Abstract
Autosomal recessive spastic ataxia of Charlevoix-Saguenay is a rare neurodegenerative disorder caused by homozygous mutations in SACSgene. We present finding on MR imaging in 2 adult Italian siblings. According to the literature we have described same of typical MRI finding of autosomal recessive spastic ataxia of Charlevoix-Saguenay disease. We found slight differences in neuroimaging pattern in our patients with a similar genotype but different age and clinical severity, this suggest that brain MRI may provide potential biomarkers to assess disease progression.
Keywords: Ataxia, Recessive ataxia, Genetics, Magnetic resonance imaging, Neuroradiology, Superior vermian atrophy
Introduction
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is a rare neurodegenerative disorder caused by homozygous mutations in SACSgene. Clinical milestones of ARSACS are the ataxic syndrome, the pyramidal involvement and the axonal neuropathy, with a typical infantile onset. Actually, the phenotype can be heterogeneous, including additional signs (eg, movement disorders, cognitive disturbances, pain, sphincters disturbances), as well as the age of onset can range from early childhood to adulthood [1].
The condition is particularly frequent in the Quebec region of Canada; however, an increasing number of cases is described in Europe, Japan and India, suggesting a worldwide diffusion of the disease [2].
Since the molecular bases of ARSACS have been mostly elucidated, it is reasonable that in the next future some specific therapy will be developed [3].
Therefore, it is necessary to identify those clinical or instrumental signs, which may allow an early diagnosis of the disease and lead the genetic analysis.
Previous reports already indicated some peculiar imaging biomarkers at brain magnetic resonance (MRI), although the neuroimaging pattern is variable in ARSACS patients [4].
Here, we describe the clinical-radiological features of 2 siblings with ARSACS, in order to highlight relevant diagnostic cues, which may support the early recognition of the condition.
Case report
Clinical features
Patient 1
Patient 1 (younger brother) is a 36 years old man, carrying compound heterozygous variants in SACS gene (c.4198T>A / c.5719C>T), whose clinical onset was at the age of 15 with an ataxic syndrome. At clinical examination he presented ataxic-spastic gait with autonomous stance and ambulance; mild weakness with muscular hypotrophy in lower limbs associated to postural deformities of the feet and bilateral Babinski sign. He also had dysarthria, nystagmus and mild tremor in upper limbs. A sensory-motor peripheral neuropathy was demonstrated. Cognition was normal. The Scale for Assessment and Rating of Ataxia score was 11.
Patient 2
Patient 2 (older sister) is a 41 years old woman, carrying compound heterozygous variants in SACS gene (c.4198T>A / c.5719C>T). Mild neurodevelopment delay was reported. ARSACS appeared at the age of 16 with a progressive ataxic syndrome responsible for the loss of ambulation at the age of 39. At clinical examination she presented severe weakness, spasticity and pyramidal signs in lower limbs and mild distal hyposthenia in upper limbs. She also had mild dysmetria, nystagmus and dysarthria. A sensory-motor peripheral neuropathy was demonstrated. Cognition was normal. The Scale for Assessment and Rating of Ataxia score was 22.
MRI findings
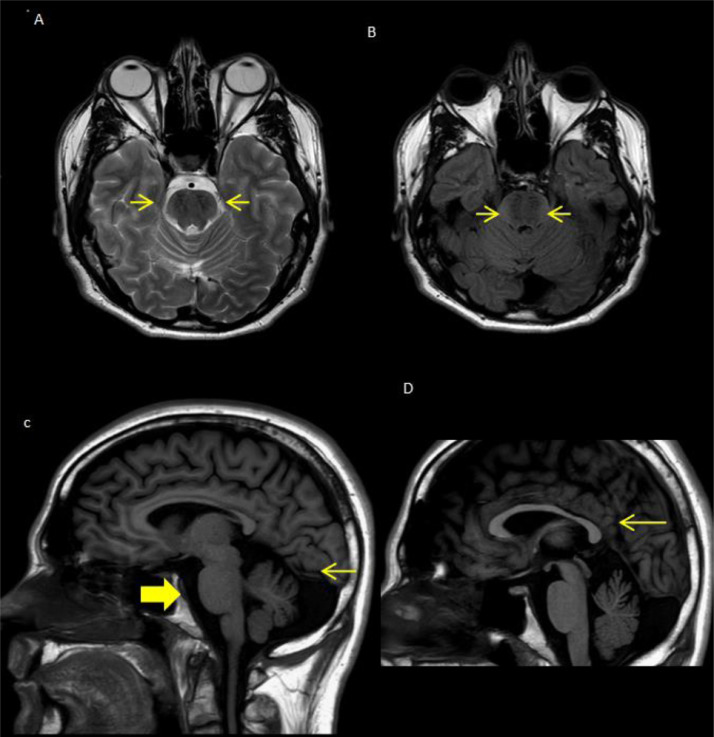
We performed an unehnaced MRI, including: Diffusion weight images, Fluid Attenuated Inversion Recovery (FLAIR) on axial plane, T2 weighted images Fast Feel Echo on axial plane, T2 weighted images Turbo Spin Echo on axial and coronal plane T1-weighted images Turbo Spin Echo with Inversion Recovery mode on axial and sagittal plane (Fig. 1, Patient 2; Fig. 2, patient 2).
Fig. 1.
Pazient 1: (A,B) Axial T2 and FLAIR show a bulky pons with linear hypointensity striation on either side of the midline “tigroid pattern” (arrows). (C) Sagittal T1-weight images shows the bulky pons (large arrow), superior vermian atrophy (arrow). (D) Sagittal T1-weight images shows thinning of the splenium of the corpus callosum (arrow).
