Highlights
-
•
Doppler imaging to measure cerebral blood flow is feasible during tilt testing.
-
•
Cerebral blood flow in ME/CFS patients is reduced during tilt testing.
-
•
90% of ME/CFS patients show abnormal cerebral blood flow reduction on tilt testing.
-
•
Cerebral blood flow reduction correlates with symptoms of orthostatic intolerance.
Keywords: Orthostatic intolerance, Cerebral blood flow, Tilt table testing, ME/CFS, Postural orthostatic tachycardia syndrome, Orthostatic hypotension
Abstract
Objective
The underlying hypothesis in orthostatic intolerance (OI) syndromes is that symptoms are associated with cerebral blood flow (CBF) reduction. Indirect CBF measurements (transcranial Doppler flow velocities), provide inconsistent support of this hypothesis. The aim of the study was to measure CBF during a 30 min head-up tilt test (HUT), using Doppler flow imaging of carotid and vertebral arteries, in individuals with chronic fatigue syndrome/myalgic encephalomyelitis (ME/CFS), a condition with a high prevalence of OI.
Methods
429 ME/CFS patients were studied: 247 had a normal heart rate (HR) and blood pressure (BP) response to HUT, 62 had delayed orthostatic hypotension (dOH), and 120 had postural orthostatic tachycardia syndrome (POTS). We also studied 44 healthy controls (HC). CBF measurements were made at mid-tilt and end-tilt. Before mid-tilt, we administered a verbal questionnaire to ascertain for 15 OI symptoms.
Results
End-tilt CBF reduction was 7% in HC versus 26% in the overall ME/CFS group, 24% in patients with a normal HR/BP response, 28% in those with dOH, and 29% in POTS patients (all P < .0005). Using a lower limit of normal of 2SD of CBF reduction in HC (13% reduction), 82% of patients with normal HR/BP response, 98% with dOH and 100% with POTS showed an abnormal CBF reduction. There was a linear correlation of summed OI symptoms with the degree of CBF reduction at mid-tilt (P < .0005).
Conclusions
During HUT, extracranial Doppler measurements demonstrate that CBF is reduced in ME/CFS patients with POTS, dOH, and even in those without HR/BP abnormalities.
Significance
This study shows that orthostatic intolerance symptoms are related to CBF reduction, and that the majority of ME/CFS patients (90%) show an abnormal cerebral flow reduction during orthostatic stress testing. This may have implications for the diagnosis and treatment of ME/CFS patients.
1. Introduction
Orthostatic intolerance is defined as a clinical condition in which symptoms worsen upon assuming and maintaining upright posture and are ameliorated by recumbency (Low et al., 2009). The ME/CFS patient population has a high prevalence of orthostatic intolerance (IOM, 2015). When orthostatic intolerance is suspected, an orthostatic stress test can be performed, usually consisting of a head-up tilt table test (Sheldon et al., 2015) or a standing test (Hyatt et al., 1975, Winker et al., 2005). Based on heart rate and blood pressure changes during these orthostatic stress tests, a variety of hemodynamic abnormalities can be diagnosed, including orthostatic hypotension, postural orthostatic tachycardia syndrome (POTS), and syncope (Freeman et al., 2011, Sheldon et al., 2015, Shen et al., 2017).
It is generally assumed that part of the OI symptomatology is related to cerebral hypoperfusion (Freeman et al., 2011, Sheldon et al., 2015, Shen et al., 2017). Indirect cerebral blood flow measurements such as those obtained by transcranial Doppler studies, provide inconsistent support of this hypothesis (Coverdale et al., 1985, Verbree et al., 1985, Park et al., 2017, Novak, 2018). A limitation of transcranial Doppler is that it measures cerebral flow velocities, not cerebral blood flow. For transcranial Doppler cerebral blood flow velocity measurement to be a valid indicator of total cerebral blood flow, an important assumption is that intracranial vessel diameters would have to remain unchanged during an intervention. Changes in vessel diameters affect flow velocities (Kantos, 1992) independent of the intervention. During head-up tilt test hypocapnia may develop (Naschitz et al., 2000, Novak, 2018). Previous studies have shown that hypocapnia can reduce intracranial vessel diameters, thereby altering the relation between flow velocity changes and hemodynamic changes (Coverdale et al., 1985, Verbree et al., 1985, Al-Khazraji et al., 2019).
Using extracranial Doppler imaging of the carotid and vertebral arteries, taking both flow velocity and vessel diameters into account, we recently demonstrated the feasibility and reproducibility of measuring total cerebral blood flow during head-up tilt test in healthy controls (van Campen et al., 2018). The aim of the current study was to use the same technique to measure whether orthostatic symptoms in ME/CFS are associated with a cerebral blood flow reduction.
2. Methods
2.1. Participants
From October 2012 to January 2018, 714 consecutive patients visited the outpatient clinic of the Stichting CardioZorg, Hoofddorp, the Netherlands, because of a suspicion of ME/CFS. This cardiology clinic specializes in diagnosing and treating adults with ME/CFS. All patients were evaluated by the same clinician (FVC). During the first visit, we determined whether participants satisfied the criteria for CFS and ME, taking the exclusion criteria into account. Patients were classified as having CFS, chronic fatigue, or no chronic fatigue as defined by Fukuda and colleagues (Fukuda et al., 1994) and as having ME or no ME as defined by Carruthers et al. (2011).
The clinician ascertained for the presence of orthostatic intolerance symptoms in daily life like dizziness/light-headedness, prior (near)-syncope, nausea, etc., as well as triggering events like standing in a line. To determine the interobserver variation of the assessment of daily life OI symptoms, a second clinician (CMCvC) reviewed the charts of 60 randomly chosen patients.
In all patients with diagnosed ME/CFS, an orthostatic stress test was performed unless the treating physician deemed a cardiopulmonary exercise test more appropriate to establish the exercise capacity (to help measure the degree of disability for social security claims). Participants in whom orthostatic testing was thought to be too taxing due to the severity of their illness did not undergo orthostatic stress testing. In a minority of patients an alternative orthostatic stress test with cerebral blood flow measurements was used (while sitting and standing), to demonstrate the effects of positional changes on cerebral blood flow (also done because of social security claims). The current use of any medication did not affect the inclusion process for tilt testing.
For comparison healthy controls were also studied. Controls were recruited from three sources: (a) announcements on ME/CFS patient advocacy websites, (b) posters in the medical clinic’s office building, and (c) healthy acquaintances of the ME/CFS participants. Controls were also asked for daily life orthostatic intolerance symptoms.
