To the Editor:
Chronic obstructive pulmonary disease (COPD) is characterized by respiratory and systemic inflammation triggered by exposure to inhaled toxicants. Over recent decades, diet has emerged as a potential factor contributing to or protecting against diseases driven by inflammation. Specifically, the essential polyunsaturated fatty acids (PUFAs) omega-3 and omega-6 have demonstrated the ability to modulate the inflammatory response and potentially influence respiratory health (1). Omega-3 PUFAs (α-linoleic acid [ALA], eicosapentaenoic acid [EPA], and docohexaenoic acid [DHA]) are the precursors of molecules known to aid in inflammation resolution (pro-resolving mediators [2]), whereas omega-6 PUFAs (linoleic acid and arachidonic acid) have notable proinflammatory properties. These fatty acids share the same metabolic enzymes, and their relative quantities in the diet may shift the inflammatory equilibrium (3). To date, epidemiologic evidence investigating a role for PUFAs and COPD morbidity is limited. We sought to investigate the relationship between omega-3 and omega-6 fatty acid intake, deliberately accounting for the opposing fatty acid, and respiratory morbidity among former smokers with moderate to severe COPD.
Methods
Study population and design
This cross-sectional analysis included data from 112 participants with COPD in Maryland who were enrolled between July 2014 and October 2018. Inclusion criteria were age 40 years or older, post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity less than 70% and FEV1 percent predicted less than 80%, former smoker (self-report of no smoking in prior 6 months and exhaled carbon monoxide ≤6 ppm [4] with at least a 10–pack-year history tobacco use). Participants were excluded for chronic oral corticosteroid use, other chronic lung diseases except those with a history of asthma, or lack of nutritional data. Nine participants did not complete the Food Frequency Questionnaire (FFQ) in the baseline examination period but were engaged to complete the FFQ over the ensuing 9 months; because exclusion did not substantially alter effect estimates, these participants are included in final analyses.
Dietary assessment
The 2007 grid form of the validated, semiquantitative Willett FFQ (5) was administered and analyzed using a comprehensive food composition database (regepi.bwh.harvard.edu/health/nutrition/) to derive average daily intake of all nutrients via food or supplement, with recall of 1 year. PUFA intake was categorized as follows: 1) total omega-3 (EPA + DHA + ALA), 2) total omega-6 (linoleic acid and arachidonic acid), and 3) omega-6/omega-3 ratio.
Respiratory health outcomes
Administered questionnaires included the modified Medical Research Council scale (functional impairment due to dyspnea), the COPD Assessment Test (CAT; impact of the disease), and the St. George’s Respiratory Questionnaire (SGRQ) and Clinical COPD Questionnaire (CCQ) (used to determine respiratory system–specific health-related quality of life). Spirometry was performed and interpreted per American Thoracic Society standards (6, 7). Participants completed 6-minute-walk distance testing per American Thoracic Society standards (8).
Analyses
Logistic and linear regression models were applied to determine the relationship between omega-3, omega-6, and omega-6/omega-3 ratio fatty acid intake and respiratory outcomes. Model 1 was adjusted only for the opposing fatty acid and total caloric intake. Model 2 was adjusted for age, sex, race, education, body mass index, total caloric intake, pack-years of smoking, the opposing fatty acid, and post-bronchodilator FEV1 percent predicted (except when FEV1 percent predicted was the exposure). A standard diagnostic procedure was run to check regression assumptions, including normality, linearity, and overall model fit. Analyses were completed using STATA version 15 software (StataCorp).
Results
Cohort characteristics are noted in Table 1. In partial and fully adjusted models, total omega-3 and total omega-6 fatty acid intake were associated with several respiratory outcomes (Table 2, Figure 1). Specifically, in fully adjusted models, every 1-g increase in total omega-3 was associated with lower CAT, SGRQ, and CCQ scores, directionally indicating beneficial associations with higher omega-3 intake. In addition, higher total omega-3 intake was related to fewer severe exacerbations in the prior 3 months and a trend toward higher lung function. Conversely, every 1-g increase in total omega-6 was associated with lower FEV1 percent predicted; higher modified Medical Research Council scale, CAT, SGRQ, and CCQ scores; and higher odds of any reported exacerbation, and specifically severe exacerbation, in the prior 3 months (Table 2, Figure 1).
Table 1.
