Abstract
The spectra presented correspond with the research article entitled “Kinetics of Formic Acid Decomposition in Subcritical and Supercritical Water – A Raman Spectroscopic Study” [1]. Data set contains in situ Raman spectra of the quenched effluent stream, which includes varied concentrations of formic acid, water, CO, CO2, and H2 as reaction products. Each spectrum is collected downstream of the subcritical or supercritical water gasification of formic acid, which occurs at a specified temperature, residence time, a constant pressure of 25 MPa, and a constant initial feedstock concentration of 3.6 wt% formic acid. Additionally, calibration spectra of formic acid in water, and spectra of pure carbon dioxide and high concentration formic acid are provided for model development. Finally, a MATLAB code used for baseline subtraction of raw data files is included with the dataset. The full dataset is hosted in Mendeley Data, https://doi.org/10.17632/hjn8xwskng.1.
Keywords: Raman spectroscopy, Supercritical water, Formic acid, Gasification, Reaction kinetics
Specifications Table
| Subject area | Analytical Chemistry |
| More specific subject area | Supercritical Water Gasification |
| Type of data | CSV files |
| How data was acquired | MarqMetrix All-In-One Raman Spectroscopic Ball Probe |
| Data format | Baseline Subtracted, Normalized, and Averaged |
| Experimental factors | Five samples were collected for each experimental condition, each consisting of 20 averages of 1000 ms integration time. These five samples were then used to create a mean sample with PEAXACT (Aachen, Germany) quantitative spectroscopy software. |
| Experimental features | Formic acid was gasified in a continuous supercritical water reactor at temperatures from 300°C to 430°C, at residence times between 4 s and 65 s, at a constant pressure of 25 MPa, and with a constant initial formic acid concentration of 3.6 wt%. Spectra were collected in the cold zone of the reactor as described inRef. [1]. |
| Data source location | Seattle, WA, USA |
| Data accessibility |
Data is hosted in Mendeley Data,https://doi.org/10.17632/hjn8xwskng.1 URL: https://doi.org/10.17632/hjn8xwskng.1 |
| Related research article |
B.R. Pinkard, D.J. Gorman, E.G. Rasmussen, J.C. Kramlich, P.G. Reinhall, I.V. Novosselov, Kinetics of Formic Acid Decomposition in Subcritical and Supercritical Water – A Raman Spectroscopic Study, Int. J. Hydrog. Energy 44 (2019) 31745–31756.https://doi.org/10.1016/j.ijhydene.2019.10.070 URL:https://doi.org/10.1016/j.ijhydene.2019.10.070 |
Value of the Data
|
1. Data
Raman spectra (.CSV files) presented in the corresponding dataset are high-resolution spectra taken of the effluent stream following the decomposition of formic acid in subcritical or supercritical water. Five replicate spectra are collected for each experimental condition, each consisting of 20 averages with 1000 ms signal integration time. The fluorescent background is removed from each spectrum using a semi-automated baseline subtraction algorithm, which provides consistent data processing over other baseline subtraction methodologies. Spectra are normalized to the major sapphire peak at 418 cm−1, to negate variations due to optical effects. The five replicate spectra are used to create a mean spectrum using PEAXACT (Aachen, Germany) quantitative spectroscopy software.
2. Experimental design, materials, and methods
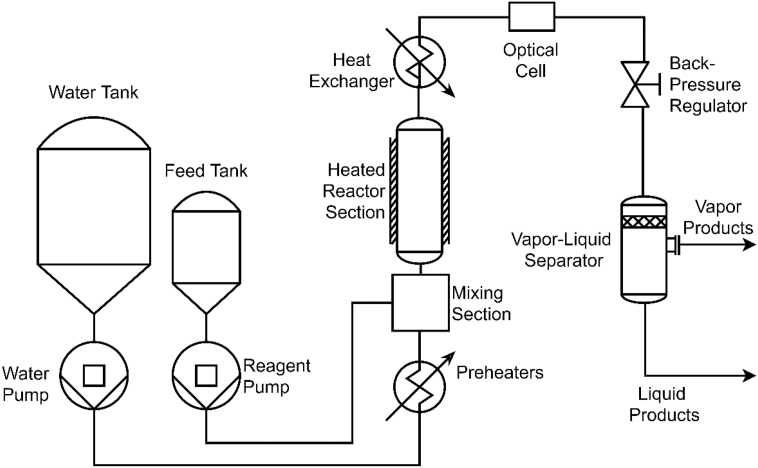
Formic acid is gasified in a continuous supercritical water reactor at temperatures between 300 and 430 °C, residence times between 4 and 65 s, a constant pressure of 25 MPa, and a constant initial formic acid concentration of 3.6 wt%. The supercritical water reactor is designed to operate at pressures up to 35 MPa and temperatures up to 560 °C. A general system schematic is shown in Fig. 1. The reactor is constructed from 6.35 mm Inconel 625 tubing with an inner diameter of 3.05 mm, and the reactor section used for these experiments has an internal volume of 18.6 mL.
