Abstract
Background and aims
Research shows inconsistent findings about the link between muscle dysmorphia (MD) and eating disorder (ED) symptomatology. The aim of this study is to synthesize the scientific evidence available on this topic, the researchers conducted a systematic review and meta-analysis.
Methods
The literature search enabled us to identify 39 published articles, which provided 36 independent estimations of the correlation between the two variables.
Results
Our analysis found a positive association between MD and ED symptoms (r+ = .36; 95% CI = 0.30, 0.41). Moderator analyses showed that the type of sample and the tools for assessing MD and ED were statistically associated with the MD–ED effect sizes. The methodological quality of the studies exhibited a positive, statistically significant association with the MD–ED effect sizes.
Conclusions
Higher levels of MD were related to greater ED symptomatology, but several study characteristics may moderate the association between the two variables. In this study, we discuss limitations and implications for clinical practice and future research.
Keywords: Adonis complex, reverse anorexia, eating behaviors, meta-analysis, muscle dysmorphia
Introduction
In a study of the psychiatric effects of steroid consumption, Pope, Katz, and Hudson (1993) described a new syndrome called “reverse anorexia,” due to its similarities with anorexia nervosa (AN) and subsequently renamed “muscle dysmorphia” (MD; Pope, Gruber, Choi, Olivardia, & Phillips, 1997). These authors suggested that MD is a form of body dysmorphic disorder (BDD) characterized by an obsessive preoccupation with the size and shape of one’s muscles, causing significant distress or impairment in daily functioning. Individuals with MD have a preoccupation with not being sufficiently lean and muscular. They believe that their muscles are smaller than they truly are (Grieve, Truba, & Bowersox, 2009; Olivardia, 2001), and they perceive themselves as puny and unattractive (Olivardia, Pope, & Hudson, 2000). Consequently, they seek even greater musculature and greater leanness to enhance the visibility of their muscularity (Choi, Pope, & Olivardia, 2002).
Additional symptoms associated with MD include the following: poor quality of life (Pope et al., 2005; Tod & Edwards, 2015a; Tod, Edwards, & Cranswick, 2016); higher rates of mood and anxiety disorders (Cafri, Olivardia, & Thompson, 2008); risk of obsessive–compulsive symptomatology and interpersonal sensitivity (Longobardi, Prino, Fabris, & Settani, 2017); lower self-esteem and self-perception (Chaney, 2008; Mitchell, Murray, Cobley, et al., 2017); increased feelings of loneliness (Chaney, 2008); impairments in social and occupational functioning (Olivardia et al., 2000; Pope, Khalsa, & Bhasin, 2017; Tod et al., 2016); and depression, neuroticism, and perfectionism (Mitchell, Murray, Cobley, et al., 2017).
MD is more prevalent in males, particularly those who engage in sports that emphasize increased muscle mass or power gain, such as weightlifting or bodybuilding (American Psychiatric Association [APA], 2013; Cella, Iannaccone, & Cotrufo, 2012; Fabris, Longobardi, Prino, & Settani, 2017; Pope, Phillips, & Olivardia, 2000). However, studies have also observed MD symptoms in females (Hale, Diehl, Weaver, & Briggs, 2013; Readdy, Cardinal, & Watkins, 2011; Robert, Monroe-Chandler, & Gammage, 2009; Tod et al., 2016). Thus, prior research has shown that both female and male bodybuilders are at extremely high risk of having MD symptoms (Hale et al., 2013). The average age of onset of MD is approximately 19–20 years (Cafri et al., 2008; Olivardia, 2001), and its etiology is not yet well known (Grieve, 2007).
At present, the American Psychiatric Association (APA, 2013) recognizes MD as a specifier for BDD in the fifth edition of Diagnostic and Statistical Manual of Mental Health Disorders (DSM-5); therefore, it belongs to the obsessive–compulsive disorder spectrum. According to the DSM-5, individuals with MD are preoccupied with the idea that their body build is too small or insufficiently lean or muscular, even though they have a normal-looking body or are quite muscular. Consequently, they perform repetitive behaviors (e.g., mirror checking, excessive grooming, skin picking, and reassurance seeking) or mental acts (e.g., comparing their appearance with that of others) in response to their appearance concerns. Therefore, the body, level of muscularity, and leanness are the obsession, and the compulsion is the desire or drive to gain more muscularity or leanness (Pope et al., 2000; Sandgren & Lavalle, 2018). Other DSM-5 diagnostic criteria specify the following necessary traits of the disorder: (a) appearance preoccupation should cause clinically significant distress or impairment in social, occupational, or other important areas of functioning; and (b) it should not be better explained by concerns with body fat or weight in an individual whose symptoms meet diagnostic criteria for an eating disorder.
Several studies have found an association between MD symptoms and higher levels of ED symptomatology (Giardino & Procidano, 2012; Goodale, Watkins, & Cardinal, 2001; Hildebrandt, Schlundt, Langenbucher, & Chung, 2006; Klimek & Hildebrandt, 2018; Lopez, Pollack, Gonzales, Pona, & Lundgren, 2015; Mitchell, Murray, Hoon, et al., 2017; Murray et al., 2012; Olivardia et al., 2000), little or no control over one’s dietary regime, compulsive exercise (Olivardia et al., 2000), diet pill use (McFarland & Kaminski, 2009), vomiting (Hildebrandt et al., 2006; McFarland & Kaminski, 2009), laxative use (Hildebrandt et al., 2006), and a prior history of EDs (Olivardia et al., 2000). Nevertheless, other studies have failed to find an association between MD and ED symptomatology (e.g., Cafri et al., 2008; Maida & Armstrong, 2005). For instance, Cafri et al. (2008) examined the link between MD and EDs in a sample of weightlifting males who met the current criteria for MD, past MD, or no history of MD. They administered the Structured Clinical Interview for DSM Disorders (SCID) to assess MD and EDs. Their findings revealed that the MD group did not show higher rates of EDs, any steroid use, or steroid abuse/dependence than controls. It is possible that the SCID was less sensitive than other assessment tools in detecting the association between MD and ED. Along the same lines, Maida and Armstrong (2005) carried out a study with a sample of males who lift weights and manifest a broad range of attitudes about their bodies, from those falling within the mainstream to those whose worries may be classified as pathological. These authors did not find a relationship between MD symptomatology and the bulimia subscale from the Eating Disorders Inventory (EDI), but they found that MD was positively related to variables measuring symptoms of body dissatisfaction, obsessive–compulsive disorder, depression, and anxiety.
This variability in the findings might be partly due to the different sampling and methodological characteristics of the studies (Nieuwoudt, Zhou, Coutts, & Booker, 2012). Across studies, differences exist in the definition of DM and the measurement tools used to assess MD and ED symptomatology (Lavender, Brown, & Murray, 2017; Lopez-Cuautle, Vazquez-Arevalo, & Mancilla-Diaz, 2016; Mitchell, Murray, Cobley, et al., 2017; Sandgreen & Lavalle, 2018). Thus, to assess MD, studies have used different self-report questionnaires and interview schedules, such as the Muscle Dysmorphic Disorder Inventory (MDDI; Hildebrandt, Langenbucher, & Schlundt, 2004), three different versions of the Muscle Dysmorphia Inventory (MDI; Lantz, Rhea, & Cornelius, 2002; Rhea, Lantz, & Cornelius, 2004; Short, 2005), the Muscle Appearance Satisfaction Scale (MASS; Mayville, Williamson, White, Netemeyer, & Drab, 2002), the Muscle Dysmorphia Symptom Questionnaire (MDSQ; Olivardia et al., 2000), Hale’s Scale (Hale, 2008), and the Muscle Dysmorphia Questionnaire (Cubberley, 2009). To assess ED symptomatology, studies have used the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994), the EDI (Garner, Olmstead, & Polivy, 1983), or the Eating Attitudes Test-26 (EAT-26; Garner, Olmstead, Bohr, & Garfinkel, 1982).
The studies also differ in the sampling procedure (e.g., convenience sample vs. probabilistic sample), the target population investigated [e.g., non-clinical samples: university students, athletes, and bodybuilders; clinical samples: MD-diagnosed participants, AN-diagnosed participants, and people who use anabolic androgenic steroid (AAS)], the gender of the participants (e.g., only males, only females, or mixed samples), the geographical location (USA, Australia, Mexico, Italy, Spain, etc.), and the ethnicity of the participants (Sandgren & Lavalle, 2018; Suffolk, Dovey, Goodwin, & Meyer, 2013).
To the best of our knowledge, no one has yet systematically studied the association between MD and ED symptomatology using a meta-analysis. A prior meta-analysis synthesized the evidence about the relationship between the drive for muscularity (DFM) and ED symptomatology, obtaining an average correlation of r+ = .27 and indicating that higher levels of DFM were associated with higher levels of ED psychopathology (Tod & Edwards, 2015b). Based on Cohen (1988), correlation coefficients of about .1, .3, and .5 can be interpreted as reflecting small, moderate, and large associations, respectively. Therefore, Todd and Edwards’s (2015b) findings showed a small to moderate association between DFM and ED symptomatology. Nevertheless, DFM and MD are different but related constructs. The former describes individual motivation to become more muscular, whereas the latter is a psychological disorder that represents the pathological pursuit of muscularity and leanness (Cafri et al., 2005). Hence, the desire to increase muscularity (i.e., DFM) does not imply distress (i.e., MD; Kimmel & Mahalik, 2004; Morrison, Morrison, & McCann, 2006). Therefore, people can present a high DFM without necessarily being distressed by perceived inadequacy or developing MD. Thus, the research has shown that individuals with MD are different from normal weightlifters in their symptom pathology and psychiatric comorbidity (Cafri et al., 2008; Olivardia et al., 2000). Recent evidence suggests that the former has a greater presence of eating disordered attitudes and beliefs than the latter (Mitchell, Murray, Hoon, et al., 2017; Murray, Nagata, et al., 2017). In addition, research also suggests that these constructs may have different neuropsychological correlates (Griffiths, Murray, & Touyz, 2013).
Therefore, we primarily aimed to conduct a systematic review and meta-analysis to synthesize the scientific evidence about the relationships between MD and ED symptomatology, given the inconsistencies in the results from empirical studies that have previously investigated this association. A second aim was to identify the study characteristics that might moderate the heterogeneous results that emerged, such as the geographic location, type of sample, measurement instruments for MD and ED, age, gender, and ethnicity, among others.
Methods
We conducted a systematic review and meta-analysis on the relationship between MD and ED symptomatology following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group, 2009). Because PRISMA guidelines were developed to be applied to meta-analyses studying intervention efficacy, some of their items were not applicable to our meta-analysis. Consequently, we also adhered to the guidelines recently proposed by the American Psychological Association Publications and Communications Board Task Force (Appelbaum et al., 2018, Table 9, p. 2123).
Study selection criteria
The studies had to meet the following inclusion criteria: (a) the study had to be published in a peer-reviewed journal, (b) it had to be an original and quantitative investigation, (c) it had to assess MD using measurement instruments that specifically evaluated the symptoms and diagnostic criteria of MD (e.g., MASS, MDDI, MDI, etc.), (d) it had to assess ED using measurement instruments that specifically evaluated the symptoms and/or diagnostic criteria of ED (e.g., EAT, EDI, and EDE), and (e) it had to empirically examine the relationship between MD and ED symptoms. We established no date, language, or participant’s age limits. We excluded qualitative studies, literature reviews, systematic reviews, meta-analyses, commentaries, editorials, and studies that did not assess MD and its relationship with ED symptomatology.