The study was carried out in accordance with the Declaration of Helsinki. All ME/CFS participants and healthy controls gave informed, written consent. The study was approved by the medical ethics committee of the Slotervaart Hospital, Amsterdam, the Netherlands.
2.2. Head-up tilt test with cerebral blood flow measurements
For the tilt test patients were instructed to continue their medication (including heart rate and blood pressure altering medication), except for patients with known or a high suspicion of POTS, as was ascertained from the referral report of the general practitioner. To confirm or establish the diagnosis of POTS these patients (n = 7) were instructed to taper off and stop the beta-blocker and/or ivabradine use for 7 days prior to the tilt test. Measurements were performed as described previously (van Campen et al., 2018). Briefly, all participants were positioned for 20 min supine before being tilted head-up to 70 degrees for a maximum of 30 min. Heart rate, systolic, and diastolic blood pressures were continuously recorded by finger plethysmography using the Nexfin device (BMeye, Amsterdam, the Netherlands). Heart rate and blood pressures were extracted from the Nexfin device and imported into an Excel spreadsheet. Internal carotid artery and vertebral artery Doppler flow velocity frames were acquired by one operator (FCV), using a Vivid-I system (GE Healthcare, Hoevelaken, the Netherlands) equipped with a 6–13 MHz linear transducer. High resolution B mode images, color Doppler images and the Doppler velocity spectrum (pulsed wave mode) were recorded in one frame. At least two consecutive series of six frames per artery were recorded. The recording time intervals of the first and last imaged artery were noted and these times were corrected to the times of a radio clock, setting the start of tilt at 0 min. Heart rate and blood pressures of the echo recording time intervals were averaged. In the supine position, image acquisition started 8(2) min prior to tilting (supine data) and during the upright position two series were acquired: one series was started at 12(3) min (mid-head-up tilt test data) and one at 22(3) min (end-head-up tilt test data). In addition, in POTS patients an extra image acquisition was performed at 5(2) min post tilt. Image acquisition for all 4 vessels at each time point (supine, mid-tilt and end-tilt) lasted 3(1) min. End-tidal PCO2 (PetCO2) was monitored using a Lifesense device (Nonin Medical, Minneapolis USA).
2.3. Orthostatic symptoms
We used two methods to ascertain orthostatic symptoms. One was the clinical interview described above, which categorized orthostatic intolerance symptoms as present or absent. The second method was included from August 2013 on. After a pilot study, ME/CFS patients and healthy controls were asked at two time points about 15 orthostatic intolerance symptoms. A written questionnaire was used and a clinician (FCV) read the questions to the patients and healthy controls. Participants were asked to respond with yes or no. The first questionnaire was completed immediately after reaching the upright position and the second at 10 min of upright position just before the mid-head-up tilt test Doppler image acquisition.
2.4. OI questionnaire during the tilt test
The symptoms that were asked for, were: dizziness or light-headedness, fatigue, palpitations, leg muscle weakness, dyspnea, blurred vision, altered hearing, neck-shoulder muscle pain, low-back pain, chest pain/chest pressure, concentration problems, perspiration, head ache/pressure on the head, tingling feeling of hands, and nausea.
2.4.1. Questionnaire at the first minute of tilt
-
1.
Did you develop, after being tilted, complaints of dizziness or lightheadedness?
-
2.
Are you, after being tilted, more fatigued in comparison to when you were lying down?
-
3.
Did you develop, after being tilted, muscle weakness of your legs?
-
4.
Did you develop, after being tilted, a feeling of dyspnea or breathlessness?
-
5.
Is your vision less sharp since you have been tilted?
-
6.
Do you hear me differently, after being tilted, in comparison to when you were lying down?
-
7.
Are you less concentrated while standing, compared to when you were lying down?
-
8.
Did you develop, after being tilted, pain in the muscles of your neck or shoulders?
-
9.
Did you develop, after being tilted, a feeling of nausea?
-
10.
Did you develop, after being tilted, a tingling feeling in your right hand*?
-
11.
Did you develop, after being tilted, a feeling of chest pain or pressure on your chest?
-
12.
Did you develop, after being tilted, low back pain?
-
13.
Did you start to sweat after being tilted?
-
14.
Did you develop, after being tilted, palpitations?
-
15.
Did you develop, after being tilted a feeling of a pressure in your head or head ache?
2.4.2. Questionnaire at the tenth minute of tilt
1A. Did you develop in the last few minutes, complaints of dizziness or lightheadedness
Or in case of a positive answer in the first minute of tilt?
1B. Are the complaints of dizziness or lightheadedness still present or have they disappeared?
2A. Did you get more fatigued in the last few minutes?
Or in case of a positive answer in the first minute of tilt
2B. Are you still more fatigued in comparison to when you were lying down or has the fatigue returned to the same level as before the tilt?
3A. Did you develop in the last few minutes, muscle weakness of your legs?
Or in case of a positive answer in the first minute of tilt
3B. Is the muscle weakness of your legs still present or did it disappear?
4A. Did you develop in the last few minutes, a feeling of dyspnea or breathlessness?
Or in case of a positive answer in the first minute of tilt
4B. Is the feeling of dyspnea or breathlessness still present or did it disappear?
5A. Has your vision become less sharp in the last few minutes?
Or in case of a positive answer in the first minute of tilt
5B. Do you still see less sharp or has your sharpness of vision returned to normal?
6A. Did you start to hear me differently in the last few minutes?
Or in case of a positive answer in the first minute of tilt
6B. Do you still hear me differently or do you hear me normally now?
7A. Has your ability to concentrate been reduced in the last few minutes?
Or in case of a positive answer in the first minute of tilt
7B. Do you still feel like your ability to concentrate is reduced, or has it now returned to normal?
8A. Did you develop in the last few minutes pain in the muscles of your neck or shoulders?
Or in case of a positive answer in the first minute of tilt
8B. Do you still have pain in the muscles of your neck or shoulders, or has the pain disappeared?
9A. Did you develop in the last few minutes a feeling of nausea?
Or in case of a positive answer in the first minute of tilt
9B. Do you still have a feeling of nausea, or has the nausea disappeared?
10A. Did you develop in the last few minutes a tingling feeling in your right hand*?
Or in case of a positive answer in the first minute of tilt
10B. Is the tingling feeling in your right hand still present or has it disappeared?
