Abstract
As part of the United States Pharmacopeia’s ongoing review of dietary supplement safety data, a new comprehensive systematic review on green tea extracts (GTE) has been completed. GTEs may contain hepatotoxic solvent residues, pesticide residues, pyrrolizidine alkaloids and elemental impurities, but no evidence of their involvement in GTE-induced liver injury was found during this review. GTE catechin profiles vary significantly with manufacturing processes. Animal and human data indicate that repeated oral administration of bolus doses of GTE during fasting significantly increases bioavailability of catechins, specifically EGCG, possibly involving saturation of first-pass elimination mechanisms. Toxicological studies show a hepatocellular pattern of liver injury. Published adverse event case reports associate hepatotoxicity with EGCG intake amounts from 140 mg to ∼1000 mg/day and substantial inter-individual variability in susceptibility, possibly due to genetic factors. Based on these findings, USP included a cautionary labeling requirement in its Powdered Decaffeinated Green Tea Extract monograph that reads as follows: “Do not take on an empty stomach. Take with food. Do not use if you have a liver problem and discontinue use and consult a healthcare practitioner if you develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice (yellowing of the skin or eyes).”
Abbreviations: ADME, Absorption, distribution, metabolism, and excretion; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; Bw, body weight; C, Catechin; CG, (+)‐catechin‐3‐gallate; CIH, Concanavalin A-induced hepatitis; CAM, causality assessment method; CMC, chemistry, manufacturing, and controls; COMT, catechol‐O‐methyltransferase; ConA, Concanavalin A; DILI, drug‐induced liver injury; DILIN, Drug‐Induced Liver Injury Network; DO, Diversity Outbred; DS, Dietary Supplement; DSAE, JS3 USP Dietary Supplements Admission Evaluations Joint Standard-Setting Subcommittee; EC, (–)‐epicatechin; ECG, (‐)‐epicatechin‐3‐gallate; EFSA, European Food Safety Authority; EGC, (–)‐epigallocatechin; EGCG, (–)‐epigallocatechin‐3‐gallate; FDA, United States Food and Drug Administration; GC, (+)‐gallocatechin; GCG, (–)‐gallocatechin‐3‐gallate; γ-GT, Gamma-glutamyl transferase; GT, green tea; GTE, green tea extract; GT(E), green tea or green tea extract; GTEH, EP Green Tea Extract Hepatotoxicity Expert Panel; HDS, herbal dietary supplement; HPMC, Hydroxypropyl methylcellulose; LD50, lethal dose, median; LT(s), Liver test(s); LFT(s), liver function test(s); MGTT, Minnesota Green Tea Trial; MIDS, multi-ingredient dietary supplement; MRL, maximum residue limit; NAA, N-acetyl aspartate; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; NOAEL, no observed adverse effect level; NTP, National Toxicology Program; OSM, online supplementary material; PAs, Pyrrolizidine Alkaloids; PDGTE, powdered decaffeinated green tea extract; PD-1, Programmed death domain-1; PK/PD, pharmacokinetics and pharmacodynamics; RUCAM, Roussel Uclaf Causality Assessment Method; SIDS, single-ingredient dietary supplement; TGF-beta, Transforming growth factor beta; USP, United States Pharmacopeia
Keywords: Green tea, Camellia sinensis, Dietary supplements, Hepatotoxicity, Liver injury, Green tea extract
1. Introduction
The tea plant (Camellia sinensis L. Kuntze, Family Theaceae) is native to Southeast Asia but is currently cultivated in more than 30 countries [1] Green tea is produced by rapidly steaming or pan-frying the leaves, thereby inactivating enzymes and preventing fermentation and oxidation that polymerizes monomeric catechins into condensed polyphenols. Thus, green tea leaves contain the highest amounts of monomeric catechins compared to black, oolong, or white tea [2].
Although traditional green tea as a beverage has a long history of consumption, the use of green tea extracts (GTEs) is a relatively recent development and has gained wide popularity as an ingredient in dietary supplements (DS), sometimes referred to as “nutraceuticals”, particularly in products marketed to aid in weight loss [3]. The term “dietary supplement” is a regulatory term that is used for a category of products marketed in the U.S. and in other countries. In the U.S., dietary supplements are defined, and their composition and claims are regulated by the Dietary Supplement Health and Education Act of 1994 (DSHEA). Herbal (or Botanical) Dietary Supplements represent a subcategory of a DS that are herbal in nature. Major constituents of GTE are polyphenolic compounds belonging to the class of catechins, including: (+)-catechin (C), (–)-catechin-3-O-gallate (CG), (–)-epicatechin (EC), (–)-epicatechin-3-O-gallate (ECG), (–)-epigallocatechin (EGC) and (–)-epigallocatechin-3-O-gallate (EGCG) [4]. GTE has been associated with potentially severe and irreversible liver injury [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]] In 2008, the United States Pharmacopeial Convention (USP) published a systematic review of GTE safety in which the authors proposed a cautionary labelling statement for inclusion into the USP monograph for Powdered Decaffeinated Green Tea Extract (PDGTE) [19]. Manufacturers claiming compliance with the USP standards for PDGTE would have been required to include this cautionary statement on their product labels. However, upon publication of the proposal, USP received various public comments, including from authors of other reviews on the topic [20], that prompted the organization to place a temporary hold on this recommendation and to monitor the literature for an additional period of time in anticipation of additional supporting evidence [21].
As part of its continuous revision practice, USP continued to monitor the literature for adverse events related to GTE intake. In 2016, the USP Dietary Supplements Admission Evaluations Joint Standard-Setting Subcommittee (USP DSAE JS3), which is responsible for determining admissibility of ingredients for USP monograph development, reviewed data on adverse effects including hepatotoxicity of GTE that had been published since 2008. Based on these data, the DSAE JS3 resolved to re-introduce the cautionary labelling statement in the USP PDGTE monograph [22]. The revised USP PDGTE monograph that contains the label caution statement became official as of March 1, 2019 [23]. The information reviewed in 2016 was insufficient to establish whether the observed GTE hepatotoxic effects were due to intrinsic factors in the GTE or to external factors such as contamination that may be introduced during the manufacturing process. Consequently, USP formed the Green Tea Extract Hepatotoxicity Expert Panel (USP GTEH EP), that was tasked with performing a comprehensive review of the literature on GTE-related hepatotoxicity to better understand the potential relationship between the reported hepatotoxicity and chemistry, manufacturing, and controls (CMC), intake of known constituents of GTE, and the pharmacokinetics and pharmacodynamics (PK/PD) of GTE.
This article reports the findings of the USP GTEH EP comprehensive review.
2. Literature search strategy
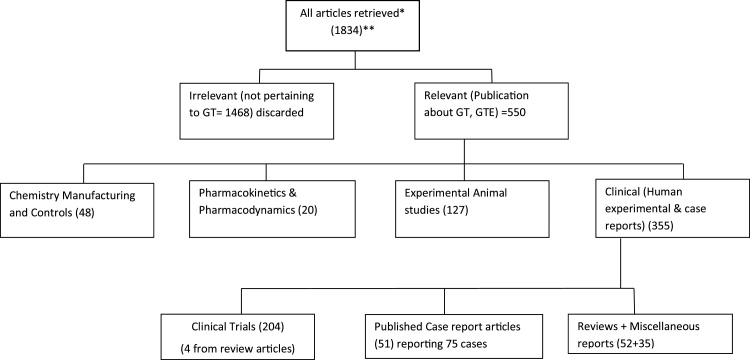
To retrieve clinical data, animal pharmacology and toxicology information, CMC, and pharmacokinetics data, systematic searches were performed in PubMed and other relevant databases covering the period from June 2008 to September 2017, using the search strategy shown in Fig. 1 and search terms listed in Table 1. The search results were supplemented/expanded with searches in Google Scholar as well as a manual search of references listed in review articles. The article titles and abstracts were reviewed by three members of the USP GTEH EP (ALR, RK and HAO-R) to identify articles relevant to GTE or green tea (considered relevant if the abstract mentioned GTE, tea polyphenols, EGCG or Polyphenon™). The selected articles were categorized into four groups based on their relevance to CMC, PK/PD, and non-clinical and clinical safety.
Fig. 1.
A summary of literature search results and categorization of retrieved articles.
* Databases searched include: PubMed, Google Scholar, NLM, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Register of Controlled Trials [CENTRAL], Agricultural Online Access [AGRICOLA], Allied and Complimentary Medicine [AMED], Computer Access to Research on Dietary Supplements [CARDS], Cumulative Index to Nursing and Allied Health Literature [CINAHL] EBSCO Health, Database of Abstracts of Reviews of Effects [DARE] PubMed Health; Embase; International Pharmaceutical Abstracts; National Technological Information Service [NTIS.
** Search strategy used a combination of [Green Tea Extract or Tea Polyphenols or EGCG] and [clinical trials or adverse reactions or adverse effects or case reports or hepatotoxicity or pharmacokinetics or liver or animals].
Table 1.
Search strategy for identifying clinical and nonclinical literature on green tea extract hepatotoxicity. Each of the green tea terms was searched in combination with each of the toxicity terms and the nonclinical descriptor “animal”.
| Green Tea Terms | Toxicity Terms | Non/Clinical Term |
|---|---|---|
| Green tea | Hepatotoxicity | Animal |
| Camellia sinensis | Liver failure | Human |
| Catechins | Liver injury | |
| Epigallocatechin gallate | Liver damage | |
| EGCG | Hepatitis | |
| Polyphenols | Hepatic necrosis | |
| Polyphenon | Hepatic fibrosis | |
| Cirrhosis and cholestasis | ||
| Adverse effects | ||
| Adverse reactions | ||
| Pharmacokinetics | ||
| Metabolism | ||
| Toxicity | ||
| Safety |
Data relevant to CMC were extracted from articles retrieved from searches in various databases as described above (see 2. Literature Search Strategy) to identify the range of manufacturing processes and the composition of the extracts resulting from various manufacturing processes, including natural constituents and contaminants (e.g., pesticide residues, toxic elemental impurities, solvent residues, pyrrolizidine alkaloids, and microbial contaminants). The Expert Panel also received unpublished information on CMC from some GTE manufacturers.
Data relevant to the absorption, distribution, metabolism, and excretion (ADME) of green tea constituents were extracted and reviewed. Data from in vitro studies and from non-clinical animal and human clinical studies were considered.
Data from clinical and non-clinical (animal) studies were extracted from a total of 204 clinical research articles and 127 non-clinical research articles published in the peer-reviewed scientific literature (Fig. 1).
Additional studies, including some published prior to 2008, were identified by exploring references in recent green tea safety review articles [[9], [10], [11],24]. For completeness, our safety data review included investigations using GTE (decaffeinated or non-decaffeinated), green tea beverage, and purified green tea polyphenols such as EGCG, although the USP monograph is for powdered decaffeinated GTE. Two reviewers (HAO-R and ALR) with training and expertise in non-clinical and clinical toxicology ranked the identified studies into one of the following three categories defined by the inclusion/exclusion criteria in Table 2: 1) not useful for safety review; 2) limited usefulness (qualitative or hazard identification level of information only); and 3) useful for risk assessment (quantitative dose-response information).
Table 2.
Inclusion/Exclusion criteria for Clinical and Animal studies. Clinical and animal studies were categorized into three groups based on the characteristics listed below.
| Group | Inclusion/Exclusion Criteria for Clinical Studies | Inclusion/Exclusion Criteria for Nonclinical Studies |
|---|---|---|
| 1 |
Group 1 - Not useful for safety review; not included in review Excluded. Efficacy study not reporting on safety endpoints/AEs # of articles: Clinical = 141; Animal = 98 |
Does not have useful/usable information, e.g.,
|
| 2 |
Group 2 - Limited usefulness (qualitative or hazard identification level of information only) Included, but do not have the best information
|
Study includes qualitative data that may be used, e.g.,
|
| 3 |
Group 3 - Useful for risk assessment purposes (quantitative dose level information useful for risk assessment) Included. Had all the required information
|
Study quantitative, includes the following information:
|
A total of 51 published case report articles reporting 75 individual cases associated with GTE intake were identified. Causality assessment for liver injury was completed by experts from the Drug-Induced Liver Injury Network (DILIN), the official name for a network that was established by the U.S. National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases in 2003 (https://dilin.org/for-researchers/dilin-overview/). The objective of DILIN is to collect and analyze cases of severe liver injury caused by prescription drugs, over-the-counter drugs, and alternative medicines, such as herbal products and dietary supplements. In this review, we use the term “DILIN” in this context and “DILIN experts” to refer to members of this network [25].
Detailed cases in published reports were examined by DILIN experts utilizing the DILIN expert opinion causality assessment method [26]. Additionally, because the Roussel Uclaf Causality Assessment Method (RUCAM) is well-recognized and used as a causality assessment method (CAM) for liver injury and validated for assessing causality in liver damage it was also used. A recent article published while this review was ongoing critically reviewed suspected herb induced liver injury by Green Tea Extracts [27]. Thus, DILIN experts also employed the RUCAM scale in assessing causality to determine the likelihood that exposure to herbal dietary supplement (HDS) products containing GTE were responsible for hepatotoxicity. The RUCAM scale is described elsewhere in the literature [[28], [29], [30]].
Following the DILIN procedure, 12 participating DILIN experts were divided into four groups with three experts per group, and within these groups of three, each group member was assigned the same cases to analyze. Each member examined the assigned cases individually and determined the causal relationship between the ingested product and the liver injury based on the DILIN scale for assessing the percent likelihood of causality [26]. Prior to determining causality, members determined case quality by considering the minimal elements for reporting drug-induced liver injury as described by Agarwal et al. [31].
Although the cases were described as liver injury due to GTE, reviewers also scored the potential causality of other agents that were ingested concomitantly. Thus, each reviewer provided three scores for each case: an Overall (drug/HDS) Score indicating the likelihood that the case represented liver injury from a drug or herbal dietary supplement; a Specific Score indicating the likelihood that GTE was a possible cause; and a third score for Other components (drug/HDS name) that was considered potentially responsible. The total score (“Overall Drug Score” for the Drug, i.e., “GTE-specific score”, plus “Other Drug Score” (drug name) did not exceed 100 % and none of the two scores was higher than the Overall score. For example, if there were two agents ingested in a case, e.g., GTE and drug x, then the combination of scores for GTE and drug x should not be greater than 100 % (e.g., two scores of possible, or one very likely and one possible, but NOT two scores of definite, or two scores of very likely). Each reviewer also provided comments explaining why he or she decided on the specific score. All data on case quality and causality scores were recorded in Excel score sheets. Severity of liver injury was graded using the criteria developed and published by the DILIN team [26]. Scoring for causality was based on the following scale: 1 = Definite [> 95 % likelihood]; 2 = Highly Likely [75-94%]; 3 = Probable [50-74%]; 4 = Possible [25-49%]; 5 = Unlikely [< 25 %]; 6 = Insufficient data as described by Fontana et al. [26].
