Graphical abstract
Keywords: Antioxidants, DPPH, FRAP, LCMS, Melia azedarach, TPC, T47D cytotoxic
Highlights
-
•
1st report on physical qualities and phytochemical content of M. azedarach wild type leaves extract and fractions.
-
•
Ethyl acetate fraction was the most active against bio-selective hormonal breast cancer T47D cell cytotoxic and antioxidant activities.
-
•
The phytochemicals content of active fraction was steroids and triterpene saponin, limonoid (toosendanin, meliarachin, salannin, salannal, 12-hydroxyamoorastatin, meliacarpinin and its derivates), and flavonoids (quercetin glycoside).
-
•
Significant (p < 0.05) correlations were observed between TPC, IC50DPPH, FRAP and IC50T47D.
Abstract
Melia azedarach L. is used widely in traditional medicine for local or systemic ailments. Although studies exist on phytochemicals and potencies of Chinese and Indian cultivars of Melia, the present study investigated in vitro antioxidant properties of Melia wild type and its cytotoxicity against T47D cell. The ethanolic extract of the Melia leaves was fractionated with n-hexane, ethyl acetate and water, and the secondary metabolites were obtained. The antioxidant properties were determined with IC50 DPPH (2,2-diphenyl-1-pycrylhydrazyl) radical and FRAP (ferric reducing antioxidant power), while the cytotoxicity was determined with the MTT method. The total phenolic (TPC) and β-sitosterol (SC) contents were also measured. The results showed that the ethyl acetate fraction had higher antioxidant and cytotoxic activities (IC50 211.89 ± 10.86 and 147.90 ± 8.49 μg/mL, respectively) than others. Significant (p < 0.05) correlations were observed between TPC, IC50DPPH, FRAP and IC50T47D. LC-EI MS analysis of the ethyl acetate fraction revealed the steroid and triterpenoid saponins, limonoids and quercetin glycosides, which influenced the medicinal properties of the Melia leaves. Melia azedarach L. wild type leaf extracts are a promising natural resource for managing breast cancer.
1. Introduction
Cancer is a global burden due to its mortality and morbidity. Its incidence and prevalence are rapidly growing worldwide. It has become a major cause of death on productive ages in all countries. Among cancer types, breast cancer is the most diagnosed and the major cause of death in females (11.6 %). The uncontrolled growth of cancer cells, resulting from genetics, infections and life styles, are complex [1]. Mutagenesis by DNA and free radical damages are related to the cancer cell initiation phase. Chemotherapies are the most widely used management technique, but they naturally kill normal and neoplastic cells. Although reactive oxygen species (ROS) play a role in cancer cell death mechanisms, they generally affect human bodies adversely. Some of the adverse ROS chemotherapies include alkyl sulfonates, ethyleneamines and hydrazines (alkylating agents), doxorubicin (anthracyclines), cisplatin and carboplatin (platinum coordination complexes), etoposides (podophyllin derivatives), and irinotecan and topotecan (camptothecins) [2]. However, intakes of antioxidant supplements, as primary and secondary steps to cancer preventions, eliminate the ROS side effects, improve tumor responses and increase patient survival rates [3].
With advances in pharmaceutical science, chemotherapy synthetic drugs are also being developed and improved to minimise these ROS side effects. Nevertheless, the array of plants available and their different phytochemicals with anticancer activities have opened research into medicinal plants to complement chemotherapy synthetic drugs for managing all types of cancer. For example, taxol from Taxus brevifolia (Taxaceae), vinblastine and vincristine from Catharanthus roseus L. G. Don (Apocynaceae), and etoposide and teniposide from Podophyllum sp. Linn (Berberidaceae), camptothecin, paclitaxel, homoharringtonine and many natural-derived compounds have been studied [4]. Their contents of terpenes, phenolics and alkaloids are examples of secondary metabolites with combined cytotoxic and antioxidant activities.
There are many other plants, however, that have not been studied to a comparable extent, and their medicinal potential is yet to be fully understood. One of these plants is a mahogany plant Chinaberry (Melia azedarach L.) that originates from Asia but now have a global spread. Three types (wild, Chinese and Indian) of M. azedarach are known based on the average size of the plants [5]. Almost all the parts of the Chinese and Indian types are used in traditional medicine, for example, their bark, seed, root and leaves are reported [[6], [7], [8]] to have antiparasitic, antifungal, diuretic, emmenagogue, antibacterial, antimalarial, hepatoprotection, antioxidant, antifertility and antipyretic properties or activities. Even though the wild type is mostly utilized for wood and ecological purposes, relatively, the cytotoxicity of the plant and its medicinal properties are yet to be fully established and understood, more so the efficacies of its extracts in different solvents on different cancer cells.
Zahoor et al. found the influence of different solvents (chloroform, butanol, hexane water and ethyl acetate) on the antibacterial, antioxidant and brine shrimp cytotoxicity of M. azedarach bark. It was found that different solvents has difference antibacterial activity, although it can be observed that ethyl acetate extract had better antioxidant and cytotoxic compare to others [8].
Furthermore, a research revealed the influence of different solvents (ethanol, petroleum ether and water) on the phytochemical content, total phenolic content (TPC) and DPPH antioxidant activity (AA) of M. azedarach leaves [9]. The ethanolic extract had the highest amount of phenolic compounds and exhibited the strongest antioxidant activity compared to petroleum ether and aqueous extracts. The toxicity of an ethanolic extract of the plant’s leaves against vero cells had an IC50 >1000 μg/mL [10]. Another study, against HT-29, A-549, MCF-7, HepG-2 and MDBK cell lines, observed that the methanolic extract of the plant’s leaves safer in term of cytotoxic activity compared to the extracts of its pulps and seeds [11]. Cytotoxic activities of the bark and root bark of M. azedarach against some cells have been reported and related to their steroid and limonoid tirucallane contents [[12], [13], [14], [15], [16]].
Based on the chemotaxonomy point of view, however, we are not aware of any in-depth study on the selective hormones of cytotoxic cells and antioxidant activities of M. azedarach leaves’ extracts and fractions, specifically on breast cancer cells. The T47D and MCF7 are hormone dependent cell lines, which are mostly used in vitro breast cancer cell line research. The T47D reveals more in screening breast cancer phytochemical targeting compounds though. It’s an ideal object for experimental progesterone-specific effects of breast cancer, as it is susceptible to progesterone in the presence of estrogen, while the MCF7 is not [17]. Using the wild type of M. azedarach, and to understand the potential activity of the plant on selective progesterone–estrogen receptor targeting breast cancer, the objectives of the study were, therefore, to investigate the cytotoxic activity against T47D cell line, antioxidant activities using DPPH and FRAP of the extracts and fractions; and examine correlations among the cytotoxic and antioxidant parameters.