Search strategy
The researchers carried out electronic searches in June 2018 in the Medline (via PubMed), PsycInfo, Science Direct, and Web of Science databases, using the following terms in ALL FIELDS: muscle dysmorph*, MDI, reverse anorexia, bigorexia, vigorexia, and Adonis complex. Furthermore, we conducted manual searches of lists of references from the retrieved studies to identify additional studies that met the selection criteria. This search yielded two additional eligible studies. In addition, the researchers screened lists of references from previous reviews and meta-analyses (e.g., Dos Santos, Tirico, Stefano, Touyz, & Claudino, 2015; Mitchell, Murray, Cobley, et al., 2017; Sandegren & Lavalle, 2018; Suffolk et al., 2013; Tod & Edwards, 2015b) to find studies that met the inclusion criteria for the present meta-analysis. Finally, in order to locate unpublished papers, the researchers sent e-mails to 12 of the most prolific authors in the field. Of the six responses we received, none led to the discovery of an unpublished study.
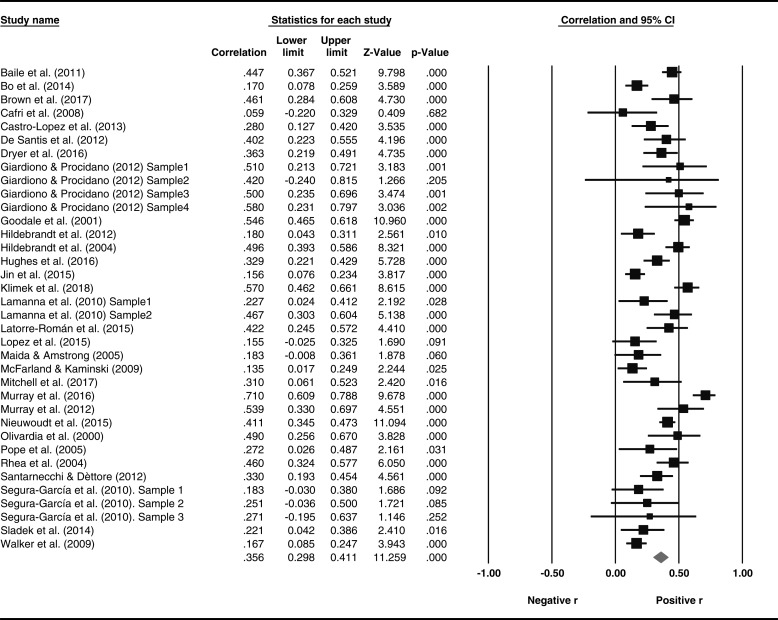
Two researchers carried out the eligibility process for this meta-analysis independently, resolving disagreements between raters by consensus. Figure 1 presents a flowchart of the study screening and selection process.
Figure 1.
Flowchart of the study selection process in the systematic review and meta-analysis of MD and ED symptomatology
Coding of the studies
The researchers produced a protocol for extracting the characteristics of the studies and applied it to each study. The following characteristics were coded: year of the study, geographical location, sampling method, type of sample (e.g., clinical, community, gymnasiums, university, and AAS users), sample size, gender, mean age, sexual orientation, ethnicity, education level, tools used for assessing MD and ED, and statistics reported to calculate the effect sizes. In addition, the researchers assessed the methodological quality of the studies by applying an ad hoc 10-item checklist, given that in the literature there is no clear methodological checklist to use in studies with a cross-sectional design where the purpose is to analyze the relationships between two variables, as in this study. Most quality checklists are designed for randomized trials and other types of research, particularly evaluations of interventions (e.g., Cochrane checklist). Therefore, their criteria focus on interventions and follow-up assessments. Other checklists are focused on cohort studies (e.g., Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies from National Institutes of Health); however, most of their items are also related to interventions and follow-up assessments. Therefore, we developed our own criteria to assess the methodological quality of primary studies.
Appendix A shows each of the 10 items on the quality checklist. Each item scored 1 when the study met the criteria and 0 otherwise. We calculated a total quality score (TQS) for each study by adding up all the corresponding quality item scores (range: 0–10), with a higher score indicating higher overall quality.
The researchers carried out the coding process in a standardized and systematic manner and two reviewers independently extracted the data. Interrater reliability was satisfactory, with a mean intraclass correlation of .98 (SD = 0.036), ranging from 0.90 to 1 for continuous variables, and with a mean κ coefficient of 0.92 (SD = 0.078), ranging from 0.81 to 1 for qualitative variables. Inconsistencies between coders were resolved by consensus.
Computing effect sizes
The effect size index was a correlation coefficient (e.g., the Pearson’s correlation coefficient, its Fisher’s Z transformation, the ϕ coefficient, the point-biserial correlation coefficient) calculated between an MD scale and an ED scale (Borenstein, Hedges, Higgins, & Rothstein, 2009). For each study, the researchers translated the Pearson’s correlations to Fisher’s Z in order to normalize their distributions and stabilize their variances. Then, the researchers back-translated the Fisher’s Z values for the individual effect sizes, as well as those for the mean effect sizes and their confidence limits, into the Pearson’s correlation metric in order to make their interpretation easier (Borenstein et al., 2009).
In studies that reported several correlations for the MD–ED relationship, because researchers used multiple measures of MD and/or ED, we calculated their average to avoid statistical dependence.
In studies that did not directly report correlation coefficients, we applied appropriate translations between effect sizes. Thus, several studied reported odds ratios (ORs; Bo et al., 2014; Cafri et al., 2008; Olivardia et al., 2000; Pope et al., 2005), and we applied a formula to transform them into correlation coefficients. For each study, the ORs were translated into d, with 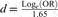 , Loge being the natural logarithm. Then, the ds were translated into r, with
, Loge being the natural logarithm. Then, the ds were translated into r, with 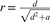 , where a was estimated with
, where a was estimated with 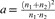 , and n1, n2 were the sample sizes in the two conditions (Sánchez-Meca, Marín-Martínez, & Chacón-Moscoso, 2003).
, and n1, n2 were the sample sizes in the two conditions (Sánchez-Meca, Marín-Martínez, & Chacón-Moscoso, 2003).
Statistical analyses
The researchers carried out a meta-analysis to assess the relationship between MD and ED symptomatology. In order to accommodate the variability shown by the effect sizes, we assumed a random-effects model (Borenstein et al., 2009; Sánchez-Meca & Marín-Martínez, 2008). We calculated a pooled correlation coefficient and its corresponding 95% confidence interval (CI). In addition, we assessed the statistical significance of the pooled correlation using the Z test.
The researchers constructed a forest plot to represent the individual and pooled effect size estimates, with their 95% CIs, and to allow visual inspection of effect size heterogeneity. We calculated both Cochran’s Q statistic and the I2 index to assess the consistency of the effect sizes (Borenstein et al., 2009; Huedo-Medina, Sánchez-Meca, Marín-Martínez, & Botella, 2006). A Q statistic with p < .05 was indicative of heterogeneity among the effect sizes. We estimated the degree of this heterogeneity using the I2 index. I2 values of around 25%, 50%, and 75% denoted low, moderate, and large heterogeneity, respectively.
To examine the influence of moderator variables on effect size variability, the researchers calculated analyses of variance (ANOVAs) and meta-regressions for categorical and continuous variables, respectively, by assuming a mixed-effects model (Borenstein et al., 2009; López-López, Marín-Martínez, Sánchez-Meca, van den Noortgate, & Viechtbauer, 2014). We assessed the statistical significance of qualitative and continuous moderators with the QB and QR statistics, respectively. In addition, we calculated an estimate of the proportion of variance accounted for by the moderator variable following Raudenbush’s (2009) proposal (López-López et al., 2014). We assessed the model misspecification with the QW and QE statistics for qualitative and continuous moderators, respectively.
Finally, to assess publication bias, the researchers used both a funnel plot with Duval and Tweedie’s trim-and-fill method for imputing missing data and the Egger’s test (Duval & Tweedie, 2000; Sterne & Egger, 2005). A funnel plot with Duval and Tweedie’s trim-and-fill method uses available data to impute missing (unreported) studies and recalculates the overall effect that would be observed with their inclusion (Duval & Tweedie, 2000). The Egger’s test is an unweighted simple regression, which takes the precision of each study as the independent variable (precision being defined as the inverse of the standard error of each effect size) and the effect size divided by its standard error as the dependent variable. A non-statistically significant result of the t-test for the hypothesis of an intercept equal to zero means that publication bias can be eliminated as a threat to the validity of the pooled effect (Sterne & Egger, 2005).
The researchers interpreted all statistical tests assuming a significance level of 5% (p < .05). The statistical analyses were performed with the Comprehensive Meta-analysis software program, version 3.0 (Biostat, Englewood, NJ, USA; Borenstein, Hedges, Higgins, & Rothstein, 2014).
Results
Study selection
The search strategy produced a total of 3,575 manuscripts. After scanning the titles and abstracts of the 3,575 identified manuscripts, the researchers preselected 544 relevant studies based on the inclusion and exclusion criteria. Then, eliminating duplicates (n = 405) left a total of 139 studies to review. After a review of the full text of the remaining articles, 39 fulfilled the selection criteria. Nevertheless, nine articles did not report a correlation between MD and ED or statistical data to calculate it. In these cases, the researchers sent e-mails to the authors of the studies to obtain these data, but none of them replied to our request. Therefore, we excluded these studies from the meta-analysis (Babusa &Túry, 2012; Babusa, Urban, Czeglédi, & Túry, 2012; Guidi, Clementi, & Grandi, 2013; Hale et al., 2013; Hildebrandt, Langenbucher, Karmin, Loeg, & Hollander, 2011; Hildebrandt et al., 2006; Hildebrandt, Walker, Alfano, Delinsky, & Bannon, 2010; Kanayama, Barry, Hudson, & Pope, 2006; Magallares, 2016). Thus, the meta-analysis included 30 published articles; all of these studies were published in a peer-reviewed journal between 2000 (Olivardia et al., 2000) and 2018 (Klimek, Murray, Brown, Gonzales, & Blashill, 2018). Three of the 30 articles provided data on four, two, and three independent samples, respectively (Giardino & Procidano, 2012; Lamanna, Grieve, Derryberry, Hakman, & McClure, 2010; Segura García et al., 2010). Thus, the data set for our meta-analysis was composed of a total of 36 independent samples.
Study characteristics
Appendix B presents descriptive characteristics of the articles included (n = 39) in the systematic review, yielding a total of 45 studies or independent samples. Overall, the majority of the studies used a convenience sample (e.g., gymnasiums, vitamin stores, bodybuilding discussion forums, Facebook, and university) and a cross-sectional design. Only one study used a probabilistic sample (Castro-Lopez, Cachón-Zagalaz, Molero, & Zagalaz-Sánchez, 2013). A large number of studies were conducted in the USA (n = 23), with 14 carried out in Europe, 4 in Australia, and 2 in Mexico; one was conducted in China, and a multinational was study carried out in the UK, the USA, Australia, and Singapore.
The 45 independent samples included 8,516 participants (mean = 189, range = 11, and 648 participants). The weighted mean age of all participants was 25.89 years. Of the 45 independent samples, 31 were composed exclusively of men and 7 of women, whereas both males and females participated in the remaining 7 samples. Overall, the majority of the participants was Caucasian, with the presence of varying percentages of other racial and ethnic groups. Finally, the majority of the participants had received at least some college education.