Subsequently, the three members of each group met and adjudicated the cases to reach a consensus on the causality assignment. Thus, the final DILIN causality scores are based on consensus expert opinion. To identify the effects that could be attributed specifically to GTE, the case reports were categorized into two groups based on whether the involved product suspected of causing liver damage contained a single-ingredient dietary supplement (SIDS) or a multi-ingredient dietary supplement (MIDS) based on the information provided in the case reports and/or available on the internet regarding the constituents of the product.
3. Possible contributions of CMC to GTE hepatotoxicity
The process of manufacturing GTE typically involves extraction of the leaves and stems of Camellia sinensis with water and/or mixtures of water with other organic solvents, most often alcohols, such as methanol or ethanol. If the process also includes decaffeination, non-toxic supercritical carbon dioxide and toxic solvents such as chloroform and dichloromethane may be used. Extracts may be further refined by filtration through a synthetic resin absorbent to remove unwanted residues [32,33]. Such processes result in GTE with much higher concentrations of catechins compared to traditional green tea (GT) beverages and may also increase the concentration of potential contaminants such as pesticide residues, toxic elemental impurities, or pyrrolizidine alkaloids. Because some of these substances have the potential to cause liver damage, we explored the potential links between their presence in GTE and the observed hepatotoxicity.
3.1. High concentration of catechins
Friedman et al. analyzed green tea raw material samples sold in the U.S. market and reported the extractable catechin concentrations after brewing the tea under standard conditions with hot water and other samples of green tea [34,35]. The results showed that there are considerable differences in catechin content of green tea on the market. Other studies show that extraction methods determine the constituents of green tea extracts [36]. GTEs contain much higher concentrations of catechins than brewed green tea beverages as shown in the examples of Polyphenon, a popular GTE on the market (Table 3: columns 2 and 3 in the table show concentration of catechins in GTE whereas column 4 shows the concentration of catechins in cut green tea leaves used in beverages/infusions). Green tea aqueous extracts can be further concentrated or purified to obtain a high-catechin fraction with some claiming to contain 80–95 % EGCG by weight [34,35].The high-catechin fraction can be prepared by extracting the leaves with organic solvents such as 80 % acetone or 70 % ethanol with or without prior extraction with water [[37], [38], [39], [40], [41]]. In the early 2000s, one of the most popular commercial GTE containing products in France and Spain was Exolise, an 80 % ethanolic dry extract standardized at 25 % catechins expressed as EGCG. Weight loss products containing Exolise reportedly caused liver injury and were subsequently banned in France and Spain in 2003 [42,43].
Table 3.
Relative percentages of catechins in dried green tea leaves (column 2) and GTE extracts ingredients (column 3) and GTE extracts in dosage forms (capsules and extracts) (column 4) on the market [34,37] and in a popular GTE on the market known as Polyphenon, e.g., Poly 30, Poly 60.
| Tea Catechins | 24 Samples of marketed Green tea leaf products [34,35] (mg/g) | Polyphenon GTEs [37] (mg/g) | Polyphenon in some dosage forms in the market [34,35] (mg/g) |
|---|---|---|---|
| (+)-Gallocatechin (+GC) | np | nd* -14 | 1-5 |
| (-)- Epigallocatechin (EGC) | nd - 14 | 30-202 | 8-20 |
| (-)-Epicatechin (EC) | 0.1 - 3 | 18-93 | 4-13 |
| (-)-Epigallocatechin gallate (EGCG) | 2.2–54 | 135-656 | 33-58 |
| (-)-Epicatechin gallate (ECG) | 1.2 - 27 | 22-125 | 7-25 |
| (-)-Gallocatechin gallate (GCG) | 0.02 – 6 | ∼30 | 2-14 |
| Total | 3-104 | 275-912 | 50-135 |
Sum of catechin contents and the content of individual catechin in mg/g; nd*: not detected; np: not provided.
3.2. Unwanted residues and contaminants in GTE
3.2.1. Residual solvents in GTE
Several studies indicate that GTEs manufactured by extraction with organic solvents may contain solvent residues in the final product. In 2011, an independent monitoring group that tested 28 GTEs and 32 finished products (supplements) from different countries (unpublished; courtesy Taiyo Kagaku Co. Ltd.) found trace amounts of different solvent residues in both GTEs and finished products [Online Supplementary Material (OSM) Table 1]. Of the 28 GTEs tested, 16 contained traces of chloroform ranging from 0.01–3.8 ppm, and two of the 28 extracts contained traces of a mixture of solvents (e.g., chloroform, dichloromethane, and ethyl acetate). Of the 32 finished products tested (GTE supplements), 17 contained traces of chloroform ranging from 0.01 to 1.6 ppm. [OSM Table 1: Range of residues of solvents, pesticides and other chemicals found in 28 extracts and 32 finished products (supplements) by a monitoring group in 2011].
These levels of solvent residues are within the permissible limits stated in the USP General Chapter <467> Residual Solvents [44]. It was not possible to establish a correlation between the presence of residual chlorinated solvents and liver damage because of the absence of data on the residual solvents in the products that were associated with liver damage in this review. Controlled studies are necessary to confirm or rule out whether residual solvents are involved in GTE associated hepatotoxicity.
3.2.2. Pesticide residues in GTE
Studies have demonstrated that pesticides can be transferred to the finished product during the tea brewing process [[45], [46], [47], [48]]. A study that tested 18 green tea commercial samples reported significant amounts of pesticide residues in some products [49]. Compliance to pesticide residue limits in tea ingredients varies by country. According to the Global MRL Database (https://globalmrl.com/home/index.html accessed 2019-03-07), the U.S. has maximum residue levels (MRLs) for 34 pesticides for tea leaves as a raw agricultural commodity. In addition, the U.S. has MRLs for 7 pesticides as processed tea leaf food products (e.g., instant tea, packaged or bagged nonperishable processed food). All others must be “non-detectable”. By comparison, Canada has MRLs for 18 pesticides in the commodity tea (dried leaves) (Health Canada MRL Database, http://pr-rp.hc-sc.gc.ca/mrl-lrm/results-eng.php accessed 2019-03-07). All other pesticides must meet the general 0.1 ppm MRL as specified in the Canadian Food and Drug Regulations.
Other regions have very different requirements, including Japan and the EU, which permit 247 and 450 pesticide residues, respectively [50] summarized the approved MRL of certain pesticides in teas as set by the EU, EPA, Japan, India, and CODEX.
There were no data about pesticides on the GTE products that were associated with hepatotoxicity. Thus, no correlations could be drawn between pesticide residues in GTE and liver injury. Compliance with the limits for pesticides may protect sufficiently against the risk for liver damage; however, more data are necessary to assess the degree of compliance. Samples reportedly involved in liver damage should be analyzed for compliance with applicable pesticide limits.
3.3. Toxic elemental impurities in GTE
Several publications have shown the presence of elemental impurities in green tea leaves [51], some of which are known to be associated with some degree of hepatotoxicity, specifically arsenic [52], cadmium [53], chromium [54,55], copper [15], lead [56], mercury [57], and manganese [[58], [59], [60]]. Because the content of toxic elements was not reported in the cases associated with liver injury, this review could not establish an association between liver damage and the presence of toxic elemental impurities.
3.3.1. Pyrrolizidine alkaloids (PAs)
Contamination of tea with PAs has been reported and is considered to result from co-harvesting the tea leaves with plants containing unsaturated PAs [61] reported that the mean concentration of PAs was 3.8 μg/L and 95th percentile was 6.1 μg/L in 310 samples of green tea infusions in their database [PAs are plant secondary metabolites that have been shown to be hepatotoxic, genotoxic, and carcinogenic] [[61], [62], [63], [64], [65], [66], [67], [68]]. In the 310 samples of green tea infusion that EFSA analyzed, the main contributors to the total PA concentration were senecionine-N-oxide (19 %), retrorsine-N-oxide (18 %), and intermedine and lycopsamine, the latter both contributing 16 % [64].
The liver and lungs are among the major target organs affected by short-term toxicity of PAs, which has also been associated with the onset of hepatic sinusoidal obstruction syndrome (SOS), also known as hepatic veno-occlusive disease. In the current review, there were no data about PAs in GTE products that were associated with hepatotoxicity. However, the characteristics of liver injury associated with GTE are hepatocellular [[9], [10], [11], [12],69], which is different from the SOS characterized liver injury induced by PAs [70].
4. Pharmacokinetics and pharmacodynamics
Our previous review by Sarma et al. [19] examined four clinical studies involving administration of concentrated GTE as purified EGCG or Polyphenon E (a decaffeinated and defined green tea catechin mixture) that delivered 200−800 mg EGCG to healthy volunteers under fed and fasting conditions [[71], [72], [73], [74], [75]]. The authors concluded that plasma concentrations of the major catechin, EGCG, were significantly increased when GTE was consumed under fasting conditions compared to fed conditions [reviewed in [19]. Another clinical study reported that relative to day 1 of administration, there was a > 60 % increase in the systemic exposure to EGCG following chronic oral administration of 800 mg EGCG or Polyphenon E once daily for four weeks [72]. The chemical interaction of polyphenols with dietary proteins has been extensively documented in the literature and is well-known to result in reduced bioavailability of tea galloylated catechins [76,77].
The potential hepatotoxicity from GTE may be linked to the pharmacokinetic properties of green tea components, particularly the catechins. Numerous reports and meta-analyses of potential GTE-mediated hepatotoxicity suggest that liver damage may occur after ingestion of GTE in high quantities (>800 mg) or for long periods of time, and the pattern of liver injury is almost always of the hepatocellular type [[9], [10], [11], [12]]. Typically, liver injury due to GTE exposure manifests within 3 months, but the latency to the onset of symptoms ranges from 10 days to seven months. Most cases present with acute hepatitis symptoms accompanied by marked hepatocellular enzyme elevations [15,69,78,79].
The pharmacokinetics of catechins is linked to GTE hepatotoxicity. Under specific conditions such as fasting, high doses, and repeated administration of GTE, systemic plasma catechin concentrations are substantially higher compared to when ingested under fed conditions and/or low or single doses [80]. These observations are further supported by a clinical study involving an oral supplement enriched in EGCG of which a 2000 mg dose was administered twice daily to non-fasted patients with chronic lymphocytic leukemia. Of the 42 enrolled patients, six (15 %) discontinued treatment after experiencing ≥ grade 2 transaminitis [81,82].
Several pharmacokinetic factors may explain the presumed GTE-mediated hepatotoxicity. All are based on the assumption that components in GTE cause hepatotoxicity (i.e., that hepatotoxicity is not due to contaminants or adulterants) and that the toxicity is directly proportional to the component concentrations exposed to the liver. Mechanisms include increased bioavailability of GTE components such as catechins, saturation of drug metabolizing enzymes, or efflux transporters that lead to increased exposure to the parent compounds and, aberrant dissolution of the product formulation which will be discussed in detail in the following sections. These proposed pharmacokinetic mechanisms do not take into account genetic variation of the patients nor idiosyncratic reactions that may be immune response-related.
4.1. Saturation of drug metabolizing enzymes or efflux transporters
One clinical study indicated that, whereas EGC and 4′-O-methyl-epigallocatechin concentrations plateaued in plasma between medium and high green tea doses (1.25 and 1.75 % infusion equivalent to 134 mg and 188 mg EGCG, respectively), EGCG and EC) concentrations did not plateau but increased proportionally with dose (80–188 mg EGCG equivalent). Regardless of dose, catechins appeared rapidly in plasma, suggesting rapid absorption through the small intestine, exposure to the liver, and minimal enterohepatic recirculation [83]. The terminal half-life of EGCG is longer than that of EGC and EC. EGC and EC, but not EGCG, were detected in the urine which may be explained by the binding of galloylated catechins to albumin, which may limit glomerular filtration of EGCG-protein complex in urine [[84], [85], [86]]. Over 90 % of total urinary EGC and EC was excreted within 8 h. When the tea exposure was increased from 1.5 to 4.5 g, the amount of EGC and EC excreted into the urine increased, but a clear dose-dependent relationship was not apparent [87].
The difference between EGC and EC versus EGCG pharmacokinetics could be explained by metabolic competition for phase II enzymes, with reduced formation of EGC conjugates at higher tea doses, coupled with competition with an intestinal efflux transporter, leading to reduced efflux of EGC into the intestinal lumen [88]. The data obtained with ileal fluid collected from healthy subjects with an intact colon who were administered Polyphenon E indicated that substantial quantities of Polyphenon E flavan-3-ols and EGCG transit from the small to the large intestine, where they are subject to breakdown by colonic bacteria. EGC and EC were present in plasma as the conjugated forms after Polyphenon E administration [89]. The blood concentration of EGCG increased at higher doses, possibly due to saturable first-pass metabolism after oral administration [71].
Based on the above observations, EGC and EC appear to be absorbed rapidly into the systemic circulation and metabolized in the intestine and liver at first pass. Comparatively, EGCG absorption was delayed. At higher GTE doses, EGCG concentrations in the plasma increased proportionally.
4.2. Bioavailability
EGCG is known to be relatively unstable and susceptible to degradation at high temperatures and at pH > 4 [[90], [91], [92]]. It is possible that taking capsules containing EGCG without food does not elicit strong responses from the stomach and the pancreas, thereby allowing EGCG to persist longer in the small intestine and enhancing the extent of absorption. Taking EGCG capsules without food could lead to the stomach producing less acidic chyme than when taken with food. The pancreas, in turn, would not secrete bicarbonate to neutralize the chyme in transit from the stomach. Slower gastric emptying in the presence of food most likely prolonged the time needed for EGCG transit to the upper portion of the small intestine. However, given that the systemic exposure to EGCG was much lower when taken with food, some of the extra time was likely spent transiting through the small intestine, where exposure to a higher pH for a longer period of time could have contributed to a higher degree of degradation [93]. In addition, a longer transit time in the presence of food likely would result in a lower Cmax and AUC, potentially subjecting the liver to lower concentrations of EGCG. Lastly, the AUC0–8h for EGCG taken without food was significantly higher (by 3.9-fold, P = 0.04) compared to that when taken with food, potentially due to a higher oral bioavailability of EGCG when taken under fasting conditions. It appears that EGCG can modulate its own systemic availability and that food may reduce the toxic potential of acute high oral doses of EGCG.
The systemic availability of EGCG as discussed is consistent with the clinical observation showing that, when GTE is administered under fasting conditions, plasma EGCG concentration increased five-fold compared to when GTE is administered with food [73]. One study involving isolated rat hepatocytes supports the observation that GTE taken with food is less likely to cause hepatotoxicity because hepatocytes exposed directly to EGCG showed a dose-dependent cellular injury and decreased hepatocyte function at concentrations of 10 μmol/L and higher. In addition, rat hepatocytes (permeabilized with the detergent digitonin) exposed to EGCG showed damage of the outer mitochondrial membrane and an uncoupling of oxidative phosphorylation [94].