2. Materials and methods
2.1. Plant material
M. azedarach dried leaves were obtained on dried season from Materia Medica Batu, which is an Indonesian government office on medicinal plants. Upon identification (No. 074/346/102.7/2017), the dried leaves were stored (herbarium No. Ma011017) prior to analysis [18] for drying shrinkage and ash, moisture and ethanol-soluble contents, and phytochemical screening [19] using standard procedures.
2.2. Preparation of the extract
The dried leaves were ground and extracted with 96 % ethanol (1:10) three times each for 24 h at room temperature. The solvent was removed in a rotary vacuum evaporator to result of the ethanolic extract (E). It was dispersed in water (1:10) and fractionated in a separating funnel with n-hexane (1:1) and ethyl acetate (1:1) to obtain n-hexane (FH), ethyl acetate (FE) and water (FW) fractions. Three replicates of the extract and fractions were done before their phytochemistry screening and analyses for cytotoxic and antioxidant activities.
2.3. MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide) Cytotoxic test of T47D cell
The T47D cell lines (epithelial cells of human ductile pleural effusion from a 54-year-old mammary gland tissue) were kindly provided by Parasitology Laboratories, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia. The cytotoxicity test, based on CCRC method [20], was conducted with the University’s ethics approval No. KE/FK/0310/EC/2018. The T47D cell was grown on RPMI with 10 % (v/v) Fetal Bovine Serum (FBS) and 1% (v/v) penicillin-streptomycin into 96-well plates. The optimized cell growth (70–80 % confluent) was treated with 100 μl of different concentrations of each of the extract and fractions (1000 to 10 μg/mL) and doxorubicin (100 to 0.1 μg/mL) before incubation for 24 h at 37 °C. MTT (10 %) was added to differentiate viable cells metabolism when purple formazan crystals were observed after 4 h in the dark before stopping the reactions and dissolving the crystals in 10 % dodecyl sulphate in a sulphuric acid solution. The cells were shaken (MRK-Retac) for 10 min and their absorbances were read (Elisa reader, Bio-Rad microplate reader Benchmark serial no.11565, Japan) at a wavelength of 595 nm. The absorbances were converted to percentage of viable cells as the following formula and graphed to obtain the IC50 (the sample concentration that inhibited 50 % of the cell growth) with linear regression analysis. Blank control (media/ Ab) experiment was conducted. A0 is absorbance of cell growth without sample, while As is absorbance of cell growth treatment with sample.
| % viable cells = (As-Ab)/ (A0-Ab) x 100 |
2.4. DPPH (2,2-diphenyl-1-pycrylhydrazyl) antioxidant activity assay
The DPPH antioxidant activity was assayed with a Microplate reader UV/VIS spectrophotometer (Multiskan Go, Thermo Scientific, Finland) using a published procedure on the concentration range sample (100–1000 μg/mL for extract and fractions; while 1,0–30 μg/mL for rutin), and the absorbances were read at a wavelength of 517 nm [21] with a solvent blank. The IC50 antioxidant activity (IC50AA) was estimated using a linear regression analysis. Rutin was used as the reference. The formula to obtain % inhibition as A0 (absorbance of DPPH without sample), and As (absorbance of DPPH with samples)
| % inhibition = ((A0-As)/ A0) x 100 |
2.5. Ferric reducing antioxidant power (FRAP)
The FRAP was carried out according to [22], in which the colored complexes of the antioxidant samples reacted with potassium ferricyanide, trichloroacetic acid and ferric chloride, and measured at 700 nm wavelength with the spectrophotometer. The samples were prepared at 100 μg/mL, while for rutin was made on concentrations range (50–200 μg/mL). A high absorbance reflects a high antioxidant potency, and this was calculated equivalent to rutin, a natural well-known glycoside flavonoid antioxidant. Solvent and reagent blanks were also analyzed.
2.6. Determination of total phenolic content (TPC)
The TPC was determined with the Folin-Ciocalteau (FC) reagent with slight modifications to the micro-plate preparation [21]. The samples were prepared at 100 μg/mL; while for gallic acid was made base on pre-experiments concentrations range (10–200 μg/mL) and absorbances were measured with the reader at 765 nm wavelength using a solvent blank. The TPC (μg GAE/ mg extract or fraction) was reported as gallic acid equivalents.
2.7. Determination of β-sitosterol content (SC)
The determination of β-sitosterol content was done according to Sutar et al. (2014) [23] with slight modifications. The extract and fractions were made at 100 μg/mL; while for β-sitosterol were prepared on concentrations range (100–1500 μg/mL) and spotted 10 μL each on TLC silica gel plates. The plates were developed with n-hexane:ethyl acetate (7:3) and sprayed with anisaldehyde-SO4 to determine the areas of β-sitosterol purple spots at 517 nm wavelength (TLC Scanner Camag 3, Switzerland). The SC is reported as μg β-sitosterol equivalent/mg of extract or fraction and calculated from a β-sitosterol calibration curve.
2.8. LCMS (Liquid Chromatography-Mass Spectrophotometry) of the ethyl acetate fraction
The ethyl acetate fraction was pre-treated in a solid-phase extraction (Qasis® HLB solvents, Waters, Milford, USA), dissolved in methanol and filtered through a 0.2 μm syringe membrane filter before chromatographic analysis [24]. The C-18 (1.8 μm, 2.1 × 100 mm) ultra-performance liquid chromatography (Acquity UPLC®-H class system, Waters, Milford, USA) was HSS column at 50 °C). The mobile phase and flow rate were followed as described [21] so was with the electrospray ionization mass spectrometry (MSsystem (Xevo G2-S QTof, Waters, Milford, USA). The results were analyzed with MassLynx 4.1 program to determine the Rt, m/z fragments and molecular formula. The predictive compounds were obtained from similarities of literature of Melia’s compounds, mass bank (Fiehnlab), pubchem or chemspider.