11A. Did you develop in the last few minutes a feeling of chest pain or pressure on your chest?
Or in case of a positive answer in the first minute of tilt
11B. Is your chest pain or pressure on your chest still present or has it disappeared?
12A. Did you develop in the last few minute low back pain?
Or in case of a positive answer in the first minute of tilt
12B. Is the low back pain still present or has it disappeared?
13A. Did you start to sweat in the last few minutes?
Or in case of a positive answer in the first minute of tilt
13B. Is your sweating still present or did it disappear?
14A. Did you develop in the last few minutes palpitations?
Or in case of a positive answer in the first minute of tilt
14B. Do you still feel palpitations or have they disappeared?
15A. Did you develop in the last few minutes a feeling of a pressure on your head or head ache?
Or in case of a positive answer in the first minute of tilt
15B. Is the pressure on your head or head ache still present or has it disappeared?
*: the left hand was not asked for as the Nexfin cuff was placed around the middle finger of the left hand.
2.5. Data analysis
The changes in heart rate and blood pressure during head-up tilt test were classified according to the consensus statement: normal heart rate and blood pressure response, classic orthostatic hypotension, delayed orthostatic hypotension, POTS, and syncope or near-syncope.
Blood flow of the internal carotid and vertebral arteries was calculated offline by an investigator (CMCvC) who was unaware of the patient or control status and unaware of the hemodynamic outcome of the head-up tilt test. Blood flow in each vessel was calculated from the mean blood flow velocities × the vessel surface area and expressed in ml/min. Flow in the individual arteries was calculated in 3–6 cardiac cycles and data were averaged. Total cerebral blood flow was calculated by adding the flow of the four arteries. We previously demonstrated that this methodology had good intra- and inter-observer variability (van Campen et al., 2018).
To assess the relation between cerebral blood flow changes during the tilt and the orthostatic intolerance symptom questionnaire, the number of positively answered orthostatic intolerance complaints at 10 min were summed, with possible values between 0 and 15. Linear regression analysis was performed between the summed orthostatic intolerance symptom score and the cerebral blood flow decrease at the mid-head-up tilt test series of measurements.
2.6. Statistical analysis
Data were analyzed using SPSS version 21 (IBM). All continuous data were tested for normal distribution using the K-S test, and presented as means (SD) or as median with the IQR. Nominal data were compared using the Chi-square test. Groups were compared using Students T test for unpaired data, or the Mann-Whitney U test, where appropriate. Within group comparison was done by the paired t-test or by the Wilcoxon signed ranks test, where appropriate. To compare the clinician ratings of the presence or absence of OI in daily life, we calculated the intra-class correlation coefficient (ICC). The McNemar test was used to determine the significance of symptom changes of the orthostatic intolerance symptom questionnaire between the 1st minute and 10th minute of tilt. Due to the number of comparisons, we choose a conservative p-value of <0.01 to be statistically significant.
3. Results
3.1. Participants
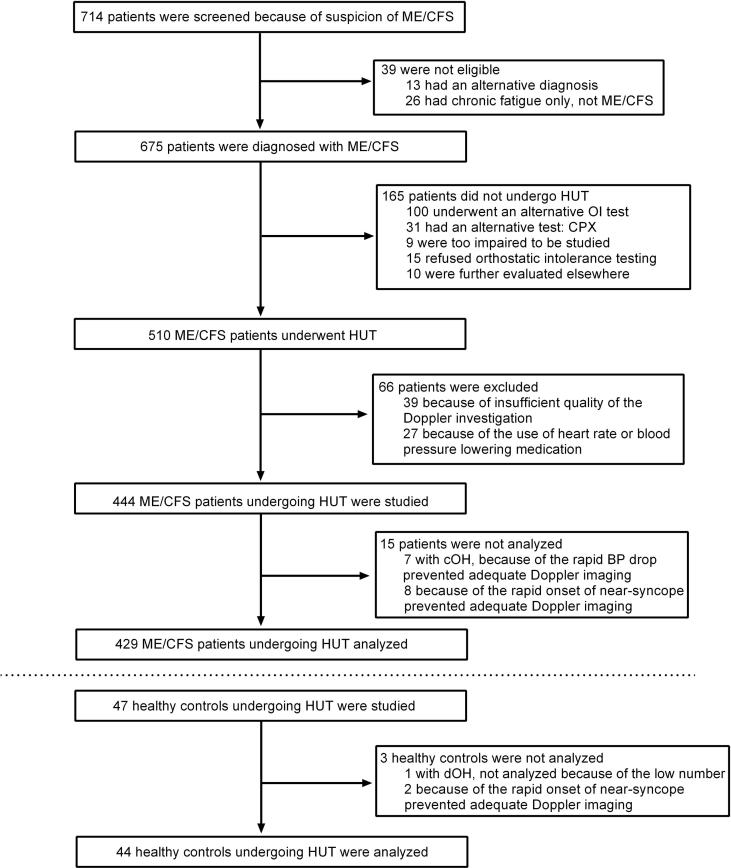
Fig. 1 illustrates the enrollment of the study participants. ME/CFS was diagnosed in 675 patients. Of those 675, 610 (90%) underwent some form of orthostatic stress testing with cerebral blood flow measurements (head-up tilt test, passive standing test, or seated test). The passive standing test or seated tests were conducted in those judged to be more severely affected. For purposes of consistency, in the present study we report only the data from the 510 patients undergoing head-up tilt test. Of these 510, 27 were excluded because of the use of heart rate and/or blood pressure lowering drugs. None of the patients used specific medication for orthostatic intolerance symptoms (e.g. fludrocortisone, desmopressin, midodrine, pyridostigmine). Eleven used beta-blockers, 3 ivabradine, 6 angiotensin converting enzyme inhibitors, 11 Angiotensin II antagonists, 2 calcium antagonists, 1 used clonidine and 3 diuretics. The indications for these medications were hypertension (n = 8), palpitations (n = 12), migraine (n = 6) and menopausal symptoms (n = 1). Because of the low numbers of specific medications and indications no subgroup analysis was performed. Fifty-four patients were also excluded for other reasons than medication use (see Fig. 1), leaving 429 ME/CFS patients to be analyzed. For comparison, we analyzed 44 healthy controls, after excluding 3 individuals. None of the controls used heart rate- or blood pressure-lowering drugs.
Fig. 1.