Systemic availability of EGCG may also depend on the amount of methylated EGCG present in the GTE. It has been demonstrated that the 3′methylated derivative of EGCG (−)-epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3′Me), is significantly more bioavailable than either the non-methylated or 4′ methylated version of EGCG. In rats, oral administration of 100 mg/kg of EGCG, EGCG3′Me or EGCG4′Me resulted in a much higher AUC for EGCG3′Me compared to the other two forms of EGCG; specifically, there was a nine-fold increase compared to EGCG. A study by Oritani et al. showed that methylation of EGCG significantly modified systemic availability following oral administration [95]. Thus, two factors are worth considering. First, GTE made using leaves from tea plant varieties containing a higher concentration of methylated EGCG, specifically EGCG3′′Me (e.g., Benifuuki, Benihomare, and Tung ting oolong tea), may result in higher systemic exposure to EGCG [96]. Second, gut microbes may play a role in the metabolism of EGCG within the gut, which may result in the conversion of EGCG to a more bioavailable form, thus increasing exposure and bioavailability.
4.3. Dissolution of product formulation
An unwanted physical interaction between the capsule shell material and polyphenol-containing extracts may occur [97]. Cellulose and polyphenols have a strong hydrophobic interaction which can significantly reduce polyphenol bioaccessibility [97]. Hydroxypropyl methylcellulose (HPMC)-based capsule shell materials, which are commonly used in human clinical trials involving plant extracts, result in an unfavorable interaction with green tea catechins [98,99]. Compared to gelatin, HPMC adversely influences capsule disintegration and dissolution characteristics of the green tea catechins, compromising the rate and extent of absorption [98,100]. Dissolution may be hampered in both the fasted and fed states, with up to one-half of the material remaining undissolved after two hours. Thus, the use of HPMC capsule shell material and cellulose filler might be an additional factor that contributes to the apparent absence of significant hepatotoxic effects of GTE compared to studies in which HPMC was not part of the capsule or filler [101]. This factor could influence the systemic exposure to EGCG and other catechins presented to the liver.
4.4. Evidence of ADME that contributes to hepatotoxicity
Our previous review [19] highlighted that systemic absorption of orally administered EGCG was higher in beagle dogs compared with rats (20 % and 1.6–14 %, respectively) [[102], [103], [104]]. In dogs, there was appreciable uptake of EGCG by the liver (17.5 % ± 4.7 %), reaching concentrations of approximately 150 μg equivalents of EGCG/g wet weight, the liver being the organ with highest accumulation. Similar observations in accumulation were noted in rat and mouse models [102,105,106]. The studies in mice showed that administration of a second dose of EGCG resulted in a significant increase in EGCG concentration in all tissues [106]. The effect of fasting on systemic exposure to EGCG was demonstrated in a series of studies with beagle dogs. Pre-fed dogs were administered recrystallized EGCG (91.8 % purity) at doses of 0, 50, 300, and 500 mg/kg bw/day (provided in two daily doses) for 13 weeks and showed no adverse effects that were deemed to be treatment related, whereas fasted dogs administered a spray-dried green tea preparation containing 80 % EGCG at 0, 50, 150, and 500 mg/kg bw/day for 13 weeks exhibited significant toxicity, including morbidity and mortality at 150 and 500 mg/kg bw/day (additional details are provided below in the non-clinical section) [107].A rat hepatocyte study showed that exposure to EGCG at concentrations of 10 μmol/L or higher during a 24 -h incubation resulted in cellular injury and reduced hepatocyte function. This study provided direct evidence that EGCG may play a role in liver injury of the hepatocellular type observed in cases associated with GTE intake. In addition, EGCG caused damage of the outer mitochondrial membrane and an uncoupling of oxidative phosphorylation in permeabilized hepatocytes (hepatocytes whose cell membranes have been made porous using chemical agents), providing a possible mechanism to explain hepatocyte toxicity [94]. Collectively, high concentrations of EGCG similar to those observed to cause hepatocyte toxicity in vitro can be achieved even at a “regular” dose/intake due to saturation of metabolism or biliary efflux, increased systemic availability from a fasting state, and/or improved bioavailability as a result of formulations with a favorable dissolution profile. Further studies are needed to confirm with certainty that the pharmacokinetics of GTE components are linked to hepatotoxicity.
5. Evidence from non-clinical toxicity studies
Non-clinical data related to GTE testing were extracted from 127 studies reported in the literature. A total of ten sub-chronic and two chronic toxicity studies of GTE were categorized as useful for risk assessment (category 3) and are detailed below.
5.1. Subacute and subchronic non-clinical toxicity studies of green tea extract
5.1.1. Mouse
Hsu et al. assessed the toxicity of an aqueous extract of green tea in 28-day oral gavage studies with male and female ICR mice (n = 10 mice per group) [108]. The mice were orally administered doses of 0, 625, 1,250, and 2,500 mg/kg (equivalent to approximately 4.5, 9.07 and 18.3 mg/kg of EGCG). Lower alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values, compared to the control, were noted in female mice at the 625 mg/kg dose of GTE (equivalent to 4.53 mg/kg of EGCG), but these findings were not considered to be dose-responsive or toxicologically relevant because they were not accompanied by liver weight or histopathological changes. Therefore, the NOAEL for this study was the high dose of 2,500 extract mg/kg (equivalent to18.1 mg/kg of EGCG) [108]. The doses evaluated in the Hsu study, when converted to human equivalent doses (HED; HED (mg/kg) = Animal dose (mg/kg)/Km ratio provided in Table 1 of [109,110]; see details in reference) correspond to 0.37, 0.74, and 1.47 mg/kg EGCG, which are much lower than typical GTE intake levels by humans. Therefore, the negative findings are not surprising and are perhaps more relevant to green tea consumption than to GTE.
The National Toxicology Program (NTP) conducted 3-month oral gavage studies with GTE (an ethanol:water extract of dried green tea leaves with deionized water vehicle) in male and female B6C3F1/N mice (n = 10 animals per group) [111]. The GTE used in these studies contained 48.4 % EGCG and was characterized for identity, purity, and stability. A total of nine constituents GC, EGC, C, EC, CG, caffeine, EGCG, GCG, and ECG were identified and quantified in the test article. Animals were dosed daily 5 days per week with 0, 62.5, 125, 250, 500, or 1000 mg/kg GTE equating to 0, 30.3, 60.5, 121, 242, 484 mg/kg EGCG; and corresponding to HEDs of 0, 2.46, 4.92, 9.84, 19.7, and 39.3 mg/kg EGCG [111]. Death was attributed to liver necrosis at 1000 mg/kg 484 mg EGCG/kg in males 6 of 10 and females 4 of 10. Glycogen depletion was noted at 250 and 500 mg/kg in males and 500 and 1000 mg/kg in females. Finally, increased incidence of centrilobular necrosis was noted at 1000 mg/kg in males and females, and increased incidence of karyomegaly at 1000 mg/kg in females only [111]. While glycogen depletion was noted in the livers of male and female mice at lower doses, it is of unknown toxicological significance, and therefore, the NOAEL for liver effects was 500 mg/kg based on centrilobular necrosis and other histopathological lesions.
5.1.2. Rat
Chengelis et al. conducted 28-day [112] toxicity studies in male and female Sprague Dawley rats (n = 5 per group) with three different green tea catechin-enriched preparations produced by Kao Corporation using a proprietary process: a heat-sterilized preparation (6.9 % EGCG), a preparation that did not undergo heat sterilization (25.1 % EGCG), and a heat-sterilized, decaffeinated preparation (6.4 % EGCG. Male and female rats were administered 0, 500, 1000, or 2000 mg/kg of the heat-sterilized preparation or the preparation without heat sterilization, or 2000 mg/kg of the heat-sterilized, decaffeinated preparation. No signs of hepatotoxicity were observed with any of the treatments, thus, the NOAEL for liver effects was 2000 mg/kg 128−502 mg/kg EGCG and HED of 20.6–81.0 mg/kg EGCG) [112].
The NTP conducted 3-month oral gavage studies in male and female F344/NTac rats (n = 10 animals per group) using the GTE and dosing scheme described above for mice [111]. Calculated HEDs for doses in rats are 4.88, 9.76, 19.5, 39.0, and 78.1 mg/kg EGCG. Liver toxicity was observed in 3 out of 10 female rats only at 1,000 mg/kg GTE. Histopathological changes in this group included hepatocyte necrosis, bile duct hyperplasia, oval cell hyperplasia, and mitosis [111]. The NOAEL for liver effects in male and female F344/NTac rats was 1000 mg/kg and 500 mg/kg GTE, respectively [111].
In another 3-month oral gavage toxicity assessment, Wang et al. (2012) evaluated Pu-erh GTE in male and female Sprague Dawley rats (n = 40 per group) at doses of 0, 1250, 2500, and 5000 mg/kg. Reported characteristics of the test article indicated that Pu-erh green tea aqueous extracts contained 7.7 % EGCG [113], resulting in doses of 96.1, 192, and 385 mg/kg EGCG and HEDs of 15.5, 31.0, and 61.0 mg/kg EGCG. Relative liver weights were increased in males and females in the 5,000 mg/kg groups. Liver enzymes (ALT and AST in males and ALT in females) were also increased. Accompanying histopathological lesions in male and female livers from animals in the 5,000 mg/kg group included minimal bile duct hyperplasia, vacuolation, and inflammation [113]. Therefore, the NOAEL in this study was 2500 mg/kg in both male and female Sprague Dawley rats.
The Japanese National Institute of Health Sciences evaluated GTE in a dosed-feed 3-month toxicity study in male and female F344 rats. The GTE test material (Sunphenon 100S™) was provided in feed at concentrations of 0 (control), 0.3%, 1.25%, and 5.0% for 3 months, resulting in average daily intakes of 180, 764, and 3525 mg/kg, respectively, for males, and 189, 820, and 3542 mg/kg, respectively, for females. The GTE used in the study contained 29.4 % EGCG, resulting in values of 52.9, 225, and 1040 mg/kg EGCG in males and 55.6, 241, and 1040 mg/kg EGCG in females. The corresponding HEDs were 8.54, 36.2, and 167 mg/kg EGCG for male rats and 8.96, 38.9, and 168 mg/kg EGCG for female rats. While no histopathological changes were observed, serum ALT and AST were increased, as were liver weights in rats administered the highest dose. Therefore, the NOAEL was determined to be 764 mg/kg and 820 mg/kg GTE in male and female F344 rats, respectively [114].
Isbrucker et al., evaluated the toxicity of a purified EGCG preparation in a 3-month study in Sprague Dawley rats [107]. Male and female rats (n = 10) were provided nominal doses of 0, 50, 150, and 500 mg/kg of EGCG (greater than 77 % purity) in feed. Additional groups were fed either 0 or 500 mg/kg of EGCG followed by a 4-week recovery period. Although there was a statistically significant increase in total serum bilirubin during post-recovery in 500 mg/kg-treated males and females, the increased bilirubin was within historical control values, and was not seen in the groups which did not have the recovery period. This finding was not considered to be toxicologically meaningful, and the NOAEL was determined to be 500 mg/kg (385 mg/kg EGCG and HED equal to 62.1 mg/kg EGCG).
Morita et al., conducted 6-month [115] toxicity studies in Sprague Dawley rats with two green tea catechin-enriched preparations produced by Kao Corporation using a proprietary process: a heat-sterilized preparation (5.9% EGCG) and a heat-sterilized, decaffeinated preparation (5.3 % EGCG). Male and female rats in the 6-month study were administered 0, 120, 400, or 1200 mg/kg of the heat sterilized preparation or 1200 mg/kg of the heat-sterilized, decaffeinated preparation via oral gavage n = 10 animals per group. Clinical chemistry and histopathological assessments were performed, and no signs of hepatotoxicity were noted. Therefore, the NOAEL for liver effects was 1200 mg/kg [115].
5.1.3. Dogs
Isbrucker et al. [107] reported the findings from a 3-month study with beagle dogs. Male and female dogs (n = 4 per group) were administered capsules containing 0, 50, 150, and 500 mg/kg EGCG (80 %) following a 15 -h fasting period, and dogs were not provided food until 3−4 hours following dosing. Two additional groups treated with 0 or 500 mg/kg were allowed to recover for 4 weeks following treatment. Liver effects recorded in this study included necrosis in two females, one at 150 mg that was sacrificed moribund, and another at 500 mg/kg that died. Other liver-related effects included increased serum bilirubin in all dogs administered the high dose, and increased ALT, AST, and gamma-glutamyl transferase (γ-GT) observed in one or more high dose dogs. The NOAEL for the fasting dog study was 50 mg/kg 80 % EGCG (or 40 mg/kg EGCG). Follow-up studies explored effects of EGCG; HED of 22 mg/kg EGCG in fed dogs and determined a NOAEL of 500 mg/kg.
A second assessment of green tea polyphenols in male and female beagle dogs was conducted by National Cancer Institute researchers [116]. Although the study was originally designed to include a 9-month exposure period in fasted dogs (n = 4–5 per group), it was terminated at 6.5 months due to extensive morbidity and mortality. The test article, Polyphenon E® contained 63.3–64.8 % EGCG. Doses included in the early-terminated study were 0, 200, 500, and 1000/800 mg/kg the maximum tolerated dose was established at 800 mg/kg after 9 days of dosing. Toxicity and deaths occurred at 1000 mg/kg. Significant hepatotoxicity was observed with increases in AST, ALT, alkaline phosphatase (ALP), total bilirubin, and triglyceride levels, centrilobular necrosis, and chronic-active inflammation with infiltration of neutrophils and mononuclear cells evident in the liver along with brown intracytoplasmic pigment in Kupffer cells. A NOAEL was not achieved as effects were observed in the lowest dose group. Therefore the NOAEL was less than 200 mg/kg (128 mg/kg EGCG; HED of 71 mg/kg) [116].
A follow-up 3-month toxicity study was conducted using a single dose of 200 mg/kg in fed (1 group) versus fasted (3 groups provided 3 lots of Polyphenon E®) male dogs (n = 3 animals per group; males were selected because they appeared to be more sensitive in the early-terminated study. Interestingly, the follow-up study resulted in less frequent and less severe toxicity than the early-terminated study, regardless of fasting state. Some signs of hepatotoxicity were noted including changes in clinical chemistry parameters and histopathological lesions in the liver in two of the three fasted groups. Notably, the lot of Polyphenon E® that elicited severe toxicity in the chronic study (see above) was the least toxic in the follow-up study, regardless of fed or fasted state. It is not clear what role the differences in test material composition played in observed response differences among the treatment groups, and the small sample size precludes definitive conclusions. The authors speculated that the lack of congruence between the chronic study and the follow-up could be due to differences in the dogs (different supplier). Despite the less severe toxicity noted in the 3-month follow-up study, the liver findings in the two groups of fasted dogs support the conclusion that a NOAEL for fasted dogs is below 200 mg/kg/day [116].