2.9. Statistical analysis
The data was presented as average values and standard deviations of triplicates. The one-way ANOVA was used for mean comparisons at a 5% significant level with SPSS version 24 and the Pearson correlation analysis was also performed.
3. Results and discussion
3.1. Physical and phytochemical profile of the extract
The M. azedarach used was the wild type, and it usually has big trees that can be up to 40 m high. This wild type is usually different from the medium-sized Chinese and Indian types [5]. Table 1 presents the physical and phytochemical quality of the extract and the fractions of the leaves. This is the first report on the quality of M azedarach dried leaves’ extracts and fractions, while earlier studies were on the plant’s bark [6]. The drying shrinkage was low (1.67 ± 0.12 %), and it showed that the leaves was dried enough for further extraction processes. The ash content (6.77 ± 0.28 %) was compared to the data of the plant’s bark. Interestingly, the ethanolic soluble content (27.28 ± 1.30 %) is much higher than about 6% reported for the plant’s bark. The extract yield was about 30 %, while the yields of the fractions ranged from 8 to 65%, and there were differences in their physical colors. The extract yield in this study that used a semi-continuous repeated percolation method, is higher than the yields of 11 % [9] and 27 % [25] reported when a maceration procedure was used. However, the extract yield is comparable to a yield of about 35 % earlier reported for dried M. azedarach fruits [26]. Apart from differences in extractions, plant parts, and plant growth conditions (e.g. environments) will affect extract yields.
Table 1.
The quality parameters of M azedarach dried leaves, extract and fractions*.
| Parameter | Dried leaves |
|---|---|
| Identity | Melia azedarach leaves |
| Macroscopic appearance | Medium and deciduous tree, leaves is opposite non-decussate phyllotaxis with specific inipinnate (imparipinnate) compound leaves, 3–8 cm long, serrated edge, typical smelling when squeezed, dark green to pale green colour |
| Ethanol soluble extract content (%) | 27.28 1.30 |
| Water soluble extract (%) | 33.85 0.56 |
| Total ash content (%) | 6.77 0.28 |
| Drying shrinkage (%) | 1.67 012 |
| Parameter | Ethanolic extract (E) | Fractions |
||
|---|---|---|---|---|
| Hexane (FH) | Ethyl acetate (FE) | Water (FW) | ||
| Appearance | Greenish black thick extract | Dark green, oily extract | Dark green thick extract | Brownish viscous extract |
| Yield (%) | 30.10.42 | 27.301.03 | 8.380.55 | 64.712.54 |
| Tannin | + | – | – | + |
| Flavonoid | + | – | + | + |
| Saponin | + | – | + | + |
| Steroid | + | + | + | – |
| Alkaloid | + | + | + | + |
| Glycoside | + | – | + | + |
| Anthraquinone | – | – | – | – |
For the yields, values with different superscripts are significantly different (p < 0.05).
+ = present, - = absent.
E = ethanolic extract, FH = hexane fraction, FE = ethyl acetate fraction and FW = water fraction.
With respect to the yields of the fractions, ethyl acetate yielded the least, possibly because of the semi polarity characteristic of the solvent, while water yielded the highest. Both the extract and the fractions contained tannins, flavonoids, saponins, steroids, alkaloids and glycosides (Table 1), and these compounds had also been reported by Ahmad et al. [9]. It can be observed in Table 1 that the fractions showed differences in the phytochemical profiles.
3.2. Cytotoxicity and antioxidant activities
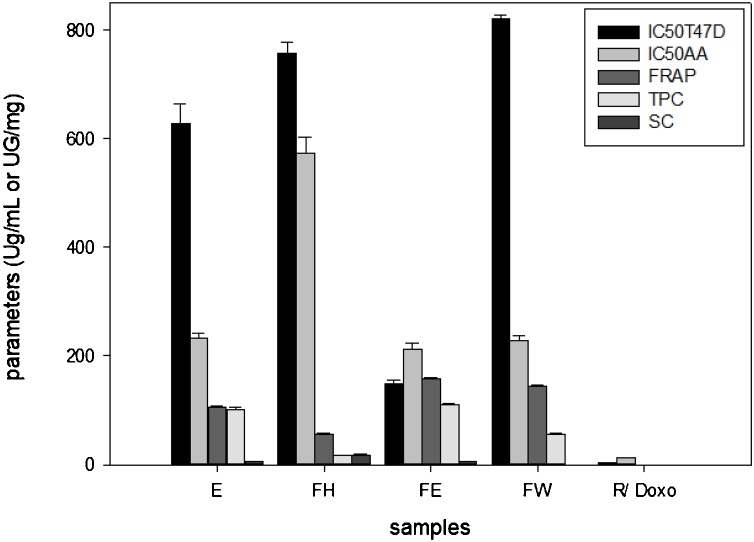
Table 2 presents the cytotoxicity (IC50T47D) of the samples that ranged from 148 (FE) to 820 μg/ml (FW). The IC50 antioxidant of the samples ranged from 212 (E) to 574 μg/ml (FH), while their FRAP was from 55 (FH) to 158 (FE) μg/mg rutin equivalent. The sequence of potential cytotoxicity was FE > E > FH and FW, while for both (DPPH and FRAP) antioxidant activities were FE > FW > E and FH (Fig. 1). The FE was effective on the T47D cells at a lower IC50 compared to vero cells (>1000 μg/mL) [10], though it was not as strong as doxorubicin (3.23 ± 0.22 μg/mL), the chemotherapy drug positive control. Wu et al. [27] found that three out of six cytotoxic steroids from ethyl acetate fractions of an ethanolic extract of M. azedarach leaves were effective against human cancer cell lines A549, H460 and U251. The three cytotoxic steroids were (20S)-5,24(28)-ergostadiene-3β,7α,16β,20-tetrol, (20S)-5-ergostene-3β,7α,16β,20-tetrol and 5-stigmastene-3,7,20-triol with an IC50 of about 12 to >80 μg/ml compared to IC50 of about 7–15 μg/mL of 5-fluorouracil. Other reported cytotoxic compounds from the plant are tirucallane triterpenes from dichloromethane and trichilin from chloroform solubles of methanolic extracts of the plant’s fruits and root barks [28,29]. Furthermore, melianones exhibited high cytotoxic effects (IC50 3.6 μg/mL), while 21-β-acetoxymelianone and 3-α- tigloylmelianol were classified as having moderate antiproliferative effects (IC50 of 100 and 91.8 μg/mL), whereas there were no reports on cytotoxic or antiproliferative effects of methyl kulonates. Among limonoids isolated from Melia root bark, it was 1-cinnamoyl-3-hydroxy-11-methoxymeliacarpinin that showed significant cytotoxic activities against P388 lymphocytic leukemia (1.5 μg/mL). The structure activity revealed the influence of C-3 and C-20 acetate substituents, though 1-deoxy structures decreased the cytotoxicity. Furthermore, the trichillins had strong cytotoxic activities in a range of 0.011–5.4 μg/mL, with 12-deacetyltrichilin being the most cytotoxic substance against P388 cells [29]. These results showed the importance of purification and isolation of potential substances to enhance cytotoxic activities, especially to selected breast cancer cell lines.