The studies used a variety of instruments to measure MD and ED. The tools most commonly used to assess MD were the MDDI (Hildebrandt et al., 2004; n = 16), the MDI (Rhea et al., 2004; n = 7), and the MASS (Mayville et al., 2002; n = 11). The assessment instruments most frequently used in the ED studies were the EAT-26 (Garner et al., 1982; n = 15), the EDI (Garner et al., 1983; n = 12), and the EDE-Q (Fairburn & Beglin, 1994; n = 10).
Assessment of methodological quality
Appendix C presents the results obtained from the assessment of the methodological quality of the studies. Specifically, the appendix shows the scores of individual studies on each quality item. The researchers also obtained a TQS by adding up the 1s and 0s for the checklist items (range =0–10). On average, the studies had a methodological quality of 5.28 (SD = 1.22), with a range of 3–7 points.
Most of the studies met the following criteria: (a) they used participant’s samples that allowed an appropriate representation of a clinical or at-risk MD or ED population (n = 31); (b) they employed measurement instruments with good psychometric properties (validity and reliability) to assess MD in the study sample (n = 30); (c) they employed measurement instruments with good psychometric properties (validity and reliability) to assess ED in the study sample (n = 28); (d) they did not dichotomize the assessment of MD and ED (n = 39); (e) they reported on results related to all the MD and ED instruments described in “Methods” section (n = 44); (f) they applied appropriate statistical tests (non-parametric vs. parametric methods) to assess the relationships between MD and ED (n = 24); and (g) they had no private financial support (n = 39). Nevertheless, most of the studies did not provide data on power analysis or report CIs around the effect sizes measured.
Finally, the following criteria were least likely to be met by the studies: (a) use of representative sampling procedures (n = 1) and (b) a priori determination of the sample size to identify an effect (n = 2). In addition, none of the studies specified whether there were dropouts or if these dropouts had similar sociodemographic characteristics to those of the final sample.
Synthesis of results
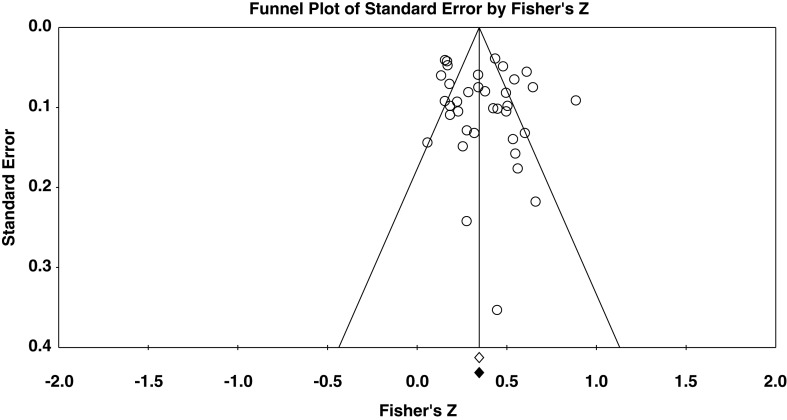
This meta-analysis estimated the relationship between MD and ED symptomatology. Figure 2 presents a forest plot of the MD–ED associations found in each individual study and their 95% CIs, as well as the mean correlation coefficient resulting from pooling all the studies and its corresponding 95% CI.
Figure 2.
Forest plot of the association between MD and ED symptomatology
As Figure 2 shows, the pooled effect size for the relationship between MD and ED was r+ = .356 (95% CI = 0.298, 0.411, κ = 36). In addition, the analysis found considerable heterogeneity among individual effect sizes, [Q(35) = 200.925, p < .0001, I2 = 82.7%].
Analysis of publication bias
Because all the studies included were published papers, we carried out analyses to determine whether publication bias might be a threat to the validity of the results of the meta-analysis. The Duval and Tweedie (2000) trim-and-fill method did not impute any effect size (see Appendix D). In addition, the Egger’s test applied to the intercept of a simple regression model of the effect sizes did not reach statistical significance [intercept = 1.09, t(34) = 1.176, p = .248]. Therefore, we can reasonably rule out publication bias as a serious threat to our meta-analytic findings.
Moderator analyses of the relationships between MD and ED symptomatology
The effect sizes showed a large amount of heterogeneity (I2 = 82.7%). Consequently, the researchers conducted statistical analyses to identify the study characteristics that might explain this heterogeneity. Specifically, we used weighted ANOVAs and simple meta-regressions for categorical and continuous moderator variables, respectively, taking the correlation coefficients between MD and ED symptomatology as the dependent variable.
Table 1 presents the results of the ANOVAs conducted on the categorical variables, such as country of the study, type of sample, MD and ED measurement instrument, and type of statistics reported. Three moderator variables showed a statistically significant association with effect sizes: (a) the type of sample used in the study, (b) the measurement instrument used to assess MD, and (c) the measurement instrument used to assess ED. The type of sample used in the study, classified as people who reported current AAS use versus other samples, accounted for 26% of the variance (p < .001). Specifically, a study found stronger MD–ED associations when it was conducted among people who used AAS (r+ = .710), compared to other samples (r+ = .342). In addition, the tools used to assess ED explained 26% of the variance (p < .001), with stronger MD–ED associations found when the EDE-Q Modified was the instrument used to assess ED (r+ = .642), in comparison with other instruments (r+ = .336). Finally, studies that used quantitative measurement instruments to assess ED symptomatology, such as EDE-Q or EAT, that had a larger mean effect size (r+ = .364) than the studies that used other instruments (r+ = .177), although this difference was only marginally statistically significant (p = .077, R2 = .01).
Table 1.
Results of the weighted ANOVAs for the influence of categorical variables on the effect sizes
| Moderator variable | k | r+ | 95% CI | ANOVA results | |
|---|---|---|---|---|---|
| rl | ru | ||||
| Geographic location | QB(1) = 0.051, p = .821 | ||||
| USA | 18 | .363 | .274 | .445 | R2 = .00 |
| Other countries | 18 | .349 | .270 | .424 | QW(34) = 195.879, p < .001 |
| Sample setting | |||||
| Gymnasiums | 18 | .359 | .284 | .430 | QB(5) = 31.793, p < .001 |
| University | 9 | .301 | .169 | .423 | R2 = .18 |
| Area metropolitan | 3 | .352 | .276 | .425 | QW(30) = 153.370, p < .001 |
| AAS users | 1 | .710 | .609 | .788 | |
| Clinical (BDD or EDS) | 2 | .272 | .055 | .464 | |
| University and other | 3 | .387 | .240 | .516 | |
| Sample setting | QB(1) = 30.137, p < .001 | ||||
| AAS users | 1 | .710 | .609 | .788 | R2 = .26 |
| Other | 35 | .342 | .287 | .394 | QW(34) = 167.137, p < .001 |
| MD measurement | |||||
| MDDI | 12 | .396 | .278 | .503 | QB(7) = 17.087, p = .017 |
| MASS | 7 | .367 | .220 | .497 | R2 = .00 |
| SCID | 3 | .285 | .031 | .504 | QW(28) = 171.339, p < .001 |
| ACQ | 4 | .398 | .320 | .471 | |
| MDS | 1 | .135 | .017 | .249 | |
| MDI | 6 | .293 | .185 | .394 | |
| MDQ | 1 | .363 | .219 | .491 | |
| MDSQ | 2 | .386 | −.012 | .678 | |
| Type of MD measurement | QB(1) = 0.385, p = .535 | ||||
| Diagnostic | 3 | .285 | .031 | .504 | R2 = .00 |
| Symptomatology | 33 | +.361 | .301 | .419 | QW(34) = 202.228, p < .001 |
| ED measurementa | |||||
| EAT | 14 | .345 | .263 | .423 | QB(6) = 30.585, p < .001 |
| EDI | 10 | .348 | .268 | .423 | R2 = .23 |
| EDE-Q | 7 | .348 | .187 | .491 | QW(30) = 141.207, p < .001 |
| EDE-Q Modified | 2 | .642 | .449 | .777 | |
| SCID | 2 | .177 | −.036 | .374 | |
| MEBBIE | 1 | .135 | .017 | .249 | |
| CHAA | 1 | .447 | .367 | .521 | |
| EDQ Modified vs. othersa | QB(1) = 8.087, p = .007 | ||||
| EDE-Q Modified | 2 | .642 | .449 | .777 | R2 = .26 |
| Others | 35 | .336 | .281 | .389 | QW(35) = 167.335, p < .001 |
| Type of ED measurementa | QB(1) = 3.129, p = .077 | ||||
| Diagnostic | 2 | .177 | −.036 | .374 | R2 = .01 |
| Symptomatology | 35 | .364 | .305 | .419 | QW(35) = 200.840, p < .001 |
| Statistics reported | QB(1) = 2.274, p = .132 | ||||
| Correlation coefficient | 32 | .368 | .308 | .426 | R2 = .03 |
| Odds ratio | 4 | .242 | .078 | .393 | QW(34) = 189.439, p < .001 |
Note. k: number of studies. r+: mean effect size. rl and ru: 95% lower and upper confidence limits around r+; QB: between-categories Q statistic; QW: within-categories Q statistic; R2: proportion of variance accounted for by the moderator variable; ED: eating disorder; EDE-Q: Eating Disorder Examination Questionnaire; MEBBIE: Male Eating Behavior and Body Image Evaluation; CHAA: Cuestionario de Hábitos de Alimentación Alterados; SCID: structured clinical Interview for DSM-IV; MDDI: Muscle Dysmorphic Disorder Inventory; MASS: Muscle Appearance Satisfaction Scale; MDI: Muscle Dysmorphia Inventory; AAS: anabolic androgenic steroid; ACQ: Adonis Complex Questionnaire; MDSQ: Muscle Dysmorphia Symptom Questionnaire; MDQ: Muscle Dysmorphia Questionnaire; CI: confidence interval; ANOVA: analysis of variance; BDD: body dysmorphic disorder.
aHughes et al.’s (2016) study used two tools for assessing EDs (EAT-26 and EDEQ).
Table 2 presents the simple meta-regressions conducted on continuous variables, such as mean age, gender (% male), and ethnicity (% Caucasian) of the sample, as well as the methodological TQS. The only moderator variable that exhibited a statistically significant association with effect sizes was the methodological TQS for the individual studies, accounting for 25% of the variance (p < .05).
Table 2.
Results of the simple weighted meta-regressions of continuous moderators on the effect sizes
| Moderator variable | k | bj | QR | QE | R2 |
|---|---|---|---|---|---|
| Mean age (years) | 36 | 0.0015 | 0.05 | 196.51** | .0 |
| Gender (% males) | 36 | 0.0000 | 0.45 | 199.26** | .0 |
| Ethnicity (% Caucasian) | 19 | −0.0003 | 0.03 | 101.58** | .0 |
| Methodology quality checklist (0–10) | 36 | 0.0776 | 10.03* | 176.31** | .25 |
Note. k: number of studies; bj: unstandardized regression coefficient; QR: Q statistic for testing the statistical significance of the moderator variable; QE: Q statistic for testing the model misspecification; R2: proportion of variance accounted for by the moderator variable.
*p < .05. **p < .001.
In addition, in order to identify the individual items on the quality checklist that were statistically associated with the effect sizes, we applied ANOVAs for each individual item (see Table 3). As Table 3 shows, items 4, 5, and 6 were statistically associated with the effect sizes. Thus, studies that reported any estimate of the reliability/validity of the MD measurement instrument (item 4) or any estimate of the reliability/validity of the ED measurement used in the study sample (item 5) showed a mean effect size (r+ = .403 and r+ = .416, respectively) larger than those that did not (r+ = .255 and r+ = .196, respectively), accounting for a large proportion of variance (R2 = .13 and R2 = .31, respectively). In addition, studies that did not dichotomize MD and/or ED variables showed a mean effect size (r+ = .376) larger than those that did so (r+ = .219), accounting for a moderate proportion of variance (R2 = .06).