Fig. 2.
Pazient 2: (A,B) Axial T2 and FLAIR show a bulky pons with linear hypointensity striation on either side of the midline “tigroid pattern” (arrows). (C) Sagittal T1-weight images shows the bulky pons (large arrow), superior vermian atrophy (arrow). (D) Sagittal T1-weight images shows thinning of the splenium of the corpus callosum (arrow).
According with the existing literature in both patients we have observed same of the characteristic neuroradiologic signs of ARSACS [5,6]. In particular we have seen an atrophic aspect of the cerebellar hemispheres mostly in the superior para-vermian and upper cerebellar vermis, bulky pons with linear hypointensity in the ponts (“tigroid pattern”) in T2 and FLAIR images (pathophysiology of which is unknown) and thinning of the splenium of corpus callosum.
Of interest, such features were more prominent in Patient 2, which was either older or more severely affected.
Discussion
ARSACS is one of the most common inherited degenerative ataxias, whose worldwide prevalence is increasing thanks to the advances in genetic analysis and the improvement of clinical classification.
Molecular bases of the disease have been recognized and the incoming availability of animal models will pave the way for disease-modifying treatments, which instead currently still lack. In this perspective, it is fundamental to identify those factors, which may support an early diagnosis of ARSACS.
Neuroimaging provides an undisputed contribution to lead genetic analysis in ataxic syndromes, since different conditions are characterized by peculiar radiological findings, such as the “molar tooth” sign in Joubert Syndrome.
Previous reports already indicated a number of brain MRI alterations, which could be commonly found in ARSACS patients, such as the superior vermian atrophy of the cerebellum, the linear hypointensity on T2- and FLAIR images of the pons (“tigroid pattern”). Further less specific findings that have been also described are the inferior vermis atrophy of the cerebellum, superior spinal cord atrophy, cerebellar hemisphere atrophy, bulky pons with T2 hypointense stripes, the thalamic T2 hyperintensities and the corpus callosum thinning.
In our small case series, including data from 2 adult siblings we found: atrophic aspect of the cerebellar hemispheres mostly in the superior para-vermian and upper cerebellar vermis, bulky pons with linear hypointensity in the ponts (“tigroid pattern”) in T2 and FLAIR images and thinning of the splenium of corpus callosum
Of interest, such abnormalities were more prominent in Patient 2, which was more severely affected and older. Given the similar genetic background of the patients, we could thus hypothesize that the imaging finds are a correlate of neurodegeneration, which may reflect the disease progression. However, larger prospective studies are necessary to validate this preliminary observation.
Conclusions
Our findings are consistent with the existing literature and highlights that features such as linear hypointensity on in the pons, superior cerebellar vermis atrophy bulky pons and thinning of the splenium of corpus callosum are strongly suggestive of ARSACS. Therefore, according to EFNS/ENS consensus on the diagnosis and management of chronic ataxias in adulthood [7], a preliminary brain MRI could be essential to assist and drive genetic analysis of ataxic syndromes, allowing early diagnosis and limiting the costs for unnecessary investigations. On the other side, the slight differences in neuroimaging pattern that we found in patients with a similar genotype but different age and clinical severity suggest that brain MRI may provide potential biomarkers to assess disease progression.
References
- 1.Briand M-M, Rodrigue X, Lessard I, Mathieu J, Brais B, Côté I, Gagnon C. Expanding the clinical description of autosomal recessive spastic ataxia of Charlevoix-Saguenay. J Neurol Sci. 2019;400:39–41. doi: 10.1016/j.jns.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Kuchay RAH, Mir YR, Zeng X, Hassan A, Musarrat J, Parwez I. ARSACS as a Worldwide Disease: Novel SACS Mutations Identified in a Consanguineous Family from the Remote Tribal Jammu and Kashmir Region in India. Cerebellum. 2019;18(4):807–812. doi: 10.1007/s12311-019-01028-2. [DOI] [PubMed] [Google Scholar]
- 3.Larivière R, Sgarioto N, Márquez BT, Gaudet R, Choquet K, McKinney RA. Sacs R272C missense homozygous mice develop an ataxia phenotype. Molecular Brain. 2019;12 doi: 10.1186/s13041-019-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Synofzik M, Soehn AS, Gburek-Augustat J, Schicks J, Karle KN. Autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS): expanding the genetic, clinical and imaging spectrum. Orphanet J Rare Dis. 2013;8:41. doi: 10.1186/1750-1172-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas A, Varman M, Yoganathan S, Subhash PK, Mani S. Teaching neuroimages: autosomal recessive spastic ataxia of Charlevoix-Saguenay Typical MRI findings First published April 2, 2018, doi: 10.1212/WNL.0000000000005252. Neurology. [DOI] [PubMed]
- 6.Prodi E., Grisoli M., Panzeri M., Minati L., Fattori F., Erbetta A. Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series. Eur J Neurol. 2013;20(1):138–146. doi: 10.1111/j.1468-1331.2012.03815.x. Epub 2012 Jul 21. [DOI] [PubMed] [Google Scholar]
- 7.van de Warrenburga B.P.C., van Gaalena J., Boeschb S., Burgunderc J.-M., Durrd A., Giuntig P. EFNS/ENS Consensus on the diagnosis and management of chronic ataxias in adulthood. Eur J Neurol. 2014;21(4):552–562. doi: 10.1111/ene.12341. Epub 2014 Jan. [DOI] [PubMed] [Google Scholar]