Table 2.
IC50T47D, IC50AA TPC and SC of the M azedarach extract and fractions*.
| Samples | (μg/mL) |
(μg/mg) |
|||
|---|---|---|---|---|---|
| IC50 T47D | IC50 AA | FRAP (RE/ sample) | TPC (GAE/ sample) | SC (βSE/ sample) | |
| E | 628.05 ± 35.69c | 232.00 ± 11.00b | 106.20±1.53b | 102.18 ± 2.59c | 5.49 ± 0.37b |
| FH | 757.09 ± 21.57d | 574.25 ± 29.71c | 55.08±2.64a | 16.33 ± 0.23a | 17.38 ± 2.47c |
| FE | 147.90 ± 8.49b | 211.89 ± 10.86b | 157.75±2.51d | 109.43 ± 3.54d | 5.04 ± 0.12b |
| FW | 820.26 ± 8.25e | 229.32 ± 8.10b | 144.84±2.67c | 55.72 ± 1.36b | nda |
| R | -- | 11.78 ± 0.74a | -- | -- | -- |
| Doxo | 3.23 ± 0.22a | -- | -- | -- | |
IC50 = inhibition concentration, T47D = T47D cell line, AA = DPPH antioxidant activity, FRAP = ferric reducing antioxidant potency, RE = rutin equivalence, TPC = total phenolic content, GAE = gallic acid equivalence, SC = equivalent to β-sitosterol content, βSE = β-sitosterol equivalence, R = rutin, Doxo = doxorubicin, nd = not detectable, -- = not tested. TPC linear regression equation y = 0.0020x+0.0144, R2 = 0.9993; SC y = 624.32x+331.10, R2 = 0.9982; while rutin linearity y = 0.0009x+0.0048, R2=0.9803). Different superscript letters between the means in the same column, are statistically different significantly (p < 0.05).
Fig. 1.
Parameters histogram of the samples result. The lower IC50AA and IC50T47D of FE showed the potent of its antioxidant and cytotoxic than others samples.
The IC50AA and FRAP values of FE were the lowest (Table 2). Previous results showed young leaves exhibited higher DPPH antioxidant activities than old leaves [14], while Orhan et al. [30] found ethyl acetate extracts of the plant’s leaves and fruits to be most notable in iron and ferrous metal-chelating assays. The flavonoid quercetin-3-O-neohesperidoside, rutin, kaempferol-3-O-rutinoside and kaempferol-O-dihexoside were found as the main radical scavengers [14]. Other studies also found that rutin, quercetin-3-O-neohesperidoside and kaempferol had potent DPPH radical scavenging activities (IC50 of 4–6 μM) [31,32]. The chemical structures of rutin and quercetin-3-rutinoside-7-rhamnoside had glycosylation position, esterified sugar type, 2,3-double bond in conjugation with 4-oxo function in the C-ring and the twist angles of the B-ring compared to the A- and C-rings that determine its ability to scavenge free radicals [33]. It was also found that kaempferol-3-O-rutinoside exhibited strong antioxidant activities. Jafari et al. [11] isolated rutin, kaempferol-3-O-robinobioside, kaempferol-3-O–rutinoside, and isoquercetin (flavonol 3-O-glycosides) also from methanolic extracts of M. azeadarach leaves. The flavonols in the leaves were highly associated with their medicinal effects.
3.3. Total phenolic and β-sitosterol contents
The results of TPC and βSE linear regression equations were y = 0.0020x+0.0144, R2 = 0.9993 and y = 624.3201x+331.1001, R2 = 0.9982). The purple spot of TLC β-sitosterol was in a good separation (Rf 0.67 and Rs 0.98–1.2) from other compounds. Table 2 also presents the TPC (16–109 μg GAE/mg) and SC (5–17 μg βSE/mg) of the extract and fractions, with the latter was not detected in the FW sample. Ethanol had been shown to better extract phenolic compounds from M. azedarach than water and petroleum ether [9]. Other studies have shown that ethanol, ethyl acetate and water also solubilize phenolic compounds, while β-sitosterol is more soluble in ethyl acetate and hexane [34]. Nampoothiri et al. [35] also found that ethyl acetate fractions exhibited higher antioxidant, DPPH, and others radical (superoxide and hydroxyl) scavenging activity than hexane and water fractions.
3.4. The LC–MS of the ethyl acetate fraction
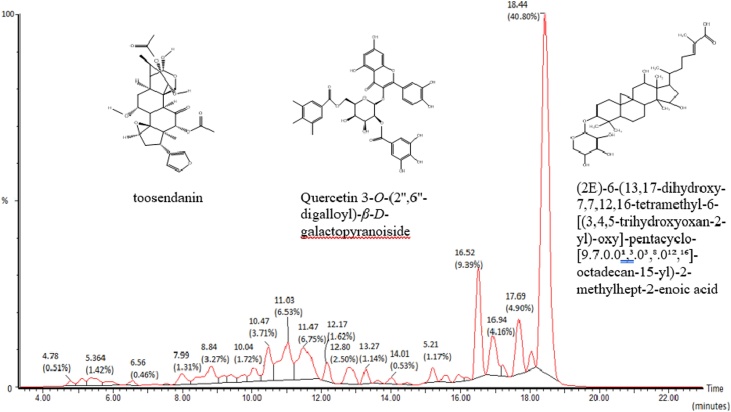
Being the most cytotoxic (Table 2), the FE sample was processed on to understand and identify its constituents. The LCMS had been used to predictive Melia’s FE fraction compounds. Though not ascertain as isolate identification, it provides more specific screening of phytochemical compounds compare to conventional method. Fig. 2 shows 32 peaks of LC separation of the FE fraction. They revealed 15 peaks with percentage area above 1% and ion fragments of the FE fraction phytocontents. The peaks were predictive as saponins (steroid glycoside), limonoids, triterpenoids and polyphenolics compounds (Table 3). The highest percentage was saponin with 40.80 % on Rt 18.438 min, while the lowest was the flavonoids with 4.68 % on Rt 5.364 and 8.841 min.
