Abstract
Background
Secondary ischaemia is a frequent cause of poor outcome in patients with subarachnoid haemorrhage (SAH). Its pathogenesis has been incompletely elucidated, but vasospasm probably is a contributing factor. Experimental studies have suggested that calcium antagonists can prevent or reverse vasospasm and have neuroprotective properties.
Objectives
To determine whether calcium antagonists improve outcome in patients with aneurysmal SAH.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched April 2006), MEDLINE (1966 to March 2006) and EMBASE (1980 to March 2006). We handsearched two Russian journals (1990 to 2003), and contacted trialists and pharmaceutical companies in 1995 and 1996.
Selection criteria
Randomised controlled trials comparing calcium antagonists with control, or a second calcium antagonist (magnesium sulphate) versus control in addition to another calcium antagonist (nimodipine) in both the intervention and control groups.
Data collection and analysis
Two review authors independently extracted the data and assessed trial quality. Trialists were contacted to obtain missing information.
Main results
Sixteen trials, involving 3361 patients, were included in the review; three of the studies were of magnesium sulphate in addition to nimodipine. Overall, calcium antagonists reduced the risk of poor outcome: the relative risk (RR) was 0.81 (95% confidence interval (CI) 0.72 to 0.92); the corresponding number of patients needed to treat was 19 (95% CI 1 to 51). For oral nimodipine alone the RR was 0.67 (95% CI 0.55 to 0.81), for other calcium antagonists or intravenous administration of nimodipine the results were not statistically significant. Calcium antagonists reduced the occurrence of secondary ischaemia and showed a favourable trend for case fatality. For magnesium in addition to standard treatment with nimodipine, the RR was 0.75 (95% CI 0.57 to 1.00) for a poor outcome and 0.66 (95% CI 0.45 to 0.96) for clinical signs of secondary ischaemia.
Authors' conclusions
Calcium antagonists reduce the risk of poor outcome and secondary ischaemia after aneurysmal SAH. The results for 'poor outcome' depend largely on a single large trial of oral nimodipine; the evidence for other calcium antagonists is inconclusive. The evidence for nimodipine is not beyond all doubt, but given the potential benefits and modest risks of this treatment, oral nimodipine is currently indicated in patients with aneurysmal SAH. Intravenous administration of calcium antagonists cannot be recommended for routine practice on the basis of the present evidence. Magnesium sulphate is a promising agent but more evidence is needed before definite conclusions can be drawn.
Plain language summary
Calcium antagonists for aneurysmal subarachnoid haemorrhage
A subarachnoid haemorrhage is a bleed in the so‐called subarachnoid space, which is the very small space between the brain and the skull, and which contains blood vessels that supply the brain. The cause of the bleeding usually is a rupture of a bulge in one of these vessels. This bulging or blister on a vessel is called an aneurysm. A subarachnoid haemorrhage is a relatively uncommon type of stroke; it accounts for about one in 20 (5%) of all strokes. Subarachnoid haemorrhage often occurs at a relatively young age: half the patients are younger than 55 years old. The outcome of patients after subarachnoid haemorrhage is generally poor: half the patients die within one month after the haemorrhage, and of those who survive the initial month, half remain dependent on someone else for help with activities of daily living (e.g. walking, dressing, bathing). One of the causes of poor outcome is a complication of subarachnoid haemorrhage called secondary ischaemia (ischaemia means lack of blood). This complication occurs four to 10 days (hence secondary) after the haemorrhage. The cause is not exactly known, but one of the factors involved is narrowing of blood vessels in the brain. Calcium antagonists are a type of drug that block calcium channels in cells and are often used for the treatment of high blood pressure. They have also been shown to counteract the narrowing of blood vessels after subarachnoid haemorrhage and to protect the brain against periods of ischaemia. This review of 16 trials, involving 3361 patients, has found that the outcome after subarachnoid haemorrhage, in terms of survival and being independent in activities of daily living, is improved by treatment with calcium channel blockers (antagonists). If the largest trial is excluded from the analysis, the results are no longer statistically significant, and therefore the evidence is not beyond all doubt. However, given the high likelihood of benefits and the modest risks associated with this treatment, the review authors conclude that calcium antagonists, in the form of oral nimodipine 60 mg every four hours, are useful in patients with subarachnoid haemorrhage from a ruptured aneurysm. Magnesium is another calcium antagonist with promising results, but larger trials with this drug are needed before we can be certain about a beneficial effect.
Background
Aneurysmal subarachnoid haemorrhage (SAH) is a subset of stroke and has an incidence of nine per 100,000 person years (de Rooij 2006). Half the patients are younger than 55 years of age (ACROSS 2000), and around 70% of patients die or remain dependent on help for activities of daily life as a result of the haemorrhage (Hop 1997). Patients who survive the initial hours after the haemorrhage are at risk of deterioration from rebleeding, secondary ischaemia, hydrocephalus and medical complications.
Secondary ischaemia occurs in one third of all patients (Brilstra 2000) and results in a poor outcome in half of the patients with this complication (Roos 2000). Secondary ischaemia develops most frequently between four to 10 days after the haemorrhage (Brilstra 2000). Its pathogenesis has been incompletely elucidated, but is often attributed to narrowing of intracranial arteries. This so‐called vasospasm may result from an increase of calcium in the vascular smooth‐muscle cell (Ljunggren 1984).
Calcium antagonists reduce the influx of calcium into the cell through blocking calcium channels. Thus, a rationale for the use of calcium antagonists for prevention of secondary ischaemia was based on the notion that these drugs can counteract the influx of calcium into the vascular smooth‐muscle cell, thereby decreasing the rate of vasospasm. After their introduction into clinical practice it was discovered that calcium antagonists also have neuroprotective properties. Another important effect of calcium antagonists is the induction of hypotension (Gaab 1985; McCalden 1989; Pickard 1990; Tettenborn 1990), which may counteract the potential benefits.
Magnesium sulphate acts as a non‐competitive antagonist of voltage‐dependent calcium channels, as a NMDA‐receptor antagonist, and has neuroprotective and vasodilatory properties (van den Bergh 2004). It has been shown to reduce cerebral vasospasm and infarct volume after experimental SAH (Ram 1991; van den Bergh 2002). Also, hypomagnesaemia occurs in more than 50% of patients with SAH and is related to the occurrence of secondary ischaemia (van den Bergh 2003). These observations have led to the hypothesis that magnesium sulphate can decrease the occurrence of secondary ischaemia in patients with aneurysmal SAH.
Several trials with nimodipine and other calcium antagonists have been performed in patients with SAH. In recent years magnesium sulphate has been added to the list of calcium antagonists that have been tested. The aim of this review is to assess the available evidence on calcium antagonists, including magnesium sulphate, in patients with aneurysmal SAH.
Objectives
To determine: (1) whether calcium antagonists improve outcome in patients with aneurysmal SAH; (2) whether calcium antagonists reduce the rate of secondary ischaemia; (3) the effects of calcium antagonists on rebleeding after aneurysmal SAH; (4) the efficacy of each calcium antagonist separately on outcome, occurrence of secondary ischaemia and occurrence of rebleeding; (5) the effect of various regimens of nimodipine administration on secondary ischaemia and outcome after aneurysmal SAH; (6) whether magnesium‐sulphate, in addition to nimodipine, improves outcome, reduces the rate of secondary ischaemia and affects the rate of rebleeding after aneurysmal SAH.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all unconfounded randomised trials comparing any calcium antagonist with control in patients with aneurysmal SAH. Trials where intervention and control groups received a calcium antagonist (nimodipine) and patients were randomised to receive either a second calcium antagonist (magnesium sulphate) or placebo were also included. We excluded uncontrolled studies, as well as quasi‐randomised controlled trials where allocation to treatment or control group was not concealed (e.g. allocation by alternation, open random number list, date of birth, day of the week, or hospital number), since foreknowledge of treatment allocation might lead to biased treatment allocation (Schulz 1995).
Types of participants
Patients of any age and either gender with SAH documented by either computerised tomography (CT) scan or cerebrospinal fluid examination were included in the analysis. A ruptured cerebral aneurysm should be proven, preferably by angiography, or at least be highly likely, judged by the pattern of haemorrhage on the CT scan. The patients could be in any clinical condition before the start of study treatment.
Types of interventions
Treatment with any calcium antagonist, including magnesium sulphate, versus control, or with a second calcium antagonist (magnesium sulphate) versus control in addition to a calcium antagonist (nimodipine) in both groups, starting within 10 days of SAH onset. Trials in which treatment was started only after the onset of symptoms from secondary ischaemia were not included.