5.1.4. Mechanistic evaluation
A study in CF-1 mice examined the effects of pretreatment with dietary EGCG on hepatotoxicity and systemic availability upon acute oral administration of EGCG [117]. Compared to control (vehicle-treated) mice, mice administered 750 mg/kg EGCG daily for three days resulted in an 80-fold increase ALT; an increase in glutathione S-transferase mRNA was also observed. Pretreatment with 3.2 mg EGCG/g diet for two weeks mitigated this response, resulting in a reduced elevation of plasma ALT by 75 %. These data suggest a putative mechanism by which low-level EGCG exposure in the GTE may mitigate pro-oxidant effects of higher levels of intake and may partly explain the observed variation in hepatotoxic response to green tea extract-containing dietary supplements [117].
5.2. Chronic toxicity and carcinogenesis study of green tea extract
The NTP conducted chronic toxicity and carcinogenesis studies with GTE in B6C3F1/N mice and Wistar Han rats [111]. The test article contained 48.4 % EGCG and doses administered were 0, 30, 100, and 300 mg/kg in B6C3F1/N mice and 0, 100, 300, or 1000 mg/kg in Wistar Han rats administered via oral gavage 5 days per week n = 50 per group for the entire duration of the study. In B6C3F1/N mice, liver findings included increased incidences of hematopoietic cell proliferation and inflammation in the liver of males administered 300 mg/kg. The NOAEL for liver effects in mice was 100 mg/kg for males, with no liver effects noted in females up to 300 mg/kg. In Wistar Han rats, liver findings included increased incidences of hepatic necrosis at 1000 mg/kg in males and females, and oval cell hyperplasia at 1000 mg/kg in females. The NOAEL for liver effects in rats was 300 mg/kg.
5.3. NOAEL for EGCG in mice, rats and dogs
The polyphenol catechins, particularly EGCG, appear to be responsible for the observed hepatotoxicity outlined above [118]. Similar to other botanical ingredients, GTE is a complex mixture with variable constituent concentrations. This complexity is compounded in the case of green tea by the existence of concentrated catechin preparations developed for increased bioactivity, such as Polyphenon E [116]. Considering the variable concentration of the putative toxic constituent, it seems appropriate to compare the NOAEL values across studies using EGCG doses, rather than the GTE doses. To do so, the GTE doses were converted into EGCG doses using information provided about the test article in the various studies (Table 4).
Table 4.
Green tea doses/intake amounts in non-clinical toxicity studies converted to EGCG doses and corresponding Human Equivalent Doses (HED).
| Study | Species | Duration | Green tea doses (mg/kg) | EGCG content (%)a | Conversion to EGCG dose (mg/kg) | Convert NOAEL to HEDb (mg/kg) |
|---|---|---|---|---|---|---|
| Hsu et al. (2011) [108]GT brew | Mice | 28-day | 625 1250 2500 |
0.725 | 4.53 9.06 18.1 (no effect) |
1.474 |
| NTP (2016) [111] GTE |
Mice | 3-month | 62.5 125 250 500 1000 |
48.4 | 30.3 60.5 121 242 NOAEL 484 |
19.7 |
| NTP (2016) [111] GTE |
Mice | 2-year | 30 100 300 |
48.4 | 14.5 48.4 NOAEL (♂) 145 (no effect ♀) |
3.93 11.8 |
| Chengelis et al. (2008) [112] Catechin preparations |
Rats | 28-day | 500 1000 2000 |
6.4-6.9 6.4-6.9 6.4-25.1 |
32.0-34.5 64.0-69.0 128-502 (no effect) |
20.6–81.0 |
| NTP (2016) [111] GTE |
Rat | 3-month | 62.5 125 250 500 1000 |
48.4 | 30.3 60.5 121 242 NOAEL (♀) 484 NOAEL (♂) |
39.0 78.1 |
| Wang et al. (2012) [113] GT brew (Pu-erh GTE) |
Rats | 3-month | 1250 2500 5000 |
7.69 | 96.1 192 NOAEL 384.5 |
31.0 |
| Takami et al. (2008) [114] GTE (Sunphenon 100S™) |
Rats | 3-month | male/female 180/189 764/820 3530/3540 |
29.4 | 52.92/55.57 225/241.08 (NOAEL) 1040/1040 |
36.4/39 |
| Isbrucker, (2006) [107] Purified EGCG |
Rats | 3-month | 50 150 500 |
77 | 38.5 116 385 (no effect) |
62.1 |
| Morita et al. 2009 [115] GTE (Catechin preparations) |
Rats | 6-month | 120 400 1200 |
5.3 | 6.36-7.08 21.2-23.6 63.6-70.8 (no effect) |
10.3–11.4 |
| Changelis et al. 2008 [112] GTE |
Rats | 28-day | 1000 2000 |
5.3 | 53 (NOAEL- localized gastric effects) 106 (NOAEL -systemic effects) |
|
| NTP (2016) [111] GTE |
Rats | 2-year | 100 300 1000 |
48.4 | 48.4 145 NOAEL 484 |
23.4 |
| Isbrucker, (2006) [107] GTE (Purified EGCG) |
Dogs (fasted) | 3-month | 50 150 500 |
80 | 40 NOAEL 120 400 |
22.2 |
| Kapetenovic et al. (2009) [116] Polyphenon E® |
Dogs (fasted) | 3-month/ 6.5-month | 200 500 1000/800 |
64 | 128 NOAEL < 128 320 640/512 |
<71.1 |
Conversion of green tea doses to EGCG doses allows for comparison of NOAEL values across studies. The NOAEL in rats and mice was relatively consistent across subchronic studies at approximately 200−300 mg/kg EGCG [111,113,114], with the exception of the Isbrucker et al., rat study where effects were not observed up to 385 mg/kg EGCG. The remaining subchronic rodent studies that failed to induce hepatotoxicity were conducted at doses well below 200 mg/kg EGCG. Morita et al. (2009) [115] used a high dose containing 70.8 mg/kg EGCG, and Hsu et al. (2011) [108] included doses ranging from 4.53 to 18.1 mg/kg EGCG. In contrast, two studies in fasted dogs revealed notably lower NOAEL levels at 40 mg/kg EGCG [107] and less than 128 mg/kg EGCG [116]. The 40 mg/kg/day dosage level is equivalent to 22.2 mg/kg/day for humans, according to the U.S. Food and Drug Administration (FDA) guidance on estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers [110]. This translates to 1554 mg in a 70-kg individual. In comparing subchronic to chronic exposures, the only notable difference in NOAELs is in male mice, where the NOAEL goes from 242 mg/kg EGCG in the subchronic to 48 in the chronic. Hepatotoxicity observed in the reviewed non-clinical studies was characterized by clinical chemistry changes, such as increases in ALT and AST and histopathological lesions in the liver.
6. Evidence from clinical studies not previously reviewed
6.1. Clinical trials
The following clinical studies, published since our 2008 article, provide further evidence in support of the clinical evidence summarized in section 4 above. A total of 204 studies were retrieved for data extraction and of these 20 studies that met the criteria for inclusion (level 3, Table 2) were included, and eight additional studies that met the inclusion criteria for level 2 (Table 2) were included as supportive data in the clinical summary. The available data from the 28 studies show that a wide variety of GTE (with different phytochemical constituents), and a wide range of doses have been studied. Durations of treatments varied ranging from a few days to 1 year. The number of subjects treated across studies also varied.
Among the studies reviewed, the Minnesota Green Tea Trial (MGTT) in postmenopausal women provided the strongest evidence for hepatotoxic effects of EGCG [17,119]. The MGTT was a randomized, placebo-controlled, double-blind trial investigating the effects of daily GTE consumption for 12 months. The objective of the trial was to observe the effects of GTE on biomarkers of breast cancer risk. The dose of GTE (4 capsules) administered in the MGTT contained 800 mg of EGCG. In subsequent MGTT reports, the potential effects of GTE on liver injury measures were analyzed [17]. This analysis included data from 1021 participants 513 in GTE and 508 in placebo arms who had normal baseline levels of liver enzymes ALT and AST. In the GTE arm of the study, serum ALT increased by 5.4 U/L [95 % confidence interval, 3.6–7.1], and AST increased by 3.8 U/L [95 % confidence interval, 2.5–5.1], which was significantly higher than those among women in the placebo arm (both P < 0.001). In total, 26 (5.1 %) of the treated subjects showed moderate or severe abnormalities in liver function tests [120]. The statistically significant odds ratio for developing liver function abnormalities was 7.0 [P = 0.0002; 95 % CI = 2.4–20.3] compared to placebo. The rise-fall pattern of liver enzyme levels that followed challenge-dechallenge-rechallenge cycles of GTE consumption in the MGTT was compelling evidence implicating the effect of high-dose GTE on potential liver injury. In addition, there were instances of positive re-challenge when a few of the subjects who had developed evidence of liver injury and who had improved after GTE had been stopped again took GTE and more rapidly re-developed liver injury. The study also considered other pos
sible factors contributing to liver injury risk including previously suggested risk factors such as catechol-O-methyltransferase genotype, use of non-steroidal anti-inflammatory drugs and statins, or alcohol consumption, which did not appear to increase the effects of green tea catechins on the liver [[120], [121], [122]].
Of the 28 clinical studies included in this review, only one multi-phase study with Polyphenon E involving a small number of patients with multiple sclerosis showed overt signs of hepatotoxicity [123]. Phase 1 of the study (n = 10 patients) was intended to assess the safety and futility (inability of a clinical trial to achieve its objectives) of Polyphenon E with the objective of correlating plasma concentrations of EGCG with neuroprotective effects through changes in N-acetyl aspartate (NAA) of Polyphenon E. Phase 2 of the study (n = 13 patients) was conducted to further assess safety and confirm the neuroprotective effects of Polyphenon E. The Polyphenon E used in these studies was a GTE containing 50 % EGCG. Two capsules containing 200 mg of EGCG per capsule were administered twice daily for a total of 800 mg/day for 6 months. ALT and AST data were collected during both phases. One patient discontinued due to grade 1 elevation of liver function tests (LFTs) during phase 1, but the study was halted at phase 2 because 5 of 7 patients in the GTE group developed abnormal LFTs. Hepatotoxicity signals were reported across phases including abdominal discomfort and elevations in liver enzymes (ALT/AST). Identifying trends in liver effects based on dose, length of dosing, and/or patient susceptibility was difficult. Notably two different lots of GTE were used in the studies, one for phase 1 and another lot for phase 2. However, quality control analyses showed similar amounts of EGCG in the different lots and showed similar characteristics in terms of appearance and moisture content. The cause of the difference in toxicity between the phases could not be attributed to differences in quality between the two lots. Differences in amounts of minor catechins between lots could account for the differential toxicity, and differences in these catechins relative to the EGCG peak across lots was noted, but no absolute quantitation was conducted. In addition, differences between lots in the number of minor unknown constituents existed. However, the authors mentioned that other trials that used the same lot as the one used in the Phase 2 study had not experienced similar liver toxicities. The authors speculated that the hepatotoxicity could be related to unique genetic background of study participants, although the demographics show that participants in Phase 2 were matched to those in Phase 1 for race, age, and sex.
Higher plasma EGCG concentrations have been observed when Polyphenon E is administered under fasting conditions: a dose of 800 mg without food is well tolerated, but 1200 mg results in frequent nausea [73]. The FDA did not allow further human studies with Polyphenon E administered under fasting conditions because of high mortality observed in fasted dogs, as mentioned previously [116]. Thus, during initiation of the studies in patients with multiple sclerosis, 400 mg twice daily with food was the highest dose the FDA allowed for one month of study.
Much higher doses of Polyphenon E enriched with EGCG have been studied in patients with chronic lymphocytic leukemia, as mentioned previously in this review [82]. In both phase 1 and phase 2 clinical studies, patients received up to 2000 mg Polyphenon E twice daily for up to 6 months. In both studies, the most common side effects were liver-related symptoms including transaminitis and abdominal pain.
In a few of the studies included in this review, LFT elevations have been reported, but levels remained within the normal range. However, other signs of potential liver effects were often reported including abdominal discomfort. For example, 3 subjects had mild abdominal discomfort after GTE treatment that resolved naturally within the first week in a randomized, double-blind, placebo-controlled trial to study the efficacy and safety of high-dose GTE on weight reduction and changes of lipid profiles in 100 women. The total daily dose of EGCG in the GTE used in this study was 856.8 mg/day, and it was taken for 12 weeks, 30 min after meals [124].
In an earlier study conducted by Chen and collaborators using the same GTE formulation and study design, one subject reported abdominal discomfort with treatment [125,126]. This adverse effect also resolved within the first week of treatment. Similarly, in a phase 2 clinical trial in patients with light-chain amyloidosis treated with 1890 mg EGCG per day for 6 months, no abnormalities in LFTs were observed [127]. However, in the majority of clinical studies reviewed, there were observations that could be interpreted as related to effects on the liver.
In summary, the doses of GTE used across the studies reviewed varied greatly, resulting in potentially large variations in systemic exposure to EGCG. The LFTs monitored across studies were inconsistent with some studies monitoring only one liver toxicity endpoint while others have a complete evaluation of LFT. However, in the majority of clinical studies reviewed, there was some indication of an effect on the liver.
6.2. Evidence from case reports
A total of 75 cases published in 51 reports were identified, of which 34 had been included in our previous review of 2008 [18]. To eliminate the possible effects of other ingredients in the involved products, only cases associated with products containing GTE as a single ingredient (35 cases out of 75 cases) were analyzed to determine the causal relationship between the product and hepatotoxicity.
Of the 51 published reports of liver injury associated with intake of products containing GTE found during the literature search, [[5], [6], [7],12,[128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156]]; fifteen articles describing 20 cases were reported in a non-English language and were translated to English by co-authors and then analyzed [42,43,45,[157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169]]. All case reports were categorized into two groups; those associated with single ingredient dietary supplements (SIDS) (N = 35) and those associated with multiple ingredient dietary supplements (MIDS) (N = 40). Causality assessment was not done for the cases involving MIDS. To rule out confounding effects from other ingredients, only case reports associated with products containing GTE as a single ingredient were analyzed to determine the causal relationship between the product and hepatotoxicity.
6.3. Assessing causal relationship between liver injury and GTE intake
Causality assessment scoring was based on parameters and methodology described by Fontana et al. [26]. Cases that had a DILIN causality score for GTE ranging from 1 to 3 were considered confirmed drug-induced liver injury (DILI) cases and were further classified to be definite (4 cases), highly likely (11 cases), or probable (14 cases), corresponding with probabilities of 95 % or greater, 75–94 % and 50–74 % respectively with the likelihood of a causal association between the liver injury event and the GTE [24] (Table 5) (Detailed results are presented in OSM Table 2, which shows causality assessment case details and results). Of the 35 cases related to SIDS products, one had insufficient data (score = 6) and could not be scored using either the DILIN method or the RUCAM scale.
Table 5.