Table 3.
Results of the weighted ANOVAs for the influence of methodological quality items on the effect sizes
| Quality item | k | r+ | 95% CI | ANOVA results | |
|---|---|---|---|---|---|
| rl | ru | ||||
| 1. Probabilistic sampling | QB(1) = 0.988, p = .320 | ||||
| Yes | 1 | .280 | .127 | .420 | R2 = .00 |
| No | 35 | .359 | .299 | .415 | QW(34) = 202.124, p < .001 |
| 2. Adequate target population | QB(1) = 1.118, p = .290 | ||||
| Yes | 24 | .380 | .310 | .446 | R2 = .01 |
| No | 12 | .316 | .215 | .411 | QW(34) = 191.814, p < .001 |
| 4. Reliability/validity MD measurement tool | QB(1) = 6.928, p = .008 | ||||
| Yes | 24 | .403 | .331 | .470 | R2 = .13 |
| No | 12 | .255 | .167 | .339 | QW(34) = 192.883, p < .001 |
| 5. Reliability/validity ED measurement tool | QB(1) = 26.172, p < .001 | ||||
| Yes | 24 | .416 | .349 | .479 | R2 = .31 |
| No | 12 | .196 | .147 | .244 | QW(34) = 159.323, p < .001 |
| 6. Absence of dichotomous DM and ED | QB(1) = 6.412, p = .011 | ||||
| Yes | 30 | .376 | .314 | .434 | R2 = .06 |
| No | 6 | .219 | .109 | .324 | QW(34) = 186.515, p < .001 |
| 8. Statistical test appropriate | QB(1) = 0.058, p = .810 | ||||
| Yes | 21 | .360 | .280 | .435 | R2 = .00 |
| No | 15 | .346 | .265 | .423 | QW(34) = 202.325, p < .001 |
| 9. Private financial support | QB(1) = 0.138, p = .711 | ||||
| Yes | 4 | .373 | .288 | .452 | R2 = .00 |
| No | 32 | .354 | .290 | .414 | QW(34) = 201.692, p < .001 |
| 10. Statistical power | QB(1) = 0.426, p = .514 | ||||
| Yes | 2 | .251 | −.101 | .548 | R2 = .00 |
| No | 34 | .361 | .302 | .418 | QW(34) = 202.397, p < .001 |
Note. Results for item 3 (dropouts were similar in socio-demographic characteristics to those of the final sample) and item 7 (absence of reporting bias) were not analyzed because all studies scored 0 and 1, respectively. k: number of studies; r+: mean effect size; rl and ru: 95% lower and upper confidence limits around r+; QB: between-categories Q statistic; QW: within-categories Q statistic; R2: proportion of variance accounted for by the moderator variable; ANOVA: analysis of variance; ED: eating disorder; MD: muscle dysmorphia.
Discussion
This is the first meta-analytic study on the relationship between MD and ED symptomatology. Our results showed that there is a positive and statistically significant relationship between MD and ED symptomatology, indicating that higher levels of MD were associated with higher levels of ED symptomatology. Following Cohen’s (1988) criteria, a correlation coefficient of r+ = .356 can be interpreted as reflecting a relationship of moderate magnitude. Our findings are consistent with the findings from Tod and Edwards’s (2015b) review, which found an association between DFM and ED symptomatology. However, the strength of the correlation was greater between MD and ED symptomatology (r = .36) than between DFM and ED (r = .27). These findings suggest that both MD and DFM share an association with EDs. People with DFM and MD want to gain lean muscle mass, which is achieved not only through physical training but also by maintaining a diet high in lean protein and using food supplements. Therefore, dietary practices are an important factor in both DFM and MD. Furthermore, research suggests that the behavior and diet subscales of the DFM predict MD characteristics (Robert et al., 2009), which might explain that both MD and DFM share an association with EDs. In other research, DFM has been identified as a precursor to the development of MD (Olivardia et al., 2000). Therefore, those who scored high on a measure of MD displayed a higher degree of DFM. From this point of view, MD might be considered an extreme variant of DFM.
We ruled out publication bias as a threat to our meta-analytic results, but we found great variability among the individual effect sizes. In order to identify the study features that could explain at least part of the effect size variability, we carried out ANOVAs and meta-regressions. Several study characteristics were statistically related to effect size, such as the type of sample, the methodological quality, and the MD and ED measurement instruments used. First, our findings showed stronger associations between MD and ED symptomatology in males reporting current AAS use than in other people (undergraduates, athletes, etc.). However, this result must be interpreted very cautiously because AAS samples were represented in only one study. Therefore, it seems plausible that males who use AAS exhibit elevated levels of MD symptomatology. Indeed, some of the most common motivations for using AAS are to increase muscle mass and strength (Cohen, Collins, Darkes, & Gwartney, 2007), to improve physical appearance (Kimergård, 2015), and to decrease body dissatisfaction and MD symptoms (Grogan, Shepherd, Evans, Wright, & Hunter, 2006). In this way, research has identified MD symptoms as a predictor of the use of substances such as anabolic steroids, prohormones, and ephedrine (Cafri, van den Berg, & Thompson, 2006). Thus, people who use AAS might be at greater risk of developing MD and ED symptomatology. These findings suggest the need to implement health promotion and prevention programs to reduce AAS use.
Furthermore, our results showed that of all the ED measurement instruments, the EDE-Q Modified (Murray et al., 2012) was the most sensitive tool in identifying relationships with MD symptomatology. Some conceptual overlapping between the EDE-Q Modified and the measures of MD is likely. It is important to note that the EDE-Q Modified is a version of the EDE-Q with the gender-relevant items identified and reversed in polarity to enhance its sensitivity in indexing male concerns (Murray et al., 2012). For instance, the item on the EDE-Q “Have you had a definite fear that you might gain weight or become fat?” was reversed on the EDE-Q Modified to “Have you had a definite fear that you might lose weight or become less muscular?” Other examples of reversed items are “Have you been deliberately trying to increase the amount of food you eat to influence your shape or weight?” and “Has thinking about food or its protein content made it more difficult to concentrate on things you are interested in; for example, reading, watching TV, or following a conversation?” (modifications italicized). Such overlaps might influence the strength of the relationships between MD and ED symptomatology. In addition, it is plausible that the strength of the association between MD and the EDE-Q Modified might be affected by the type of sample used to examine this relationship. In this regard, the only two studies that applied the EDE-Q Modified (Murray, Griffiths, Mond, Kean, & Blashill, 2016; Murray et al., 2012) used samples of participants who were not from a community population. In one of them; the participants were AAS users (Murray et al., 2016), and in the other study, they were clinical participants (Murray et al., 2012). Therefore, clinicians should be aware of this fact and take it into account when identifying co-occurrence.
Moreover, our findings revealed that of all the ED assessment instruments, the SCID was the least sensitive in identifying relationships between MD and ED symptomatology, which might explain why the study by Cafri et al. (2008) did not reveal a relationship between MD and ED symptomatology. One possible explanation is that the SCID provides a more thorough and conservative assessment of an ED, having less overlap with MD than other ED measures. This might suggest that MD is associated with certain ED symptoms but not strongly or consistently with a separate ED diagnosis.
Finally, our findings suggest that studies with higher methodological quality are more sensitive in identifying relationships between MD and ED symptomatology. In addition, we found stronger MD–ED associations in the studies that assessed the psychometric properties of the MD and ED instruments with the data found than in those that did not assess these properties. These results agree with psychometric theory, which proposes that using measurement instruments with good psychometric properties will provide greater sensitivity in revealing statistical associations between variables than using non-reliable and/or non-valid instruments. Finally, we found lower MD–ED associations in the studies that dichotomized the MD and ED measures (i.e., reporting ORs instead of Pearson’s correlation coefficients). These results agree with statistical theory, which proposes that dichotomizing variables produce a loss of sensitivity in detecting statistical associations among variables (Schmidt & Hunter, 2015). Therefore, one would expect higher correlations in studies with higher methodological quality that used quantitative measurement instruments for MD assessment, such as MASS and MDDI, and for ED assessment, such as EAT or EDE-Q.
Limitations
This meta-analysis has several limitations. Five studies could not be included in the final analysis because the statistical data reported did not allow us to calculate the effect sizes, and their authors did not reply to the request for data. Furthermore, most of the studies included were conducted in the USA, which might limit the generalization of the results. In addition, given the existence of great heterogeneity among the effect sizes, it is possible that other moderator variables not considered in our meta-analysis might be relevant in explaining this heterogeneity (Menees, Grieve, Mienaltowski, & Pope, 2013). These moderators are likely to be psychosocial constructs such as socially prescribed perfectionism (Dryer, Farr, Hiramatsu, & Quinton, 2016) or masculinity-related constructs (Tod et al., 2016). Increased understanding and awareness of MD and ED symptomatology might contribute to identifying and referring people at risk, so that they can receive help from mental health services. Finally, it is important to remember that all the studies included in this meta-analysis were cross-sectional, which means we cannot draw causal inferences about the relationship between MD and ED symptomatology.
Recommendations for future research
Most research on MD–ED has used non-clinical samples, often university students, who, in general, have low psychopathology. Thus, future research should investigate the MD–ED association in clinical samples, that is, in people diagnosed with MD and/or ED. These studies might improve our knowledge to develop more optimal treatment approaches. On other hand, to find out whether the EDE-Q Modified (Murray et al., 2012) is really the most sensitive tool for assessing the association between MD and ED symptomatology, additional studies are needed to analyze this relationship in community samples.
Moreover, given that most of the studies used cross-sectional designs, which do not allow us to draw causal inferences about the relationship between MD and ED symptomatology, future research should implement longitudinal studies. These studies might help to clarify the relationship between MD and ED symptomatology in chronological terms, for instance, whether ED precedes MD or vice versa, and they might clarify the stability of MD and/or ED symptomatology.
Finally, given that most of the studies were conducted in the USA and with male participants, future research should study this topic in other social contexts and in the female population in order to investigate potential differences based on cultural factors and/or sex differences.
Conclusions
This is the first meta-analytic study on the relationship between MD and ED symptomatology, contributing to a more accurate view of this phenomenon, despite the limitations described above. Furthermore, our findings may have some implications for clinical practice. They suggest that the symptoms and behaviors characteristic of MD tend to co-occur with ED symptomatology in males and females. Thus, when evaluating individuals presenting with MD, clinicians should investigate the possibility of the presence of ED, and vice versa, particularly in males. In this regard, a recent case study showed the transition from thinness-oriented to muscularity-oriented disordered eating during the course of treatment for AN (Murray, Griffiths, Mitchison, & Mond, 2017). As the authors of this study pointed out, the emergence of muscularity-oriented disordered eating during the treatment of AN might be misinterpreted as healthy, due to the move away from dangerously low body weight, which is the objective of AN treatment.