Types of outcome measures
The primary outcome measurement was poor outcome (death or dependence on help for activities of daily life), assessed within six months after the haemorrhage.
Other outcome measurements were:
case fatality;
secondary ischaemia (clinical signs of cerebral ischaemia, as well as CT‐documented cerebral infarction alone);
rebleeding within six month of SAH;
adverse effects of the treatment.
Since most studies provided an imprecise definition of secondary ischaemia, we used the number of patients with secondary ischaemia as given by the authors of the trials included in the review. We did not adjust these numbers to a predefined definition of secondary ischaemia (van der Schaaf 2002). Poor outcome (death or dependency) was based on the information provided by the trial authors.
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Review Group Co‐ordinator in April 2006. In addition, we searched MEDLINE (1966 to May 2006) and EMBASE (1980 to May 2006) (Appendix 1).
We searched the reference lists of all relevant trials and contacted stroke trialists in an effort to identify further published, ongoing and unpublished studies.
For previous versions of this review, we handsearched two Russian neurological and neurosurgical journals (Zhurnal Nevropatologii i Psikhiatrii Imeni SS Korsakova and Terapevticheskii arkhiv) from 1990 to 2003. In 1995 and 1996, we also contacted 18 pharmaceutical companies asking them to provide any other relevant randomised controlled trials, published or unpublished. Sixteen of the 18 companies replied to our request: Bayer AG, Synthelabo Pharmacie, Sandoz BV, Astra, Bristol‐Myers Squibb, WYETH, Boehringer Ingelheim, Pfizer BV, Akzo Nobel, Centocor BV, Schering Nederland BV, Yamanouchi Europe BV, Amersham Healthcare, Parke‐Davis, Zambon Nederland BV, and UCB Pharma BV.
Data collection and analysis
Data extraction and trial quality assessment
Two review authors independently extracted details of randomisation methods, blinding of treatments and outcome assessments. We aimed to extract from each trial the outcome assessments at the end of follow up for all patients who were originally allocated to each treatment group to perform an intention‐to‐treat analysis. On the basis of the extracted data the review authors decided whether intention‐to‐treat analysis was possible from the published data, and whether treatment groups were comparable with regard to major prognostic risk factors for outcome, the number of patients who were excluded or lost to follow up, definition of outcome events, and entry and exclusion criteria. In addition, we recorded study drug dose, route and timing of the drug administration, type and timing of aneurysm treatment, duration of follow up, number of deaths, severe disability, CT‐documented infarction, episodes of rebleeding, whether angiography was performed before randomisation, and adverse effects of study drugs. Functional outcome was dichotomised into poor outcome (death or dependency for daily activities) and good outcome (independent for daily activities) based on the information of the individual trials. For the trials of magnesium sulphate we recorded whether study medication was given in addition to another calcium antagonist (nimodipine) or not. If patients were excluded or lost to follow up after randomisation (trials with so‐called explanatory analysis) or if any of the above data were not available from the publications, we sought further information by contacting the trialists to allow an intention‐to‐treat analysis. If the data about patients who were excluded or lost to follow up remained unavailable, a decision whether to include this particular trial in the review was made by all review authors. Quality of allocation concealment was rated as follows: A ‐ adequate, double‐blinded treatment concealment; B ‐ unknown whether treatment concealment was adequate, C ‐ single‐blinded or no blinding of treatment concealment for outcome assessment.
Data analysis
We based the primary analyses on the intention‐to‐treat results (if available) of the individual trials, for 'poor outcome' (death or dependence on help for activities of daily life), and for case fatality. We calculated an estimate of the treatment effect across trials (relative risk (RR) with a 95% confidence interval (CI)) using standard methods. We also calculated absolute risk reductions and numbers needed to treat. To quantify inconsistency across trials, we used the I‐squared statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity. A value greater than 50% may be considered substantial heterogeneity.
If primary analyses suggested a beneficial effect, secondary analyses were performed according to the worst‐case‐scenario method. A worst‐case‐scenario analysis assumes that those patients who had been excluded or lost to follow up in the treatment group have the worst possible outcome while those patients who had been excluded or lost to follow up in the control group have the best possible outcome. If the effects of primary and secondary meta‐analyses were of the same direction and magnitude, we could make a definitive conclusion regarding the treatment effectiveness, otherwise no definitive conclusion was made. Worst‐case‐scenario analysis was also performed if efficacy was found for the prevention of clinical events, but not for para‐clinical outcome measurements, such as cerebral infarction documented by CT scan, since para‐clinical outcomes are usually assessed in patients with a clinical deterioration only.
After previous versions of this review were published, new trials of magnesium sulphate have been performed. Since nimodipine is now the standard treatment in many countries, trials have been performed with magnesium sulphate in addition to a standard regimen with another calcium antagonist (nimodipine). Therefore, studies that assessed magnesium sulphate in the absence of another calcium antagonist (nimodipine) were included in the main analysis; studies that assessed magnesium sulphate in the presence of another calcium antagonist (nimodipine) were analysed separately.
Additional pre‐specified analyses were based on: (1) the methodological quality of the trials (exclusion of trials in which blinding for treatment allocation or outcome assessment was not performed or reported); (2) the time between SAH and randomisation (exclusion of trials enrolling patients after four days); (3) the time of the outcome assessment; (4) separate assessment of the efficacy of each agent and route of administration; (5) exclusion of the largest trial.
To test for a difference between subgroups, the Deeks method was used (Deeks 2001).
Results
Description of studies
We identified 27 trials of treatment with calcium antagonists in patients with subarachnoid hemorrhage: 20 trials of any calcium antagonist versus placebo (Allen 1983; Cabral 1991; Cadoux‐Hudson 1999; Ferro 1990; Haley 1993; Han 1993; Islekel 1999; Jan 1988; Luo 1996; Maldonado 1990; Messeter 1987; Neil‐Dwyer 1987; Ohman 1991; Petruk 1988; Philippon 1986; Pickard 1989; Shibuya 1992; Wang 1995; Xie 1994; Zhu 2001); and seven trials of magnesium sulphate in addition to nimodipine (IMASH; MASH‐II; Prevedello 2006; van den Bergh 2005; Veyna 2002; Wong 2006; Zhu 1996). The trials were performed between 1983 and 2006. Sixteen of these 27 trials were included in the review, including three trials of magnesium sulphate in addition to nimodipine (Veyna 2002; van den Bergh 2005; Wong 2006). Details of these trials are given in the 'Characteristics of included studies' table. The other 11 trials were excluded for the following reasons: two trials of nimodipine were excluded because nimodipine was started only after the development of secondary ischemia (Jan 1988, 188 patients) or after vasospasm (Zhu 1996, 84 patients); four trials totalling 561 patients were excluded because allocation to the treatment and control groups was not randomised (Islekel 1999; Maldonado 1990; Wang 1995) or the allocation to the treatment or control groups was not concealed (Prevedello 2006); another trial of magnesium sulphate was excluded because it was not a randomised trial (Cadoux‐Hudson 1999); one trial of 30 patients was excluded because outcome was given only for those patients with secondary ischaemia (Cabral 1991); and another study with 32 patients was excluded because there were no data on any of the outcome measures (Xie 1994). Two trials of magnesium in addition to nimodipine, both aiming to include several hundreds of patients, are presently ongoing (IMASH; MASH‐II).
Size of the trials and treatment modes
The meta‐analysis included 16 studies with a total of 3361 patients (1665 in the treatment group and 1696 in the control group). In three trials, with 383 patients, magnesium sulphate versus placebo was studied in addition to nimodipine. The number of patients per trial ranged from 20 (Messeter 1987) to 906 (Haley 1993). The mean ages of the patients ranged from 44 to 56 years. In 13 trials, the study treatment was started within four days after onset of SAH; in three trials it was started within 10 days after onset of SAH (Haley 1993; Han 1993; Ohman 1991). In these three trials, drug therapy was started in 69% to 86% patients within three days after the most recent SAH.