Summary of causality assessment results for case reports.
| CAUSALITY ASSESSMENT RESULTS FOR CASES RELATED TO SIDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DILIN causality Scale | OVERALL (HDS) score | % of total | Agent-Specific (GTE) Score | % of total | Specific Score - OTHER (drug name) | % of total | RUCAM scale | RUCAM score | % of total |
| 1 = definite | 4 | 11.4 | 4 | 11.4 | 0 | 0.0 | 0.0 | ||
| 2 = highly likely | 11 | 31.4 | 11 | 31.4 | 0 | 0.0 | Highly Probable: * or >8 | 6 | 17.1 |
| 3 = probable | 14 | 40.0 | 14 | 40.0 | 0 | 0.0 | Probably:6-8 | 23 | 62.9 |
| 4 = possible | 5 | 14.3 | 5 | 14.3 | 5 | 12.5 | Possible: 3-5 | 6 | 17.1 |
| 5 = unlikely | 0 | 0 | 0 | 0 | 29 | 87.5 | Unlikely: 1-2 | 0 | 0.0 |
| 6 = insufficient data | 1 | 2.9 | 1 | 2.9 | 1 | 0 | Excluded: 0 or <0 | 1 | 2.9 |
| 35 | 100.0 | 35 | 100.0 | 35 | 100.0 | 35 | 100.0 | ||
SIDS: single-ingredient dietary supplement, containing only green tea extract as ingredient.
DILIN: Drug‐Induced Liver Injury Network.
HDS: herbal dietary supplement.
GTE: green tea extract.
Drug name: different drugs were involved, some used alone or concurrently with others.
DILIN experts concluded that there was positive liver injury in 29 cases (Table 5), scoring them as probably related to GTE (14 cases), highly likely related to GTE (11 cases) or definitely related to GTE (4 cases). Only four cases that had positive de-challenge and positive re-challenge were scored as definite.
Twenty-six cases involved concentrated GTE, nineteen of which excluded the involvement of other drugs. One case involved a 46-year-old woman who developed jaundice and had severe hepatocellular injury seven months after starting daily intake of extracts of Chinese green teas. Other possible causes such as underlying liver disease were ruled out. The amount of GTE used was not provided in the article. One of the authors, HL Bonkovsky, who was among the DILIN experts performing causality assessment in this review, communicated that all alternative explanations were reasonably ruled out and that the latency and recovery and clinical picture were typical of GTE hepatotoxicity [6].
The Molinari case [146] involved a 44-year-old white woman who took 720 mg of GTE daily for 6 months and developed fulminant liver failure. All other possible causes were ruled out, and her liver injury was typical of other reported GTE-induced DILI in that the liver showed severe, albeit variable, hepatic necrosis with areas of relatively preserved hepatic parenchyma, areas showing centrilobular (zone 3) necrosis and bridging necrosis, and in other areas, panlobular or multilobular necrosis.
The Porcel case [148] of a 53-year old female who ingested 3 capsules daily of Fitofruit grasas acumuladas (the label indicated the product contained GTE but the amount was not provided) for 2 weeks one month prior to her liver injury also ruled out other risk factors such as hepatitis A, B, and C viruses, Epstein-Barr virus, cytomegalovirus, and lacked serologic evidence of auto-immune liver injury (negative antinuclear and antimitochondrial antibodies). However, no medical history was provided, and the course of illness was not clearly outlined.
Four cases were part of a case series reported by Björnsson and Olsson in 2007 [130] involving the intake of Cuur®, an herbal weight-loss supplement containing 82 % ethanolic dry extract of green tea leaves. The duration of treatment before the manifestation of liver injury was 5–20 weeks, although the amount of extract ingested was not provided. Twelve of the cases involved the use of Exolise®; (Arkopharma), which, as already mentioned, reportedly caused liver injury and was subsequently banned in France and Spain in 2003 [42,43].
The DILIN experts also analyzed the cases using the RUCAM scale [[28], [29], [30],170] and determined that 29 cases had a RUCAM score of 6 or higher, corresponding to a probable or highly probable causality (same number of cases identified by DILIN methods assigned causality as, definite, highly likely or probable). These were not all the same as the cases that were identified by DILIN (with score of 3 or below described above). Three cases were recognized as probable by DILIN scoring but not by RUCAM: [138,146,148]. All three cases were well described but were missing information, had incomplete medical histories, and involved concurrent use of other medications. Although the use of other medications could be considered less likely to be involved because these medications had been used for long periods of time without adverse effects, their involvement and/or interaction with GTE could not be completely ruled out.
6.4. Liver injury associated with GTE
Most of the liver injury cases 22 out of 34 analyzed here occurred in women (Supplementary Information-OSM Table 2), raising the question of the role sex may play in influencing whether exposure to GT results in liver injury. Of the 34 cases that were judged to be causally related to GT preparations, twenty-seven were related to preparations containing GTE. Nine cases (out of 22 involving women) involved the use of GTE for weight-loss, whereas in one case GTE was used as therapy to prevent breast cancer recurrence. Notably, not all cases reported explicitly the reason for GT use. That most reported cases of liver injury involved women is probably a reflection of the higher use of dietary supplements by women as shown by a recent study of DS use among military service personnel [[171], [172], [173]], as with previous studies that showed similar trends. Furthermore, surveys have shown that the use of dietary supplements for weight loss is more prevalent among women than men [174,175]. Thus, this set of data does not give any indication that a particular sex or population is more susceptible to GTE-induced hepatotoxicity. Characteristics of liver injury observed in all cases reviewed here were hepatocellular or hepatitis, consistent with previous observations [13] (Supplementary Information CR-3). The median age of these patients was 51 years (20−81 years), however, no other demographic information was provided.
In her review of published cases of liver injury associated with herbs, Brown (2017) ranked green tea as the fourth herb among the herbs and dietary supplements associated with liver toxicity [176]. However, Brown’s review did not perform causality assessment.
It can be estimated that millions of people ingested products containing GTE since early 2000, but only hundreds of DILI cases have been reported in the same period. Hu et al. reviewed data from 48 clinical studies that monitored adverse events related to the hepato-biliary system. They concluded that incidence of hepatotoxicity due to GT was approximately 4.9 %, calculated from elevated liver functions biomarkers in 111 events out of 2269 subjects who were consuming green tea preparations (including green tea, GTE, or individual catechins) [11]. However, the accuracy of any numerical estimation of prevalence is questionable because severe underreporting and product mislabeling are known to occur. Thus, there is not enough evidence to suggest a dose below which hepatotoxicity does not occur. In a recent review published at the same time as our review was being developed, Teschke and Xuan [27], re-analyzed cases of suspected liver injury associated with GTE (many of which we have also analyzed here) and categorized the cases into three groups as “idiosyncratic” or “intrinsic herb induced liver injury” or “liver adaptation.” The authors concluded that the benefit-to-risk assessment is negative and thus the use of GTE cannot be recommended, but they recommended no restrictions for the use of GT beverages [27].
Navarro et al. reported that 40 % of 73 HDS that were linked to herbal-induced liver injury contained GTE; yet GTE was not always declared on the product labels [13]. Furthermore, it is notable that liver injury due to drugs or dietary supplements is severely under-reported. On the basis of 3667 cases identified from 2004 through 2013, it was estimated that 23,005 cases of emergency department visits were due to dietary supplements [177,178]. However, this finding was criticized because the analysis included products such as homeopathic products, human growth hormone, and human chorionic gonadotropin, which are not dietary supplements [179]. The United States FDA generally estimates that at most, 10 % of adverse events for drugs are reported, with reporting of AERs associated with DS being much lower [180]. Some reasons for underreporting include the following: 1) the true cause of liver injury was not discovered; 2) observers of such reactions did not publish the results; 3) reports were submitted to journals but were rejected for publication; 4) observers do not report all instances of DS-induced liver injury to the FDA MedWatch reporting system. Additionally, it is also possible that some health care providers may not associate GTE with liver injury due to the general presumption of green tea safety.
As is the case for other drugs that cause idiosyncratic DILI [27,[181], [182], [183]], it is reasonable to expect that a patient’s genetic predisposition plays a significant role in whether or not an individual develops liver injury following the intake of GTE. This expectation is supported by the results of a study in Diversity Outbred (DO) mice, a genetically heterogeneous mouse population, exposed to EGCG (50 mg/kg; daily for three days). The study showed that the EGCG was well tolerated in the majority of the mice; however, some of the mice (16 %; 43/272) developed severe hepatotoxicity (10–86.8 % liver necrosis). This is an indication that a minor but appreciable percentage of the mouse population was susceptible to liver damage when exposed to EGCG at levels not harmful to the majority of the population [184]. If genetic factors affect the sensitivity to EGCG in humans similarly to what is observed in the DO mice, this may offer an explanation for the infrequently-observed severe DILI with jaundice. Further evidence supporting the hypothesis that patients’ genetic predisposition may play a significant role in GTE induced liver injury was provided by a recent study in programmed death domain-1 (PD-1) knock-out mice engineered to mimic idiosyncratic drug-induced liver injury, which showed that EGCG can cause immune-mediated liver injury. In the study, PD-1 knockout mice were administered 250 mg/kg or 500 mg/kg of GTE (Applied Nutrition© Maximum Strength Green Tea Triple Fat Burner in rodent meal) for 6 weeks. PD-1 knock-out mice treated with the higher GTE dose of 500 mg/kg developed delayed onset increase in ALT, which was not observed in similarly treated wild-type mice [185].
Interestingly, an earlier study that investigated the effects of EGCG on concanavalin A (ConA)-induced hepatitis (CIH) (a murine model of immune-mediated liver injury) showed that EGCG had a protective effect compared to non-treated mice. Mice that were pretreated with EGCG had lower ALT levels, reduced inflammatory infiltration, and hepatocyte apoptosis in the liver. Other studies have also demonstrated similar hepatoprotective effects of EGCG against ConA-induced liver injury, which has been hypothesized to be due to EGCG’s anti-inflammatory and anti-oxidant properties [186]. Mice with CIH are an experimental model of immune-mediated liver disease in humans [187], and the liver is characterized by massive hepatocellular degeneration and lymphoid infiltration [188]. It is important to point out that these observations have not been corroborated in humans. EGCG appears to display both damaging and protective effects as a cytokine modulator, which is not unusual and may be an indication of the involvement of other master cytokine regulators such as transforming growth factor beta (TGF beta). TGF beta plays a central role in modulating oxidative stress in the liver and human body and is known to participate in many stages of liver disease progression, including injury through inflammation and fibrosis, cirrhosis, and cancer [189].
On the other hand, a recent study by Lambert in CF-1 mice examined the effect of pre-treatment with dietary EGCG on hepatotoxicity and systemic availability upon acute oral administration of EGCG [117]. Compared to control (vehicle-treated) mice, mice administered 750 mg/kg EGCG daily for three days showed an 80-fold increase in ALT. An increase in glutathione S-transferase mRNA was also observed. Pre-treatment with 3.2 mg EGCG/g diet for two weeks mitigated this response, resulting in a reduced elevation of plasma ALT by 75 %. These data suggest a putative mechanism by which EGCG in the GTE may mitigate pro-oxidant effects and may partly explain the observed variation in hepatotoxic response to GTE-containing dietary supplements [117].
7. Discussion
The human cases reviewed herein involved the use of preparations containing green tea in a wide range of doses. GTE intake amounts ranged from 500 mg to 3000 mg which is about 250−1800 mg EGCG daily). The median intake amount was estimated at 720 mg/day (delivering 623 mg of EGCG daily). In most cases, the GTE had been taken daily for two or more weeks before onset of the acute liver injury, which in some cases occurred up to a month after stopping the intake of GTE. Most subjects involved in the DILI cases (21 out of 35) were using GTE for weight loss and likely had reduced food intake as well. This may be significant given the increased hepatotoxicity of EGCG preparations in fasted compared to fed dogs [107]. Individuals trying to lose weight are also likely be eating less food which may inadvertently mimic fasted conditions resulting in significantly increased bioavailability of EGCG.
Clinical studies have shown that when GTE is taken on an empty stomach, the bioavailability of EGCG is much higher than when taken with food. For example, in healthy subjects (ten per group), administered Polyphenon E at 800 mg as a single dose showed an unbound Cmax of EGCG in the fasting condition to be more than five times that obtained after administration of the same dose with food [72]. Other studies have shown that the AUC0–8h for EGCG taken without food was significantly (P = 0.04) higher (by 3.9-fold) compared to when taken with food, potentially due to a higher oral bioavailability of EGCG when taken under fasting conditions [72,93]. In the Minnesota study involving postmenopausal women (MGTT), the authors concluded that the phase 2 clinical trial clearly demonstrated that high-dose of GTE intake for approximately 12 months was associated with liver enzyme elevation; the intake of 843 mg of EGCG was well tolerated but 6.7 % of the women experienced an increase in ALT levels and 1.3 % experienced significant elevation in ALT. In other studies reviewed, the authors noted that in many of the cases showing increased ALT levels, other possible risk factors that may have contributed to the increased liver enzymes could have been involved [119,120,190]. The public should be informed about the potential risk of liver injury with a high, sustained dose of GTE as a dietary supplement, especially for those who are obese or have preexisting liver disease [17]. Obesity increases the risk of developing nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH) [191] which may exacerbate the GTE induced liver injury. In the US, a study of 420 patients with NAFLD found that liver-related complications were the third most common cause of death among these patients [192].
The information reviewed here, together with our previous review of 34 case reports [19] other publications on hepatotoxicity associated with products containing green tea [20,133], including a recent review by Teschke and Xuan, 2019 [27] and other reviews of case reports [12,193], indicate that GTE can cause liver injury. What remains unclear is the exact mechanism(s) by which GTE induces liver injury, and the level below which no liver injury occurs.
Previous clinical, animal and in vitro toxicology evaluations have implicated EGCG as the main GT constituent likely responsible for the hepatotoxicity of GTE. For example, Lambert et al. demonstrated in CF-1 mice that a single dose of EGCG 1500 mg/kg increased plasma ALT by 138-fold and reduced survival by 85 %. In CF-1 mice, dosing with EGCG once-daily caused hepatotoxicity and decreased survival; ALT levels were increased 184- fold following two once-daily doses of 750 mg/kg of EGCG. Treatment with high doses of EGCG resulted in moderate to severe hepatic necrosis [194]. A clinical study reported that relative to the day of administration, a > 60 % increase was observed in systemic exposure to EGCG following chronic oral administration of 800 mg EGCG or Polyphenon E once-daily for four weeks [72]. in vitro studies have also demonstrated that rat hepatocytes exposed to increasing EGCG concentrations during short periods of time caused cytotoxicity and increased cell death [92].
The analysis of published case reports has its limitations in terms of incompleteness of information related to the onset of adverse events and ingestion of suspect product. Questions concerning intake amounts, duration of intake, and the temporal relationship between intake and onset of injury frequently remain unanswered. Most cases do not indicate the exact amount of GTE that was ingested nor the composition of the product. Additionally, the patient’s underlying medical information or information on concomitant use of other medicines or dietary supplements is frequently missing.