People with MD symptomatology may also have ED symptomatology, which appears to be associated with greater psychopathology (Chandler, Grieve, Derryberry, & Pegg, 2009; Ebbeck, Watkins, Concepcion, Cardinal, & Hammermeister, 2009; Grieve & Shacklette, 2012; Pope et al., 2005). This situation, in addition to the serious limitations in social and work performance caused by MD and the associated unhealthy behaviors, makes it necessary to use strategies for identifying at-risk individuals and for prevention, such as inviting them to participate in psychotherapy. Because MD and ED symptomatology, and the co-occurrence of these symptoms, can occur more often among athletes, the development of such intervention strategies may be of special interest for mental health professionals working in the sports area. We do not believe that participation in sports is inherently pathological, but it is likely that some individuals take up sports for reasons (which may be pathological) that correlate with MD and ED symptomatology or increase the risk of developing symptoms of these two disorders. A psychological assessment conducted by an experienced sports psychologist could identify the athletes in need of psychological treatment. Based on our data, we recommend that clinicians use the MDDI or MASS to assess MD and the EDE-Q Modified to assess ED when examining the co-occurrence of MD and ED symptomatology. These are the most sensitive measurement instruments in identifying relationships between MD and ED (considering the previous discussion about the EDEQ Modified).
Appendix A: Checklist for assessing the methodological quality of the studies
| Item | Description |
|---|---|
| 1 | Representative sampling procedures (Yes: 1; No: 0) |
| 2 | The inclusion and exclusion criteria of participants were suitable to represent clinical or at risk population for MD and ED (Yes: 1; No: 0) |
| 3 | Dropouts were similar in sociodemographic characteristics to those of the final sample (completers) (Yes: 1; No: 0; Unable to determine: 0) |
| 4 | The measurement instrument used to assess MD show good psychometric properties (validity and reliability) in the sample of study (Yes: 1; No: 0; Unable to determine: 0) |
| 5 | The measurement instrument used to assess ED shows good psychometric properties (validity and reliability) in the sample of study (Yes: 1; No: 0; Unable to determine: 0) |
| 6 | The assessment of DM and ED was not dichotomized (Yes: 1; No: 0) |
| 7 | Absence of reporting bias: results for all MD and ED instruments described in “Methods” section were reported (Yes: 1; No: 0) |
| 8 | The statistical test used to assess the relationships between MD and ED was appropriate to the data (non-parametric methods vs. parametric methods) (Yes: 1; No: 0; Unable to determine: 0) |
| 9 | Absence of private financial support (Yes: 1; No: 0) |
| 10 | Statistical power: sample sizes have been calculated to detect an effect (Yes: 1; No: 0; Unable to determine: 0) |
Note. The methodological quality checklist consisted of 10 criteria with a dichotomous response scale. The presence of the criterion is given 1 point and its absence 0 points. The total score was 10 points. MD: muscle dysmorphia; ED: eating disorders.
Appendix B: Characteristics of the studies or independent samples included in the systematic review and/or meta-analysis (n = 45)
| Study/country | N | Sampling method | Age (mean) | Males (%) | Sexual orientation (%) | Ethnicity (%) | Education level (%) | MD | ED | ES (r) |
|---|---|---|---|---|---|---|---|---|---|---|
| Babusa and Tury (2012)/Hungary | 120 | Convenience sample of bodybuilders recruited in gymnasiums and undergraduates non bodybuilders | 27.75 | 100 | NR | NR | NR | MASS | EDI | NR |
| Babusa et al. (2012)/Hungary | 529 | Convenience sample of weight lifters and undergraduates | 24.37 | 100 | NR | NR | >Undergraduate | MASS | EDI | NR |
| Baile, González, Ramírez, and Suárez (2011)/Spain | 418 | Convenience sample from gymnasiums and university | 20.86 | 100 | NR | NR | >Undergraduate (81.3) | ACQ | CHAA | .447 |
| Bo et al. (2014)/Italy | 440 | Convenience sample from university | 19.80 | 45.9 | NR | NR | >Undergraduate | MDDI | EAT-26 | .170 |
| Brown, Forney, Pinner, and Keel (2017)/USA | 93 | Convenience sample from university and local community | 20.37 | 100 | >H (90.3) | NR | >Undergraduate (86) | MDDI | EDE-Q | .461 |
| Cafri et al. (2008)/USA | 51 | Convenience sample of weightlifters from gymnasiums and vitamin stores | 25.71, Range: 18–40 | 100 | >H (96.1) | >C (80.4) | NR | SCID-NP | SCID-NP | .059 |
| Castro-López et al. (2013)/Spain | 154 | Probabilistic sample from bodybuilding gymnasiums | 24.97 | 92.2 | NR | NR | NR | ACQ | EDI-2 | .280 |
| De Santis et al. (2011)/USA | 100 | Convenience sample from bars, clubs, and beaches | 32.47, Range: 18–51 | 100 | G = 100 | LH = 100 | NR | ACQ | EAT-26 | .402 |
| Dryer et al. (2016)/Australia | 158 | Convenience sample from metropolitan area | 26.94, Range: 18–36 | 100 | H = 76 G = 15 B = 9 |
NR | University degree (45) | MDQ | EDI-3 | .363 |
| Sample 1: Giardino and Procidano (2012)/Mexico | 35 | Convenience sample of weightlifters from university gymnasiums | 23.34, Range: 19–35 | 100 | NR | LH = 71.7 MR = 8.7 C = 4.3 |
>Undergraduate (89.2) | MASS | EAT-26 | .510 |
| Sample 2: Giardino and Procidano (2012)/Mexico | 11 | Convenience sample of weightlifters from university gymnasiums | 22.18, Range: 18–27 | 0 | NR | LH = 71.7 MR = 8.7 C = 4.3 |
>Undergraduate (89.2) | MASS | EAT-26 | .420 |
| Sample 3: Giardino and Procidano (2012)/USA | 43 | Convenience sample of weightlifters from university gymnasiums | 20.47, Range: 18–30 | 100 | NR | C = 77.5 LH = 6 AA = 6 O = 10.5 |
>Undergraduate (91.2) | MASS | EAT-26 | .500 |
| Sample 4: Giardino and Procidano (2012)/USA | 24 | Convenience sample of weightlifters from university gymnasiums | 20.17, Range: 18–22 | 0 | NR | C = 77.5 LH = 6 AA = 6 O = 10.5 |
>Undergraduate (91.2) | MASS | EAT-26 | .580 |
| Goodale et al. (2001)/USA | 323 | Convenience sample from university | 20.0, Range: 18–41 | 34.9 | NR | >C (80.4) | Undergraduate | MDSQ | EDE-Q | .546 |
| Guidi et al. (2013)/Italy | 79 | Convenience sample from gymnasiums | 30 | 43 | NR | NR | Undergraduate (30.4) | MDQ | EDI-2 | NR |
| Hale et al. (2013)/USA | 74 | Convenience sample from gymnasiums | Range: 18–48 | 0 | NR | NR | NR | MDI | EDI-2 (drive for thinness scale) | NR |
| Hildebrandt Harty, and Langenbucher (2012)/USA | 201 | Convenience sample from University | 19.17 | 49.8 | >H (97.5) | C = 55.7 AA = 19.9 AsA = 15.9 O = 8.5 |
Undergraduate | MDDI | EDE–Q | .180 |
| Hildebrandt et al. (2011)/USA | 85 | Convenience sample from gymnasiums, nutritional supplements stores, discussion boards, newspaper advertisements, and general volunteer websites to complete a series of assessments | 36.63 | 83.7 | H = 82.3 G = 11.9 B = 5.8 |
C = 54.5 LH = 21.7 AA = 15.6 AsA = 6.9 O = 1.3 |
Undergraduate (28.1) | MDDI | EDE-Q | NR |
| Hildebrandt et al. (2004)/USA | 237 | Convenience sample of weightlifters from gymnasiums and vitamin stores | 32.64, Range: 18–72 | 100 | NR | NR | NR | MDDI | EDI | .496 |
| Hildebrandt et al. (2006)/USA | 237 | Convenience sample of weightlifters from gymnasiums and vitamin stores | 32.64, Range: 18–72 | 100 | NR | NR | NR | MDDI | EDI | NR |
| Hildebrandt et al. (2010)/USA | 342 | Convenience sample from university | 19.76 | 57.3 | NR | C = 53.3 LH = 17 AA = 7.6 O = 22.2 | Undergradate | MDDI | EDE-Q EAT-26 | NR |
| Hughes, Dean, and Allen (2016)/Australia | 284 | Convenience sample from metropolitan area | 32.6, Range: 19–84 | 100 | NR | C = 84.9 A = 9.9 I = 1.7 O = 3.5 |
NR | MDI | EAT-26 EDE-Q |
.387 .271 |
| Jin et al. (2015)/China | 592 | Convenience sample from fitness centers and gymnasiums | 27, Range: 18–58 | 100 | NR | NR | NR | MASS | EAT-26 | .156 |
| Kanayama et al. (2006)/USA | 89 | Convenience sample from gymnasiums | 29.67 | 100 | NR | >C (80.9) | College graduate (42.7) | Own | EDI | NR |
| Klimek et al. (2018)/USA | 180 | Convenience sample from university | 19.6, Range: 18–33 | 100 | 87.2 | C = 55 | Undergraduate | MDDI | EDE-Q | .570 |
| Sample 1: Lamanna et al. (2010)/USA | 93 | Convenience sample from university | 19.13, Range: 17–44 (data from all sample) | 100 | NR | C = 87.4 AA = 6 LH = 1.1 NA = 1.1 AsA = 1.1 O = 3.3 (data from all sample) |
Undergraduate | MDDI | EAT-26 | .227 |
| Sample 2: Lamanna et al. (2010)/USA | 106 | Convenience sample from university | 19.13, Range: 17–44 (data from all sample) | 0 | NR | C = 87.4 AA = 6 LH = 1.1 NA = 1.1 AsA = 1.1 O = 3.3 (data from all sample) |
Undergraduate | MDDI | EAT-26 | .467 |
| Latorre-Román et al. (2015)/Spain | 99 | Convenience sample from bodybuilding gymnasiums | 25.45 | 100 | NR | NR | NR | ACQ | EAT-26 | .422 |
| Lopez et al. (2015)/USA | 120 | Convenience sample from University | 23.24 | 100 | NR | C = 60 AA = 20 LH = 8.3 O = 11.7 |
Undergraduate | MDI | EDE-Q | .155 |
| Magallares (2016)/Spain | 615 | Convenience sample from university | 25.56, Range: 18–30 | 0 | NR | NR | Undergraduate | MASS | EDI-2 | NR |
| Maida and Armstrong (2005)/USA | 106 | Convenience sample of weightlifters from gymnasiums | Range: 18–45 | 100 | NR | NR | >had attended college for 1–3 years (56) | MDSQ | EDI | .183 |
| McFarland and Kaminski (2009)/USA | 276 | Convenience sample from university | Median: 20 (data from all sample) | 100 | H = 89.8 G/B = 7.5 |
EA = 66.7 LH = 12.2 AA = 10.5 |
Undergraduate | MDS | MEBBIE | .135 |
| Mitchell, Murray, Hoon, et al. ( 2017)/Australia | 60 | Convenience sample of competitive natural bodybuilders | 29.6 | 100 | NR | NR | NR | MDDI | EAT | .31 |
| Murray et al. (2016)/England | 122 | Convenience sample of people reporting current anabolic androgenic steroid use from bodybuilding gymnasiums and specialist needle exchange for self-administered anabolic–androgenic steroids injections | 29.38 Range: 17–48 | 100 | H = 83.6 G/B = 13.1 |
NR | NR | MDDI | EDE-Q Modified | .710 |
| Murray et al. (2012)/UK/USA/Australia/Singapore | 60 | Convenience sample from gymnasiums and clinical settings | 26.58 | 100 | NR | NR | NR | MDDI | EDE-Q Modified | .539 |
| Nieuwoudt, Zhou, Coutts, and Booker (2015)/Australia | 648 | Convenience sample from weightlifting/bodybuilding discussion forums, facebook | 29.5 Range: 18–65 | 100 | >H (93.4) | >C (85.3) | NR | MASS | EAT-26 | .411 |
| Olivardia et al. (2000)/USA | 54 | Convenience sample from gymnasiums | 25.4 | 100 | NR | >C (90.7) | Graduate: 48.14 | SCID-P | EDI | .490 |
| Pope et al. (2005)/USA | 63 | Convenience sample from clinical settings and advertisement | 34.91 | 100 | NR | >C (90.5) | >at least some college (over 69.8) | SCID-NP | SCID-NP | .272 |
| Rhea et al. (2004)/USA | 151 | Convenience sample from bodybuilders (n = 58) and weight lifters (n = 93) | 28.48 | 91.4 | NR | C = 90 O = 10 |
NR | MDI | EDI | .460 |
| Santarnecchi and Dèttore (2012)/Italy | 180 | Convenience sample from gymnasiums | 32.67 Range: 23–41 | 100 | NR | NR | NR | MDDI | EDI | .330 |