Nimodipine was given orally every four hours in a total daily dose of 360 mg in five trials (593 treatment group patients including 246 patients who received oral medication after seven to 10 days of continuous intravenous infusion) (Han 1993; Neil‐Dwyer 1987; Ohman 1991; Philippon 1986; Pickard 1989), in a total daily dose of 540 mg in one trial (91 patients) (Petruk 1988), and in a total daily dose of 2.1 mg/kg in another trial (58 patients) (Allen 1983). In one small study (Messeter 1987), 13 patients received a continuous intravenous infusion of nimodipine in a dose of 2 mg/hour. One study first administered nimodipine five to eight drops per minute of 10 mg per 50 ml solution for eight to 10 hours daily intravenously for 15 days, followed by 90 mg orally for another 15 days (exact dosage in mg unknown) (Zhu 2001). In two trials where nimodipine was given orally or through a continuous intravenous infusion (95 patients), this drug was also administered intraoperatively into the basal cisterns near the exposed arterial segments (Messeter 1987; Neil‐Dwyer 1987). Duration of the nimodipine treatment was 21 days in all trials but two. In one trial (Messeter 1987), the treatment duration was at least nine days whereas the overall treatment period was not specified, in another trial the treatment period was 30 days (Zhu 2001). There were two trials of nicardipine treatment after aneurysmal SAH; one trial with oral nicardipine three doses of 60 mg per day for 21 days (Ferro 1990) and one with intravenous nicardipine 0.15 mg/kg/hr for 14 days after the SAH (Haley 1993). Another trial studied AT877 given in three doses of 30 mg intravenously per day for 14 days (Shibuya 1992). One study assessed magnesium sulphate versus control in the absence of another calcium antagonist as standard treatment; in this study magnesium was given intravenously, 25 mg in 500 cc of 5% glucose, 20 to 40 drops per minute, one to two times a day for two to three weeks (exact dosage in mg unknown) (Luo 1996).
In all three trials where patients were randomised to receive either magnesium sulphate or placebo in addition to standard nimodipine treatment, magnesium sulphate was administered intravenously (Veyna 2002; van den Bergh 2005; Wong 2006). One trial used a bolus infusion of 6 g followed by continuous infusion at 2 g/hour for 10 days and dosage adjustments were made to maintain magnesium levels between 4 and 5.5 mg/dl (between 1.7 and 2.3 mmol/L). Patients in the placebo group received routine infusions of magnesium sulphate if serum magnesium was less than 2 mg/dl (0.8 mmol/L) (Veyna 2002). A second trial used magnesium sulphate 64 mmol/day continuously for 14 days after occlusion of the aneurysm or for 18 days after the SAH (van den Bergh 2005); a third trial used a bolus injection of 20 mmol followed by 80 mmol magnesium sulphate per day continuously for 14 days (Wong 2006).
Aneurysm treatment
In 11 of the 13 trials of calcium antagonists versus placebo, the aneurysm was treated by surgical clipping; in six trials, the aneurysm was clipped in all patients, in the other five trials 30% to 60% of patients had their aneurysm clipped. Two studies did not mention the method of aneurysm treatment (Luo 1996; Zhu 2001). In the three trials of magnesium sulphate versus placebo in addition to nimodipine, aneurysms were treated with surgical clipping (52% to 66% of all patients) as well as endovascular coiling (24% to 28% of all patients) (van den Bergh 2005; Veyna 2002; Wong 2006). In none of these three trials were results given separately according to the way the aneurysm was occluded.
Inclusion and exclusion criteria
In 2741 (82%) of the patients, aneurysms were confirmed by angiography or autopsy. In three trials angiography was not mandatory before randomisation; in one of these two trials an aneurysm was demonstrated by angiography or autopsy in 77% of patients (Neil‐Dwyer 1987), in the other trial in 66% of patients (Pickard 1989). One trial included patients with an aneurysmal pattern on CT scan, and in the absence of an aneurysmal pattern on CT an aneurysm had to be confirmed by angiography (van den Bergh 2005); another trial included patients with a SAH on CT scan, without further specification (Luo 1996). Five trials (Allen 1983; Han 1993; Messeter 1987; Ohman 1991; Philippon 1986) enrolled only patients in good or fairly good clinical condition on admission (Hunt and Hess grades I to III).
Outcome measures and follow‐up duration
In 10 trials poor outcome (death or dependence) was assessed at one to six months. Two trials measured functional outcome as early as after three weeks when most patients with aneurysmal SAH are still hospitalised and complications may still supervene (Ferro 1990; Philippon 1986). One trial report did not specify case fatality separately from poor outcome (Philippon 1986). Duration of follow up was 21 days in three trials (Allen 1983; Ferro 1990; Philippon 1986); duration of hospital stay in one trial (Luo 1996), one month in two trials (Shibuya 1992; Zhu 2001); two months in one trial (Messeter 1987); three months in six trials (Haley 1993; Neil‐Dwyer 1987; Petruk 1988; Pickard 1989; van den Bergh 2005; Veyna 2002), three to six months (mean duration 114 days) in one trial (Han 1993); six months in one trial (Wong 2006), and at six months and also at one to three years in one trial (Ohman 1991).
Data on secondary ischaemia (ischaemic neurological deficit by clinical criteria or CT‐scan‐documented cerebral infarction) were available for all 16 trials included in the analysis. Rebleeding during the follow‐up period was reported in nine studies (Allen 1983; Ferro 1990; Haley 1993; Neil‐Dwyer 1987; Petruk 1988; Pickard 1989; Shibuya 1992; Zhu 2001; van den Bergh 2005).
Risk of bias in included studies
Method of randomisation and data analysis
Six trials used envelopes (sealed, opaque, and sequentially numbered) as the method of randomisation (Haley 1993; Han 1993; Neil‐Dwyer 1987; Ohman 1991; Petruk 1988; Wong 2006). In five trials (Allen 1983; Ferro 1990; Messeter 1987; Shibuya 1992; van den Bergh 2005), numbered or coded containers administered sequentially to the enrolled participants were used to randomise patients to either the treatment or control group. In one trial (Pickard 1989), patients were randomised by means of separate randomisation lists (type unspecified) for each centre, balanced in blocks of four. In four trials the exact method of randomisation was not specified (Luo 1996; Philippon 1986; Veyna 2002; Zhu 2001).
Outcome assessment and comparability of the treatment groups
A double‐blind placebo‐controlled design was used in 12 of the 16 trials. In one trial outcome assessment was unblinded (Veyna 2002), and for another it remained unknown whether the outcome assessment was blinded (Han 1993). Two studies did not use a placebo and did not mention whether outcome assessment was blinded (Luo 1996; Zhu 2001). Data on outcome were incomplete in seven of the 16 included studies, mostly because patients with a different diagnosis or other protocol violations had been excluded from the analysis after randomisation (Allen 1983; Ferro 1990; Haley 1993; Petruk 1988; Philippon 1986; Shibuya 1992; Veyna 2002). The number of patients without data on follow up totalled 47 for the treatment group (5.7% of all patients in the treatment group of the seven trials with incomplete follow up, and 2.8% for all 16 trials); this number was 38 for the control group (4.5% of the control patients in the seven trials with incomplete follow up, and 2.2% for all 16 trials). Treatment and control groups were well balanced for major prognostic factors in 14 of the trials; two trials did not give information on baseline characteristics other than age and gender (Luo 1996; Zhu 1996).
Effects of interventions
Comparison 01: Poor outcome (death or dependence)
Calcium antagonists versus placebo
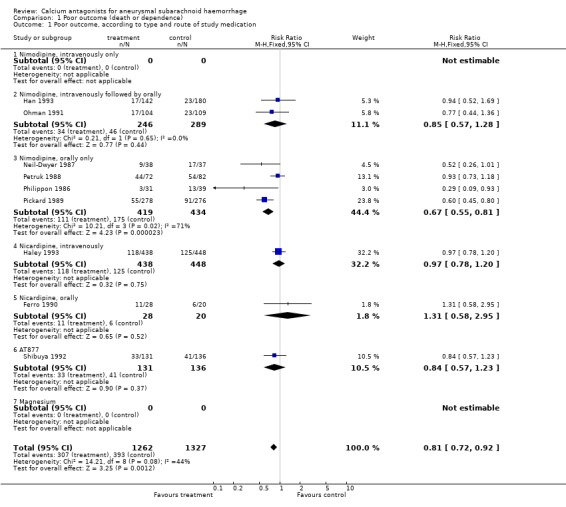
Nine trials adequately reported functional outcome within six months after the SAH, totalling 2589 patients (Ferro 1990; Haley 1993; Han 1993; Neil‐Dwyer 1987; Ohman 1991; Petruk 1988; Philippon 1986; Pickard 1989; Shibuya 1992) (Comparison 01.01). The relative risk (RR) for poor outcome (death or dependency) was 0.81 (95% confidence interval (CI) 0.72 to 0.92). The absolute risk reduction was 5.3%; the corresponding number of patients needed to treat to benefit (NNTB) to prevent a single poor outcome event was 19 (95% CI 1 to 51).