The information that could be extracted from the case reports reviewed herein indicate that consumption of dietary supplements and food supplements containing GTE could lead to adverse effects on the liver, and these effects can occur within a wide range of EGCG intake amounts ranging from approximately 140 mg to ∼1000 mg/day EGCG. These intake levels are representative of the amounts of EGCG found in dietary supplement products. However, most published cases did not provide sufficient information to determine the actual intake levels of the GTE or EGCG, thus the relationship between levels of GTE/EGCG intake and liver injury could not be established. The range of intake of EGCG from dietary supplements containing concentrated GTE based on information from labels of products in the DSLD is 45-1575 mg/day 100 -4500 mg of GTE per day. These ranges lie within the intake levels that have been shown to be associated with DILI in the published case reports reviewed herein.
Based on our assessment, it is reasonable to conclude that risks of hepatotoxicity due to GTE intake are real, and exposure may lead to liver injury, including serious liver injury. Furthermore, the rather infrequent published reports of liver injury with jaundice, implying greater severity, and multiple factors impacting this outcome, contribute to the unpredictability of the association between liver injury and GTE intake. Genetic variability and bioavailability may explain the susceptibility to a wide range of doses reported in cases of induced liver injury following GTE intake and indicate that GTE induced liver injury may also be idiosyncratic in susceptible individuals, for example in the case of mother and daughter [134]. A recent review by Teschke and Xuan (2019) [27] also arrived at a similar conclusion.
Our study supports the inclusion of a statement in the USP GTE monograph cautioning users of GTE about the potential liver damaging effects of GTE especially when ingested under fasting conditions or in cases where there is underlying liver vulnerability. Given that animal studies show a clear association between liver injury and EGCG intake, product labels should also declare the amount of EGCG. Our review did not find sufficient data to determine a minimum level of GTE or EGCG that would cause liver injury in humans following the typical pattern of consumption in dietary supplements.
In 2017, Health Canada reviewed the potential risk of hepatotoxicity associated with GTE and strengthened the cautionary risk statement in their GTE monographs [195]. Norway also reviewed possible safety concerns associated with consumption of GTEs and concluded that intake of more than 0.4 mg EGCG/kg bw per day as a bolus may cause adverse biological effects and noted that there is increased susceptibility to toxicity when GTE or green tea infusion is taken following fasting [196].
The 2018 EFSA review of catechins in GTE concluded that clinical studies indicate that intake of GTE as a food supplement delivering EGCG amounts equal or above 800 mg daily increased serum transaminases, which is indicative of liver injury. The review further concluded that it was not possible to identify an EGCG dose from GTEs that could be considered safe because in one case, intake of 375 mg of 80 % ethanolic extract resulted in hepatotoxicity [10].
8. Concluding remarks
Our review shows a clear occurrence of severe hepatotoxicity from ingestion of GTE in humans albeit with very low frequency and further suggests that specific GT constituents, particularly EGCG and other catechins found in GTE, are likely to be involved in the observed hepatotoxicity. The recognized factors that can contribute to the hepatotoxic effects are the concentration of catechins in certain GTE-containing products [10,34,78], the bolus dose provided by certain dosage forms such as capsules and tablets, and the intake of GTE under fasting conditions which increases the absorption of GTE constituents which has been observed in clinical [73] and animals studies [116]. Furthermore, genetic susceptibility, idiosyncrasy, and/or underlying liver health may play a role in whether the hepatotoxic effects are manifested. While dosing data and NOAELs from animal studies exist, the human evidence regarding dose-response is less robust, with hepatotoxicity occurring at doses below those observed in animal studies. The setting of a dose below which toxicity will not occur was deemed impractical due to a possible role of genetic factors and the idiosyncratic nature of hepatotoxicity in humans. Therefore, the overall weight-of-evidence from this review justifies inclusion of a label cautionary statement in the USP PDGTE ingredient monograph, which reads as follows: “Dosage forms prepared with this article should bear the following statement: ‘Do not take on an empty stomach. Take with food. Do not use if you have a liver problem and discontinue use and consult a healthcare practitioner if you develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice (yellowing of the skin or eyes).’”
Compliance with this label caution statement is mandatory only for products that claim compliance with USP quality standards for GTE such as PDGTE. Our findings are consistent with recently published reviews by EFSA [10] and Hu et al. [11], and other global government authorities including Health Canada [195]194] and Norwegian Food Safety Authority (NFSA) [196].
CRediT authorship contribution statement
Hellen A. Oketch-Rabah: Conceptualization, Project administration, Investigation, Supervision, Methodology, Visualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Amy L. Roe: Conceptualization, Supervision, Methodology, Visualization, Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Cynthia V. Rider: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Herbert L. Bonkovsky: Formal analysis, Writing - original draft, Writing - review & editing. Gabriel I. Giancaspro: Conceptualization, Resources, Writing - review & editing. Victor Navarro: Conceptualization, Formal analysis, Writing - review & editing. Mary F. Paine: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Joseph M. Betz: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Robin J. Marles: Conceptualization, Writing - review & editing. Steven Casper: Conceptualization, Writing - review & editing. Bill Gurley: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Scott A. Jordan: Conceptualization, Writing - review & editing. Kan He: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Mahendra P. Kapoor: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Theertham P. Rao: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Averell H. Sherker: Formal analysis, Writing - review & editing. Robert J. Fontana: Formal analysis, Writing - review & editing. Simona Rossi: Formal analysis, Writing - review & editing. Raj Vuppalanchi: Formal analysis, Writing - review & editing. Leonard B. Seeff: Formal analysis, Writing - review & editing. Andrew Stolz: Formal analysis, Writing - review & editing. Jawad Ahmad: Formal analysis, Writing - review & editing. Christopher Koh: Formal analysis, Writing - review & editing. Jose Serrano: Formal analysis, Writing - review & editing. Tieraona Low Dog: Conceptualization, Supervision, Methodology, Visualization, Writing - review & editing. Richard Ko: Conceptualization, Supervision, Methodology, Visualization, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors have declared the following:
HAO-R and GIG are employed by USP.
The below listed authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: Hellen A Oketch-Rabah, Gabriel I. Giancaspro, Jawad Ahmad, Steven Casper, Bill Gurley, Cynthia V. Rider, Leonard B Seef, Jose Serrano, Joseph M. Betz, Christopher Koh, Kan He, Mary F. Paine, Raj Vuppalanchi, Robin J. Marles, Averell H. Sherker, Simona Rossi, Scott Jordan, Victor J. Navarro, Andrew Stolz.
The below listed authors declare the described financial interests/personal relationships which may be considered as potential competing interests:
Tieraona Low Dog is a Consultant at MegaFoods; Herbert L. Bonkovsky has been and continues to be an investigator in the NIH-sponsored US Drug-Induced Liver Injury Network since 2003 and is supported by a cooperative agreement between NIDDK and UNC & Wake Forest University, U01 DK 065201; Amy L. Roe works for a company that manufactures and distributes dietary supplements; including some that may contain green tea extract; Richard Ko is the founder and owner of Herbal Synergy, a company that consults for the dietary supplement, food, and pharmaceutical companies; Kan He is employed by Herbalife, a company that manufactures and distributes dietary supplement that may contain green tea or green tea extracts; Robert J. Fontana receives research support from Gilead, Abbvie, and BristolMyerSquibb, and also provides consulting for Alynam and AstraZeneca; Mahendra P. Kapoor is an employee of Taiyo Kagaku Co Ltd, a company that manufactures several nutraceutical ingredients including green tea extracts; Theertham Pradyumna Rao is an employee of Taiyo Kagaku Co Ltd, a company that manufactures several nutraceutical ingredients including green tea extracts.
Acknowledgements
The authors wish to thank the members of the 2015-2020 USP Dietary Supplements Admission Evaluations Joint Standard-Setting Subcommittee for valuable discussions of the review; other USP Staff: Dr. Kristi Muldoon Jacobs for her contribution during initial discussions on selecting articles for review; Dr. Jaap Venema and Alissa Jijon, JD for critically reviewing the manuscript; and Ms. Eleonora d’Amore (USP) for copyediting the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.02.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chaturvedula V.S.P., Prakash I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011;5(11):2110–2124. https://academicjournals.org/journal/JMPR/article-full-text-pdf/65ACCB520061 [Google Scholar]
- 2.Juneja L.R. CRC Press; 2013. Green Tea Polyphenols: Nutraceuticals of Modern Life. [Google Scholar]
- 3.Jurgens T.M. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst. Rev. 2012;(12) doi: 10.1002/14651858.CD008650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka T., Matsuo Y., Kouno I. In: Biochemical and Physicochemical Characteristics of Green Tea Polyphenols, in Green Tea Polyphenols: Nutraceuticals of Modern Life. Juneja L., editor. CRC Press, Taylor& Francis Group; Boca Raton, FL: 2013. pp. 19–38. [Google Scholar]
- 5.Bonkovsky H.L. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann. Intern. Med. 2006;144(1):68–69. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 6.Javaid A., Bonkovsky H.L. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): a growing concern. J. Hepatol. 2006;45(2):334–335. doi: 10.1016/j.jhep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Araujo J.L., Worman H.J. Acute liver injury associated with a newer formulation of the herbal weight loss supplement Hydroxycut. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-210303. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown A.L. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br. J. Nutr. 2008;101(6):886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekant W. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017;277:104–108. doi: 10.1016/j.toxlet.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 10.EFSA Scientific opinion on the safety of green tea catechins by panel on food additives nutrient sources added to food (Younes, Maged; Aggett, Peter; Aguilar, Fernando; Crebelli, Riccardo; Dusemund, Birgit; filipič, Metka; frutos, Maria Jose; Galtier, Pierre; Gott, david) EFSA J. 2018;16(4):e05239. doi: 10.2903/j.efsa.2018.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J. The safety of green tea and green tea extract consumption in adults–results of a systematic review. Regul. Toxicol. Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Mazzanti G. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009;65(4):331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 13.Navarro V.J. Catechins in dietary supplements and hepatotoxicity. Dig. Dis. Sci. 2013;58(9):2682–2690. doi: 10.1007/s10620-013-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S.S. Green tea extract: a potential cause of acute liver failure. World J. Gastroenterol. 2013;19(31):5174–5177. doi: 10.3748/wjg.v19.i31.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NIH:National Institute of Diabetes and . Livertox: Clinical and Research Information on Drug-induced Liver Injury. US National Library of Medicine; Bethesda, MD: 2017. Digestive and kidney diseases (NIDDKD)https://www.ncbi.nlm.nih.gov/books/NBK547852/ Accessed on December 19, 2019. [Google Scholar]
- 16.Yates A.A. Bioactive nutrients-time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017;84:94–101. doi: 10.1016/j.yrtph.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev. Res. Phila. (Phila) 2017;10(10):571–579. doi: 10.1158/1940-6207.CAPR-17-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. National Library of Medicine . 2019. Green Tea (Cammelia Sinensis) in LIVERTOX DATABASE.https://livertox.nih.gov/GreenTea.htm [cited 2019 December 21]; 2018:[Available from: [Google Scholar]
- 19.Sarma D.N. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf. 2008;31(6):469–484. doi: 10.2165/00002018-200831060-00003. 200831060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Teschke R., Schulze J. Suspected herbal hepatotoxicity: requirements for appropriate causality assessment by the US Pharmacopeia. Drug Saf. 2012;35(12):1091–1097. doi: 10.2165/11631960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.USP . 2009. USP Update on the USP Green Tea Extract Monograph.https://www.uspnf.com/notices/retired-compendial-notices/update-usp-green-tea-extract-20monograph available at. [Google Scholar]
- 22.USP In-Process Revision: Powdered Decaffeinated Green Tea Extract available at: 22 http://www.usppf.com/pf/pub/index.html. Pharmacopeal Forum Online 2017 [cited 2019 December 12].