This shows the flow of recruitment and patient flow explaining reasons for exclusion and number of patients analyzed. ME/CFS: myalgic encephalomyelitis/chronic fatigue syndrome; cOH: classic orthostatic hypotension; dOH: delayed orthostatic hypotension; HUT: head-up tilt test; OI: orthostatic intolerance.
Table 1 shows the baseline demographic and clinical characteristics of the study participants. All patients met the Fukuda criteria for CFS, and 70% met the criteria for ME. The interobserver reliability to assess the presence of daily life orthostatic intolerance symptoms was good, with an ICC of 0.80. Daily life orthostatic intolerance symptoms were reported by 369/429 (86%) ME/CFS patients; 193/247 (78%) in those with a normal heart rate/blood pressure response, 57/62 (92%) in those with delayed orthostatic hypotension, and 119/120 (99%) in those with POTS.
Table 1.
Demographic data.
| Patients (n = 429) | Healthy controls (n = 44) | P | |
|---|---|---|---|
| Age (years) | 39(12) | 37(14) | ns |
| Female gender | 372/429 (87%) | 39/44 (87%) | ns |
| BMI (kg/m2) | 23.4 (20.7–26.8) | 23.4 (21.3–26.9) | ns |
| BSA (duBois; m2) | 1.84(0.20) | 1.87(0.19) | ns |
| Daily life OI symptoms | 369/429 (86%) | 3/44 (7%) | <0.0005 |
| CFS | 429/429 (100%) | ||
| ME | 301/429 (70%) | ||
| Disease duration (years) | 10 (5–16) | ||
| Tilt test results: | |||
| Normal HR/BP response | 247/429 (58%) | 44/44 (100%) | <0.0005 |
| Delayed OH | 62/429 (14%) | ||
| POTS | 120/429 (28%) |
BMI: body mass index; BP: blood pressure; BSA: body surface area; duBois: BSA formula of Dubois; HR: heart rate; OH: orthostatic hypotension; OI: orthostatic intolerance; POTS: postural orthostatic tachycardia syndrome.
3.2. Head-up tilt table testing
Table 2 shows the supine, mid-tilt and end-tilt measurements of heart rate, systolic blood pressure, diastolic blood pressure, PetCO2 and cerebral blood flow for each group. Data from controls with a normal heart rate and blood pressure response were compared with those of ME/CFS patients with a normal heart rate and blood pressure. Furthermore, the three ME/CFS groups were compared. Heart rate increased significantly during the upright position in all groups. By definition POTS patients showed the highest heart rate increase. Systolic blood pressure of all groups decreased significantly during the tilt. By definition systolic blood pressures of delayed orthostatic hypotension patients were significantly lower at the end of the tilt period. End-tilt diastolic blood pressure increased in the groups except in delayed orthostatic hypotension patients. The PetCO2 at the end-tilt decreased in all groups, being lowest in POTS patients.
Table 2.
Tilt table test data of ME/CFS participants and healthy controls.
| Group 1 HC with norm HR/BP N = 44 |
Group 2 ME/CFS with norm HR/BP N = 247 |
Group 3 ME/CFS with dOH N = 62 |
Group 4 ME/CFS with POTS N = 120 |
Group 1 vsGroup 2P | Group 2 vsGroup 3P | Group 2 vsGroup 4P | Group 3 vsGroup 4P | |
|---|---|---|---|---|---|---|---|---|
| HR supine (bpm) | 65 (13) | 70 (10) | 71 (11) | 77 (14) | <0.005 | <0.0005 | <0.005 | |
| HR end-tilt (bpm) | 83 (15) | 88 (12) | 93 (14) | 117 (16) | <0.01 | <0.0005 | <0.0005 | |
| SBP supine (mmHg) | 132 (14) | 135 (17) | 142 (17) | 132 (15) | <0.005 | <0.0005 | ||
| SBP end-tilt (mmHg) | 125 (13) | 133 (17) | 119 (14) | 126 (15) | <0.01 | <0.0005 | <0.0005 | <0.01 |
| DBP supine (mmHg) | 78 (7) | 79 (9) | 79 (9) | 78 (8) | ||||
| DBP end-tilt (mmHg) | 81 (8) | 86 (9) | 79 (8) | 86 (9) | <0.005 | <0.0005 | <0.0005 | |
| PetCO2 supine (mmHg) | 37 (35–39) | 37 (35–38) | 37 (35–39) | 36 (34–38) | ||||
| PetCO2 end-tilt (mmHg) | 36 (34–38) | 32 (28–35) | 32 (28–35) | 26 (23–31) | <0.0005 | <0.0005 | <0.0005 | |
| CBF supine (ml/min) | 625 (79) | 613 (108) | 609 (108) | 623 (97) | ||||
| CBF mid-tilt (ml/min) | 594 (77) | 505 (107) | 460 (85) | 462 (85) | <0.0005 | <0.005 | <0.001 | |
| CBF mid-tilt %change | −5.1 (2.6)% | −19.1 (10.2)% | −25.4 (6.7)% | −26.1 (6.5)% | <0.0005 | <0.0005 | <0.001 | |
| CBF end-tilt (ml/min) | 581 (76) | 467 (102) | 440 (84) | 443 (76) | <0.0005 | |||
| CBF end-tilt %change | −6.7 (3.1)% | −23.6 (10.3)% | −27.7 (6.0)% | −28.9 (5.8)% | <0.0005 | <0.0005 | <0.0005 |
BP: blood pressure; DBP: diastolic blood pressure; CBF: cerebral blood flow; dOH: delayed orthostatic hypotension; HR: heart rate; HC: healthy controls; norm: normal; PetCO2: end-tidal CO2 pressure; POTS: postural orthostatic tachycardia syndrome; SBP: systolic blood pressure; %change: percent change from supine data.
All within group supine values differ from end-tilt values at P < 0.001 except for SBP and DBP in HC which differ at P < 0.005, and DBP in dOH which did not differ.