In a sensitivity analysis for trials of good methodological quality, we excluded four of the seven trials: one in which the method of blinding treatment allocation was not reported (Han 1993), and three in which it was uncertain whether assessment of outcome was blinded (Ferro 1990; Petruk 1988; Philippon 1986). The estimated effect without these trials was essentially unchanged (RR 0.74; 95% CI 0.62 to 0.90). The RR was just no longer statistically significant in the worst case scenario (RR 0.92; 95% CI 0.81 to 1.03) or when the largest trial (Pickard 1989) was excluded from the analysis (RR 0.88, 95% CI 0.77 to 1.03).
When two trials that reported on outcome as early as 21 days (Ferro 1990; Philippon 1986) were excluded, the RR remained essentially the same (RR 0.82, 95% CI 0.72 to 0.93).
Also a subgroup analysis of patients admitted within four days of SAH, with the exclusion of three trials in which patients were randomised until seven days after the SAH (Haley 1993; Han 1993; Ohman 1991), did not appreciably change the result (RR 0.71; 95% CI 0.63 to 0.80). Analysis for poor outcome according to type of calcium antagonist and route of administration showed that the results are statistically significant only for oral administration of nimodipine (RR 0.67, 95% CI 0.55 to 0.81). For the trials that administered nimodipine first intravenously and then orally, no statistically significant effect was found (RR 0.85; 95% CI 0.57 to 1.28). For the other calcium antagonists, the results were inconclusive: in the AT877 trial the relative risk for poor outcome was 0.84 (95% CI 0.57 to 1.23); for the trial of intravenous nicardipine 0.97 (95% CI: 0.78 to 1.20); and for one very small trial of oral nicardipine 1.31 (95% CI 0.58 to 2.95) (Comparison 01.01).
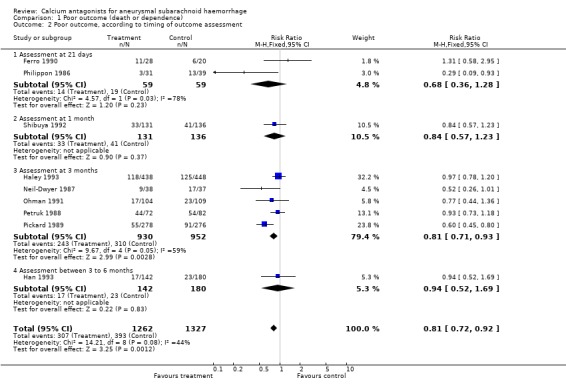
Comparison 01.02 shows poor outcome according to the timing of outcome assessment. There were no statistical differences between the subgroups of different calcium antagonists (P = 0.43) or between the subgroups according to timing of outcome assessment (P = 1.0) as determined by the Deeks method (Deeks 2001).
Magnesium sulphate versus placebo in addition to nimodipine
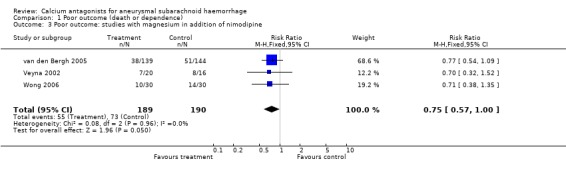
Data on poor outcome were available for all three trials totalling 379 patients (Wong 2006; van den Bergh 2005; Veyna 2002) (Comparison 01.03). The relative risk for poor outcome was borderline statistically significant: RR 0.75 (95% CI 0.57 to 1.00). The absolute risk reduction was 9% and the NNTB 11 (95% CI 5 to infinity). In the worst‐case analysis the relative risk was 0.77 (95% CI 0.58 to 1.03), and after exclusion of one methodologically poor trial (Veyna 2002) the relative risk was 0.76 (95% CI 0.56 to 1.03).
Comparison 02: Case fatality
Calcium antagonists versus placebo
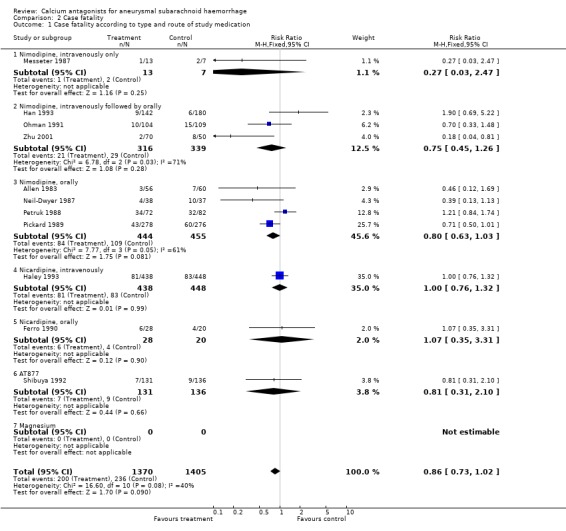
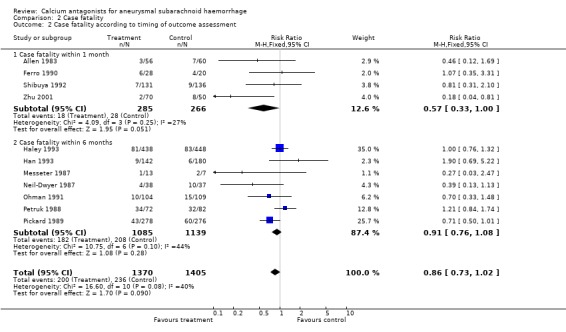
Case fatality was adequately reported in 11 trials totalling 2775 patients (Allen 1983; Ferro 1990; Haley 1993; Han 1993; Messeter 1987; Neil‐Dwyer 1987; Ohman 1991; Petruk 1988; Pickard 1989; Shibuya 1992; Zhu 2001) (Comparison 02.01). For all studies, the relative risk was 0.87 (95% CI 0.73 to 1.02). Case fatality according to timing of outcome assessment yielded similar results: for studies with outcome assessment within one month after the SAH the relative risk was 0.57 (95% CI 0.33 to 1.00), and for studies with outcome assessment up to six months the relative risk was 0.91 (95% CI 0.76 to 1.08) (Comparison 02.02). When poor quality studies (Han 1993; Petruk 1988; Zhu 2001) were excluded the result was just statistically significant (RR 0.82, 95% CI 0.67 to 0.99), as when three studies were excluded that randomised patients up to seven days after the SAH (Haley 1993; Han 1993; Ohman 1991) (RR 0.76, 95% CI 0.61 to 0.95). For separate drugs and routes of administration, the results were all not statistically significant, the relative risk for oral nimodipine was 0.80 (95% CI 0.63 to 1.03) (Comparison 02.01). There were no statistical differences between the subgroups of different calcium antagonists (P = 1.0) or between the subgroups according to timing of outcome assessment (P = 0.84).
Magnesium sulphate in addition to nimodipine
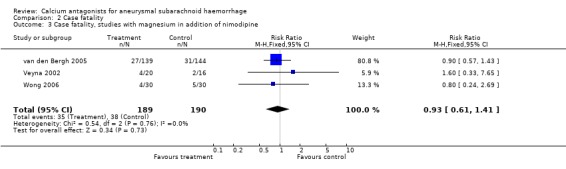
Case fatality was adequately reported in all three trials totalling 379 patients (van den Bergh 2005; Veyna 2002; Wong 2006) (Comparison 02.03). The relative risk was 0.93 (95% CI 0.61 to 1.41) for all studies, and when one poor quality study (Veyna 2002) was excluded it was 0.89 (95% CI 0.58 to 1.37).