Baseline characteristics
| Demographics | |
| Age, yr | 66.4 ± 8.3 |
| Male sex, % | 56.3 |
| Race, % | |
| White | 66.1 |
| Black | 29.5 |
| Others | 4.5 |
| Income, % | |
| <$10,000–$19,999 | 35 |
| $20,000–$39,999 | 27 |
| $40,000–$69,999 | 15 |
| >$70,000 | 12 |
| Don’t know/refused | 11 |
| Education, above high school, % | 60.7 |
| Smoking history, pack-years | 51.7 ± 33.6 |
| BMI, kg/m2 | 31.3 ± 8.0 |
| Dietary intake | |
| Energy, kcal | 2,039 ± 801 |
| Omega-3, g, median (IQR) | 1.89 (1.29) |
| ALA | 1.60 (1.00) |
| EPA + DHA | 0.20 (0.22) |
| Omega-6, g, median (IQR) | 13.58 (8.06) |
| Omega-6/omega-3 ratio | 7.5 ± 1.7 |
| Respiratory health outcomes | |
| FEV1, % predicted | 54.0 ± 16.9 |
| mMRC score ≥2, % | 49.6 |
| CAT score | 17.2 ± 7.8 |
| SGRQ total | 44.8 ± 17.4 |
| CCQ | 2 ± 1 |
| 6MWD, ft | 773 ± 388 |
| Any exacerbation, %* | 23.2 |
| Severe exacerbation, %* | 9.8 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ALA = α-linolenic acid; BMI = body mass index; CAT = COPD Assessment Test; CCQ = Clinical COPD Questionnaire; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; FEV1 = forced expiratory volume in 1 second; IQR = interquartile range; mMRC = modified Medical Research Council dyspnea scale; SGRQ = St. George’s Respiratory Questionnaire.
Data are presented as mean ± SD unless otherwise noted.
In the 3 months before baseline assessment.
Table 2.
Relationship between total omega-3, omega-6, and respiratory outcomes
| Total Omega-3 |
Total Omega-6 |
Omega-6/Omega-3 Ratio |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||
| Coefficient | P Value | Coefficient | P Value | Coefficient | P Value | Coefficient | P Value | Coefficient | P Value | Coefficient | P Value | |
| FEV1, % predicted* | 5.99 (−0.73 to 12.72) | 0.08 | 6.90 (−0.09 to 13.89) | 0.05 | −1.39 (−2.45 to −0.34) | <0.01 | −1.50 (−2.57 to −0.43) | 0.01 | −3.09 (−6.25 to 0.08) | 0.06 | −3.51 (−6.73 to −0.29) | 0.04 |
| CAT score† | −4.83 (−7.85 to −1.81) | <0.01 | −4.54 (−7.76 to −1.31) | 0.01 | 0.93 (0.46 to 1.40) | <0.01 | 0.84 (0.34 to 1.34) | <0.01 | 2.14 (0.70 to 3.57) | <0.01 | 1.95 (0.45 to 3.45) | 0.01 |
| SGRQ total‡ | −11.57 (−18.23 to −4.91) | <0.01 | −11.11 (−18.19 to −4.03) | <0.01 | 2.16 (1.11 to 3.20) | <0.01 | 1.95 (0.85 to 3.05) | <0.01 | 5.28 (2.13 to 8.44) | <0.01 | 4.93 (1.66 to 8.20) | <0.01 |
| CCQ‡ | −0.60 (−1.00 to −0.20) | <0.01 | −0.58 (−1.00 to −0.15) | 0.01 | 0.09 (0.03 to 0.16) | <0.01 | 0.09 (0.02 to 0.15) | 0.01 | 0.24 (0.05 to 0.43) | 0.01 | 0.23 (0.04 to 0.43) | 0.02 |
| 6MWD§ | 194.10 (−15.51 to 403.70) | 0.07 | 178.74 (−43.82 to 401.30) | 0.11 | −32.06 (−63.39 to −0.72) | 0.05 | −30.96 (−64.84 to 2.92) | 0.07 | −78.65 (−172.73 to 15.44) | 0.07 | −79.41 (−176.28 to 17.46) | 0.11 |
| mMRC, OR‡ | 0.48 (0.19 to 1.22) | 0.12 | 0.42 (0.15 to 1.22) | 0.11 | 1.19 (1.02 to 1.39) | 0.02 | 1.23 (1.04 to 1.47) | 0.02 | 1.46 (0.97 to 2.19) | 0.07 | 1.52 (0.96 to 2.42) | 0.07 |
| Severe exacerbation, OR¶ | 0.06 (0.01 to 0.54) | 0.01 | 0.08 (0.01 to 0.73) | 0.03 | 1.58 (1.12 to 2.21) | <0.01 | 1.48 (1.05 to 2.09) | 0.02 | 2.53 (1.29 to 4.99) | <0.01 | 2.25 (1.09 to 4.64) | 0.03 |
| Any exacerbation, OR¶ | 0.33 (0.09 to 1.27) | 0.11 | 0.31 (0.07 to 1.38) | 0.13 | 1.30 (1.06 to 1.61) | 0.01 | 1.30 (1.03 to 1.64) | 0.02 | 1.47 (0.93 to 2.32) | 0.10 | 1.41 (0.84 to 2.38) | 0.19 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CAT = COPD Assessment Test; CCQ = Clinical COPD Questionnaire; FEV1 = forced expiratory volume in 1 second; mMRC = modified Medical Research Council dyspnea scale; OR = odds ratio; SGRQ = St. George’s Respiratory Questionnaire.