Fig. 2.
LC- EI MS chromatogram results analysis and predictive phytochemicals content from the M. azedarach leaves ethyl acetate fraction. It contents of limonoid (toosendanin, meliacarpinin, 12-hydroxyamoorastatin and their derivates, meliarachin, salannin and salannal), flavonoid glycosides (quercetin-7-O-β-d-glucopyranoside and quercetin 3-O-(2′',6′'-digalloyl)-β-d-galactopyranoiside), saponins (triterpene and steroid glycosides), and triterpene aglycone.
Table 3.
Predictive compounds of FE M. azedarach.
| Peak No | Rt (minutes) | % area | m/z [M+H] | Predictive compounds [molecular formula, M] | References |
|---|---|---|---|---|---|
| 4 | 5.364 | 1.42 | 465 | Quercetin-7-O-β-d-glucopyranoside [C21H20O12, 464.4] | [37,38] |
| 8 | 7.987 | 1.31 | 613 | Salannal [C34H44O10, 612.716] | [37] |
| 9 | 8.841 | 3.27 | 763 | Quercetin 3-O-(2′',6′'-digalloyl)-Beta-d-galactopyranoiside [C38H34O17, 762.7] | [37] |
| 13 | 10.041 | 1.72 | 573 | Meliarachin B [C30H36O11, 572.6] | [37] |
| 14 | 10.474 | 3.71 | 683 | (2R,3S,4S,5R,6S)-3,4-dihydroxy-6-[3,7,8-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxochromen-5-yl]-oxy-5-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]-oxyoxan-2-yl]-methyl acetate [C30H34O18, 682.1745] | [36] |
| 15 | 11.032 | 6.53 | 575 | Toosendanin [C30H38O11, 574.616] | [15,39] |
| 16 | 11.474 | 6.75 | 697 | Methyl (23S)-7,14,23-trihydroxy-4-methoxy-6,16,22-trimethyl-25-[(E)-3-phenylprop-2-enoyl]-oxy-3,9,11,17,20-pentaoxaoctacyclo [17.6.1.18,15.01,5.06,18.07,16.010,14.022,26]-heptacos-12-ene-4-carboxylate (1-cinnamoyl-3-hydroxy-11-methoxymeliacarpinin) [C30H49O18, 696.7] | [37] |
| 17 | 12.169 | 1.62 | 557,497 | toosendanin derivates [M–H2O+H]+ at 557.4 and [M–CH3COO+H]+ at 497.2 | [15,39] |
| 18 | 12.802 | 2.50 | 537 | Cyclopenta[c]pyran-4-carboxylic acid, 1-(β-d-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-5-hydroxy-7-methyl-6-[(2E)-1-oxo-3-phenyl-2-propen-1-yl]-oxy- methyl ester, [C26H32O12, 536.1894] | [36] |
| 19 | 13.265 | 1.14 | 597 | Salannin [C34H44O9, 596.7] | [37,41] |
| 23 | 15.214 | 1.17 | 547 | Meliarachin H/I [C29H38O10, 546.6] | [37] |
| 27 | 16.521 | 9.39 | 593, 533 | 12-hydroxyamoorastatin – acetyl derivate [C30H40O12, 592.23] | [37,40] |
| 28 | 16.942 | 4.16 | 533 | 12-hydroxyamoorastatin [C28H36O10, 532.2308] | [37,40] |
| 30 | 17.68 | 4.90 | 637 | 5-[17-(5,6-dihydroxy-6-methylheptan-2-yl)-3,12-dihydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-2-yl]-oxy-3-hydroxy-3-methyl-5-oxopentanoic acid [C36H60O9, 636.4237] | [36] |
| 32 | 18.438 | 40.80 | 621 | (2E)-6-(13,17-dihydroxy-7,7,12,16-tetramethyl-6-[(3,4,5-trihydroxyoxan-2-yl)-oxy]-pentacyclo-[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]-octadecan-15-yl)-2-methylhept-2-enoic acid [C35H56O9, 620.3924] | [36] |
The quercetin was obtained with m/z [M+H] 303. Flavonoid quercetin-7-O-β-d-glucopyranoside was obtained with ion parents 465 compare to its data on [M] 464.6 [36]. The compounds on Rt 8.841 min had similar fragmentation pattern, but higher m/z value on 763 which was assumed as quercetin-3-O-(2″,6″-digalloyl)-β-d-galactopyranoside [M] 762.7 [[36], [37], [38]]. The MS result can not differentiate C3 or C7 bond glycoside on the aglycone flavonoid, accordingly though. Two peaks revealed toosendanin and its derivate, which have m/z at 557 (Rt 11.032 min, 6.53 % and 12.169 min, 1.62 %) [15,39]. It was also observed the ions fragmentation at m/z 497, 479, 437, and 419; which were identified as existence of two acetoxyl and two hydroxyl groups in toosendanin as the fragments of [M+H–H2O − CH3COOH]+, [M+H−2H2O − CH3COOH]+, [M+H–H2O−2CH3COOH]+, and [M+H−2H2O−2CH3COOH]+, respectively [39]. The 1-cinnamoyl-3-hydroxy-11-methoxymeliacarpinin with m/z [M+H] 697 was observed on Rt 11.474 min with 6.53 % [37,38]. The 12-hydroxyamoorastatin and its acetyl derivate were on Rt 16.942 and 16.521min with 4.16 % and 9.39 % respectively [40]. Highly percentage of steroids and terpen saponins compounds were detected on 18.438 min (40.80 %) and Rt 12.802 (2.50 %), while triterpenoids aglycone was on Rt 17.68 min (4.90 %). Sterol fragment was observed at Rt 18.438 min (m/z of 275 with (M+-C3H7O)18-CH3+side chain) with 16-ketosteroids cleavage, which was identified as β-sitosterol glucoside [M+H] 577 compound [12,36,37]. Others compounds were analyzed as meliarachin, salannin, and salannal as listed in Table 3. Salannin and salannal (Rt 13.265 min, 1.14 %; 7.987 min, 1.31 %) [41], and meliacarpinin derivates (Rt 11.474 min, 6.35 %) [37,42,43]. The identification process based on comparison with the most identical fragments to available references data, though others may have similarity only some part to quite different. The varieties on the technical analysis method and the limitation of the data were leading to the used of references available on the experiment or the importance of isolation to identification of the substances further. The environment influence and local variety of the M. azedarach wild type provide opportunity to obtain new or modified of its chemical entities. For example quercetin glycoside at Rt 8.841 min was found have longer glycoside and not identical with the references available. Others of saponin and triterpen at Rt 18.438, 17.680 and 12.802 min were identical with references data [[36], [37], [38]], but had not been reported on the Melia’s content yet. Some compounds resulted specific Rt and fragmentations ion pattern such as meliatoxin, meliarachin, β-sitosterol and flavonoids, which were suggested to be used as biomarker for Melia extracts [12].
