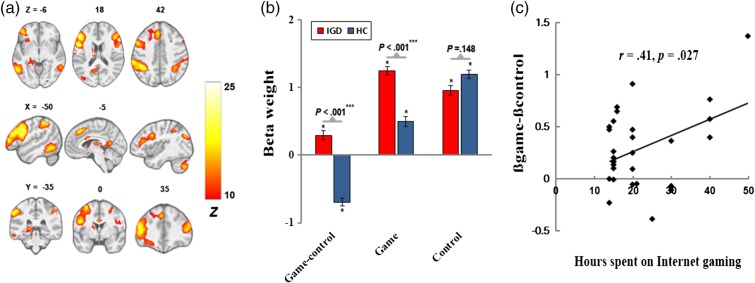
A frontoparietal network (Figure 3) incorporated regions involved in multiple frontal and attentional networks of Allen et al. (2011), including DLPFC, middle frontal cortex and inferior frontal cortex, middle temporal gyrus, and inferior parietal lobe (IPL). We found higher response to “game-control” contrast in this network and its positive correlation with hours spent on Internet gaming in IGD (r = .41, p = .027).
Figure 3.
The frontoparietal network. (a) Spatial map displayed at a threshold of pFWE < .0001 and cluster size (κ) > 100; (b) greater engagement for “game-control” contrast in IGD; and (c) positive correlation of cue-induced FN engagement with hours spent on Internet gaming
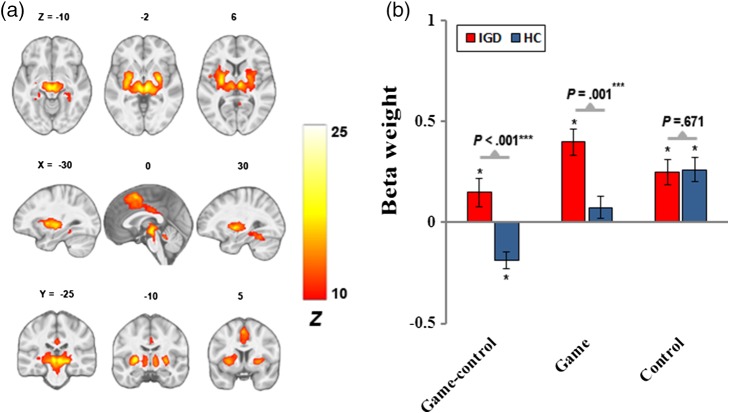
A dorsal-limbic network (Figure 4), similar to IC21 in Allen et al. (2011) and IC3 of Laird et al. (2011), composed of coordinated signals in the putamen, thalamus, midbrain, and dorsal ACC. IGD showed greater engagement of this network in the “game-control” contrast than HC.
Figure 4.
The dorsal-limbic network. (a) Spatial map displayed at a threshold of pFWE < .0001 and cluster size (κ) > 100; (b) greater engagement for “game-control” contrast in IGD
The exploratory between FN correlational analyses of “game-control” beta weights revealed that greater engagement of the frontoparietal network was positively associated with engagement of the temporo-occipital network (r = .60, p < .001) and the dorsal-limbic network (r = .44, p = .016), and negatively associated with disengagement of the temporo-insular network (r = −.37, p = .048). In addition, engagement of the dorsal-limbic network was positively correlated with engagement of the temporo-occipital network (r = .37, p = .048) (see Supplementary Figure S1).
Discussion
This study investigated functional brain networks associated with cue-reactivity in IGD. We identified four FNs that differed in engagement during Internet gaming stimuli as compared to general Internet surfing stimuli (game-control), and differed in engagement in IGD as compared to HC. Specifically in IGD, game-control engagement of the temporo-occipital and frontoparietal network was associated with severity of IGD (CIAS scores and hours spent on gaming, respectively). Game-control disengagement of a temporo-insular network was associated with craving. However, engagement of a dorsal-limbic was not associated with any clinical measures. These results provide insights into cue-induced FN activity and possible mechanisms of craving and addictive behaviors in IGD. The identified four FNs were discussed in details as follows.