Fig. 1.
Schematic of the University of Washington supercritical water gasification reactor.
HPLC pumps provide user-defined flow rates of reagent and water into the reaction environment, while a back pressure regulator controls internal pressure. Water is preheated prior to reagent injection, to establish a clear reaction starting point, and a custom mixing section ensures rapid heating and mixing of the reagent. A radiant cylinder heater surrounds the coiled reactor section to maintain isothermal conditions, which are monitored with Type-K thermocouples in contact with the flow at multiple locations. A vertically oriented heat exchanger quenches high-temperature reactions after the reactor section. A more thorough discussion on the reactor design can be found in Ref. [1].
A Raman immersion ball probe from MarqMetrix (Seattle, WA) is located immediately after the heat exchanger for in situ monitoring of product species in the quenched effluent stream. The Raman spectroscopic cell is positioned in the cold reactor zone (25 °C, 25 MPa) to avoid issues with thermal expansion and sealing, and to simplify quantitative spectroscopy. A full discussion on the difficulties of placing the Raman cell in the hot zone can be found elsewhere [[2], [3], [4]]. A 785 nm fiber-optic Raman laser is operated at 300 mW to excite the molecules in the optical volume, and spectra are collected in the backscatter configuration. A sapphire ball lens focuses the excitation light 0.6 mm in front of the lens, which protrudes 0.5 mm into the flow. Five replicate spectra, each with a total integration time of 20 s, are collected and averaged for each operational condition. This reduces noise and ensures that minor spectral constituents are accurately represented. The dark signal is automatically subtracted by the MarqMetrix system, which results from the spontaneous generation of electrons in the detector and is measured by the spectrometer under no laser excitation [5]. The fluorescent background is subtracted semi-manually using a MATLAB routine.
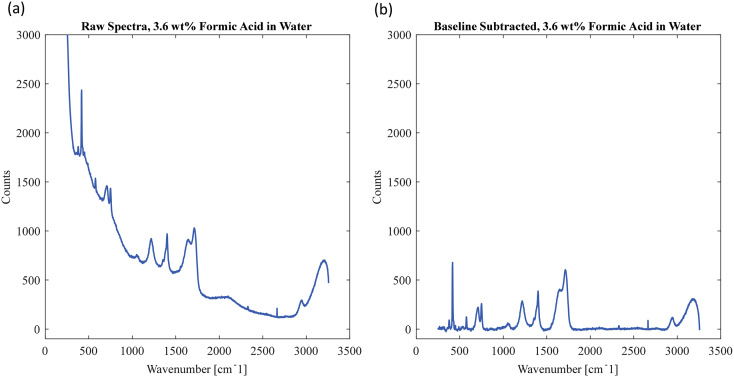
Raman spectra of certain molecules contain large fluorescent background signals which must be subtracted to obtain useful data. Water generates significant fluorescence; thus, fluorescent background subtraction is needed to analyze aqueous mixtures [5]. The background signal follows a linear shape, with a maximum value at low wavenumbers and a minimum value at high wavenumbers, but linear subtractions generate large errors. Various algorithms and methods exist for fluorescent background subtraction, including polynomial fits, wavelet algorithms, manual subtractions, and others [[6], [7], [8]]. For consistent data analysis, a semi-manual background subtraction algorithm was implemented in MATLAB, based on the knowledge that only Raman peaks from sapphire, water, formic acid, CO, CO2, and H2 exist in the Raman spectra. The Raman spectral intensity should theoretically be zero at wavenumbers where known Raman peaks do not exist. The MATLAB code defines anchor points where the Raman spectrum should be zero and interpolates between these points to form a baseline. This baseline is then directly subtracted from the measured spectrum. Additionally, each spectrum is normalized by the major sapphire peak, which should be constant for all collected data, but could vary slightly due to optical variations. By normalizing, the effects of these optical variations on the collected spectra are removed. The MATLAB code used for this semi-manual subtraction is included with the data files and can be generalized for spectra of different mixtures. Fig. 2 demonstrates the efficacy of the baseline subtraction algorithm.