Coefficient represents predicted change in outcome, or odds ratio (OR) where noted, with 95% confidence interval per 1-g increase in total omega-3 or omega-6 intake. Model 1 is adjusted for alternate fatty acid. Model 2 includes the following covariates: age, sex, race (white vs. others), education (high school or less vs. more than high school), body mass index, total calories, pack-years of smoking, FEV1 percent predicted (except when FEV1 percent predicted is an outcome), and alternate fatty acid. Coefficients reaching statistical significance (P < 0.05) are shown in boldface.
Model 1, n = 111; model 2, n = 111.
Model 1, n = 107; model 2, n = 106.
Model 1, n = 111; model 2, n = 110.
n = 76.
Model 1, n = 112; model 2, n = 111.
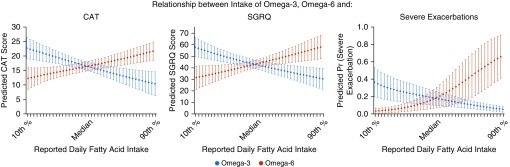
Figure 1.
Representative respiratory outcomes demonstrating the opposing relationships between omega-3 and omega-6 intake and predicted COPD Assessment Test CAT score (impact of disease), predicted St. George’s Respiratory Questionnaire (SGRQ) score (health-related quality of life), and predicted probability of reported severe exacerbation in the prior 3 months. Point estimates and 95% confidence intervals depict average predicted level of outcome at the observed level of omega fatty acid between the 10th and 90th percentiles of the population range, adjusting for other covariates, constructed using a fractional polynomial model to more flexibly identify omega fatty acid–outcome functional shape, allowing potentially nonlinear relationships. Covariates include age, sex, race (white vs. others), education (high school or less versus more than high school), body mass index, total calories, pack-years of smoking, forced expiratory volume in 1 second percent predicted, and alternate fatty acid held at their observed values. CAT = COPD Assessment Test; Pr = probability.
The omega-6/omega-3 ratio was likewise associated with several respiratory outcomes (Table 2). A higher omega-6/omega-3 ratio was linked with higher adverse COPD impact defined by higher CAT scores and worse quality of life determined by higher total SGRQ and CCQ scores, as well as lower FEV1 percent predicted and higher odds of severe exacerbation.
Discussion
In a population of former smokers with moderate to severe COPD, accounting for the opposing fatty acid, higher total omega-3 intake was associated with less COPD morbidity (lower risk of severe exacerbations, better health-related quality of life, and fewer respiratory symptoms), whereas higher omega-6 intake was associated with higher COPD morbidity (lower lung function; worse dyspnea, quality of life, and respiratory health status; and higher odds of exacerbations). Associations found when examining the omega-6/omega-3 ratio were consistent with individual effects. This is one of the few investigations demonstrating a potential beneficial association of omega-3 intake and COPD outcomes and, to our knowledge, the first study demonstrating a potential adverse association between omega-6 and omega-6/omega-3 ratio with respiratory symptoms and functional status in a cohort with COPD.
PUFAs have emerged as a dietary component capable of arbitrating inflammation, and as such, they may be critical in diseases driven by inflammation, including COPD. Omega-3 PUFAs are the source of pro-resolving mediators, including resolvins, protectins, and maresins (2). Pro-resolving mediators, derived from EPA and DHA, actively resolve inflammation through enhancement of macrophage-clearing function, mitigation of inflammatory cell recruitment, modulation of proinflammatory cytokines, and promotion of cell differentiation (9). ALA, also a precursor of EPA and DHA, may itself have primary effects on inflammation and has been shown to downregulate nitric oxide and tumor necrosis factor-α in murine macrophages (10). Omega-6 PUFAs are the sources of several compounds, including lipoxins (generally antiinflammatory [3]), but also eicosanoids such as cysteinyl leukotrienes, thromboxanes, and prostaglandins (proinflammatory [11]). Although omega-6 PUFAs are a more complex milieu of compounds, it is reasonable to suspect that the proinflammatory effects of omega-6 could predominate in susceptible populations.