3.5. Correlation analysis among activities and contents
Correlation analysis between the activity parameters revealed negative and positive significant outcomes. TPC significantly (p < 0.05) correlated with parameters IC50AA (-0.845), FRAP (0.695) and IC50T47D (-0.709), while IC50T47D significantly correlated with IC50AA (0.671), but there were no significant correlations between SC and others. Though they have small percentage in the fraction, phenolic compounds play important roles in cytotoxicity and antioxidant activities. The significant correlations reported were consistent with these relationships. Positive correlations had been reported for pomegranate cytotoxicity and TPC on MDA-MB-231 (0.980); and total flavonoid content to HT-29 cell line cytotoxic (0.864) [44]. Grigalius and Petrikaite [45] suggested the structure-activity relationships of both antioxidant and anticancer activities were due to ortho-dihydroxy group in ring C of flavonoids. The ortho-dihydroxy was obtained (about 4.68 %) in FE as quercetin glycoside (Table 3). Quercetin was found as powerfull hypochlorous acid, chloramines, nitric oxide, and superoxide scavengers; and also cytotoxic to red blood cell haemolysis, compared than that of kaempferol and isoquercitrin [46]. Quercetin significantly inhibited human breast cancer cells (MCF-7 and MDA-MB231), and moreover it mentioned has the cytoprotective role against oxidative stress through antioxidant effect, motivating apoptotic cell death via prooxidant activity, and inhibiting tumourigenesis [47].
Furthermore, Ashraf et al. [48] found that steroids and triterpene saponins were cytotoxic against MCF-7. Podolak et al. [49] highlighted the potential of saponins as anticancer. He found the important factors responsible for improving the cytotoxicity including structural feature, number and the sequence of sugar residues in a carbohydrate chain, also the position of sugar attachment to the aglycone. The cytotoxicity was enhance with the prolonged of the sugars chain. The saponins were stimulate apoptotic process in tumor cells, in intrinsic pathway mostly. Non apoptotic processes were also involved as cell cycle arrestment, autophagic cell, death stimulation, inhibiting of metastasis and cytoskeleton, including disintegration of the cell. Saponins are also promising as inhibiting tumor cells angiogenesis and recombinant protein toxins. Furthermore saponins have physiologically binding to nuclear receptors activity, including to conventional steroid hormone receptors (estrogen receptor, glucocorticoid receptor, mineralocorticoid receptor, and androgen receptor) and the orphan receptors (peroxisome proliferatoractivated receptor (PPAR), liver X receptor (LXR), farnesoid X receptor (FXR), and Pregnane X receptor (PXR)) [50].
Akhihisa et al. [51] revealed limonoid trichillin type of meliarachin C and salannin type of 3-O-deacetyl-40-demethyl-28-oxosalannin are cytotoxic to HL-60 cells by inducing apoptotic cell death. Zhou et al. [43] observed limonoids tetracyclic sendanin, trichillin and C-seco limonoid types with 14,15-epoxide ring and a C-19/C-29 acetal bridge exhibit very strong and strong cytotoxicity antiproliferation against Hela S3 (human epithelial cancer) cell line and against P388 cells. Yadav et al. [52] found the role of neem limonoids in mitochondria oxidative phosphorylation complexes, though not effective to p53 and Bax-independent. Neem limonoids are, however, useful for multiple cancers, including cancer-drug-resistant ones and as a novel for solid cancer therapy.
4. Conclusions
The ethyl acetate fraction from the M. azedarach L. wild type leaves ethanolic extract showed the most T47D bio-selective hormonal cytotoxicity and antioxidant activities. Significant correlations among TPC, IC50T47D, IC50AA and FRAP activities were obtained to lend credence to compound-antioxidant-cytotoxicity relationships. The ethyl acetate fraction contain steroid and triterpenoid saponins, triterpenoid, ortho-dihydroxy flavonols of quercetin glycosides, limonoid toosendanin and its derivate, 12-hydroxyamoorastatin and its acetyl derivate, 1-cinnamoyl-3-hydroxy-11-methoxymeliacarpinin, meliarachin, salannin and salannal. The compounds were potent against the breast cancer cells and showed promises in breast cancer managements.
Ethical statement for biotechnology reports
Hereby, I /insert author name/ consciously assure that for the manuscript /insert title/ the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
4)
The paper properly credits the meaningful contributions of co-authors and co-researchers.
-
5)
The results are appropriately placed in the context of prior and existing research.
-
6)
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
-
7)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
The violation of the Ethical Statement rules may result in severe consequences.
To verify originality, your article may be checked by the originality detection software iThenticate. See also http://www.elsevier.com/editors/plagdetect.