- 23.2019. USP USP-NF Monographs for Powdered Decaffeinated Green Tea Extract. Official Mar 1. [Google Scholar]
- 24.Bedrood Z., Rameshrad M., Hosseinzadeh H. Toxicological effects of Camellia sinensis (green tea): a review. Phytother. Res. 2018;32(7):1163–1180. doi: 10.1002/ptr.6063. [DOI] [PubMed] [Google Scholar]
- 25.DILIN . 2011. What Is Drug-Induced Liver Injury Network (DILIN)? Bethesda.https://dilin.org/ [cited 2019 October 5]; Available from: [Google Scholar]
- 26.Fontana R.J. Drug-induced liver injury network (DILIN) prospective study. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teschke R., Xuan T. Suspected herb induced liver injury by green tea extracts: critical review and case 2 analysis applying RUCAM for causality assessment. Jpn. J. Gastroenterol. Hepatol. 2019;1(6):1–16. https://www.jjgastrohepto.org/pdf/JJGH-v2-1030.pdf [Google Scholar]
- 28.Danan G., Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol. 1993;46(11):1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 29.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci. 2015;17(1) doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochon J. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48(4):1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal V.K. Important elements for the diagnosis of drug-induced liver injury. Clin. Gastroenterol. Hepatol. 2010;8(5):463–470. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor . In: Green Tea: History, Processing Techniques, Principles, Traditions, Features and Attractions in Green Tea Polyphenols, Nutraceuticals of Modern Life. LR J., editor. CRS press, Taylor & Francis group; NY, USA: 2013. pp. 1–18. [Google Scholar]
- 33.Vuong Q.V. Improved extraction of green tea components from teabags using the microwave oven. J. Food Compos. Anal. 2012;27(1):95–101. https://doi.org/810.1080/87559129.2011.563397. [Google Scholar]
- 34.Friedman M. Distribution of catechins, theaflavins, caffeine, and theobromine in 77 teas consumed in the United States. J. Food Sci. 2005;70(9) [Google Scholar]
- 35.Friedman M. HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: comparison of water and 80% ethanol/water extracts. J. Food Sci. 2006;71(6) https://doi.org/10.1111/j.1365-2621.2005.tb08304.x. [Google Scholar]
- 36.Banerjee S., Chatterjee J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015;52(6):3158–3168. doi: 10.1007/s13197-014-1487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara Y. CRC press; 2001. Green Tea: Health Benefits and Applications. [Google Scholar]
- 38.Lorenzo J.M., Munekata P.E.S. Phenolic compounds of green tea: health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016;6(8):709–719. [Google Scholar]
- 39.Ninomiya . In: Chemical and Physiochemical Properties of Green Tea Polyphenols. Yamamoto, editor. CRS Press LLC; USA: 1997. [Google Scholar]
- 40.Perva-Uzunalić A. Extraction of active ingredients from green tea (Camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chem. 2006;96(4):597–605. [Google Scholar]
- 41.Ziaedini A., Jafari A., Zakeri A. Extraction of antioxidants and caffeine from green tea (Camelia sinensis) leaves: kinetics and modeling. Rev. Agaroquimica Y Tecnol. Aliment. 2010;16(6):505–510. doi: 10.1177/1082013210367567. [DOI] [PubMed] [Google Scholar]
- 42.Seddik M. Y at-il des risques d’hépatotoxicité avec Exolise®. Gastroenterol. Clin. Biol. 2001;25:834–835. https://doi.org/GCB-08-2001-25-8-9-0399-8320-101019-ART24. [PubMed] [Google Scholar]
- 43.Vial T. 2003. Hépatite Aiguë Imputable à l’Exolise®(Camellia Sinensis) https://doi.org/GCB-12-2003-27-12-0399-8320-101019-ART19. [PubMed] [Google Scholar]
- 44.USP USP-NF . 2019. General Chapter <467> Residual Solvents., Official Mar 1. 2019. [Google Scholar]
- 45.el Abu, Wafa Y. Anales De Medicina Interna. SciELO; Espana: 2005. Hepatitis aguda inducida por Camellia sinens (té verde) [DOI] [PubMed] [Google Scholar]
- 46.Paramasivam M., Chandrasekaran S. Persistence behaviour of deltamethrin on tea and its transfer from processed tea to infusion. Chemosphere. 2014;111:291–295. doi: 10.1016/j.chemosphere.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 47.Wang J., Cheung W., Leung D. Determination of pesticide residue transfer rates (percent) from dried tea leaves to brewed tea. J. Agric. Food Chem. 2014;62(4):966–983. doi: 10.1021/jf404123h. [DOI] [PubMed] [Google Scholar]
- 48.Xue J. Transfer of difenoconazole and azoxystrobin residues from chrysanthemum flower tea to its infusion. Food Addit. Contam. Part A. 2014;31(4):666–675. doi: 10.1080/19440049.2014.882020. [DOI] [PubMed] [Google Scholar]
- 49.Hayward D.G., Wong J.W., Park H.Y. Determinations for pesticides on Black, Green, Oolong, and White Teas by gas chromatography triple-quadrupole mass spectrometry. J. Agric. Food Chem. 2015;63(37):8116–8124. doi: 10.1021/acs.jafc.5b02860. [DOI] [PubMed] [Google Scholar]
- 50.Muraleedharan N. Strategies for reducing pesticide residues in tea. Int. J. Tea Sci. 2004;3(3 and 4) [Google Scholar]
- 51.Fernández-Cáceres P.L. Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. J. Agric. Food Chem. 2001;49(10):4775–4779. doi: 10.1021/jf0106143. [DOI] [PubMed] [Google Scholar]
- 52.Ratnaike R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003;79(933):391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Järup L. Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998:1–51. https://www.jstor.org/stable/40967243 [PubMed] [Google Scholar]
- 54.Vincent J.B. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003;33(3):213–230. doi: 10.2165/00007256-200333030-00004. [DOI] [PubMed] [Google Scholar]
- 55.Cerulli J. Chromium picolinate toxicity. Ann. Pharmacother. 1998;32(4):428–431. doi: 10.1345/aph.17327. [DOI] [PubMed] [Google Scholar]
- 56.Farmand F. Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ. Res. 2005;98(1):33–39. doi: 10.1016/j.envres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Lee M.R. Blood mercury concentrations are associated with decline in liver function in an elderly population: a panel study. Environ. Health. 2017;16(1):17. doi: 10.1186/s12940-017-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spangler J.G. Air manganese levels and chronic liver disease mortality in North Carolina counties: an ecological study. Int. J. Environ. Res. Public Health. 2012;9(9):3258–3263. doi: 10.3390/ijerph9093258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Nino W.R., Pedraza-Chaverri J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014;69:182–201. doi: 10.1016/j.fct.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Jaishankar M. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.EFSA Dietary exposure assessment to pyrrolizidine alkaloids in the European population. Efsa J. 2016;14(8):e04572. [Google Scholar]
- 62.Bodi D. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31(11):1886–1895. doi: 10.1080/19440049.2014.964337. [DOI] [PubMed] [Google Scholar]
- 63.EFSA Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012;10(5):2663. [Google Scholar]
- 64.EFSA Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017;15(7):e04908. doi: 10.2903/j.efsa.2017.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madge I. Pyrrolizidine alkaloids in herbal teas for infants, pregnant or lactating women. Food Chem. 2015;187:491–498. doi: 10.1016/j.foodchem.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 66.Merz K.H., Schrenk D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016;263:44–57. doi: 10.1016/j.toxlet.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Mulder P.P.J. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: results of a survey across Europe. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35(1):118–133. doi: 10.1080/19440049.2017.1382726. [DOI] [PubMed] [Google Scholar]
- 68.Shimshoni J.A. Pyrrolizidine and tropane alkaloids in teas and the herbal teas peppermint, rooibos and chamomile in the Israeli market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015;32(12):2058–2067. doi: 10.1080/19440049.2015.1087651. [DOI] [PubMed] [Google Scholar]
- 69.Navarro V.J. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuman M.G. Hepatotoxicity of pyrrolizidine alkaloids. J. Pharm. Pharm. Sci. 2015;18(4):825–843. doi: 10.18433/j3bg7j. [DOI] [PubMed] [Google Scholar]
- 71.Chow H.H. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomarkers Prev. 2001;10(1):53–58. https://cebp.aacrjournals.org/content/10/1/53.long [PubMed] [Google Scholar]
- 72.Chow H.H. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003;9(9):3312–3319. https://clincancerres.aacrjournals.org/content/9/9/3312.long [PubMed] [Google Scholar]
- 73.Chow H.H. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005;11(12):4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 74.Ullmann U. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int. J. Vitam. Nutr. Res. 2004;74(4):269–278. doi: 10.1024/0300-9831.74.4.269. [DOI] [PubMed] [Google Scholar]
- 75.Ullmann U. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003;31(2):88–101. doi: 10.1177/147323000303100205. https://doi.org/710.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 76.Egert S. Simultaneous ingestion of dietary proteins reduces the bioavailability of galloylated catechins from green tea in humans. Eur. J. Nutr. 2013;52(1):281–288. doi: 10.1007/s00394-012-0330-8. [DOI] [PubMed] [Google Scholar]
- 77.Rashidinejad A. Addition of milk to tea infusions: helpful or harmful? Evidence from in vitro and in vivo studies on antioxidant properties. Crit. Rev. Food Sci. Nutr. 2017;57(15):3188–3196. doi: 10.1080/10408398.2015.1099515. https://doi.org/10.1080/10408398.2015.1099515. [DOI] [PubMed] [Google Scholar]
- 78.Navarro V. Liver injury from herbal and dietary supplements: an introduction. Clin. Liver Dis. (Hoboken) 2019;14(2):43–44. doi: 10.1002/cld.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DILIN . Liver Tox Database, Green Tea. NIH; Bethesda, MD: 2018. DILIN clinical and research information on drug induced liver injury.https://livertox.nlm.nih.gov/GreenTea.htm (Camelia sinensis). Available at: [Google Scholar]
- 80.Isomura T. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur. J. Clin. Nutr. 2016;70(11):1340. doi: 10.1038/ejcn.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shanafelt T.D. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119(2):363–370. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shanafelt T.D. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol. 2009;27(23):3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Renouf M. Dose-response plasma appearance of green tea catechins in adults. Mol. Nutr. Food Res. 2013;57(5):833–839. doi: 10.1002/mnfr.201200512. https://doi.org/2910.1002/mnfr.201200512. [DOI] [PubMed] [Google Scholar]
- 84.Bae M.J. Albumin stabilizes (–)‐epigallocatechin gallate in human serum: binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009;53(6):709–715. doi: 10.1002/mnfr.200800274. [DOI] [PubMed] [Google Scholar]
- 85.Ishii T. Human serum albumin as an antioxidant in the oxidation of (−)-epigallocatechin gallate: participation of reversible covalent binding for interaction and stabilization. Biosci. Biotechnol. Biochem. 2011;75(1):100–106. doi: 10.1271/bbb.100600. [DOI] [PubMed] [Google Scholar]
- 86.Lee M.-J. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiology and Prevention Biomarkers. 2002;11(10):1025–1032. https://cebp.aacrjournals.org/content/11/10/1025.full-text.pdf [PubMed] [Google Scholar]
- 87.Yang C.S. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiology and Prevention Biomarkers. 1998;7(4):351–354. https://cebp.aacrjournals.org/content/7/4/351.long [PubMed] [Google Scholar]
- 88.Clifford M.N., van der Hooft J.J., Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013;98(6 Suppl):1619S–1630S. doi: 10.3945/ajcn.113.058958. [DOI] [PubMed] [Google Scholar]
- 89.Auger C. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J. Nutr. 2008;138(8):1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 90.Proniuk S., Liederer B.M., Blanchard J. Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer prevention. J. Pharm. Sci. 2002;91(1):111–116. doi: 10.1002/jps.10009. [DOI] [PubMed] [Google Scholar]
- 91.Roach P.D. We-P14: 429 despite its instability, epigallocatechin gallate effective lowers serum cholesterol in the hypercholesterolaemic rabbit. Atheroscler. Suppl. 2006;7(3):441. [Google Scholar]
- 92.Wang R., Zhou W., Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008;56(8):2694–2701. doi: 10.1021/jf0730338. https://doi.org/1410.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- 93.Naumovski N., Blades B.L., Roach P.D. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants Basel (Basel) 2015;4(2):373–393. doi: 10.3390/antiox4020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kucera O. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid. Med. Cell. Longev. 2015:476180. doi: 10.1155/2015/476180. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oritani Y. Comparison of (-)-epigallocatechin-3-O-gallate (EGCG) and O-methyl EGCG bioavailability in rats. Biol. Pharm. Bull. 2013;36(10):1577–1582. doi: 10.1248/bpb.b13-00349. [DOI] [PubMed] [Google Scholar]
- 96.Sano M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999;47(5):1906–1910. doi: 10.1021/jf981114l. [DOI] [PubMed] [Google Scholar]
- 97.Phan A.D. Binding of dietary polyphenols to cellulose: structural and nutritional aspects. Food Chem. 2015;171:388–396. doi: 10.1016/j.foodchem.2014.08.118. [DOI] [PubMed] [Google Scholar]
- 98.Glube N., Moos L., Duchateau G. Capsule shell material impacts the in vitro disintegration and dissolution behaviour of a green tea extract. Results Pharma Sci. 2013;3:1–6. doi: 10.1016/j.rinphs.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.United States Pharmacopeial Convention . 2019. (USP) USP42-NF37. General Chapter <1094> Capsules—Dissolution Testing And Related Qualitry Attributes; p. 7592. [Google Scholar]
- 100.Son Y.R. Combinational enhancing effects of formulation and encapsulation on digestive stability and intestinal transport of green tea catechins. J. Microencapsul. 2016;33(2):183–190. doi: 10.3109/02652048.2016.1144816. [DOI] [PubMed] [Google Scholar]
- 101.Janssens P.L., Hursel R., Westerterp-Plantenga M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015;145(5):864–870. doi: 10.3945/jn.114.207829. [DOI] [PubMed] [Google Scholar]
- 102.Chen L. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997;25(9):1045–1050. http://dmd.aspetjournals.org/content/25/9/1045.long [PubMed] [Google Scholar]
- 103.Zhu B.T. Rapid conversion of tea catechins to monomethylated products by rat liver cytosolic catechol-O-methyltransferase. Xenobiotica. 2001;31(12):879–890. doi: 10.1080/00498250110079798. [DOI] [PubMed] [Google Scholar]
- 104.Zhu M., Chen Y., Li R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000;66(5):444–447. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]
- 105.Lambert J.D. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003;133(12):4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 106.Suganuma M. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 107.Isbrucker R. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem. Toxicol. 2006;44(5):626–635. doi: 10.1016/j.fct.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 108.Hsu Y.W. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food Chem. Toxicol. 2011;49(10):2624–2630. doi: 10.1016/j.fct.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 109.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7(2):27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.FDA Center for Drug Evaluation and Research (CDER) 2005. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers.https://www.fda.gov/media/72309/download Available at. [Google Scholar]
- 111.National Toxicology Program (NTP) Toxicology studies of green tea extract in F344/NTac rats and B6C3F1/N mice and Toxicology and carcinogenesis studies of green tea extract in Wistar Han [Crl: wi (Han)] rats and B6C3F1/N mice. Toxicol. Program Tech. Rep. Ser. 2016;585 doi: 10.22427/NTP-TR-585. https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr585_508.pdf Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chengelis C.P. 28-Day oral (gavage) toxicity studies of green tea catechins prepared for beverages in rats. Food Chem. Toxicol. 2008;46(3):978–989. doi: 10.1016/j.fct.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 113.Wang D. Evaluation of oral subchronic toxicity of Pu-erh green tea (camellia sinensis var. assamica) extract in Sprague Dawley rats. J. Ethnopharmacol. 2012;142(3):836–844. doi: 10.1016/j.jep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 114.Takami S. Evaluation of toxicity of green tea catechins with 90-day dietary administration to F344 rats. Food Chem. Toxicol. 2008;46(6):2224–2229. doi: 10.1016/j.fct.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 115.Morita O. Safety assessment of heat-sterilized green tea catechin preparation: a 6-month repeat-dose study in rats. Food Chem. Toxicol. 2009;47(8):1760–1770. doi: 10.1016/j.fct.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 116.Kapetanovic I.M. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260(1-3):28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.James K.D., Forester S.C., Lambert J.D. Dietary pretreatment with green tea polyphenol, (-)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (-)-epigallocatechin-3-gallate. Food Chem. Toxicol. 2015;76:103–108. doi: 10.1016/j.fct.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu K.M., Yao J., Boring D. Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. Int. J. Toxicol. 2011;30(1):19–20. doi: 10.1177/1091581810387445. [DOI] [PubMed] [Google Scholar]