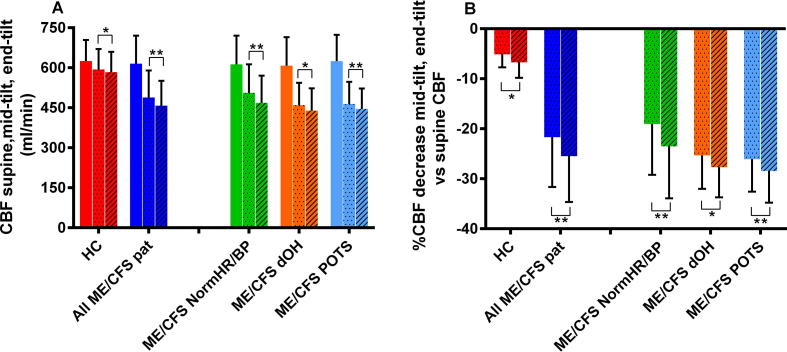
Supine cerebral blood flow was not different between ME/CFS patients and controls. In the total group of ME/CFS patients cerebral blood flow decreased from 615(105) ml/min at supine, to 488(101) ml/min at mid-tilt, and to 456(94) ml/min at end-tilt. In the controls cerebral blood flow decreased 5% at mid-tilt, and 7% at end-tilt. In all ME/CFS patient groups the % cerebral blood flow decrease was larger, ranging between 19 and 26% at mid-tilt and between 24 and 29% at end-tilt. Fig. 2 shows the graphical presentation of these results.
Fig. 2.
(A) CBF in ml/min of healthy controls and the 3 patient groups during head-up tilt. The left colored column is supine, the dotted column is mid-tilt, and the hatched column is end-tilt. (B) This shows the % decrease from supine for mid and end tilt data in patients and controls. CBF: cerebral blood flow; HC: healthy controls; ME/CFS dOH: ME/CFS patients with delayed orthostatic hypotension; ME/CFS NormHR/BP: ME/CFS patients with a normal heart rate and blood pressure response; ME/CFS POTS: ME/CFS patients with postural orthostatic tachycardia syndrome. Comparison between mid and end tilt data: *=P < .005; **=P < .0005.
The end-tilt % cerebral blood flow decrease in controls was 7(3)%. Assuming a lower limit of normal of 2 SD below the mean for healthy controls, the lower limit of a normal % cerebral blood flow decrease during the tilt was 13%. Using this cut-off value, 384/429 ME/CFS patients (90%) showed a more than 13% reduction: 82% of patients with a normal heart rate and blood pressure response, 98% of delayed orthostatic hypotension patients, and 100% of POTS patients.
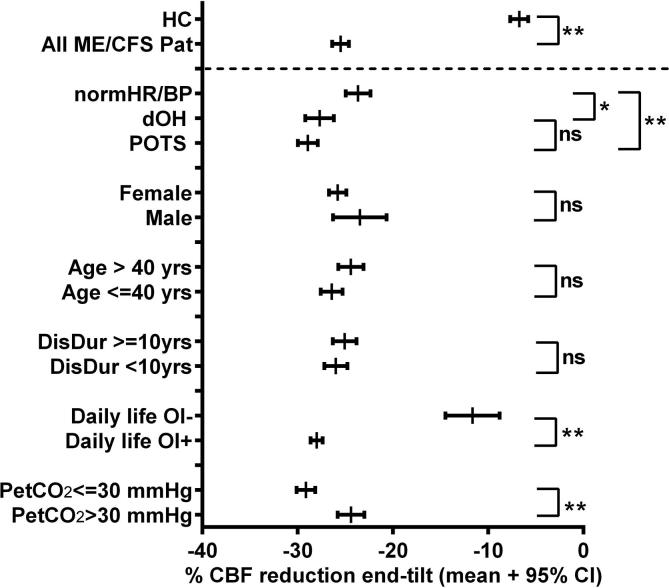
Fig. 3 shows the 95% CI of the % cerebral blood flow decrease at the end of the tilt for the controls and the different ME/CFS groups. The % cerebral blood flow decrease for all ME/CFS groups was significantly greater than that of the controls. Among ME/CFS participants, patients with daily life orthostatic intolerance symptoms, and those with an end-tilt PetCO2 ≤ 30 mmHg showed a greater % cerebral blood flow decrease. There was no difference in the % cerebral blood flow reduction based on age, sex and disease duration dichotomized at 10 years.
Fig. 3.
95% confidence intervals of the %decrease in cerebral blood flow of healthy controls and different ME/CFS patient groups at end tilt. The %decrease of all ME/CFS patient groups and subgroups was significantly larger than that of healthy controls. Abbreviations: Atyp: atypical; CBF: cerebral blood flow; CI: confidence interval; Daily life OI: clinician estimate of orthostatic intolerance symptoms; DisDur: disease duration; dOH: delayed orthostatic hypotension; HC: healthy controls; NormHR/BP: patients with a normal heart rate and blood pressure response; Pat: patients; PetCO2: end-tidal CO2 pressure; POTS: postural orthostatic tachycardia syndrome; yrs: years. **: P < .0005; *: P < .01 between groups.
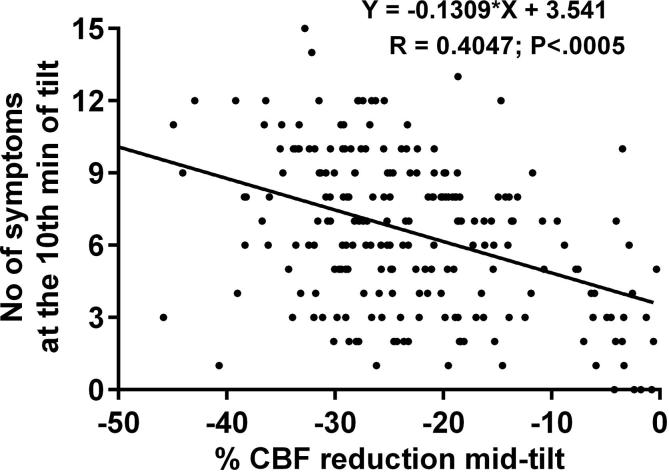
Fig. 4 presents the relation between the sum of positively answered symptom questions per patient and the % cerebral blood flow decrease at mid HUT. The linear regression shows that a greater % cerebral blood flow decrease was significantly associated with a larger number of symptoms (P < .0005).
Fig. 4.
Linear regression analysis of the sum of positively answered OI questions per patient and the % CBF reduction at mid-tilt. The linear regression shows that a higher % CBF decrease during HUT was associated with a larger number of symptoms: P < .0005.