The temporo-occipital network
The temporo-occipital network has been linked to motivation and visual processing, and associated with viewing complex, often emotional, stimuli (Laird et al., 2011). In IGD, greater engagement of this network in response to the “game-control” contrast was consistent with the greater visual processing in the middle occipital cortex and fusiform gyrus induced by addiction-related cues as compared to evocative control stimuli in IGD (Liu et al., 2017; Lorenz et al., 2013), and during the “cannabis-food” contrast in cannabis addicts (Charboneau et al., 2013). In addition, the bilateral temporo-occipital coupling was also found to be higher in obese as compared to control subjects (Olde Dubbelink et al., 2008).
We found that engagement of the temporo-occipital FN was positively correlated with the CIAS scores among IGD, which is consistent with positive correlations between occipital responses to addictive cues and the severity of alcohol/nicotine dependence (Claus, Ewing, Filbey, Sabbineni, & Hutchison, 2011; Smolka et al., 2006). Overall, these results suggest that IGD showed enhanced visual processing of Internet gaming cues (e.g., greater visual scanning or processing of the image details), and this visual processing became more salient with the severity of IGD increased.
The temporo-insular network
Negative engagement, or disengagement, of the temporo-insula network has been linked to the processing of salient visual or affective stimuli (Laird et al., 2011). For example, insula has been implicated in avoidance learning and fear conditioning following exposure to aversive stimuli (Samanez-Larkin, Hollon, Carstensen, & Knutson, 2008). Thus, the insula and STG were deactivated by addiction-related cues in cannabis abusers (Charboneau et al., 2013), obesity (Garcia-Garcia et al., 2014), and IGD (Liu, Hospadaruk, Zhu, & Gardner, 2011). Moreover, we found that greater disengagement of the temporo-insula FN was correlated with increased game-craving rating within IGD. This association is in conflict with the positive correlation between activity of insula and craving reported in previous research (Abdolahi et al., 2015; Bonson et al., 2002; Naqvi, Rudrauf, Damasio, & Bechara, 2007). This discrepancy may be due to the core methodological differences between the GLM and ICA approaches and further research is needed to confirm this finding.
These results regarding the temporo-insula network suggest that response to gaming-cues in IGD may be similar to the substance users’ response to drug cue (Franken, 2003). According to the conditioned model, the Internet gaming cues, as consequence of conditioning process, would become condition stimuli for IGD, inducing a conditioned craving response to gaming-related stimuli. Thus, IGD would engage more inhibition of auditory functioning to facilitate the detection and processing of visual addiction-relevant cues, perhaps resulting in higher craving (Ryan, 2002).
The frontoparietal network
The frontoparietal network has been implicated in multiple cognitive and executive processes, including attention, goal-direction, working memory, and cognitive control (Laird et al., 2011). Correspondingly, the greater activation of DLPFC and the IPL during addiction-related cues compared with other evocative stimuli has been previously identified in IGD (Sun et al., 2012; Weinstein & Lejoyeux, 2015) as well as in cocaine abusers (Garavan et al., 2000; Wilcox, Teshiba, Merideth, Ling, & Mayer, 2011).
The importance of this network in cue-reactivity can be reflected by the cognitive model that suggests addiction-related cues can trigger positive expectations and thus may encourage addictive behaviors (Skinner & Aubin, 2010). The positive correlation between cue-induced frontoparietal network engagement and hours spent on Internet gaming in IGD may indicate that IGD who spent more time on gaming may exhibit more memories of prior gaming experiences when seeing Internet gaming cues.
An alternative explanation for greater activation of the frontoparietal network might be greater identification with the virtual world. Studies found that massively multiplayer online role-playing games addiction has been associated with self-concept impairments and increased identification with ones’ own avatar. Internet gamers display greater activity in parietal regions (especially in the left angular gyrus) during avatar reflection, and this response is associated with symptom severity (Lemenager et al., 2014, 2016). Correspondingly, multiple areas in the frontoparietal network, such as IPL (including the angular gyrus) and prefrontal cortex, were found to be involved in theory of mind and self-other distinction (Frith & Frith, 2006; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Schurz, Tholen, Perner, Mars, & Sallet, 2017; van Veluw & Chance, 2014). Thus, individuals with IGD may be more inclined to form an identification with the virtual world that could be triggered by the gaming-related cues. In addition, the alternative explanation for the association between higher engagement of the frontoparietal network and dorsal limbic network may be that the identification with the virtual world was related to a motivation to escape into the virtual world in individuals with IGD. Future research is needed to verify and confirm these group difference and correlation findings.