I agree with the above statements and declare that this submission follows the policies of Solid State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Declaration of Competing Interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
The authors are grateful to the Directorate General of Higher Education, Ministry of National Education, Republic of Indonesia for PUPT Ristek DIKTI 2019 and PKPI-sandwich like program 2019.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00437.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I. 2018. Global Cancer Statistics 2018 : GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries; pp. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H. Systemic chemotherapy for cancer: from weapon to treatment. Lancet Oncol. 2008;9:304. doi: 10.1016/S1470-2045(08)70075-5. [DOI] [PubMed] [Google Scholar]
- 3.Conklin K.A. Chemotherapy associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 4.Prakash A., Kumar O., Kumar A.P. Anticancer potential of plants and natural products: a review. Am. J. Pharmacol. Sci. 2013;1:104–115. [Google Scholar]
- 5.Mabberley D.J. A monograph of Melia in Asia and the Pacific. The history of white cedar and Persian lilac. Gard. Bull. 1984;37:49–64. http://www.cabi.org/ISC/abstract/19840326280 [Google Scholar]
- 6.Sharma D., Singla Y.P. Preliminary and pharmacological profile of Melia azedarach L.: an overview. Int. J. Appl. Pharm. Sci. Res. 2013;3:133–138. [Google Scholar]
- 7.Ervina M., Sukardiman A review: Melia azedarach L. as a potent anticancer drug. Pharmacogn. Rev. 2018;12:94–102. [Google Scholar]
- 8.Zahoor M., Ahmed M., Naz S., Ayaz M. Cytotoxic, antibacterial and antioxidant activities of extracts of the bark of Melia azedarach (China Berry) J. Nat. Prod. Res. 2015;29(12):1170–1172. doi: 10.1080/14786419.2014.982649. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad M.F., Rao A.S., Ahemad S.R., Ibrahim M. Phytochemical studies and antioxidant activity of Melia azedarach Linn. Leaves by DPPH scavenging assay. Int. J. Pharm. App. 2012;3:271–276. [Google Scholar]
- 10.Srinivasan V., Panneerselvam R., Gunasekaran S., Palani S. Nephro-protective activity of ethanolic extract of Melia azadirach against H2O2 induced toxicity in vero cell line. Int. J. App. Biol. Pharm. Tech. 2015;6:44–49. [Google Scholar]
- 11.Jafari M., Saeidnia S., Hajimehdipoor S., Ardekani H., Faramarzi M.R.S., Hadjiakhoondi M.A., Khanavi A. Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation, DARU. J. Pharm. Sci. 2013;21 doi: 10.1186/2008-2231-21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inada A., Kobayashi M., Nakanishi T. Phytochemical studies on Meliaceous plants. III structures of two new pregnane steroids, toosendansterols A and B, from leaves of Melia toosendan et Zucc. Chem. Pharm. Bull. 1998;36:609–612. [Google Scholar]
- 13.Cornelio V.E., Forim M.R., Perlatti B., Fernandes J.B., Vieira P.C., Napolitano M.P., Yost R.A., Da Silva M.F.G.F. Identification of meliatoxins in Melia azedarach extracts using mass spectrometry for quality control. Planta Med. 2017;83:312–317. doi: 10.1055/s-0042-115773. [DOI] [PubMed] [Google Scholar]
- 14.M’rabet Y., Rokbeni N., Cluzet S., Boulila A., Richard T., Krisa S., Marzouki L., Casabianca H., Hosni K. Profiling of phenolic compounds and antioxidant activity of Melia azedarach L. leaves and fruits at two stages of maturity. Ind. Crops Prod. 2017;107:232–243. [Google Scholar]
- 15.Ong E.S., Ong C.M. Qualitative and quantitative analysis of toosendanin in Melia toosendan Sieb. et Zucc (Meliaceae) with liquid chromatography/ tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:589–598. doi: 10.1002/rcm.2878. [DOI] [PubMed] [Google Scholar]
- 16.Takeya K., Qiao Z.S., Hirobe C., Itokawa H. Cytotoxic azadirachtin-type limonoids from Melia azedarach. Phytochemistry. 1996;42:709–712. doi: 10.1016/0031-9422(96)00044-1. [DOI] [PubMed] [Google Scholar]
- 17.Yu S., Kim T., Yoo K.H., Kang K. The T47D cell line is an ideal experimental model to elucidate the progesterone-specific effects of a luminal a subtype of breast cancer. Biochem. Biophys. Res. Commun. 2017;486:752–758. doi: 10.1016/j.bbrc.2017.03.114. [DOI] [PubMed] [Google Scholar]
- 18.WHO . 2011. Quality Control Methods for Herbal Materials. [Google Scholar]
- 19.Farnsworth N.R. Biological and phytochemical screening of plants. J. Pharm. Sci. 1966;69:225–268. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Chemoprevention Research Center (CCRC) Faculty of Pharmacy Gajah Mada University; 2013. Cytotoxic Test With MTT Method. [Google Scholar]
- 21.Ervina M., Lie H.S., Diva J., Caroline, Tewfik S., Tewfik I. Optimization of water extract of Cinnamomum burmannii bark to ascertain its in vitro antidiabetic and antioxidant activities. Biocatal. Agric. Biotechnol. 2019;19 [Google Scholar]
- 22.Vijayalakshmi M., Ruckmani K. 2016. Ferric Reducing Anti-oxidant Power Assay in Plant Extract; pp. 570–572. [Google Scholar]
- 23.Sutar R.C., Kasture S.B., Kalaichelvan V.K. Identification, quantification and validation of β-sitosterol from Holoptelea integrifolia (roxb.) planch using high performance thin layer chromatography method. Int. J. Pharm. Pharm. Sci. 2014;6:249–252. [Google Scholar]
- 24.Danaceau J. 2013. Successful Sample Preparation Strategies for LC/MS/MS Analysis of Drugs in Complex Technical Biological Matrices for Forensic Toxicology Applications. http://www.waters.com/webassets/cms/events/docs/FINALSamplePrep StrategiesClinical_Forensic 11_ 5_13.pdf, accessed 10 September 2018. [Google Scholar]
- 25.Irshan Y., Suparto I.H., Sulistiyani S. Cytotoxic and antiproliferative activities of Melia azedarach leaves ethanolic extract on A549 human lung cancer cells. J. Jamu Indones. 2017;2:17–24. [Google Scholar]
- 26.Carpinella M.C., Herrero G.G., Alonso R.A., Palacios S.M. Antifungal activity of Melia azedarach fruit extract. Fitoterapia. 1999;70:296–298. [Google Scholar]