Fig. 2.
3.6 wt% formic acid in DI water (a) before fluorescent background subtraction and (b) after baseline subtraction.
Quantitative spectroscopic techniques are implemented using PEAXACT to extract concentration measurements from Raman spectra. The magnitude of a molecule's Raman spectrum scales linearly with its molar concentration, thus peak heights and peak areas are directly proportional to molar concentration. In order to accurately measure peak height or area, a compound must have a dominant characteristic peak in a “spectral window,” where no other Raman peaks exist. For regions in which multiple characteristic peaks of separate compounds are convoluted, hard modeling or indirect hard modeling (IHM) can be used to calculate molar concentrations. Hard modeling relies on modeling characteristic peaks as pseudo-Voigt curves, and each compound is assigned a pure spectrum which contains all of its respective peaks. The pure spectra are then superposed to create a best-fit of the analyzed spectrum, with the resulting weightings corresponding to molar concentrations [9,10]. Accurate calibration is needed for all quantitative spectroscopy techniques. Corresponding product yields extracted from each Raman spectra are presented in Ref. [1], following a calibration method proposed by Beumers et al. [11].
Acknowledgments
Funding for this work was provided by the Defense Threat Reduction Agency (DTRA) – Grant HDTRA1-17-1-0001.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2020.105312.
Contributor Information
Brian R. Pinkard, Email: pinkardb@uw.edu.
David J. Gorman, Email: dgorman7@uw.edu.
Elizabeth G. Rasmussen, Email: egwohlfo@uw.edu.
Vedant Maheshwari, Email: vm9295@uw.edu.
John C. Kramlich, Email: kramlich@uw.edu.
Per G. Reinhall, Email: reinhall@uw.edu.
Igor V. Novosselov, Email: ivn@uw.edu.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pinkard B.R., Gorman D.J., Rasmussen E.G., Kramlich J.C., Reinhall P.G., Novosselov I.V. Kinetics of formic acid decomposition in subcritical and supercritical water – a Raman spectroscopic Study. Int. J. Hydrogen Energy. 2019;44:31745–31756. [Google Scholar]
- 2.Pinkard B.R., Gorman D.J., Tiwari K., Kramlich J.C., Reinhall P.G., Novosselov I.V. Review of gasification of organic compounds in continuous-flow, supercritical water reactors. Ind. Eng. Chem. Res. 2018;57:3471–3481. [Google Scholar]
- 3.Pinkard B.R., Gorman D.J., Tiwari K., Kramlich J.C., Reinhall P.G., Novosselov I.V. Supercritical water gasification: practical design strategies and operational challenges for lab-scale, continuous flow reactors. Heliyon. 2019;5:e01269. doi: 10.1016/j.heliyon.2019.e01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braeuer A.S. Prospects: facing current challenges in high pressure high temperature process engineering with in situ Raman measurements. J. Supercrit. Fluids. 2018;134:80–87. [Google Scholar]
- 5.McCreery R.L. vol. 225. John Wiley & Sons; 2005. (Raman Spectroscopy for Chemical Analysis). [Google Scholar]
- 6.Gan F., Ruan G., Mo J. Baseline correction by improved iterative polynomial fitting with automatic threshold. Chemometr. Intell. Lab. Syst. 2006;82:59–65. [Google Scholar]
- 7.Ruckstuhl A.F., Jacobson M.P., Field R.W., Dodd J.A. Baseline subtraction using robust local regression estimation. J. Quant. Spectrosc. Radiat. Transf. 2001;68:179–193. [Google Scholar]
- 8.Liland K.H., Almøy T., Mevik B.H. Optimal choice of baseline correction for multivariate calibration of spectra. Appl. Spectrosc. 2010;64:1007–1016. doi: 10.1366/000370210792434350. [DOI] [PubMed] [Google Scholar]
- 9.Kriesten E., Alsmeyer F., Bardow A., Marquardt W. Fully automated indirect hard modeling of mixture spectra. Chemometr. Intell. Lab. Syst. 2008;91:181–193. [Google Scholar]
- 10.Alsmeyer F., Koß H.J., Marquardt W. Indirect spectral hard modeling for the analysis of reactive and interacting mixtures. Appl. Spectrosc. 2004;58:975–985. doi: 10.1366/0003702041655368. [DOI] [PubMed] [Google Scholar]
- 11.Beumers P., Brands T., Koss H.J., Bardow A. Model-free calibration of Raman measurements of reactive systems: application to monoethanolamine/water/CO2. Fluid Phase Equil. 2016;424:52–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.