The dorsal-limbic network
The limbic network has been associated with reward, motivation, and emotional processing (Laird et al., 2011). In this study, IGD displayed greater cue-induced engagement of an FN comprising mostly dorsal structures of the limbic system. For example, the dorsal striatum is considered to be closely associated with incentive salience for conditioned rewarding stimuli (Berridge & Robinson, 2003), and is implicated in the habituation and automatization of compulsive drug-seeking (Everitt & Robbins, 2013).
According to the incentive salience theory, the salience of gaming and gaming-related stimuli would increase through Pavlovian conditioning (Robinson & Berridge, 1993). Consequently, the presence of gaming-related cues alone can act as a conditioned reinforcer (Dickinson et al., 2000) and then induces higher activation of reward system and reward processing (Volkow, Wang, & Baler, 2011). Consistently, greater activation in mesolimbic dopamine reward circuits was also found in drug addicts and IGD (Claus et al., 2011; Due, Huettel, Hall, & Rubin, 2002; Garavan et al., 2000; Liu et al., 2011).
The lack of associations between the engagement of this network and the severity of IGD or the craving for Internet gaming might be explained by the neural mechanisms of transition from goal-directed drug use to habitual, compulsive drug use, and possible mediation of dorsal striatum for the habitual compulsive behavior (Everitt & Robbins, 2013).
General discussion
Neurobiological models of addiction posit that addiction-related cues provoke enhanced activation of the visual, memory, reward, and motivation processing that may commandeer execution control systems (Volkow et al., 2010). Consistent with these theories of substance-related addiction, current findings of viewing Internet gaming cues relative to general Internet surfing cues indicated greater engagement of visual, attentional, motivational, reward, and executive systems and greater disengagement of the auditory processing system in IGD. This pattern of FN engagement indicates IGD’s attentional and emotional bias toward gaming cues, which may elicit increased craving and ultimately can lead to addictive gaming behaviors. The consistent findings, with previous GLM-based research on cue-reactivity in IGD (Ko et al., 2013; Weinstein & Lejoyeux, 2015), obesity (Wiemerslage et al., 2016), and substance addictions (Volkow et al., 2010), provide further evidence for that the mechanisms of behavioral addiction resemble those in substance addiction (Potenza, 2008).
Furthermore, the interactions between these FNs may provide further insights for the understanding of the cue-induced craving and Internet gaming behaviors in IGD, and perhaps addictive behaviors more broadly. Exploratory correlational analyses revealed that the frontoparietal network was positively correlated with the temporo-occipital and dorsal-limbic networks, and negatively associated with the temporo-insular network. These correlations may indicate a coordination among these networks in experiencing the Internet gaming cues toward final planning for, and partaking in, Internet gaming behaviors. Specifically, the frontoparietal network may draw attention to addiction-related cues through integration of sensory input from the tempo-occipital and temporo-insular networks, linking the stimuli to gaming memories (Goldman-rakic & Leung, 2002), and planning gaming behaviors through interactions with the reward and motivational processing in the dorsal-limbic network (Mitchell, 2011; Skinner & Aubin, 2010).
Several theoretical models have stressed the important role of reward sensitivity and craving underlying the development and maintenance of IGD. These models suggest enhanced craving responses are associated with reduced executive functioning and inhibitory control, and the reductions in executive control in turn lead to diminished inhibition of motivation-seeking and craving. Such imbalances would promote maladaptive decision-making, resulting in excessive Internet gaming and eventually IGD. Furthermore, the excessive Internet gaming and the gratification perceived would lead to an increase in cue-reactivity and craving, through conditioning and reinforcement processes. A vicious cycle is formed to heavy the severity of IGD (Brand, Young, Laier, Wolfling, & Potenza, 2016; Dong & Potenza, 2014).