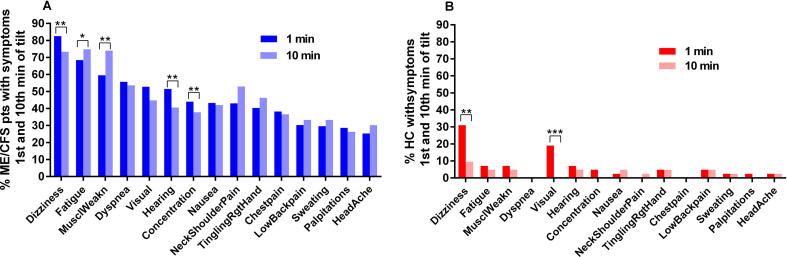
Fig. 5 presents the prevalence of specific OI symptoms in the ME/CFS patients (5A) and the healthy controls (5B). The percentage of those with ME/CFS reporting symptoms of fatigue, leg muscle weakness, and shoulder/neck muscle pain increased significantly at the 10th min. In contrast, dizziness/light-headedness, blurred vision, and altered hearing decreased significantly at the 10th min of tilt compared to the 1st min of tilt. Among controls dizziness/light-headedness and blurred vision decreased significantly by the 10 min point.
Fig. 5.
OI symptoms in ME/CFS patients at the first minute and at the 10th minute (A) of tilt, and healthy controls at the first minute and at the 10th minute of tilt (B). MusclWeakn: leg muscle weakness; TinglingRgtHand: tingling feeling of the right hand. The percentage of positively answered symptom questions of patients were all significantly higher than of HC, both at the 1st and the 10th minute of tilt. Comparison between the 1st minute and 10th minute of tilt: **:P < .0005, *:P < .005.
The summed orthostatic intolerance symptom scores of patients with a normal heart rate/blood pressure response were 6.5(3.0), in delayed orthostatic hypotension patients 6.0(2.7) and in POTS patients 8.0(2.9) at the 1st minute of tilt, and 6.4(3.1), 5.9(2.9), and 8.8(2.7), respectively, at the 10th minute of tilt. The differences between POTS patients and the two other groups were highly significant both at the 1st and 10th minute of tilt (all P < .001). The summed orthostatic intolerance symptom score in controls was 1.0(1.0) at the 1st minute and 0.5(0.9) at the 10th minute. This score was significantly lower than the orthostatic intolerance symptom score in all three patient groups (P all <.001).
4. Discussion
The main finding of this study is that extracranial Doppler imaging demonstrates a significant reduction in cerebral blood flow during head-up tilt test in ME/CFS patients compared to healthy controls. While this reduction might be expected in ME/CFS patients with POTS and delayed orthostatic hypotension, a novel finding of this study is that those who had a normal heart rate and blood pressure response on head-up tilt also experienced a comparable cerebral blood flow reduction. Moreover, the clinical importance of this observation is reflected in the strong association identified between the number of orthostatic symptoms reported during head-up tilt test and the degree of cerebral blood flow reduction.
Most prior studies on orthostatic intolerance in different diseases have focused on demonstrable abnormalities of heart rate and blood pressure (orthostatic hypotension, POTS, syncope) (Purewal and Watkins, 1995, Siennicki-Lantz et al., 1999, Kong and Chuo, 2003, Fuente Mora et al., 2017, Park et al., 2017). A number of studies have investigated the relation between these hemodynamic abnormalities and cerebral hypoperfusion using transcranial Doppler. Some studies observed a cerebral flow velocity reduction in the different diseases compared to healthy controls (Mankovsky et al., 2003, Treger et al., 2005, Haubrich et al., 2010), while others did not (Razumovsky et al., 2003, van Beek et al., 2010, Fuente Mora et al., 2017).
Cerebral flow velocity changes not only depend on the hemodynamic changes of orthostatic hypotension, POTS, and syncope, but also on diameter changes of the intracranial vessels. Intracranial vessel diameter changes are susceptible to changes in PetCO2 (Coverdale et al., 1985, Verbree et al., 1985, Al-Khazraji et al., 2019). However, published data on intracranial vessel diameter changes are inconsistent, ranging from large changes in diameter of individual intra- and extra-cranial vessels to non-significant changes. To address the limitations associated with transcranial Doppler measurement of cerebral blood flow velocity, we studied total cerebral blood flow during an orthostatic stress, measuring both flow velocity and vessel diameters.
Several points about the study findings deserve emphasis. First, our study shows that in response to head-up tilt, ME/CFS patients with either delayed orthostatic hypotension or POTS develop a cerebral blood flow reduction of 28%, and 29%, respectively, at the end of the upright position. This is highly significantly different from the 7% reduction in controls with a normal tilt test. It clearly demonstrates that reduced cerebral blood flow is a cardinal contributor to orthostatic intolerance symptoms. The exact cause of cerebral blood flow reduction remains to be determined, but may involve reductions in cardiac output and the presence of hypocapnia. Similarly, the pathophysiology of orthostatic intolerance symptoms (possibly related to increased catecholamines, metabolic changes or inflammatory changes) needs to be addressed in future studies.
Second, recent transcranial Doppler studies have pointed out that orthostatic intolerance complaints may be present without heart rate and blood pressure abnormalities (Shin et al., 2016, Brooks et al., 2017, Novak, 2018). In three different patient groups with orthostatic intolerance (orthostatic intolerance patients (Shin et al., 2016), Parkinson patients with orthostatic intolerance (Park et al., 2017) and in those with orthostatic intolerance and hypocapnia (Novak, 2018)) the cerebral flow velocity reduction of patients with a normal head-up tilt test, but with orthostatic intolerance symptoms, was similar to the reduction among POTS and orthostatic hypotension patients, and significantly more than in patients without orthostatic intolerance. Our results are consistent with and extend these findings: patients with orthostatic intolerance symptoms in daily life have a significantly larger cerebral blood flow decrease (28%) than patients without orthostatic intolerance symptoms in daily life (12% decrease). This confirms the conclusion of Shin et al. that patients with orthostatic intolerance symptoms share a pathophysiologic similarity with those who have confirmed POTS or orthostatic hypotension, namely reduced cerebral perfusion (Shin et al., 2016).
Third, orthostatic intolerance symptomatology is diverse. This is evident from the 15 very different symptoms that were ascertained during the tilt test. Despite the variability in individual symptom reports, there is a linear relation between the summed orthostatic intolerance score and the degree of cerebral blood flow decrease during the tilt test: a higher summed score is related to a larger cerebral blood flow decrease (see Fig. 4). However, we would caution that clinical symptoms alone may be insufficient to diagnose orthostatic intolerance, as some individuals who did not endorse orthostatic symptoms before the study nonetheless had substantial reductions in cerebral blood flow. This is evident from Fig. 3 in which patients, denying orthostatic intolerance symptoms in daily life, still had a mean cerebral blood flow reduction of 12% (reduction range between 1 and 46%).