Comparison 03: Secondary ischaemia
Calcium antagonists versus placebo
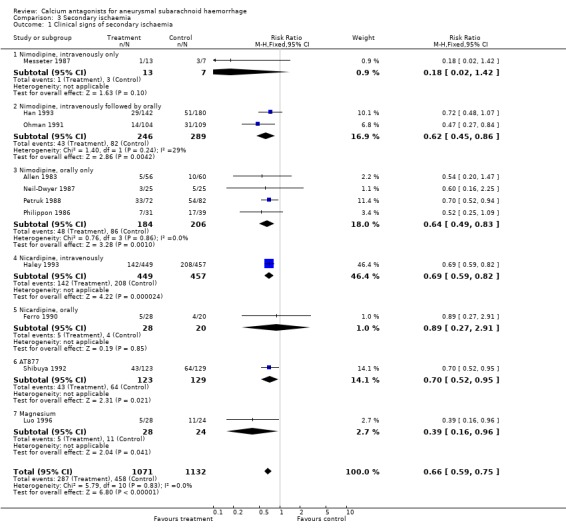
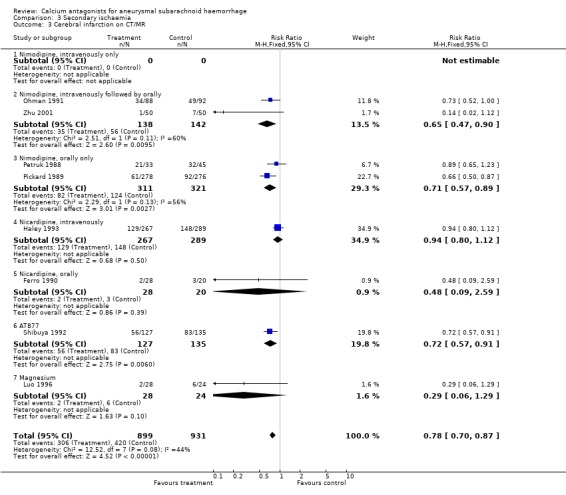
Data on clinical signs of secondary ischaemia were available for 11 trials (2303 patients) (Allen 1983; Ferro 1990; Haley 1993; Han 1993; Luo 1996; Messeter 1987; Neil‐Dwyer 1987; Ohman 1991; Petruk 1988; Philippon 1986; Shibuya 1992) (Comparison 03.01). The pooled relative risk was statistically significant: 0.66 (95% CI 0.59 to 0.75). Data on CT‐confirmed cerebral infarction were available in eight trials totalling 1830 patients (Ferro 1990; Haley 1993; Luo 1996; Ohman 1991; Petruk 1988; Pickard 1989; Shibuya 1992; Zhu 2001) (Comparison 03.03). The relative risk for CT‐confirmed infarction was 0.78 (95% CI 0.70 to 0.87). The absolute risk reduction was 14% for clinical signs of secondary ischaemia and 11% for infarction on CT. The corresponding numbers needed to treat were seven patients (95% CI 6 to 10) to prevent a clinical episode of secondary ischaemia, and nine patients (95% CI 6 to 15) to prevent a CT‐confirmed infarct. Also in the worst‐case scenario analyses the relative risk for clinical signs of secondary ischaemia was statistically significant (RR 0.78; 95% CI 0.70 to 0.87), and also when poor quality studies were excluded (RR 0.66, 95% CI 0.58 to 0.76) or after exclusion of three studies that included patients up to seven days after the SAH were excluded (Haley 1993; Han 1993; Ohman 1991) (RR 0.57, 95% CI 0.45 to 0.72). For oral nimodipine separately, the relative risk for a clinical episode of secondary ischaemia was 0.64 (95% CI 0.49 to 0.83), and for a CT‐confirmed infarct 0.71 (95% CI 0.57 to 0.89). Analyses by other routes of administration of nimodipine showed no significant differences for the protective effect on secondary ischaemia.
Nicardipine (RR 0.69, 95% CI 0.59 to 0.82), AT877 (RR 0.70, 95% CI 0.52 to 0.95) and magnesium sulphate (RR 0.29, 95% CI 0.06 to 0.87) yielded statistically significant results for clinical signs of secondary ischaemia. There were no statistical differences between the subgroups of different calcium antagonists for occurrence of secondary ischemia (P = 0.73).
Magnesium sulphate in addition to nimodipine
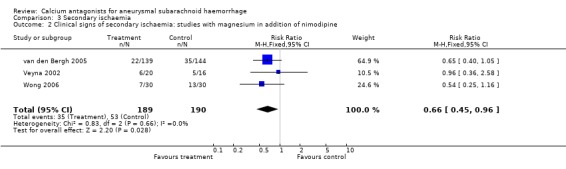
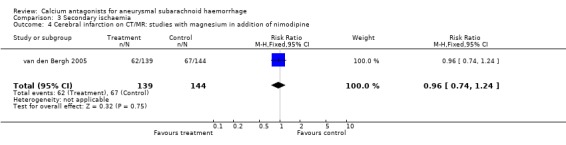
Data on clinical signs of secondary ischaemia were available for all three trials, totalling 379 patients (van den Bergh 2005; Veyna 2002; Wong 2006) (Comparison 03.02). The relative risk was 0.66 (95% CI 0.45 to 0.96). The absolute risk reduction was 11% and the corresponding NNTB 11patients (95% CI 6 to 108). The results were essentially the same in the worst‐case scenario (RR 0.68, 95% CI 0.46 to 0.98) and when one poor quality study was excluded (Veyna 2002), RR 0.62 (95% CI 0.41 to 0.93). Data on infarction on CT or magnetic resonance imaging (MRI) scan were not available.
Comparison 04: Rebleeding
Calcium antagonists versus placebo
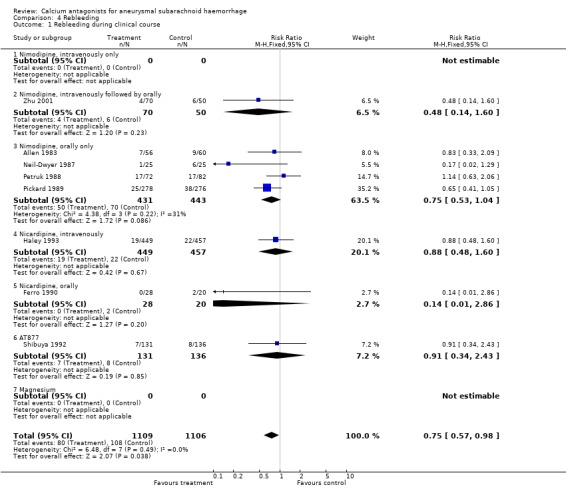
Data were available in eight studies totalling 2215 patients (Allen 1983; Ferro 1990; Haley 1993; Neil‐Dwyer 1987; Petruk 1988; Pickard 1989; Shibuya 1992; Zhu 2001) (Comparison 04.01). There was a statistically significant reduction in the frequency of rebleeding among patients allocated to calcium antagonist treatment: RR 0.75 (95% CI 0.57 to 0.98). The absolute risk reduction was 3% and the corresponding NNTB 39 (95% CI 21 to 431). There were no statistical differences between the subgroups of different calcium antagonists (P = 0.72).
Magnesium sulphate in addition to nimodipine
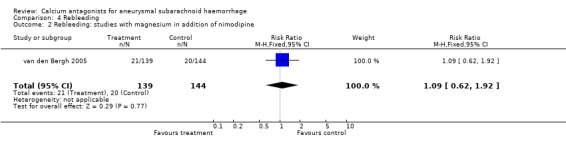
Data on rebleeding were available for one trial totalling 283 patients, with a relative risk of 1.09 (95% CI 0.62 to 1.92) (van den Bergh 2005) (Comparison 04.01).
Adverse effects
Calcium antagonists versus placebo
Adverse effects were reported in six of the 13 trials (Allen 1983; Haley 1993; Petruk 1988; Philippon 1986; Pickard 1989; Shibuya 1992 ), but the actual number of patients with adverse events was available only in two nimodipine trials (Petruk 1988; Pickard 1989) and in one nicardipine trial (Haley 1993). Two reports (Allen 1983; Philippon 1986) stated that there were no side effects or adverse reactions attributable to nimodipine. No serious adverse effects have been reported with AT877 given intravenously (30 mg over 30 minutes, three times a day) (Shibuya 1992). In the two nimodipine trials with actual numbers of serious adverse events, hypotension was reported in 2.1% of nimodipine treated patients and in 1.4% of controls, and reversible dysfunction of the liver/biliary system in 1.4% of nimodipine treated patients and in 1.8% of controls. All other reported adverse events had occurred in only one or two patients. Nicardipine treatment was associated with hypotension (34% versus 5% in controls), phlebitis at the injection site (22% versus 5% in controls), and with pulmonary oedema in combination with azotemia (6% versus 2% in controls).
Magnesium sulphate in addition to nimodipine
Two studies reported on adverse effects. In one study none of the patients had a serious adverse event, three patients reported a warm feeling during the start of study drug infusion, and another patient receiving magnesium sulphate complained of pain during injection (Wong 2006). Another study reported no serious side effects, but mentioned that in the treatment group eight patients discontinued study medication because of side effects (hypotension 1, bradycardia 2, atrial fibrillation 1, hypermagnesaemia 3, renal failure 1) versus two patients in the control group (phlebitis 1, intracranial haematoma 1) (van den Bergh 2005).
Statistical homogeneity
The assumption of statistical homogeneity between studies was not violated in any of the main analyses. However, in some sub‐analyses the I‐squared statistic was higher than 50% (oral nimodipine in analyses for poor outcome, case fatality and infarction on CT, intravenous nimodipine followed by oral administration in analyses for case fatality and infarction on CT, outcome assessment at 21 days and at three months in poor outcome analysis) and sensitivity analyses (worst outcome analyses for poor outcome, clinical signs of secondary ischaemia and rebleeding).