Note that our findings using the ICA approach revealed some inconsistencies in brain activity associated with cue-reactivity relative to prior GLM-based studies of cue-reactivity in IGD. This discrepancy is likely due to the core methodological differences between the GLM and ICA approaches. That is, ICA identifies components of regional signals that contribute to large-scale distributed networks, whereas GLM examines the aggregate, emergent BOLD signal in a particular region. This outlines the complexity of regional activations and the necessity to consider interactions with other structures to provide nuanced understanding of the function of a certain region, particularly as it may integrate into multiple functional networks.
Limitations
Features of this study may limit the generalizability of the current findings to other IGD populations. First, given that we only recruited male participants, future studies involving both male and female participants are needed to identify the potential unique cue-reactivity processes among female IGD subjects. Second, IGD participants in this study represented a sample of students enrolled in Chinese universities and thus the findings may not be generalized to a broader clinical population. Finally, as this was a cross-sectional study, it cannot be determined if the observed alterations in FN engagement represent a consequence of IGD or a vulnerability factor that may have predisposed participants to developing IGD.
Conclusions
To our knowledge, this is the first report identifying alterations in the functional brain networks involved in the cue-reactivity in IGD. Compared with HC, individuals with IGD displayed altered engagement of four distinct FNs, involved in sensory processing, attention, memory, motivation, and executive functioning, in processing Internet gaming cues relative to general Internet surfing cues. These findings are consistent with cue-reactivity alterations reported in individuals with substance-related addictions and provide further evidence that IGD may represent a behavioral addiction.
Funding Statement
Funding sources: This work was supported by the National Natural Science Foundation of China (nos. 31170990, 31871122, and 31700966), Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (to Sarah, W. Yip and Sheng, Zhang), and the China Postdoctoral Science Foundation (no. 2017M620655).
Authors’ contribution
J-TZ and X-YF were responsible for the study concept and design. LL, L-JW, BL, and Y-WY contributed to the data acquisition. SWY, J-SX, and SZ assisted with data analysis. S-SM drafted the manuscript. J-TZ, PDW, NZ, and X-YF provided critical revision of the manuscript. All authors critically reviewed the content and approved final version of the manuscript for publication.
Conflict of interest
The authors declare no conflict of interest.
References
- Abdolahi A., Williams G. C., Benesch C. G., Wang H. Z., Spitzer E. M., Scott B. E., Block R. C., van Wijngaarden E. (2015). Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction, 110(12), 1994–2003. doi: 10.1111/add.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Elseoud A., Starck T., Remes J., Nikkinen J., Tervonen O., Kiviniemi V. (2010). The effect of model order selection in group PICA. Human Brain Mapping, 31(8), 1207–1216. doi: 10.1002/hbm.20929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. A., Erhardt E. B., Damaraju E., Gruner W., Segall J. M., Silva R. F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A. M., Caprihan A., Turner J. A., Eichele T., Adelsheim S., Bryan A. D., Bustillo J., Clark V. P., Feldstein Ewing S. W., Filbey F., Ford C. C., Hutchison K., Jung R. E., Kiehl K. A., Kodituwakku P., Komesu Y. M., Mayer A. R., Pearlson G. D., Phillips J. P., Sadek J. R., Stevens M., Teuscher U., Thoma R. J., Calhoun V. D. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. doi: 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Beck A. T., Brown G., Epstein N., Steer R. A. (1988). An inventory for measuring clinical anxiety – Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. doi: 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Erbaugh J., Ward C. H., Mock J., Mendelsohn M. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bell A. J., Sejnowski T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. doi: 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- Berridge K. C., Robinson T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. doi: 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Bonson K. R., Grant S. J., Contoreggi C. S., Links J. M., Metcalfe J., Weyl H. L., Kurian V., Ernst M., London E. D. (2002). Neural systems and cue-induced cocaine craving. Neuropsychopharmacology, 26(3), 376–386. doi: 10.1016/S0893-133X(01)00371-2 [DOI] [PubMed] [Google Scholar]