Fourth, using the lower limit of normal in cerebral blood flow reduction, 90% ME/CFS (95% CI 87–92%) patients showed a more than 13% reduction. The findings confirm and extend previous reports of a high prevalence of orthostatic intolerance in the ME/CFS population. Our results show that 98% of delayed orthostatic hypotension patients and 100% of POTS patients had a more than 13% cerebral blood flow reduction, inferring that in those with documented delayed orthostatic hypotension and POTS, clinically important reductions in cerebral blood flow could be assumed to be present. On the other hand, the largest group of ME/CFS patients were those with a normal heart rate/blood pressure response. Of this group, 82% had an abnormal cerebral blood flow decrease as defined by the lower limits of normal. These patients would have been misclassified as having normal hemodynamics using just heart rate and blood pressure changes during head-up tilt test. Our data suggest that for a more comprehensive categorization of the circulatory dysfunction in ME/CFS patients cerebral blood flow measurements should be included.
Fifth, our data extend the observation from transcranial Doppler studies that hypocapnia reduces cerebral flow velocities (Immink et al., 1985, Novak, 2018). Using more direct imaging by extracranial Doppler of the internal carotid and vertebral arteries, we showed for the first time that total cerebral blood flow in ME/CFS is also reduced in the presence of hypocapnia.
Sixth, we prospectively studied OI in 429 adults with ME/CFS, a substantially higher sample size than in earlier studies. In the Institute of Medicine review on orthostatic intolerance in ME/CFS (IOM, 2015), the median sample size of included studies was 28 participants (range 10–78). Our large sample size narrows the 95% CI around the estimated proportion of ME/CFS patients with orthostatic intolerance, and the proportion of the different types of hemodynamic response.
Finally, most ME/CFS case definitions have recognized that there are heterogeneous precipitating and perpetuating factors, and most case definitions have recommended subtyping of the illness to define more homogenous samples (Fukuda et al., 1994, Carruthers et al., 2011). Our data suggest that the cerebral blood flow reduction is a common denominator of symptoms across subgroups in this population.
We excluded patients using heart rate and blood pressure lowering drugs to assess the pure effect of the disease on cerebral blood flow during orthostatic stress testing. Although the hemodynamic drugs have been shown to decrease heart rate and blood pressure (van Zwieten et al., 1984, Gee et al., 2018, Williams et al., 2018), little is known about the effects of the various drugs on cerebral perfusion. Most studies using transcranial Doppler have focused on the effect of various antihypertensive drugs in patients with hypertension (Lipsitz et al., 2005, Claassen et al., 2009, Seifert et al., 2009, Hajjar et al., 2013). Angiotensin II blockers were shown to increase cerebral blood flow velocities in healthy controls (Krejcy et al., 1997). Calcium blockers did not change cerebral blood flow velocities in healthy controls (Lemkuil et al., 2016). The effects of these drugs on the results of orthostatic stress testing are unknown and need to be studied in future.
4.1. Limitations
We acknowledge that referral bias by the general practitioner may have played a role, selectively referring patients with orthostatic symptoms. Conversely, our study did not enroll those who were bedbound, and we elected not to expose those with more severe functional impairments to tilt testing. Whether disease severity differences lead to differences in cerebral blood flow reduction needs to be studied in the future. Individuals with ME/CFS have been reported to have variable function from day to day and week to week. Future studies can evaluate whether the cerebral blood flow measurements differ on good versus bad days.
The findings of this study would be strengthened by demonstration of a dose response relationship between cerebral blood flow and end-tidal CO2 and by confirmation that the cerebral blood flow reductions and symptoms also correlate with other measures of hypoperfusion. Our focus was on the prevalence of reductions of cerebral blood flow and therefore investigations of cerebral autoregulation and regional cerebral blood flow were beyond the scope of this study. These topics would be important to investigate in the future.
Finally, the use of extracranial Doppler flow to measure cerebral blood flow has to be replicated by others and in different patient groups. It is unclear how much the orthostatic intolerance of ME/CFS patients differs from other forms of circulatory dysfunction such as those characterized by autonomic neuropathy.
5. Conclusion
This study demonstrates that a clinically significant reduction in cerebral blood flow during head-up tilt test is present in patients with ME/CFS and that the degree of reduction is strongly associated with the provocation of orthostatic intolerance symptoms during head-up tilt test and in daily life. Cerebral blood flow measurements, using Doppler imaging of the internal carotid and vertebral arteries during tilt testing, have the potential to improve the orthostatic intolerance assessment, especially in patients with orthostatic intolerance and a normal heart rate and blood pressure response during head-up tilt test.
Acknowledgments
Acknowledgement
Dr Rowe is supported by the Sunshine Natural Wellbeing Foundation Professorship.
Conflict of interest statement
None for all authors.