| Sample 1: Segura-García et al. (2010)/Italy | 86 | Convenience sample from fitness center | 27.67 | 100 | NR | NR | NR | MDI | EDI-2 | .183 |
| Sample 2: Segura-García et al. (2010)/Italy | 48 | Convenience sample from fitness center | 28.6 | 0 | NR | NR | NR | MDI | EDI-2 | .251 |
| Sample 3: Segura-García et al. (2010)/Italy | 20 | Convenience sample from clinical setting (women with EDs) | 22.1 | 0 | NR | NR | NR | MDI | EDI-2 | .271 |
| Sladek, Engeln, and Miller (2014)/USA | 160 | Convenience sample from university and via Amazon’s Mechanical Turk website | 26.62 Range: 18–64 | 100 | >H (97.1) | >C (68.8) | >Undergraduate (57.2) | MASS | EAT-26 | NR |
| Walker, Anderson, and Hildebrandt (2009)/Albania | 550 | Convenience sample from university | 18.98 Range: 16–30 | 100 | NR | C = 68.9 AA = 8.7 A = 7.7 H = 5.8 MR = 4.6 O = 4.3 |
Undergraduate | MDDI | EDE-Q | .167 |
Note. MD: muscle dysmorphia; ED: eating disorder; ES: effect size statistics; NR: not reported. Ethnicity: A: Asian; AA: African American; AsA: Asian American; C: Caucasian; EA: European American; H: Hispanic; I: Indian; LH: Latin Hispanic; MR: multiracial; NA: Native American; O: others. Sexual Orientation: H: heterosexual; G: gay, B: bisexual. Muscle dysmorphia: ACQ: Adonis Complex Questionnaire (Pope et al., 2000); MDS-Q: Muscle Dysmorphia Symptom Questionnaire (Olivardia et al., 2000); MASS: Muscle Appearance Satisfaction Scale (Mayville et al., 2002); MDDI: Muscle Dysmorphic Disorder Inventory (Hildebrandt et al., 2004); MDI: Muscle Dysmorphia Inventory (Rhea et al., 2004); MD-Q: Muscle Dysmorphia Symptom Questionnaire (Grieve et al., 2012); MDS: Muscle Dysmorphia Scale (Kaminski, McFarland, & Chapman, 2008); SCID-NP: structured clinical Interview for DSM-IV – Non patient version; SCID-P: structured clinical interview for DSM-IV – Patient version. Eating disorders: CHAA: Cuestionario de Hábitos de Alimentación Alterados; EAT-26: Eating Attitudes Test (Garner, Olmstead, Bohr, & Garfinkel, 1982); EDE-Q: Eating Disorder Examination Questionnaire (Fairburn & Beglin, 1994); EDE-Q Modified: Eating Disorder Examination Questionnaire Modified (Murray et al., 2012); EDI: Eating Disorders Inventory (Garner et al., 1983); MEBBIE: Male Eating Behavior and Body Image Evaluation (Kaminski et al., 2002).
Appendix C: Methodological quality of the studies
| Study | Methodological quality criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total (0–10) | |
| Babusa and Túry (2012) | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Babusa et al. (2012) | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Baile et al. (2011) | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 5 |
| Bo et al. (2014) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 3 |
| Brown et al. (2017) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Cafri et al. (2008) | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Castro López et al. (2013) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 5 |
| De Santis et al. (2011) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Dryer et al. (2016) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Giardino and Procidano (2012): Study 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Giardino and Procidano (2012): Study 2 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Giardino and Procidano (2012): Study 3 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Giardino and Procidano (2012): Study 4 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Goodale et al. (2001) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Guidi et al. (2013) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Hale et al. (2013) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Hildebrandt et al. (2004) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Hildebrandt et al. (2006) | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Hildebrandt et al (2010) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| Hildelbrandt et al. (2011) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Hildebrandt et al. (2012) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Hughes et al. (2016) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Jin et al. (2015) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Kanayama et al. (2006) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 |
| Klimek et al. (2018) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Lamanna et al. (2010): Study 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Lamanna et al. (2010): Study 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Latorre-Román et al. (2014) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Lopez et al. (2015) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| Magallares (2016) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 |
| Maida et al. (2005) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| McFarland and Kaminski (2009) | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Mitchell, Murray, Cobley, et al. (2017) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Murray et al. (2012) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Murray et al. (2016) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Nieuwoudt et al. (2015) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Olivardia et al. (2000) | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 |
| Pope et al. (2005) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Rhea et al. (2004) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Santarnecchi and Dettore (2012) | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Segura-García et al. (2010): Sample 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 |
| Segura-García et al. (2010): Sample 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 |
| Segura-García et al. (2010): Sample 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 4 |
| Sladek et al. (2014) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Walker et al. (2009) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
Note. The methodological quality checklist consists of 10 criteria with a dichotomous response scale. The presence of the criterion is given 1 point and its absence 0 points. The total quality score for each study was obtained by summing the 1s and 0s through the 10 quality items (see Appendix A for a description of the quality items).
Appendix D
Funnel plot of the MD–ED effect sizes to assess publication bias. White circles represent each of the studies included. The absence of black circles indicates that the trim-and-fill method had not to impute missing data to symmetrize the funnel plot.
Funding Statement
Funding sources: This research is not funded by a specific project grant.
Authors’ contribution
CL and MAF conceived of the presented idea and developed the theory. LB-R and MR-A performed the computations and statistical analysis. JS-M verified the analytical methods and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Conflict of interest
The authors declare no potential conflict of interest with regard to the research, authorship, and/or publication of this article.
References
- References marked with an asterisk indicate studies included in the meta-analysis. [Google Scholar]
- American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Appelbaum M., Cooper H., Kline R. B., Mayo-Wilson E., Nezu A. M., Rao S. M. (2018). Journal article reporting standards for quantitative research in psychology: The APA Publications and Communications Board Task Force report. American Psychologist, 73(1), 3–25. doi: 10.1037/amp0000191 [DOI] [PubMed] [Google Scholar]
- *Babusa B., Túry F. (2012). Muscle dysmorphia in Hungarian non-competitive male bodybuilders. Eating Weight Disorders, 17, e49–e53. doi: 10.1007/BF03325327 [DOI] [PubMed] [Google Scholar]
- *Babusa B., Urbán R., Czeglédi E., Túry F. (2012). Psychometric properties and construct validity of the Muscle Appearance Satisfaction Scale among Hungarian men. Body Image, 9(1), 155–162. doi: 10.1016/j.bodyim.2011.08.005 [DOI] [PubMed] [Google Scholar]
- *Baile J. I., González A., Ramírez C., Suárez P. (2011). Imagen corporal, hábitos alimentarios y hábitos de ejercicio físico en hombres usuarios de gimnasio y hombres universitarios no usuarios [Body image, eating behaviours and exercise training among male gym users and male non-users at the university]. Revista de Psicología del Deporte, 20, 353–366. Retrieved from http://www.rpd-online.com/article/view/783 [Google Scholar]
- *Bo S., Zoccali R., Ponzo V., Soldati L., De Carli L., Benso A., Fea E., Rainoldi A., Durazzo M., Fassino S., Abbate-Daga G. (2014). University courses, eating problems and muscle dysmorphia: Are there any associations? Journal of Translational Medicine, 12, 221. doi: 10.1186/s12967-014-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M. J, Hedges L. V., Higgins J. P., Rothstein H. R. (2009). Introduction to meta-analysis. Chichester, UK: Wiley. [Google Scholar]
- Borenstein M. J., Hedges L. V., Higgins J., Rothstein H. R. (2014). Comprehensive meta-analysis (version 3). Englewood, NJ: Biostat. [Google Scholar]
- *Brown T. A., Forney K. J., Pinner D., Keel P. K. (2017). A randomized controlled trial of The Body Project: More Than Muscles for men with body dissatisfaction. International Journal of Eating Disorders, 50, 873–883. doi: 10.1002/eat.22724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cafri G., Olivardia R., Thompson J. K. (2008). Symptom characteristics and psychiatric comorbidity among males with muscle dysmorphia. Comprehensive Psychiatry, 49, 374–379. doi: 10.1016/j.comppsych.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Cafri G., Thompson J. K., Ricciardelli L., McCabe M., Smolak L., Yesalis C. (2005). Pursuit if the muscular ideal: Physical and psychological consequences and putative risk factors. Clinical Psychology Review, 25(2), 215–239. doi: 10.1016/j.cpr.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Cafri G., van den Berg P., Thompson J. K. (2006). Pursuit of muscularity in adolescent boys: Relations among biopsychosocial variables and clinical outcomes. Journal of Clinical Child and Adolescent Psychology, 35(2), 283–291. doi: 10.1207/s15374424jccp3502_12 [DOI] [PubMed] [Google Scholar]
- *Castro-Lopez R., Cachón-Zagalaz J., Molero D., Zagalaz-Sánchez M. L. (2013) Dismorfia muscular y su relación con síntomas de trastornos de la conducta alimentaria [Muscle dysmorphia and its relationship with the symptoms of eating disorders]. Revista Mexicana de Trastornos Alimentarios [Mexican Journal of Eating Disorders], 4, 31–36. Retrieved from http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-15232013000100004&lng=es [Google Scholar]
- Cella S., Iannaccone M., Cotrufo P. (2012). Muscle dysmorphia: A comparison between competitive bodybuilders and fitness practitioners. Journal of Nutritional Therapeutics, 1, 12–18. Retrieved from http://www.lifescienceglobal.com/pms/index.php/jnt/article/view/451/pdf [Google Scholar]