- Brand M., Young K. S., Laier C., Wolfling K., Potenza M. N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience and Biobehavioral Reviews, 71, 252–266. doi: 10.1016/j.neubiorev.2016.08.033 [DOI] [PubMed] [Google Scholar]
- Calhoun V., Adali T., Pearlson G., Pekar J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. doi: 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charboneau E. J., Dietrich M. S., Park S., Cao A., Watkins T. J., Blackford J. U., Benningfield M. M., Martin P. R., Buchowski M. S., Cowan R. L. (2013). Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: Preliminary results. Psychiatry Research-Neuroimaging, 214(2), 122–131. doi: 10.1016/j.pscychresns.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Weng L., Su Y., Wu H., Yang P. (2003). Development of a Chinese Internet Addiction Scale and its psychometric study. Chinese Journal of Psychology, 45(3), 279. doi: 10.1037/t44491-000 [DOI] [Google Scholar]
- Claus E. D., Ewing S. W., Filbey F. M., Sabbineni A., Hutchison K. E. (2011). Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology, 36(10), 2086–2096. doi: 10.1038/npp.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A., Smith J., Mirenowicz J. (2000). Dissociation of pavlovian and instrumental incentive learning under dopamine antagonists. Behavioral Neuroscience, 114(3), 468–483. doi: 10.1037/0735-7044.114.3.468 [DOI] [PubMed] [Google Scholar]
- Dong G. H., Potenza M. N. (2014). A cognitive-behavioral model of Internet gaming disorder: Theoretical underpinnings and clinical implications. Journal of Psychiatric Research, 58, 7–11. doi: 10.1016/j.jpsychires.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due D. L., Huettel S. A., Hall W. G., Rubin D. C. (2002). Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. The American Journal of Psychiatry, 159(6), 954–960. doi: 10.1176/appi.ajp.159.6.954 [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Robbins T. W. (2013). From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience and Biobehavioral Reviews, 37(9 Pt. A), 1946–1954. doi: 10.1016/j.neubiorev.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Franken I. H. (2003). Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27(4), 563–579. doi: 10.1016/S0278-5846(03)00081-2 [DOI] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534. doi: 10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., Cho J. K., Sperry L., Ross T. J., Salmeron B. J., Risinger R., Kelley D., Stein E. A. (2000). Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. The American Journal of Psychiatry, 157(11), 1789–1798. doi: 10.1176/appi.ajp.157.11.1789 [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia I., Horstmann A., Jurado M. A., Garolera M., Chaudhry S. J., Margulies D. S., Villringer A., Neumann J. (2014). Reward processing in obesity, substance addiction and non-substance addiction. Obesity Reviews, 15(11), 853–869. doi: 10.1111/obr.12221 [DOI] [PubMed] [Google Scholar]
- Goldman-rakic P. S., Leung H. C. (2002). Functional architecture of the dorsolateral prefrontal cortex in monkeys and humans. In D. T. Stuss, R. T. Knight. (Eds.), Principles of frontal lobe function (pp. 85–95). New York, NY: Oxford University Press. [Google Scholar]
- Heinz A., Beck A., Grusser S. M., Grace A. A., Wrase J. (2009). Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction Biology, 14(1), 108–118. doi: 10.1111/j.1369-1600.2008.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., Beck A., Wrase J., Mohr J., Obermayer K., Gallinat J., Puls I. (2009). Neurotransmitter systems in alcohol dependence. Pharmacopsychiatry, 42, S95–S101. doi: 10.1055/s-0029-1214395 [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A., Esposito F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage, 22(3), 1214–1222. doi: 10.1016/j.neuroimage.2004.03.027 [DOI] [PubMed] [Google Scholar]
- Holden C. (2001). Compulsive behaviors: “Behavioral’ addictions: Do they exist? Science, 294(5544), 980–982. doi: 10.1126/science.294.5544.980 [DOI] [PubMed] [Google Scholar]
- Ko C. H., Liu G. C., Yen J. Y., Chen C. Y., Yen C. F., Chen C. S. (2013). Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addiction Biology, 18(3), 559–569. doi: 10.1111/j.1369-1600.2011.00405.x [DOI] [PubMed] [Google Scholar]