Discussion
Effect of prophylactic calcium antagonists on clinical outcome and secondary ischaemia
Calcium antagonists versus placebo
Our aggregation of all calcium antagonist trials suggests a reduction of poor outcome (defined as death or dependency in activities of daily life) after SAH, though the reduction of case fatality alone is not statistically significant. In addition, calcium antagonists reduce the frequency of secondary ischaemia; this is probably the most likely intermediate factor through which calcium antagonists exert their beneficial effect on outcome. The data also indicate that oral nimodipine improves the overall outcome, in agreement with the conclusions of previous overviews (Barker 1996; Mascio 1994; Robinson 1990; Tettenborn 1990). There is no evidence that nimodipine or other calcium antagonists improve outcome when given intravenously. In the worst‐case‐scenario analysis and after exclusion of the largest trial with oral nimodipine, the benefit in terms of reduction in 'poor outcome' is no longer statistically significant, which implies that the benefits of nimodipine are not beyond all reasonable doubt. Also, there was statistical heterogeneity between the studies for oral nimodipine and the beneficial effect on poor outcome for oral nimodipine was mainly based on one large study (Pickard 1989). Another issue that must be addressed is that in none of these studies were aneurysms treated by endovascular coiling, so strictly there is no information on calcium antagonist therapy in patients treated with this technique. Nevertheless, since the point estimates remain essentially the same in the sensitivity analyses, our data strongly suggest that oral nimodipine (60 mg every four hours) is efficacious as initial treatment in patients with aneurysmal SAH. Intravenous administration of calcium antagonists, which is more expensive and has a substantial risk of induced hypotension (Haley 1993), cannot be recommended on the basis of the present evidence. However, the lack of statistical significance of intravenous calcium antagonists could be part due to the small number of patients studied with this strategy. For magnesium in addition to nimodipine, the results of this meta‐analysis are very promising. The relative risk for both poor outcome and the occurrence of clinical secondary ischemia were statistically significant, and the relative risk for case fatality showed a favourable trend. However, the number of included patients is small and these results need to be confirmed by larger trials, of which two are currently being conducted (IMASH; MASH‐II).
Effect of calcium antagonists on rebleeding
Our data suggest that the use of calcium antagonists in patients with SAH is not associated with an increased frequency of rebleeding; in contrast, we found a statistically significant reduction in the rate of rebleeding. This reduction is independent from the clinical condition on admission of patients and from the type of calcium antagonists used, but it may well be a confounding effect through the protection against ischaemia, which allows earlier operation. Only one magnesium study reported on rebleeding and the result is inconclusive.
Adverse effects
In the current review, dose‐related effects of calcium antagonist drugs other than nimodipine could not be addressed because of the lack of sufficient data. In a randomised double‐blind dose‐comparing study of nicardipine (Haley 1994), the beneficial clinical effects of high‐dose (0.15 mg/kg/hr) and low‐dose (0.075 mg/kg/hr) intravenous nicardipine treatment were virtually equivalent, but administration of low‐dose nicardipine was attended by fewer serious side effects. Similar results have been obtained in another dose‐ranging study (Massiou 1992) where the tolerability of four doses of intravenous nicardipine (0.03, 0.08, 0.11, and 0.15 mg/kg/hr) was assessed. A dose escalating open clinical trial (Shibuya 1990) comparing various doses of AT877 has indicated that AT877 has its most favourable effects at a dosage of 90 mg per day. Two of the magnesium studies reported adverse effects; there were no serious adverse effects.
Methodological issues of the present overview
We have examined all randomised controlled trials in which any type of calcium antagonist was compared with control in patients with SAH. The trials were comparable with respect to study design and selection of patients, and most had a well‐balanced distribution of prognostic factors for poor outcome between treatment and control groups, which adds to the validity of the results. Sensitivity analyses showed that the beneficial results in terms of risk reduction for poor outcome were robust in that the results did not appreciably change after exclusion of trials with questionable blinding or trials with randomisation of patients more than four days after SAH, or after inclusion of trials with very late enrolment or very early assessment. The benefits were no longer statistically significant when a worst‐case‐scenario analysis was applied to patients who had been randomised but not accounted for in the published reports, because of erroneous diagnosis or protocol violations.
Since the mechanisms of action differ for the calcium antagonists included in the review, we separately assessed the efficacy of each drug. The reason for including AT877 in the overview on calcium antagonists was that, apart from protein kinase inhibiting properties (Seto 1995), this drug has also an intracellular calcium antagonist action (Satoh 1992; Takizawa 1993).
Authors' conclusions
Implications for practice.
Based on our conclusions, we recommend oral nimodipine (60 mg every four hours, to be continued for three weeks) as standard treatment in patients with aneurysmal subarachnoid haemorrhage. Although the evidence about the beneficial effect of nimodipine is not beyond all doubt, and is mainly based on one large study where aneurysms were treated with surgical clipping, we recommend oral nimodipine given the potential benefits and modest risks associated with it. Intravenous administration of calcium antagonists is more expensive and potentially hazardous in view of hypotensive effects, and is therefore not recommended. There is no evidence that nicardipine or AT877 has a significant effect on functional outcome after aneurysmal subarachnoid haemorrhage.
Implications for research.
Given that the evidence for the protective effect of calcium antagonists against secondary ischaemia and poor outcome after aneurysmal subarachnoid haemorrhage is no longer statistically significant after exclusion of a single large trial with oral nimodipine, and that most studies have been performed in the era before coiling was available, further placebo‐controlled trials of oral nimodipine could provide a more definitive conclusion. In practice, the support for a confirmatory study is bound to be modest. Even on the assumption that the benefits of oral nimodipine after SAH are beyond any doubt and are also valid in patients with aneurysm occlusion by means of coiling, there are other unresolved issues: the advantages and disadvantages in patients in poor clinical condition on admission or in patients with established cerebral ischaemia, the optimal dose, the optimal time window, and the question whether other types of calcium antagonists offer better protection. Magnesium sulphate is a potent calcium antagonist and a promising agent in patients with SAH; further trials are needed and under way.
Feedback
Results for nimodipine separately
Summary
This review pools data from trials of nimodipine, nicardipine and AT877 in patients with subarachnoid haemorrhage (SAH). Since nimodipine alone is widely used in SAH, the results for its effects need to be reported separately for all outcomes, not for just some as in the review now.
Reply
In the update of November 2001, also for secondary ischaemia, the results of nimodipine are reported separately. Therefore, the updated version provides results for nimodipine separately for all outcomes (for poor outcome and rebleeding in the analyses figures, for the other outcomes in the text). All data are provided in such a way that consumers can make additional calculations if they want to.
Contributors
Comment: R Schuurman, MD, Dept of Neurosurgery, Academic Medical Centre, Amsterdam, The Netherlands
Reply: GJE Rinkel
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 27 October 2006 | New search has been performed | The protocol of the review has been slightly changed because trials have subsequently been published where patients were randomised to receive a second calcium antagonist (magnesium sulphate) versus control in addition to another calcium antagonist (nimodipine) in both the treatment and control groups. These trials were analysed separately. Eight new trials were identified, and four trials were included in the review. These included one trial of nimodipine and three trials of magnesium sulphate. The main results and conclusions of the review are unchanged. Phrasing has been amended throughout the text. |
| 27 October 2006 | New citation required but conclusions have not changed | Change of authorship. |
Acknowledgements
We would like to thank Dr C Counsell, Professor PAG Sandercock, and Professor CP Warlow for their valuable comments, which helped us with preparation of the original review, and we greatly appreciate the assistance of the Cochrane Stroke Group in identifying all randomised controlled trials of the use of calcium antagonists in patients with subarachnoid haemorrhage. We are grateful to Dr SH Lee for providing us with extra data from the trial by Dr DH Han. The authors also wish to thank Dr JM Ferro for providing us with unpublished data from his trial and Drs K Mee, K Messeter, J Öhman, KC Petruk, JD Pickard, J Philippon, W Poon, TJ Preziosi, M Shibuya, and JC Torner for taking the trouble to reply to our correspondence. The authors would also like to thank Dr TE Vinogradova for her help in handsearching relevant randomised controlled trials of stroke in Russia and Dr S Tan for translating Chinese articles.
Appendices
Appendix 1. MEDLINE search strategy
The following search strategy, using a combination of controlled vocabulary and free text terms, was used for MEDLINE and modified for EMBASE.