Investigations examining associations between omega-3 and omega-6 intake and COPD morbidity are limited but generally consistent with the findings in the present study. Some studies suggest that dietary omega-3 is associated with lower risk of incident COPD (12, 13) and lower levels of systemic inflammatory markers (14). We have previously demonstrated a protective association between omega-3 intake and respiratory symptoms among susceptible subgroups with COPD, including smokers and those with low education (15). Investigations into the relationship between omega-6 and COPD health are further limited, though higher intake of several omega-6 PUFAs has been associated with COPD prevalence (16) and pulmonary inflammation (17). Our study adds to a growing body of evidence linking omega-3 intake with lower COPD morbidity and provides new, consistent associations regarding the potentially harmful association between omega-6 intake and COPD-related respiratory outcomes.
Though omega-3 and omega-6 exposure were obtained by FFQ, which can be affected by recall bias and/or misreporting, the Willett FFQ has been widely validated as a reliable tool (18–20). Relationships may be influenced by distribution of diet in the study population; over half of participants demonstrated an omega-6/omega-3 ratio below 10 (a ratio of ∼10:1 has been defined in a U.S. population, representing Western diet [21]), perhaps suggesting healthier lifestyle choices. These same choices may confound associations at the individual level as well, where omega-3 or omega-6 may be a marker of a healthier or less healthy overall diet, respectively.
Our study has notable strengths. We demonstrate consistent results across multiple outcomes spanning physiologic markers of disease morbidity, qualitative outcomes, and healthcare use and for the first time report an observation of an adverse association of omega-6 and omega-6/omega-3 ratio with respiratory symptoms and functional status in COPD. Our results suggest that nutrition may represent a modifiable risk factor for respiratory morbidity among patients with COPD.
Footnotes
Supported by grants from the National Institute on Minority Health and Health Disparities (NIMHD) P50MD010431/the U.S. Environmental Protection Agency (EPA) 83615001 (N.H.), the National Institute of Environmental Health Sciences (NIEHS) R01ES022607 (N.H.), NIEHS K23ES029105 (E.B.), NIEHS F32ES029789 (A.K.), and the National Heart, Lung, and Blood Institute (NHLBI) T32HL007534 (A.K.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Berthon BS, Wood LG. Nutrition and respiratory health—feature review. Nutrients. 2015;7:1618–1643. doi: 10.3390/nu7031618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [Discussion, pp. 1127–1136.] [DOI] [PubMed] [Google Scholar]
- 6.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 8.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren J, Chung SH. Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55:5073–5080. doi: 10.1021/jf0702693. [DOI] [PubMed] [Google Scholar]
- 11.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahar E, Folsom AR, Melnick SL, Tockman MS, Comstock GW, Gennaro V, et al. Atherosclerosis Risk in Communities Study Investigators. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. N Engl J Med. 1994;331:228–233. doi: 10.1056/NEJM199407283310403. [DOI] [PubMed] [Google Scholar]
- 13.Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH Atherosclerosis Risk in Communities Study Investigators. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1780–1785. doi: 10.1164/ajrccm.159.6.9810068. [DOI] [PubMed] [Google Scholar]
- 14.de Batlle J, Sauleda J, Balcells E, Gómez FP, Méndez M, Rodriguez E, et al. PAC-COPD Study Group. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem. 2012;23:817–821. doi: 10.1016/j.jnutbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Lemoine Soto CM, Brigham E, Woo H, Hanson C, McCormack MC, Koch A, et al. Omega-3 fatty acid intake and prevalent respiratory symptoms among US adults with COPD. BMC Pulm Med. 2019;19:97. doi: 10.1186/s12890-019-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeever TM, Lewis SA, Cassano PA, Ocké M, Burney P, Britton J, et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63:208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutting S, Papanicolaou M, Xenaki D, Wood LG, Mullin AM, Hansbro PM, et al. Dietary ω-6 polyunsaturated fatty acid arachidonic acid increases inflammation, but inhibits ECM protein expression in COPD. Respir Res. 2018;19:211. doi: 10.1186/s12931-018-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longnecker MP, Lissner L, Holden JM, Flack VF, Taylor PR, Stampfer MJ, et al. The reproducibility and validity of a self-administered semiquantitative food frequency questionnaire in subjects from South Dakota and Wyoming. Epidemiology. 1993;4:356–365. doi: 10.1097/00001648-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev. 1998;7:283–290. [PubMed] [Google Scholar]
- 21.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]