- Ko C.-H., Yen J.-Y., Chen S.-H., Yang M.-J., Lin H.-C., Yen C.-F. (2009). Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Comprehensive Psychiatry, 50(4), 378–384. doi: 10.1016/j.comppsych.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Laird A. R., Fox P. M., Eickhoff S. B., Turner J. A., Ray K. L., McKay D. R., Glahn D. C., Beckmann C. F., Smith S. M., Fox P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. doi: 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemenager T., Dieter J., Hill H., Hoffmann S., Reinhard I., Beutel M., Vollstädt-Klein S., Kiefer F., Mann K. (2016). Exploring the neural basis of avatar identification in pathological Internet gamers and of self-reflection in pathological social network users. Journal of Behavioral Addictions, 5(3), 485–499. doi: 10.1556/2006.5.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemenager T., Dieter J., Hill H., Koopmann A., Reinhard I., Sell M., Kiefer F., Vollstädt-Klein S., Mann K. (2014). Neurobiological correlates of physical self-concept and self-identification with avatars in addicted players of massively multiplayer online role-playing games (MMORPGs). Addictive Behaviors, 39(12), 1789–1797. doi: 10.1016/j.addbeh.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Liu L., Yip S. W., Zhang J. T., Wang L. J., Shen Z. J., Liu B., Ma S. S., Yao Y. W., Fang X. Y. (2017). Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addiction Biology, 22(3), 791–801. doi: 10.1111/adb.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. S., Hospadaruk L., Zhu D. C., Gardner J. L. (2011). Feature-specific attentional priority signals in human cortex. Journal of Neuroscience, 31(12), 4484–4495. doi: 10.1523/JNEUROSCI.5745-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. C., Kruger J. K., Neumann B., Schott B. H., Kaufmann C., Heinz A., Wustenberg T. (2013). Cue reactivity and its inhibition in pathological computer game players. Addiction Biology, 18(1), 134–146. doi: 10.1111/j.1369-1600.2012.00491.x [DOI] [PubMed] [Google Scholar]
- Mitchell D. G. V. (2011). The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behavioural Brain Research, 217(1), 215–231. doi: 10.1016/j.bbr.2010.10.030 [DOI] [PubMed] [Google Scholar]
- Naqvi N. H., Rudrauf D., Damasio H., Bechara A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. doi: 10.1126/science.1135926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Dubbelink K. T., Felius A., Verbunt J. P., van Dijk B. W., Berendse H. W., Stam C. J., Delemarre-van de Waal H. A. (2008). Increased resting-state functional connectivity in obese adolescents; a magnetoencephalographic pilot study. PLoS One, 3(7), e2827. doi: 10.1371/journal.pone.0002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. J., Friston K. J. (2013). Structural and functional brain networks: From connections to cognition. Science, 342(6158), 1238411. doi: 10.1126/science.1238411 [DOI] [PubMed] [Google Scholar]
- Pelchat M. L., Johnson A., Chan R., Valdez J., Ragland J. D. (2004). Images of desire: Food-craving activation during fMRI. Neuroimage, 23(4), 1486–1493. doi: 10.1016/j.neuroimage.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Potenza M. N. (2008). The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philosophical Transactions of the Royal Society B-Biological Sciences, 363(1507), 3181–3189. doi: 10.1098/rstb.2008.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M. N., Steinberg M. A., Skudlarski P., Fulbright R. K., Lacadie C. M., Wilber M. K., Rounsaville B. J., Gore J. C., Wexler B. E. (2003). Gambling urges in pathological gambling – A functional magnetic resonance imaging study. Archives of General Psychiatry, 60(8), 828–836. doi: 10.1001/archpsyc.60.8.828 [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18(3), 247–291. doi: 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Ryan F. (2002). Detected, selected, and sometimes neglected: Cognitive processing of cues in addiction. Experimental and Clinical Psychopharmacology, 10(2), 67–76. doi: 10.1037/1064-1297.10.2.67 [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G. R., Hollon N. G., Carstensen L. L., Knutson B. (2008). Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychological Science, 19(4), 320–323. doi: 10.1111/j.1467-9280.2008.02087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Schurz M., Tholen M. G., Perner J., Mars R. B., Sallet J. (2017). Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: A review using probabilistic atlases from different imaging modalities. Human Brain Mapping, 38(9), 4788–4805. doi: 10.1002/hbm.23675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Li C. S. R. (2007). Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review, 26(1), 25–31. doi: 10.1080/09595230601036960 [DOI] [PubMed] [Google Scholar]