MEDLINE (Ovid) 1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. Vasospasm, Intracranial/ 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. exp Calcium Channel Blockers/ 15. Calcium/ai [Antagonists & Inhibitors] 16. calcium antagonist$.tw. 17. (amlodipine or amrinone or bencyclan$ or bepridil or AT877 or AT 877 or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sul$ or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil).tw. 18. 14 or 15 or 16 or 17 19. 13 and 18 20. limit 19 to human
Data and analyses
Comparison 1. Poor outcome (death or dependence).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor outcome, according to type and route of study medication | 9 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.92] |
| 1.1 Nimodipine, intravenously only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Nimodipine, intravenously followed by orally | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.28] |
| 1.3 Nimodipine, orally only | 4 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.81] |
| 1.4 Nicardipine, intravenously | 1 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.20] |
| 1.5 Nicardipine, orally | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.95] |
| 1.6 AT877 | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.23] |
| 1.7 Magnesium | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Poor outcome, according to timing of outcome assessment | 9 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.92] |
| 2.1 Assessment at 21 days | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.28] |
| 2.2 Assessment at 1 month | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.23] |
| 2.3 Assessment at 3 months | 5 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.93] |
| 2.4 Assessment between 3 to 6 months | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.52, 1.69] |
| 3 Poor outcome: studies with magnesium in addition of nimodipine | 3 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 1.00] |
1.1. Analysis.
Comparison 1 Poor outcome (death or dependence), Outcome 1 Poor outcome, according to type and route of study medication.
1.2. Analysis.
Comparison 1 Poor outcome (death or dependence), Outcome 2 Poor outcome, according to timing of outcome assessment.
1.3. Analysis.
Comparison 1 Poor outcome (death or dependence), Outcome 3 Poor outcome: studies with magnesium in addition of nimodipine.
Comparison 2. Case fatality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality according to type and route of study medication | 11 | 2775 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.02] |
| 1.1 Nimodipine, intravenously only | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.47] |
| 1.2 Nimodipine, intravenously followed by orally | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.26] |
| 1.3 Nimodipine, orally | 4 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.03] |
| 1.4 Nicardipine, intravenously | 1 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| 1.5 Nicardipine, orally | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.35, 3.31] |
| 1.6 AT877 | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.31, 2.10] |
| 1.7 Magnesium | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Case fatality according to timing of outcome assessment | 11 | 2775 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.02] |
| 2.1 Case fatality within 1 month | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 1.00] |
| 2.2 Case fatality within 6 months | 7 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 3 Case fatality, studies with magnesium in addition of nimodipine | 3 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.41] |
2.1. Analysis.
Comparison 2 Case fatality, Outcome 1 Case fatality according to type and route of study medication.
2.2. Analysis.
Comparison 2 Case fatality, Outcome 2 Case fatality according to timing of outcome assessment.
2.3. Analysis.
Comparison 2 Case fatality, Outcome 3 Case fatality, studies with magnesium in addition of nimodipine.
Comparison 3. Secondary ischaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical signs of secondary ischaemia | 11 | 2203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.59, 0.75] |
| 1.1 Nimodipine, intravenously only | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.42] |
| 1.2 Nimodipine, intravenously followed by orally | 2 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.86] |
| 1.3 Nimodipine, orally only | 4 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.83] |
| 1.4 Nicardipine, intravenously | 1 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.82] |
| 1.5 Nicardipine, orally | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.27, 2.91] |
| 1.6 AT877 | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 1.7 Magnesium | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.96] |
| 2 Clinical signs of secondary ischaemia: studies with magnesium in addition of nimodipine | 3 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.96] |
| 3 Cerebral infarction on CT/MR | 8 | 1830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 3.1 Nimodipine, intravenously only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Nimodipine, intravenously followed by orally | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 3.3 Nimodipine, orally only | 2 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.57, 0.89] |
| 3.4 Nicardipine, intravenously | 1 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.12] |
| 3.5 Nicardipine, orally | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.59] |
| 3.6 AT877 | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 3.7 Magnesium | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.29] |
| 4 Cerebral infarction on CT/MR: studies with magnesium in addition of nimodipine | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.24] |
3.1. Analysis.
Comparison 3 Secondary ischaemia, Outcome 1 Clinical signs of secondary ischaemia.
3.2. Analysis.
Comparison 3 Secondary ischaemia, Outcome 2 Clinical signs of secondary ischaemia: studies with magnesium in addition of nimodipine.
3.3. Analysis.
Comparison 3 Secondary ischaemia, Outcome 3 Cerebral infarction on CT/MR.
3.4. Analysis.
Comparison 3 Secondary ischaemia, Outcome 4 Cerebral infarction on CT/MR: studies with magnesium in addition of nimodipine.
Comparison 4. Rebleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rebleeding during clinical course | 8 | 2215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.98] |
| 1.1 Nimodipine, intravenously only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Nimodipine, intravenously followed by orally | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.60] |
| 1.3 Nimodipine, orally only | 4 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.04] |
| 1.4 Nicardipine, intravenously | 1 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.60] |
| 1.5 Nicardipine, orally | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.86] |
| 1.6 AT877 | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.34, 2.43] |
| 1.7 Magnesium | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Rebleeding: studies with magnesium in addition of nimodipine | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.62, 1.92] |
4.1. Analysis.
Comparison 4 Rebleeding, Outcome 1 Rebleeding during clinical course.
4.2. Analysis.
Comparison 4 Rebleeding, Outcome 2 Rebleeding: studies with magnesium in addition of nimodipine.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 1983.
| Methods | Method of randomisation: numbered or coded containers administered sequentially to enrolled participants Blinding: partly double‐blind design Analysis: after exclusion of protocol violations (9 of 125 patients) Post‐randomisation exclusions: 2 of 58 patients in treatment and 3 of 63 patients in control group Definition of outcomes: stated | |
| Participants | Location: 5 university centres from the USA and Canada Rx: 58 (male to female ratio 0.6) Controls: 63 (male to female ratio 0.5) Age range: 17 to 79 years Entry criteria: documented aneurysmal SAH by either CT scan or CSF examination, and angiography; SAH within 96 hours of the start of nimodipine treatment; Hunt and Hess grade I or II just before the start of medication Timing of surgery: within 14 days of entry (not further specified) Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: gelatin capsules containing nimodipine (0.7 mg/kg initially and followed by 0.35 mg/kg 6 times per day) for 21 days Controls: gelatin capsules containing placebo for 21 days | |
| Outcomes | Clinical outcomes: development of a neurological deficit from cerebral arterial spasm, and the severity of the deficit after 21 days of treatment (including death) Additional measures: CT scan rankings according to neurological outcome; quantitative assessment of the severity of vasospasm by angiography; evaluation of the concentration of nimodipine in CSF and blood at the time of surgery and the hemodynamic effects | |
| Notes | Exclusion criteria: neurological deficit on entry Follow‐up duration: 21 days Adverse effects: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ferro 1990.
| Methods | Method of randomisation: numbered containers administered sequentially to enrolled participants Blinding: double‐blind treatment; masking of outcome assessment not stated Analysis: on‐treatment analysis Post‐randomisation exclusions: 2 (both from Rx group) because of incorrect diagnosis (no SAH) Definition of outcomes: stated | |
| Participants | Location: Portugal Rx: 28 (male to female ratio 0.6) Controls: 20 (male to female ratio 1.2) Age range: 21 to 69 years (mean 51 years) Entry criteria: age 21 to 69 years, SAH within 96 hours of onset confirmed by CT scan or CSF examination or both Timing of surgery: after the seventh day of SAH onset (16 of 28 Rx group patients and 8 of 20 controls had aneurysm surgery) Comparability of treatment groups: fairly good for major prognostic factors | |
| Interventions | Rx: nicardipine (60 mg) orally daily every 8 hours for 21 days Controls: placebo orally every 8 hours for 21 days Steroids and phenobarbital were given to all patients; symptomatic vasospasm was managed with volume expansion therapy | |
| Outcomes | Clinical outcomes: death, rebleeds, delayed cerebral ischaemia, and modified Rankin scale at 21 days after SAH Additional measurements: severity of angiographic vasospasm, new infarcts on CT scan, and neuropsychological evaluation | |
| Notes | Exclusion criteria: non‐aneurysmal causes of SAH as defined by CT scan, patients who were taking anticoagulants, severe systemic illnesses (cancer, renal or hepatic failure), comatose patients (Hunt and Hess grade V) Follow‐up duration: 21 days Adverse effects: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Haley 1993.