- Chandler C. G., Grieve F. G., Derryberry W. P., Pegg P. O. (2009). Are anxiety and obsessive-compulsive symptoms related to muscle dysmorphia? International Journal of Men’s Health, 8, 143–154. Retrieved from http://www.mensstudies.info/OJS/index.php/IJMH/article/view/568 [Google Scholar]
- Chaney M. P. (2008). Muscle dysmorphia, self-esteem, and loneliness among gay and bisexual men. International Journal of Men’s Health, 7(2), 157–170. doi: 10.3149/jmh.0702.157 [DOI] [Google Scholar]
- Choi P. Y. L., Pope H., Olivardia R. (2002). Muscle dysmorphia: A new syndrome in weightlifters. British Journal of Sports Medicine, 36(5), 375–376. doi: 10.1136/bjsm.36.5.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cohen J., Collins R., Darkes J., Gwartney D. (2007). A league of their own: Demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. Journal of the International Society of Sports Nutrition, 4(1), 12. doi: 10.1186/1550-2783-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubberley R. (2009). Evaluating the reliability and validity of the Muscle Dysmorphia Inventory (Master’s thesis). Western Kentucky University, Bowling Green, Kentucky. Retrieved from http://digitalcommons.wku.edu/theses/121/ [Google Scholar]
- *De Santis J. P., Layerla D. M., Barroso S., Gattamorta K. A., Sanchez M., Prado G. J. (2011). Predictors of eating attitudes and behaviors among gay Hispanic men. Archives of Psychiatric Nursing, 26, 11–126. doi: 10.1016/j.apnu.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Dos Santos C. A., Tirico P. P., Stefano S. C., Touyz S. W., Claudino A. M. (2015). Systematic review of the diagnostic category muscle dysmorphia. Australian and New Zealand Journal of Psychiatry, 50(4), 322–333. doi: 10.1177/0004867415614106 [DOI] [PubMed] [Google Scholar]
- *Dryer R., Farr M., Hiramatsu I., Quinton S. (2016). The role of sociocultural influences on symptoms of muscle dysmorphia and eating disorders in men, and the mediating effects of perfectionism. Behavioral Medicine, 42(3), 174–182. doi: 10.1080/08964289.2015.1122570 [DOI] [PubMed] [Google Scholar]
- Duval S., Tweedie R. (2000). Trim and fill: A simple funnel- plot- based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Ebbeck V., Watkins P. L., Concepcion R. Y., Cardinal B. J., Hammermeister J. (2009). Muscle dysmorphia symptoms and their relationships to self-concept and negative affect among college recreational exercisers. Journal of Applied Sport Psychology, 21(3), 262–275. doi: 10.1080/10413200903019376 [DOI] [Google Scholar]
- Fabris M. A., Longobardi C., Prino L. E., Settani M. (2017). Attachment style and risk of muscle dysmorphia in a sample of male bodybuilders. Psychology of Men & Masculinity, 19(2), 273–281. doi: 10.1037/men0000096 [DOI] [Google Scholar]
- Fairburn C. G., Beglin S. J. (1994). Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders, 16(4), 363–370. doi: [DOI] [PubMed] [Google Scholar]
- Garner D. M., Olmstead M. P., Bohr Y., Garfinkel P. E. (1982). The Eating Attitudes Test: Psychometric features and clinical correlates. Psychological Medicine, 12(4), 871–878. doi: 10.1017/S0033291700049163 [DOI] [PubMed] [Google Scholar]
- Garner D., Olmstead M. P., Polivy J. (1983). Development and validation of a Multidimensional Eating Disorder Inventory for anorexia nervosa and bulimia. International Journal of Eating Disorders, 2(2), 15–34. doi: [DOI] [Google Scholar]
- *Giardino J. C., Procidano M. E. (2012). Muscle dysmorphia symptomatology: A cross-cultural study in Mexico and the United States. International Journal of Men’s Health, 11(1), 83–103. doi: 10.3149/jmh.1101.83 [DOI] [Google Scholar]
- *Goodale K. R., Watkins P. L., Cardinal B. J. (2001). Muscle dysmorphia: A new form of eating disorder? American Journal of Health Education, 32(5), 260–266. doi: 10.1080/19325037.2001.10603480 [DOI] [Google Scholar]
- Grieve F. G. (2007). A conceptual model of factors contributing to the development of muscle dysmorphia. Eating Disorders, 15(1), 63–80. doi: 10.1080/10640260601044535 [DOI] [PubMed] [Google Scholar]
- Grieve F. G., Shacklette M. D. (2012). Brief report on men’s bodies and mood: Correlates between depressive symptoms and muscle dysmorphia symptoms. North American Journal of Psychology, 14, 563 Retrieved from http://www.freepatentsonline.com/article/North-American-Journal-Psychology/312401777.html [Google Scholar]
- Grieve F. G., Truba N., Bowersox S. (2009). Etiology, assessment, and treatment of muscle dysmorphia. Journal of Cognitive Psychotherapy, 23(4), 306–314. doi: 10.1891/0889-8391.23.4.306 [DOI] [Google Scholar]
- Griffiths S., Murray S., Touyz S. (2013). Drive for muscularity and muscularity-oriented disordered eating in men: The role of set shifting difficulties and weak central coherence. Body Image, 10(4), 636–639. doi: 10.1016/j.bodyim.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Grogan S., Shepherd S., Evans R., Wright S., Hunter G. (2006). Experiences of anabolic steroid use: In-depth interviews with men and women body builders. Journal of Health Psychology, 11(6), 845–856. doi: 10.1177/1359105306069080 [DOI] [PubMed] [Google Scholar]
- *Guidi J., Clementi C., Grandi C. (2013). Valutazione del disagio psicologico e delle caratteristiche di personalità nella dipendenza da esercizio fisico primaria [Psychological distress and personality characteristics among individuals with primary exercise dependence]. Rivista di Psichiatria, 48, 121–129. doi: 10.1708/1272.14036 [DOI] [PubMed] [Google Scholar]
- Hale W. D. (2008). Scale development in muscle dysmorphia (Unpublished doctoral dissertation). Oklahoma State University, Stillwater, OK. [Google Scholar]
- *Hale B., Diehl D., Weaver K., Briggs M. (2013). Exercise dependence and muscle dysmorphia in novice and experienced female bodybuilders. Journal of Behavioral Addictions, 2(4), 244–248. doi: 10.1556/JBA.2.2013.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hildebrandt T., Harty S., Langenbucher J. W. (2012). Fitness supplements as a gateway substance for anabolic-androgenic steroid use. Psychology of Addictive Behaviors, 26(4), 955–962. doi: 10.1037/a0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hildebrandt T., Langenbucher J. W., Karmin J. K., Loeg K. L., Hollander E. (2011). Development and validation of Appearance and Performance Enhancing Drug Use Schedule. Addictive Behaviors, 36(10), 949–958. doi: 10.1016/j.addbeh.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hildebrandt T., Langenbucher J., Schlundt D. G. (2004). Muscularity concerns among men: Development of attitudinal and perceptual measures. Body Image, 1(2), 169–181. doi: 10.1016/j.bodyim.2004.01.001 [DOI] [PubMed] [Google Scholar]
- *Hildebrandt T., Schlundt D., Langenbucher J., Chung T. (2006). Presence of muscle dysmorphia symptomatology among male weightlifters. Comprehensive Psychiatry, 47(2), 127–135. doi: 10.1016/j.comppsych.2005.06.001 [DOI] [PubMed] [Google Scholar]
- *Hildebrandt T., Walker C. D., Alfano L., Delinsky S., Bannon K. (2010). Development and validation of a Male Specific Body Checking Questionnaire. International Journal of Eating Disorders, 43, 77–87. doi: 10.1002/eat.20669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina T. B., Sánchez-Meca J., Marín-Martínez F., Botella J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. doi: 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- *Hughes E. K., Dean C., Allen J. S. (2016). Measures of eating disorder symptoms, drive for muscularity, and muscle dysmorphia: Norms and typologies of Australian men. Australian Journal of Psychology, 68(4), 270–280. doi: 10.1111/ajpy.12105 [DOI] [Google Scholar]
- *Jin X., Jin Y., Zhou S., Li X., Yang S., Yang D., Nieuwoudt J. E., Yao J. (2015). The Muscle Appearance Satisfaction Scale: A factorial analysis of validity and reliability for its use on adult Chinese male weightlifters. Body Image, 14, 94–101. doi: 10.1016/j.bodyim.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Kaminski P. L., McFarland M. B., Chapman B. P. (2008). The Muscle Dysmorphia Scale (MDS). Available from the author: Department of Psychology, University of North Texas, Denton, TX. [Google Scholar]
- Kaminski P. L., Slaton S. R., Caster J., Own L., Baker K., Chapman B. P. (2002, November). The Male Eating Behavior and Body Image Evaluation (MEBBIE): A scale to measure eating, exercise, and body image concerns in men. Poster presented at the annual convention of the Texas Psychological Association, San Antonio, TX. [Google Scholar]
- *Kanayama G., Barry S., Hudson J. I., Pope H. G. (2006). Body image and attitudes toward male roles in anabolic-androgenic steroid users. American Journal of Psychiatry, 163(4), 697–703. doi: 10.1176/ajp.2006.163.4.697 [DOI] [PubMed] [Google Scholar]
- Kimergård A. (2015). A qualitative study of anabolic steroid use amongst gym users in the United Kingdom: Motives, beliefs and experiences. Journal of Substance Use, 20(4), 288–294. doi: 10.3109/14659891.2014.911977 [DOI] [Google Scholar]
- Kimmel S. B., Mahalik J. R. (2004). Measuring masculine body ideal distress: Development of a measure. International Journal of Men’s Health, 3(1), 1–10. doi: 10.3149/jmh.0301.1 [DOI] [Google Scholar]
- Klimek P., Hildebrandt T. (2018). Psychosocial correlates of gap time to anabolic-androgenic steroid use. International Journal of Eating Disorders, 51(6), 535–541. doi: 10.1002/eat.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Klimek P., Murray S. B., Brown T., Gonzales M., Blashill A. J. (2018). Thinnes and muscularity internalization: Associations with disordered eating and muscle dysmorphia in men. International Journal of Eating Disorders, 51(4), 352–357. doi: 10.1002/eat.22844 [DOI] [PubMed] [Google Scholar]
- *Lamanna J., Grieve F. G., Derryberry W. P., Hakman M., McClure A. (2010). Antecedents of eating disorders and muscle dysmorphia in a non-clinical sample. Eating Weight Disorders, 15(1-2), e23–e33. doi: 10.1007/BF03325277 [DOI] [PubMed] [Google Scholar]
- Lantz C. D., Rhea D. J., Cornelius A. E. (2002). Muscle dysmorphia in elite-level power lifters and bodybuilders: A test of differences within a conceptual model. Journal of Strength and Conditioning Research, 16(4), 649e655. doi: 10.1519/00124278-200211000-00026 [DOI] [PubMed] [Google Scholar]
- *Latorre-Román P. A., Garrido-Ruiz A., García-Pinillos F. (2015). Versión española del cuestionario del complejo de Adonis: un cuestionario para el análisis del dimorfismo muscular o vigorexia [Spanish version of the Adonis Complex Questionnaire: A questionnaire for the analysis of muscle dysmorphia or bigorexia]. Nutrición Hospitalaria, 31, 1246–1253. doi: 10.3305/nh.2015.31.3.8292 [DOI] [PubMed] [Google Scholar]
- Lavender J. M., Brown T. A., Murray S. B. (2017). Men, muscles, and eating disorders: An overview of traditional and muscularity-oriented disordered eating. Current Psychiatry Reports, 19(6), 32. doi: 10.1007/s11920-017-0787-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi C., Prino L. E., Fabris M. A., Settani M. (2017). Muscle dysmorphia and psychopathology: Findings from an Italian sample of male bodybuilders. Psychiatric Research, 256, 231–236. doi: 10.1016/j.psychres.2017.06.065 [DOI] [PubMed] [Google Scholar]