- 119.Samavat H. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes Control. 2015;26(10):1405–1419. doi: 10.1007/s10552-015-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dostal A.M. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015;83:26–35. doi: 10.1016/j.fct.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dostal A.M. Green tea extract and catechol-O-methyltransferase genotype modify the post-prandial serum insulin response in a randomised trial of overweight and obese post-menopausal women. J. Hum. Nutr. Diet. 2017;30(2):166–176. doi: 10.1111/jhn.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dostal A.M. Green tea extract and Catechol-O-Methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J. Nutr. 2016;146(1):38–45. doi: 10.3945/jn.115.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lovera J. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015;358(1-2):46–52. doi: 10.1016/j.jns.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen I.J. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016;35(3):592–599. doi: 10.1016/j.clnu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Liu C.Y. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PLoS One. 2014;9(3):e91163. doi: 10.1371/journal.pone.0091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu K. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am. J. Clin. Nutr. 2013;98(2):340–348. doi: 10.3945/ajcn.112.052746. [DOI] [PubMed] [Google Scholar]
- 127.Meshitsuka S. Phase 2 trial of daily, oral epigallocatechin gallate in patients with light-chain amyloidosis. Int. J. Hematol. 2017;105(3):295–308. doi: 10.1007/s12185-016-2112-1. [DOI] [PubMed] [Google Scholar]
- 128.Arzenton E. Acute hepatitis caused by green tea infusion: a case report. Adv. Pharmacoepidemiol. Drug Saf. 2012;(3):170. [Google Scholar]
- 129.Bergman J., Schjøtt J. Hepatitis Caused by Lotus‐f3? Basic Clin. Pharmacol. Toxicol. 2009;104(5):414–416. doi: 10.1111/j.1742-7843.2009.00385.x. [DOI] [PubMed] [Google Scholar]
- 130.Bjornsson E., Olsson R. Serious adverse liver reactions associated with herbal weight-loss supplements. J. Hepatol. 2007;47(2):295–297. doi: 10.1016/j.jhep.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 131.Chen G.C. Acute liver injury induced by weight-loss herbal supplements. World J. Hepatol. 2010;2(11):410. doi: 10.4254/wjh.v2.i11.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cruz A.C.D. Mo1814 fulminant liver failure secondary to “Saba appetite control and energy” weight loss supplement. Gastroenterology. 2014;146(5) p. S-1002. [Google Scholar]
- 133.Dara L., Hewett J., Lim J.K. Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World J. Gastroenterol.: WJG. 2008;14(45):6999. doi: 10.3748/wjg.14.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Federico A., Tiso A., Loguercio C. A case of hepatotoxicity caused by green tea. Free Radic. Biol. Med. 2007;43(3):474. doi: 10.1016/j.freeradbiomed.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 135.Fernandez J. Three cases of liver toxicity with a dietary supplement intended to stop hair loss. Rev. Esp. Enferm. Dig. 2014;106(8):552–555. Avalable in [Eng.] at: http://www.grupoaran.com/mrmUpdate/lecturaPDFfromXML.asp?IdArt=4621030&TO=R1VN&Eng=1. Accessed on Octber 8, 2019. [PubMed] [Google Scholar]
- 136.Fong T.-L. Hepatotoxicity due to hydroxycut®: a case series. Am. J. Gastroenterol. 2010;105(7) doi: 10.1038/ajg.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gallo E. Is green tea a potential trigger for autoimmune hepatitis? Phytomedicine. 2013;20(13):1186–1189. doi: 10.1016/j.phymed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 138.Gloro R. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur. J. Gastroenterol. Hepatol. 2005;17(10):1135–1137. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- 139.Haimowitz S. Liver failure after Hydroxycut™ use in a patient with undiagnosed hereditary coproporphyria. J. Gen. Intern. Med. 2015;30(6):856–859. doi: 10.1007/s11606-014-3153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiménez-Encarnación E. Euforia-induced acute hepatitis in a patient with scleroderma. BMJ Case Rep. 2012 doi: 10.1136/bcr-2012-006907. 2012:bcr2012006907. https://doi.org/10.1136/bcr-2012-006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jimenez-Saenz M., del Carmen Martinez-Sanchez M. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006;44(3):616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 142.Jones F.J., Andrews A.H. Acute liver injury associated with the herbal supplement hydroxycut in a soldier deployed to Iraq. Am. J. Gastroenterol. 2007;102(10):2357. doi: 10.1111/j.1572-0241.2007.01353_10.x. [DOI] [PubMed] [Google Scholar]
- 143.Kanda T. Severe hepatotoxicity associated with chinese diet product ‘Onshidou-Genbi-Kounou’. J. Gastroenterol. Hepatol. 2003;18(3):354–355. doi: 10.1046/j.1440-1746.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- 144.Kaswala D. Hydroxycut-induced liver toxicity. Ann. Med. Health Sci. Res. 2014;4(1):143–145. doi: 10.4103/2141-9248.126627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McDonnell W.M., Bhattacharya R., Halldorson J.B. Fulminant hepatic failure after use of the herbal weight-loss supplement exilis. Ann. Intern. Med. 2009;151(9):673–674. doi: 10.7326/0003-4819-151-9-200911030-00021. [DOI] [PubMed] [Google Scholar]
- 146.Molinari M. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl. 2006;12(12):1892–1895. doi: 10.1002/lt.21021. https://doi.org/ 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- 147.Pillukat M.H. Concentrated green tea extract induces severe acute hepatitis in a 63-year-old woman--a case report with pharmaceutical analysis. J. Ethnopharmacol. 2014;155(1):165–170. doi: 10.1016/j.jep.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 148.Porcel J. Hepatotoxicity associated with green tea extracts. Ann. Intern. Med. 2005 [Google Scholar]
- 149.Radha Krishna Y. Acute liver failure caused by ‘fat burners’ and dietary supplements: a case report and literature review. Can. J. Gastroenterol. Hepatol. 2011;25(3):157–160. doi: 10.1155/2011/174978. https://doi.org/10.0.4.131/2011/174978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma T. Hydroxycut®(herbal weight loss supplement) induced hepatotoxicity: a case report and review of literature. Hawaii Med. J. 2010;69(8):188. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3118021/pdf/hmj6908_0188.pdf [PMC free article] [PubMed] [Google Scholar]
- 151.Stevens T., Qadri A., Zein N.N. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut [7] Ann. Intern. Med. 2005;142(6):477–478. doi: 10.7326/0003-4819-142-6-200503150-00026. [DOI] [PubMed] [Google Scholar]
- 152.Stickel F. Liver injury from herbal and dietary supplements. Dtsch. Med. Wochenschr. 2015;140(12):908–911. doi: 10.1055/s-0041-102437. [DOI] [PubMed] [Google Scholar]
- 153.Vanstraelen S., Rahier J., Geubel A.P. Jaundice as a misadventure of a green tea (camellia sinensis) lover: a case report. Acta Gastroenterol. Belg. 2008;71(4):409–412. https://dial.uclouvain.be/pr/boreal/object/boreal:22101 [PubMed] [Google Scholar]
- 154.Verhelst X. Acute hepatitis after treatment for hair loss with oral green tea extracts (Camellia Sinensis) Acta Gastroenterol. Belg. 2009;72(2):262–264. http://hdl.handle.net/1854/LU-2106824 [PubMed] [Google Scholar]
- 155.Weinstein D.H. SlimQuick™-associated hepatotoxicity in a woman with alpha-1 antitrypsin heterozygosity. World J. Hepatol. 2012;4(4):154. doi: 10.4254/wjh.v4.i4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Whitsett M., Halegoua-De Marzio D., Rossi S. SlimQuick™-associated hepatotoxicity resulting in fulminant liver failure and orthotopic liver transplantation. ACG Case Rep. J. 2014;1(4):220. doi: 10.14309/crj.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.2003. Agence Francese de Securite Sancaire des Product Sante Communiques oe Presse: Suspension de l’autorisation de mlse sur le marche de la specialite pharmaceutique Excolise® (gallate d’epigallocatechol)https://ansm.sante.fr/S-informer/Communiques-Communiques-Points-presse/Suspension-de-l-autorisation-de-mise-sur-le-marche-de-la-specialite-pharmaceutique-EXOLISE-R-gallate-d-epigallocatechol Available at: [Google Scholar]
- 158.de Paula J.M.P., Barquero J.G., Hidalgo S.F. Hepatitis tóxica por Camellia sinensis. Gastroenterol. Hepatol. 2008;31(6):402. doi: 10.1157/13123613. [DOI] [PubMed] [Google Scholar]
- 159.García-Cortés M. Liver injury induced by" natural remedies": an analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev. Esp. Enferm. Dig. 2008;100(11):688. doi: 10.4321/s1130-01082008001100004. [DOI] [PubMed] [Google Scholar]
- 160.García-Morán S. Hepatitis aguda asociada a ingestión de camellia thea y orthosiphon stamineus. Gastroenterol. Hepatol. 2004;27(9):559–560. doi: 10.1016/s0210-5705(03)70527-5. [DOI] [PubMed] [Google Scholar]
- 161.Gavilan J. Phytotherapy and hepatitis. Rev. Clin. Esp. 1999;199(10):693–694. [PubMed] [Google Scholar]
- 162.Lorenzo-Almorós A. Hepatitis aguda por extracto de té verde. Gastroenterología y Hepatología. 2015;38(1):44–45. doi: 10.1016/j.gastrohep.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Martínez-Sierra C., Unceta P.R., Herrera L.M. Hepatitis aguda tras ingestión de té verde. Medicina Clínica. 2006;127(3):119. doi: 10.1157/13090268. [DOI] [PubMed] [Google Scholar]
- 164.Sadornil C.D., Puigtió S.F., Durández R. Hepatotoxicidad por Camelia sinensis. Med. Clin. 2004;122(17):677–678. doi: 10.1157/13061393. [DOI] [PubMed] [Google Scholar]
- 165.Thiolet C. Cytolyse aiguë après prise d’un thé chinois. Gastroentérologie clinique et biologique. 2002;26(10):939–940. https://doi.org/GCB-10-2002-26-10-0399-8320-18101019-ART30. [PubMed] [Google Scholar]
- 166.Rohde J., Jacobsen C., Kromann-Andersen H. Toksisk hepatitis udløst af grøn te. Ugeskr Læger. 2011;173:3. [PubMed] [Google Scholar]
- 167.Mathieu N. Hépatotoxicité probable de l’X-elles® utilisé en phytothérapie. Gastroentã©rologie Clin. Biol. 2005;29(11):1188–1189. doi: 10.1016/s0399-8320(05)82193-1. [DOI] [PubMed] [Google Scholar]
- 168.Pedrós C. Hepatotoxicidad por extracto etanólico seco de Camellia sinensis. Med Clin (Barc) 2003;121(15):598–599. doi: 10.1016/s0025-7753(03)74026-3. [DOI] [PubMed] [Google Scholar]
- 169.Peyrin-Biroulet L. Hépatotoxicité probable du gallate d’épigallocatéchol utilisé en phytothérapie. Gastroentérologie clinique et biologique. 2004;28(4):404–406. doi: 10.1016/s0399-8320(04)94944-5. https://doi.org/GCB-04-2004-28-4-0399-8320-101019-ART11. [DOI] [PubMed] [Google Scholar]
- 170.Benichou C., Danan G., Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J. Clin. Epidemiol. 1993;46(11):1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 171.Oria M., Greenwood M.R.C., editors. Use of Dietary Supplements by Military Personnel. National Academies Press; Washington DC: 2008. Institute of medicine (IOM) [PubMed] [Google Scholar]
- 172.Qato D.M. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Knapik J.J. Dietary supplement use in a large, representative sample of the US armed forces. J. Acad. Nutr. Diet. 2018;118(8):1370–1388. doi: 10.1016/j.jand.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 174.Bailey R.L. Why US adults use dietary supplements. JAMA Intern. Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 175.Crescioli G. Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Intern. Emerg. Med. 2018;13(6):857–872. doi: 10.1007/s11739-018-1880-4. [DOI] [PubMed] [Google Scholar]
- 176.Brown A.C. Liver toxicity related to herbs and dietary supplements: online table of case reports. Part 2 of 5 series. Food Chem. Toxicol. 2017;107(Pt A):472–501. doi: 10.1016/j.fct.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 177.Cohen P.A. Emergency department visits and hospitalisations for adverse events related to dietary supplements are common. Evid. Med. 2016;21(2):79. doi: 10.1136/ebmed-2015-110362. [DOI] [PubMed] [Google Scholar]
- 178.Geller A.I. Emergency department visits for adverse events related to dietary supplements. N. Engl. J. Med. 2015;373(16):1531–1540. doi: 10.1056/NEJMsa1504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.MacKay D. Emergency department visits related to dietary supplements. N. Engl. J. Med. 2016;374(7):694–695. doi: 10.1056/NEJMc1514454. [DOI] [PubMed] [Google Scholar]
- 180.Pascale B. Dietary supplements: knowledge and adverse event reporting among American Medical Society for Sports Medicine physicians. Clin. J. Sport. Med. 2016;26(2):139–144. doi: 10.1097/JSM.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 181.Cirulli E.T. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156(6):1707–1716. doi: 10.1053/j.gastro.2019.01.034. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nicoletti P. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152(5):1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Urban T.J. Minocycline hepatotoxicity: clinical characterization and identification of HLA-B *35:02 as a risk factor. J. Hepatol. 2017;67(1):137–144. doi: 10.1016/j.jhep.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Church R.J. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015;76:19–26. doi: 10.1016/j.fct.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uetrecht J. Using PD-1 knockout mice to test the potential of Green tea extract and (-)-epigallocatechin gallate (EGCG) to cause idiosyncratic drug-induced liver injury (IDILI). Society of Toxicology, 57th Annual Meeting and ToxExpo™; San Antonio, Texas: Oxford University Press; 2018. Abstract no. 1099. Available at. [Google Scholar]
- 186.Liu D. Epigallocatechin-3-gallate (EGCG) attenuates concanavalin A-induced hepatic injury in mice. Acta Histochem. 2014;116(4):654–662. doi: 10.1016/j.acthis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 187.Kaneko Y. Augmentation of Vα14 NKT cell–mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A–induced hepatitis. J. Exp. Med. 2000;191(1):105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miyazawa Y. Involvement of intrasinusoidal hemostasis in the development of concanavalin A-induced hepatic injury in mice. Hepatology. 1998;27(2):497–506. doi: 10.1002/hep.510270225. [DOI] [PubMed] [Google Scholar]
- 189.Fabregat I. TGF-beta signalling and liver disease. FEBS J. 2016;283(12):2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 190.Webster A.D. Quality of life among postmenopausal women enrolled in the Minnesota Green Tea Trial. Maturitas. 2018;108:1–6. doi: 10.1016/j.maturitas.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007;65(suppl_1):S57–S63. doi: 10.1111/j.1753-4887.2007.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 193.Mazzanti G., Di Sotto A., Vitalone A. Hepatotoxicity of green tea: an update. Arch. Toxicol. 2015;89(8):1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 194.Lambert J.D. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010;48(1):409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Health Canada . Dear Healthcare Professional Letter. 2017. Green tea extract-containing natural health products - rare risk of serious liver injury.http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2017/65100a-eng.php Available at. [Google Scholar]
- 196.Norwegian Institute of Public Health, Safety assessment on levels of (-)-Epigallocatechin-3-gallate (EGCG) in green tea extracts used in food supplements. Nowrwegian Institute of Public Health, Oslo. Available at https://www.mattilsynet.no/mat_og_vann/spesialmat_og_kosttilskudd/kosttilskudd/norwegian_institute_of_public_health_safety_assessment_on_levels_of_egcg_in_green_tea_extracts_used_in_food_supplements.22068/binary/Norwegian%20Institute%20of%20Public%20Health:%20Safety%20assessment%20on%20levels%20of%20EGCG%20in%20green%20tea%20extracts%20used%20in%20food%20supplements. Accessed on October 8, 2019. 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.