- 27.Wu S.B., Ji Y.P., Zhu J.J., Zhao Y., Xia G., Hu Y.H., Hu J.F. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids. 2009;74:761–765. doi: 10.1016/j.steroids.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Ntalli N.G., Cottiglia F., Bueno C.A., Alché L.E., Leonti M., Vargiu S., Bifulco E., Menkissoglu-Spiroudi U., Caboni P. Cytotoxic tirucallane triterpenoids from Melia azedarach fruits. Molecules. 2010;15:5866–5877. doi: 10.3390/molecules15095866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeya K., Qiao Z.S., Hirobe C., Itokawa H. Cytotoxic trichilin-type limonoids from Melia azedarach. Bioorg. Med. Chem. Lett. 1996;4:1355–1359. doi: 10.1016/0968-0896(96)00128-9. [DOI] [PubMed] [Google Scholar]
- 30.Orhan I.E., Guner E., Ozturk N., Senol F.S., Erdem S.A., Kartal M., Sener B. Enzyme inhibitory and antioxidant activity of Melia azedarach L. naturalized in Anatolia and its phenolic acid and fatty acid composition. Ind. Crop. Prod. 2012;37:213–218. [Google Scholar]
- 31.Matsuda H., Morikawa T., Ishiwada T., Managi H., Kagawa M., Higashi Y., Yoshikawa M. Medicinal flowers:VIII. Radical scavenging constituents from the flowers of Prunus mume: structure of prunose III. Chem. Pharm. Bull. 2003;51:440–443. doi: 10.1248/cpb.51.440. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R., Zhang B.L., He T., Yi T., Yang J.P., He B. Increase of rutin antioxidant activity by generating maillard reaction products with lysine. Bioorg. Med. Chem. Lett. 2016;26:2680–2684. doi: 10.1016/j.bmcl.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Su D., Ti H., Zhang R., Zhang M., Wei D., Deng Y., Guo J. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 2014;158:385–391. doi: 10.1016/j.foodchem.2014.02.134. [DOI] [PubMed] [Google Scholar]
- 34.Wei D., Wang L., Liu C., Wang B. β-Sitosterol solubility in selected organic solvents. J. Chem. Eng. Data. 2010;55:2917–2919. [Google Scholar]
- 35.Nampoothiri S.V., Binil Raj S.S., Prathapan A., Abhilash P.A., Arumughan C., Sundaresan A. In vitro antioxidant activities of the methanol extract and its different solvent fractions obtained from the fruit pericarp of Terminalia bellerica. Nat. Prod. Res. 2011;25:277–287. doi: 10.1080/14786419.2010.482053. [DOI] [PubMed] [Google Scholar]
- 36.2020. Mona-Mass Bank of North America.https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QEHF010860 VF-NPL-QEHF023440, VF-NPL-QEHF023122, CCMSLIB00000855190. [Google Scholar]
- 37.U.S. National Library of Medicine . 2020. National Center for Biotechnology Information, Pubchem.https://pubchem.ncbi.nlm.nih.gov/ [Google Scholar]
- 38.Chemspider; 2020. Royal Society of Chemistry.http://www.chemspider.com/ [Google Scholar]
- 39.Wu J.L., Leung E.L.H., Zhou H., Liu L., Li N. Metabolite analysis of toosendanin by an ultra-high performance liquid chromatography-quadrupole-time of flight mass spectrometry technique. Molecules. 2013;18:12144–12153. doi: 10.3390/molecules181012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polonsky J., Varon Z., Marazano C., Arnoux B., Pettit G.R., Schmid J.M., Ochi M., Kotsuki H. The structure of amoorastatone and the cytotoxic limonoid 12-hydroxyamoorastatin. Experientia. 1979;35:987–1130. doi: 10.1007/BF01949897. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J., Lu J., Fan X., Wu X., Diao R., Yua H., Xu R., Zi H. A new cytotoxic salannin-class limonoid alkaloid from seeds of Azadirachta indica A. Juss. Chin. Chem. Lett. 2018;29:1261–1263. [Google Scholar]
- 42.Zhou J.B., Tadera K., Minami Y., Yagi F., Kurawaki J., Takezaki K., Nakatani M. New limonoids from Melia toosendan. Biosci. Biotechnol. Biochem. 1998;62:496–500. doi: 10.1271/bbb.62.496. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H., Hamazaki A., Fontana J.D., Takahashi H., Wandscheer C.B., Fukuyama Y. Cytotoxic limonoids from Brazilian Melia azedarach. Chem. Pharm. Bull. 2005;53:1362–1365. doi: 10.1248/cpb.53.1362. [DOI] [PubMed] [Google Scholar]
- 44.Eddebbagh M., Massoudi M., Abourriche A., Berrada M., Attaleb M., Benbacer L., Bennamara A. Correlation of the cytotoxic and antioxidant activities of Moroccan pomegranate (Punica granatum) with phenolic and flavonoid contents. J. Pharm. Pharmacol. 2016;4:511–519. [Google Scholar]
- 45.Grigalius I., Petrikaite V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules. 2017;22 doi: 10.3390/molecules22122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vellosa J.C.R., Regasini L.O., Khalil N.M., Bolzani V.S., Khalil O.A.K., Manente F.A., Netto H.P., Oliveira O.M.M.F. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Ecl. Quím. 2011;36:7–20. [Google Scholar]
- 47.Ranganathan S., Halagowder D., Sivasithambaram N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One. 2015 doi: 10.1371/journal.pone.0141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashraf M.F., Aziz M.A., Stanslas J., Ismail I., Kadir M.A. Assessment of antioxidant and cytotoxicity activities of saponin and crude extracts of Chlorophytum borivilianum. The Sci.World J. 2013;216894:7. doi: 10.1155/2013/216894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podolak I., Galanty A., Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem. Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T., Zhong S., Li T., Zhang J. Saponins as modulators of nuclear receptors. Cri. Rev. Food Sci. & Nut. 2018 doi: 10.1080/10408398.2018.1514580. [DOI] [PubMed] [Google Scholar]
- 51.Akihisa T., Pan X., Nakamura Y., Kikuchi T., Takahashi N., Matsumoto M., Ogihara E., Fukatsu M., Koike K., Tokuda H. Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry. 2013;89:59–70. doi: 10.1016/j.phytochem.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Yadav N., Kumar S., Kumar R., Srivastava P., Sun L., Rapali P. Mechanism of neem limonoids-induced cell death in cancer: role of oxidative phosphorylation. Free Radic. Biol. Med. 2016;90:261–271. doi: 10.1016/j.freeradbiomed.2015.11.028. 028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.