- *Lopez A., Pollack L., Gonzales S., Pona A. A., Lundgren J. D. (2015). Psychosocial correlates of muscle dysmorphia among collegiate males. Journal of Psychological Inquiry, 20, 58–66. [Google Scholar]
- Lopez-Cuautle C., Vazquez-Arevalo R., Mancilla-Diaz J. M. (2016). Evaluación diagnóstica de la dismorfia muscular [Diagnostic evaluation of muscular dysmorphia]. Anales de Psicología, 32(2), 405–4016. doi: 10.6018/analesps.32.2.203871 [DOI] [Google Scholar]
- López-López J. A., Marín-Martínez F., Sánchez-Meca J., van den Noortgate W., Viechtbauer W. (2014). Estimation of the predictive power of the model in mixed-effects meta-regression: A simulation study. British Journal of Mathematical and Statistical Psychology, 67(1), 30–48. doi: 10.1111/bmsp.12002 [DOI] [PubMed] [Google Scholar]
- *Magallares A. (2016). Drive for thinness and pursuit of muscularity: The role of gender ideologies. Universitas Psychologica, 15(2), 353–360. doi: 10.11144/Javeriana.upsy15-2.dtpm [DOI] [Google Scholar]
- *Maida D. M., Armstrong S. L. (2005). The classification of muscle dysmorphia. International Journal of Men’s Health, 4(1), 73–91. doi: 10.3149/jmh.0401.73 [DOI] [Google Scholar]
- Mayville S. B., Williamson D. A., White M. A., Netemeyer R. G., Drab D. L. (2002). Development of the Muscle Appearance Satisfaction Scale: A self-report measure for the assessment of muscle dysmorphia symptoms. Assessment, 9(4), 351–360. doi: 10.1177/1073191102238156 [DOI] [PubMed] [Google Scholar]
- *McFarland M. B., Kaminski P. L. (2009). Men, muscles and mood: The relation between self-concept, dysphoria, and body image disturbances. Eating Behaviors, 10(1), 68–70. doi: 10.1016/j.eatbeh.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Menees L., Grieve F. G., Mienaltowski A., Pope J. (2013). Critical comments about the body and muscle dysmorphia symptoms in collegiate men. International Journal of Men’s Health, 12(1), 17–28. doi: 10.3149/jmh.1201.17 [DOI] [Google Scholar]
- Mitchell L., Murray S. B., Cobley S., Hackett D., Gifford J., Capling L., O’Connor H. (2017). Muscle dysmorphia symptomatology and associated psychological features in bodybuilders and non-bodybuilder resistance trainers: A systematic review and meta-analysis. Sports Medicine, 47(2), 233–259. doi: 10.1007/s40279-016-0564-3 [DOI] [PubMed] [Google Scholar]
- *Mitchell L., Murray S. B., Hoon M., Hackett D., Prvan T., O’Connor H. (2017). Correlates of muscle dysmorphia symptomatology in natural bodybuilders: Distinguishing factors in the pursuit of hyper-muscularity. Body Image, 22, 1–5. doi: 10.1016/j.bodyim.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Morrison M. A., McCann L. (2006). Striving for bodily perfection? An overview of the drive for muscularity In Kindes M. V. (Ed.), Body image: New research (pp. 1–34). Hauppauge, NY: Nova Science. [Google Scholar]
- Murray S. B., Griffiths S., Mitchison D., Mond J. M. (2017). The transition from thinness-oriented to muscularity-oriented disordered eating in adolescent males: A clinical observation. Journal of Adolescent Health, 60(3), 353–355. doi: 10.1016/j.jadohealth.2016.10.014 [DOI] [PubMed] [Google Scholar]
- *Murray S. B., Griffiths S., Mond J. M., Kean J., Blashill A. J. (2016). Anabolic steroid use and body image psychopathology in men: Delineating between appearance-versus performance-driven motivations. Drug and Alcohol Dependence, 165, 198–202. doi: 10.1016/j.drugalcdep.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Murray S. B., Nagata J. M., Griffiths S., Calzo J. P., Brown T. A., Mitchison D., Blashill A. J., Mond J. M. (2017). The enigma of male eating disorders: A critical review and synthesis. Clinical Psychology Review, 57, 1–11. doi: 10.1016/j.cpr.2017.08.001 [DOI] [PubMed] [Google Scholar]
- *Murray S. B., Rieger E., Hildebrand T., Karlov L., Russell J., Boon E., Dawson R. T., Touyz S. W. (2012). A comparison of eating, exercise, shape, and weight related symptomatology in males with muscle dysmorphia and anorexia nervosa. Body Image, 9(2), 193–200. doi: 10.1016/j.bodyim.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Nieuwoudt J. E., Zhou S., Coutts R. A., Booker R. (2012). Muscle dysmorphia: Current research and potential classification as a disorder. Psychology of Sport and Exercise, 13(5), 569–577. doi: 10.1016/j.psychsport.2012.03.006 [DOI] [Google Scholar]
- *Nieuwoudt J. E., Zhou S., Coutts R. A., Booker R. (2015). Symptoms of muscle dysmorphia, body dysmorphic disorder, and eating disorder in a nonclinical population of adult male weightlifters of Australia. Journal of Strength and Conditioning Research, 29(5), 1406–1414. doi: 10.1519/JSC.0000000000000763 [DOI] [PubMed] [Google Scholar]
- Olivardia R. (2001). Mirror, mirror on the wall, who’s the largest of them all? The features and phenomenology of muscle dysmorphia. Harvard Review of Psychiatry, 9(5), 254–259. doi: 10.1080/10673220127900 [DOI] [PubMed] [Google Scholar]
- *Olivardia R., Pope H. G., Hudson J. I. (2000). Muscle dysmorphia in male weightlifters: A case-control study. American Journal of Psychiatry, 157(8), 1291–1296. doi: 10.1176/appi.ajp.157.8.1291 [DOI] [PubMed] [Google Scholar]
- Pope H. G., Gruber A. J., Choi P., Olivardia R., Phillips K. A. (1997). Muscle dysmorphia: An underrecognized form of body dysmorphic disorder. Psychosomatics, 38(6), 548–557. doi: 10.1016/S0033-3182(97)71400-2 [DOI] [PubMed] [Google Scholar]
- Pope H. G., Katz D. L., Hudson J. I. (1993). Anorexia nervosa and “reverse anorexia” among 108 male bodybuilders. Comprehensive Psychiatry, 34(6), 406–409. doi: 10.1016/0010-440X(93)90066-D [DOI] [PubMed] [Google Scholar]
- Pope H. G., Khalsa J. H., Bhasin S. (2017). Body image disorders and abuse of anabolic-androgenic steroids among men. JAMA-Journal of the American Medical Association, 317(1), 23–24. doi: 10.1001/jama.2016.17441 [DOI] [PubMed] [Google Scholar]
- Pope H. G., Phillips K. A., Olivardia R. (2000). The Adonis complex: The secret crisis of male body obsession. New York, NY: The Free Press. [Google Scholar]
- *Pope C. G., Pope H. G., Menard W., Fay C., Olivardia R., Phillips K. A. (2005). Clinical features of muscle dysmorphia among males with body dysmorphic disorder. Body Image, 2, 395–400. doi: 10.1016/j.bodyim.2005.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W. (2009). Analyzing effect sizes: Random-effects models In Cooper H., Hedges L. V., Valentine J. C. (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 295–315). New York: Russell Sage Foundation. [Google Scholar]
- Readdy T., Cardinal B. J., Watkins P. L. (2011). Muscle dysmorphia, gender role stress, and sociocultural influences: An exploratory study. Research Quarterly for Exercise and Sport, 82, 310–319. doi: 10.1080/02701367.2011.10599759 [DOI] [PubMed] [Google Scholar]
- *Rhea D. J., Lantz C. D., Cornelius A. E. (2004). Development of the Muscle Dysmorphia Inventory (MDI). Journal of Sports Medicine and Physical Fitness, 44, 428–435. Retrieved from http://www.drdebbierhea.com/wp-content/uploads/2013/05/Development-of-the-MDI-2005.pdf [PubMed] [Google Scholar]
- Robert C. A., Munroe-Chandler K. J., Gammage K. L. (2009). The relationship between the drive for muscularity and muscle dysmorphia in male and female weight trainers. The Journal of Strength and Conditioning Research, 23(6), 1656–1662. doi: 10.1519/JSC.0b013e3181b3dc2f [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J., Marín-Martínez F. (2008). Confidence intervals for the overall effect size in random-effects meta-analysis. Psychological Methods, 13(1), 31–48. doi: 10.1037/1082-989X.13.1.31 [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J., Marín-Martínez F., Chacón-Moscoso S. (2003). Effect-size indices for dichotomized outcomes in meta-analysis. Psychological Methods, 8(4), 448–467. doi: 10.1037/1082-989X.8.4.448 [DOI] [PubMed] [Google Scholar]
- Sandgren S. S., Lavallee D. (2018). Muscle dysmorphia research neglects DSM-5 diagnostic criteria. Journal of Loss and Trauma, 23(3), 211–243. doi: 10.1080/15325024.2018.1428484 [DOI] [Google Scholar]
- *Santarnecchi E., Dèttore D. (2012). Muscle dysmorphia in different degrees of bodybuilding activities: Validation of the Italian version of Muscle Dysmorphia Disorder Inventory and bodybuilder image grid. Body Image, 9(3), 396–403. doi: 10.1016/j.bodyim.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Schmidt F. L., Hunter J. E. (2015). Methods of meta-analysis: Correcting error and bias in research synthesis (3rd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- *Segura-García C., Ammendolia A., Procopio L., Papaianni M. C., Sinopoli F., Bianco C., De Fazio P., Capranica L. (2010). Body uneasiness, eating disorders and muscle dysmorphia in individuals who overexercise. Journal of Strength and Conditioning Research, 24(11), 3098–3104. doi: 10.1519/JSC.0b013e3181d0a575 [DOI] [PubMed] [Google Scholar]
- Short J. (2005). Creating a tool to measure muscle dysmorphia (Unpublished master’s thesis). Western Kentucky University, Bowling Green, KY. [Google Scholar]
- *Sladek M. R., Engeln R., Miller S. A. (2014). Development and validation of the Male Body Talk Scale: A psychometric investigation. Body Image, 11(3), 233–244. doi: 10.1016/j.bodyim.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C., Egger M. (2005). Regression methods to detect publication and other bias in meta-analysis In Rothstein H. R., Sutton A. J., Borenstein M. (Eds.), Publication bias in meta-analysis: Prevention, assessment and adjustments (pp. 99–100). Chichester, UK: Wiley. [Google Scholar]
- Suffolk M. T., Dovey T. M., Goodwin H., Meyer C. (2013). Muscle dysmorphia: Methodological issues, implication for research. Eating Disorders, 21(5), 437–457. doi: 10.1080/10640266.2013.828520 [DOI] [PubMed] [Google Scholar]
- Tod D., Edwards C. (2015a). Relationships among muscle dysmorphia characteristics, body image, quality of life, and coping in males. Journal of Science and Medicine in Sport, 18(5), 585–589. doi: 10.1016/j.jsams.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Tod D., Edwards C. (2015b). A meta-analysis of the drive for muscularity’s relationships with exercise behaviour, disordered eating, supplement consumption, and exercise dependence. International Review of Sport and Exercise Psychology, 8(1), 185–203. doi: 10.1080/1750984X.2015.1052089 [DOI] [Google Scholar]
- Tod D., Edwards C., Cranswick I. (2016). Muscle dysmorphia: Current insights. Journal of Psychology Research and Behavior Management, 9, 179–188. doi: 10.2147/PRBM.S97404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Walker D. C., Anderson D. A., Hildebrandt T. (2009). Body checking behaviors in men. Body Image, 63, 164–170. doi: 10.1016/j.bodyim.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]