- Skinner M. D., Aubin H. J. (2010). Craving’s place in addiction theory: Contributions of the major models. Neuroscience and Biobehavioral Reviews, 34(4), 606–623. doi: 10.1016/j.neubiorev.2009.11.024 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Fox P. T., Miller K. L., Glahn D. C., Fox P. M., Mackay C. E., Filippini N., Watkins K. E., Toro R., Laird A. R., Beckmann C. F. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka M. N., Buhler M., Klein S., Zimmermann U., Mann K., Heinz A., Braus D. F. (2006). Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology, 184(3–4), 577–588. doi: 10.1007/s00213-005-0080-x [DOI] [PubMed] [Google Scholar]
- Sun Y. J., Ying H., Seetohul R. M., Xuemei W., Ya Z., Qian L., Guoqing X., Ye S. (2012). Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents). Behavioural Brain Research, 233(2), 563–576. doi: 10.1016/j.bbr.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Tiffany S. T., Conklin C. A. (2000). A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction, 95(8 Suppl. 2), 145–153. doi: 10.1080/09652140050111717 [DOI] [PubMed] [Google Scholar]
- van Veluw S. J., Chance S. A. (2014). Differentiating between self and others: An ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging and Behavior, 8(1), 24–38. doi: 10.1007/s11682-013-9266-8 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Baler R. D. (2011). Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences, 15(1), 37–46. doi: 10.1016/j.tics.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Fowler J. S., Tomasi D., Telang F., Baler R. (2010). Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays, 32(9), 748–755. doi: 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wu L., Lin X., Zhang Y., Zhou H., Du X., Dong G. (2016). Dysfunctional default mode network and executive control network in people with Internet gaming disorder: Independent component analysis under a probability discounting task. European Psychiatry, 34, 36–42. doi: 10.1016/j.eurpsy.2016.01.2424 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang Y., Lin X., Zhou H., Du X., Dong G. (2017). Group independent component analysis reveals alternation of right executive control network in Internet gaming disorder. CNS Spectrums, 23(5), 300–310. doi: 10.1017/S1092852917000360 [DOI] [PubMed] [Google Scholar]
- Weinstein A., Lejoyeux M. (2015). New developments on the neurobiological and pharmaco-genetic mechanisms underlying Internet and videogame addiction. The American Journal on Addictions, 24(2), 117–125. doi: 10.1111/ajad.12110 [DOI] [PubMed] [Google Scholar]
- Wiemerslage L., Nilsson E. K., Dahlberg L. S., Ence-Eriksson F., Castillo S., Larsen A. L., Bylund S. B., Hogenkamp P. S., Olivo G., Bandstein M., Titova O. E., Larsson E. M., Benedict C., Brooks S. J., Schioth H. B. (2016). An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. European Journal of Neuroscience, 43(9), 1173–1180. doi: 10.1111/ejn.13177 [DOI] [PubMed] [Google Scholar]
- Wilcox C. E., Teshiba T. M., Merideth F., Ling J., Mayer A. R. (2011). Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and Alcohol Dependence, 115(1–2), 137–144. doi: 10.1016/j.drugalcdep.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. J., Sayette M. A., Fiez J. A. (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7(3), 211–214. doi: 10.1038/nn1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. S., Calhoun V. D., Potenza M. N. (2015). The absence of task-related increases in BOLD signal does not equate to absence of task-related brain activation. Journal of Neuroscience Methods, 240, 125–127. doi: 10.1016/j.jneumeth.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. S., Zhang S., Calhoun V. D., Monterosso J., Li C. S. R., Worhunsky P. D., Stevens M., Pearlson G. D., Potenza M. N. (2013). Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. Neuroimage, 79, 62–71. doi: 10.1016/j.neuroimage.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. T., Ma S. S., Li C. R., Liu L., Xia C. C., Lan J., Wang L. J., Liu B., Yao Y. W., Fang X. Y. (2016). Craving behavioral intervention for Internet gaming disorder: Remediation of functional connectivity of the ventral striatum. Addiction Biology, 23(1), 337–346. doi: 10.1111/adb.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]