References
- Al-Khazraji B.K., Shoemaker L.N., Gati J.S., Szekeres T., Shoemaker J.K. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J. Cereb. Blood Flow Metab. 2019;39:1204–1214. doi: 10.1177/0271678X18762880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.K., Chalder T., Rimes K.A. Chronic fatigue syndrome: cognitive, behavioural and emotional processing vulnerability factors. Behav. Cogn. Psychother. 2017;45:156–169. doi: 10.1017/S1352465816000631. [DOI] [PubMed] [Google Scholar]
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T. Myalgic encephalomyelitis: international consensus criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J.A., Levine B.D., Zhang R. Cerebral vasomotor reactivity before and after blood pressure reduction in hypertensive patients. Am. J. Hypertens. 2009;22:384–391. doi: 10.1038/ajh.2009.2. [DOI] [PubMed] [Google Scholar]
- Coverdale N.S., Gati J.S., Opalevych O., Perrotta A., Shoemaker J.K. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J. Appl. Physiol. 1985;2014(117):1090–1096. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Fuente Mora C., Palma J.A., Kaufmann H., Norcliffe-Kaufmann L. Cerebral autoregulation and symptoms of orthostatic hypotension in familial dysautonomia. J. Cereb. Blood Flow Metab. 2017;37:2414–2422. doi: 10.1177/0271678X16667524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Int. Chronic Fatigue Syndrome Study Group Ann. Intern. Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gee M.E., Watkins A.K., Brown J.N., Young E.J.A. Ivabradine for the treatment of postural orthostatic tachycardia syndrome: a systematic review. Am J Cardiovasc Drugs. 2018;18:195–204. doi: 10.1007/s40256-017-0252-1. [DOI] [PubMed] [Google Scholar]
- Hajjar I., Hart M., Chen Y.L., Mack W., Novak V., Chui H C. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. J. Am. Geriatr. Soc. 2013;61:194–201. doi: 10.1111/jgs.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich C., Pies K., Dafotakis M., Block F., Kloetzsch C., Diehl R.R. Transcranial Doppler monitoring in Parkinson's disease: cerebrovascular compensation of orthostatic hypotension. Ultrasound Med. Biol. 2010;36:1581–1587. doi: 10.1016/j.ultrasmedbio.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Hyatt K.H., Jacobson L.B., Schneider V.S. Comparison of 70 degrees tilt, LBNP, and passive standing as measrues of orthostatic tolerance. Aviat. Space Environ. Med. 1975;46:801–808. [PubMed] [Google Scholar]
- Immink R.V., Pott F.C., Secher N.H., van Lieshout J.J. Hyperventilation, cerebral perfusion, and syncope. J. Appl. Physiol. 1985;2014(116):844–851. doi: 10.1152/japplphysiol.00637.2013. [DOI] [PubMed] [Google Scholar]
- IOM . The National Academies Press; Washington DC: 2015. Beyond mayalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. [PubMed] [Google Scholar]
- Kantos H. Assessment of cerebral autoregulation dynamics. Stroke. 1992;23:1031. [PubMed] [Google Scholar]
- Kong K.H., Chuo A.M. Incidence and outcome of orthostatic hypotension in stroke patients undergoing rehabilitation. Arch. Phys. Med. Rehabil. 2003;84:559–562. doi: 10.1053/apmr.2003.50040. [DOI] [PubMed] [Google Scholar]
- Krejcy K., Wolzt M., Kreuzer C., Breiteneder H., Schutz W., Eichler H.G. Characterization of angiotensin-II effects on cerebral and ocular circulation by noninvasive methods. Br. J. Clin. Pharmacol. 1997;43:501–508. doi: 10.1046/j.1365-2125.1997.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkuil B.P., Gierl B.T., Patel P.M., Pearn M.L., Nguyen L.C., Minokadeh A. The effect of clevidipine on cerebral blood flow velocity and carbon dioxide reactivity in human volunteers. J. Neurosurg. Anesthesiol. 2016;28:337–340. doi: 10.1097/ANA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Lipsitz L.A., Gagnon M., Vyas M., Iloputaife I., Kiely D.K., Sorond F. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- Low P.A., Sandroni P., Joyner M., Shen W.K. Postural tachycardia syndrome (POTS) J. Cardiovasc. Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankovsky B.N., Piolot R., Mankovsky O.L., Ziegler D. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabet. Med. 2003;20:119–126. doi: 10.1046/j.1464-5491.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- Naschitz J.E., Rosner I., Rozenbaum M., Gaitini L., Bistritzki I., Zuckerman E. The capnography head-up tilt test for evaluation of chronic fatigue syndrome. Semin. Arthritis Rheum. 2000;30:79–86. doi: 10.1053/sarh.2000.9201. [DOI] [PubMed] [Google Scholar]
- Novak P. Hypocapnic cerebral hypoperfusion: a biomarker of orthostatic intolerance. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0204419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kim H.T., Park K.M., Ha S.Y., Kim S.E., Shin K.J. Orthostatic dizziness in Parkinson's disease is attributed to cerebral hypoperfusion: a transcranial doppler study. J. Clin. Ultrasound. 2017;45:337–342. doi: 10.1002/jcu.22452. [DOI] [PubMed] [Google Scholar]
- Purewal T.S., Watkins P.J. Postural hypotension in diabetic autonomic neuropathy: a review. Diabet. Med. 1995;12:192–200. doi: 10.1111/j.1464-5491.1995.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Razumovsky A.Y., DeBusk K., Calkins H., Snader S., Lucas K.E., Vyas P. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J. Neuroimaging. 2003;13:57–67. [PubMed] [Google Scholar]
- Seifert T., Rasmussen P., Secher N.H., Nielsen H.B. Cerebral oxygenation decreases during exercise in humans with beta-adrenergic blockade. Acta Physiol. (Oxf.) 2009;196:295–302. doi: 10.1111/j.1748-1716.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- Sheldon R.S., Grubb B.P., 2nd, Olshansky B., Shen W.K., Calkins H., Brignole M. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.K., Sheldon R.S., Benditt D.G., Cohen M.I., Forman D.E., Goldberger Z.D. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2017;70:620–663. doi: 10.1016/j.jacc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Shin K.J., Kim S.E., Park K.M., Park J., Ha S.Y., Kim S.E. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol. Scand. 2016;134:108–115. doi: 10.1111/ane.12516. [DOI] [PubMed] [Google Scholar]
- Siennicki-Lantz A., Lilja B., Elmstahl S. Orthostatic hypotension in Alzheimer's disease: result or cause of brain dysfunction? Aging (Milano) 1999;11:155–160. [PubMed] [Google Scholar]
- Treger I., Shafir O., Keren O., Ring H. Cerebral blood flow velocity during postural changes on tilt table in stroke patients. Eura Medicophys. 2005;41:293–296. [PubMed] [Google Scholar]
- van Beek A.H., Sijbesma J.C., Jansen R.W., Rikkert M.G., Claassen J.A. Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J. Alzheimers Dis. 2010;21:519–526. doi: 10.3233/JAD-2010-100288. [DOI] [PubMed] [Google Scholar]
- van Campen C., Verheugt F.W.A., Visser F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin. Neurophysiol. Pract. 2018;3:91–95. doi: 10.1016/j.cnp.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwieten P.A., Thoolen M.J., Timmermans P.B. The hypotensive activity and side effects of methyldopa, clonidine, and guanfacine. Hypertension. 1984;6:II28-33. doi: 10.1161/01.hyp.6.5_pt_2.ii28. [DOI] [PubMed] [Google Scholar]
- Verbree J., Bronzwaer A.S., Ghariq E., Versluis M.J., Daemen M.J., van Buchem M.A. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J. Appl. Physiol. 1985;2014(117):1084–1089. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- Winker R., Prager W., Haider A., Salameh B., Rudiger H.W. Schellong test in orthostatic dysregulation: a comparison with tilt-table testing. Wien. Klin. Wochenschr. 2005;117:36–41. doi: 10.1007/s00508-004-0288-5. [DOI] [PubMed] [Google Scholar]