| Methods | Method of randomisation: numbered or coded containers administered sequentially to enrolled participants Blinding: double‐blind treatment and blinded assessment of all outcomes Analysis: after exclusion of protocol violations (110 of 906 patients) Post‐randomisation exclusions due to protocol violations: 11 of 449 patients in treatment and 9 of 457 patients in control group, only for clinical outcome and for case fatality, not for secondary ischemia and for rebleeding Definition of outcomes: stated | |
| Participants | Location: 41 North American neurosurgical centres Rx: 449 (male to female ratio 0.6) Controls: 457 (male to female ratio 0.5) Age range: 18 years or older (mean age for Rx 49.7 years, for controls 50.1 years) Entry criteria: angiographically documented saccular aneurysmal SAH within 7 days of the start of nicardipine treatment Timing of surgery: 0 to 3 days ‐ 68.5% in nicardipine treated patients and 65.8% in controls; 4 to 6 days ‐ 14.6% and 17.2%; 7 to 10 days ‐ 5.7% and 6.4%; 11 to 14 days ‐ 6.7% and 5.4%; 15+ days ‐ 4.5% and 5.2% respectively Comparability of treatment groups: good for major prognostic factors 265 (29%) of patients in poor clinical condition (WFNS IV or V) | |
| Interventions | Rx: continuous intravenous infusion of high‐dose nicardipine (0.15 mg/kg/hr) for up to 14 days Controls: continuous intravenous infusion of placebo for up to 14 days | |
| Outcomes | Clinical outcomes: a good recovery according to the GOS, mortality, disability, development of infarction and delayed ischemic deficit due to vasospasm at 3 months following SAH Additional measurements: graded neurological examination according to National Institutes of Health Stroke Scale and Folstein Mini‐Mental State; follow‐up cerebral angiography and transcranial Doppler ultrasonography | |
| Notes | Exclusion criteria: complicating illness, prior use of calcium antagonists, discharge within 14 days, other therapy for vasospasm such as reserpine treatment, angioplasty Follow‐up duration: 3 months Adverse effects: mild or moderate hypotension in 34.5% of cases and in 17.5 % of controls (life‐threatening hypotension in 3% of either group); phlebitis at the injection site in 22.3% of cases and in 5% of controls; pulmonary oedema in combination with azotemia in 6% of nicardipine‐treated patients and in 2.4% of controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Han 1993.
| Methods | Method of randomisation: sequentially numbered sealed opaque envelopes Blinding: not stated Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcomes: stated | |
| Participants | Location: USA Rx: 142 Controls: 180 Sex: males/females (actual number of patients by sex are not provided) Age range: 24 to 79 years of age for the treatment group (mean age 50.4 years); 18 to 70 years of age for the control group (mean 49.6 years) Entry criteria: all patients who underwent surgery for intracranial ruptured aneurysm during the study period Timing of surgery: 0 to 3 days ‐ 19.7% in nimodipine treated patients and 11.1% in controls; 4 to 14 days ‐ 59.1% and 35.5%; more than 14 days ‐ 21.2% and 43.3% respectively Comparability of treatment group: good for major prognostic factors | |
| Interventions | Rx: nimodipine intravenously at 30 microgram/kg/hr for the first week beginning on the day of admission; then given orally at 360 mg/day for the following two weeks Controls: treatment without nimodipine during the same period | |
| Outcomes | Clinical outcome measures: death and poor outcome (GOS equal to or more than grade 3) from all causes, death due to delayed ischaemic deficit, development of delayed ischaemic deficit | |
| Notes | Exclusion criteria: not stated Follow‐up duration: 3 to 6 months (mean 3.8 months) Adverse effects: not reported Unpublished data were provided by Dr Sun Ho Lee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Luo 1996.
| Methods | Method of randomisation: not stated Blinding: not stated, but no placebo used Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcomes: stated | |
| Participants | Location: Guangdong, China Rx: 28 Controls: 24 Sex: 24 male in Rx group and 18 male in control group Age range: 20 to 70 years of age for the treatment group (mean age 53 years); 15 to 80 years of age for the control group (mean 50 years) Entry criteria: SAH on CT scan Timing of aneurysm treatment: not mentioned Comparability of treatment group: no information | |
| Interventions | Rx: Magnesium sulphate 25 mg in 500 cc of 5% glucose, intravenously 20 to 40 drops per minute for 1 to 2 times a day during 2 to 3 weeks Controls: regular treatment | |
| Outcomes | (1) Clinical symptoms of vasospasm (2) Infarction on CT (3) Death | |
| Notes | Exclusion criteria: abnormal densities brain tissue on admission CT Follow‐up duration: hospital stay Adverse effects: not registered NB: After development of clinical symptoms, patients in the control group were also given magnesium sulphate; data on death therefore not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Messeter 1987.
| Methods | Method of randomisation: numbered or coded containers administered sequentially to enrolled participants Blinding: not stated Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcomes: not stated for functional outcomes | |
| Participants | Location: University Hospital, Sweden Rx: 13 (male to female ratio 0.5) Controls: 7 (male to female ratio 0.7) Age range: 23 to 59 years (mean 44 years) Entry criteria: non‐consecutive series of good grade patients (Hunt and Hess grade I‐III) with aneurysmal SAH documented by angiography who were subjected to early aneurysm surgery within 72 hours after SAH Timing of surgery: aneurysm surgery within 72 hours after SAH Comparability of treatment groups: fairly good for major prognostic factors | |
| Interventions | Rx: nimodipine intraoperatively to the exposed arterial segment followed by intravenously at approximately 2 mg/hr for at least 9 days Controls: nimodipine was not given, but in every other respect the management was the same as in nimodipine‐treated patients | |
| Outcomes | Clinical outcomes: delayed ischaemic deterioration and death, functional outcome only in broad terms without time indication Additional measurements: hemispheric CBF (xenon method) during anaesthesia before craniotomy | |
| Notes | Exclusion criteria: not specified Follow‐up duration: two months Adverse effects: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Neil‐Dwyer 1987.
| Methods | Method of randomisation: sequentially numbered sealed opaque envelopes Blinding: double‐blinded treatment and blinded assessment of all outcomes Analysis: both intention‐to‐treat analysis and analysis after exclusion of protocol violations (25 of 75 patients) Losses to follow up: none Definition of outcome events: stated | |
| Participants | Location: London, UK Rx: 38 (male to female ratio 0.5) Controls: 37 (male to female ratio 0.5) Age range: 18 to 65 years (mean age for Rx 47 years, for controls 50 years) Entry criteria: patients of all neurological grades with documented SAH by CT scan or CSF examination within 96 hours after SAH (angiographically verified ruptured aneurysms were found in 58 (77%) of all randomised patients) Timing of surgery: mean day, 13 in nimodipine treated patients and 9 in controls Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: nimodipine (two 30 mg tablets) orally every 4 hours for 21 days Controls: two placebo tablets orally every 4 hours for 21 days In 17 of 40 patients, nimodipine or placebo vehicle was installed into the basal cisterns intraoperatively after aneurysm clipping | |
| Outcomes | Clinical outcomes: death, delayed ischaemic neurological deficit, good or poor functional outcome, rebleeding, operative complications Additional measurements: CBF evaluation using the xenon‐133 inhalation method; mean arterial blood pressure | |
| Notes | Exclusion criteria: no aneurysm at angiography, death within 72 hours from aneurysm rupture, withdrawn owing to condition, nimodipine treatment less than 21 days Follow‐up duration: 3 months Adverse effects: initial fall of the mean blood pressure of approximately 5 mmHg over the first 24 hours of nimodipine treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ohman 1991.
| Methods | Method of randomisation: sequentially numbered sealed opaque envelopes Blinding: double‐blinded treatment and blinded assessment of late outcomes, including radiological assessments Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcome events: stated | |
| Participants | Location: Helsinki University Central Hospital, Finland Rx: 104 (male to female ratio 1.0) Controls: 109 (male to female ratio 0.9) Age range: 16 to 70 years (mean age for Rx 44 years, for controls 44 years) Entry criteria: patients with aneurysmal SAH documented by CT scan and angiography who were in Hunt and Hess grades I‐III on admission Timing of surgery: early surgery was performed in 27 of 91 nimodipine treated patients and in 27 of 92 placebo treated patients, subacute surgery in 31 of 91 and in 29 of 92, and late surgery in 33 of 91 and in 36 of 92 patients respectively Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: nimodipine 0.25 microgram/kg/min intravenously for 2 hours followed by 0.5 microgram/kg/min continuous intravenous infusion for 7 to 10 days and then orally 60 mg tablets every 4 hours until 21 days after SAH Controls: placebo by continuous intravenous infusion for 7 to 10 days followed by orally administered placebo tablets every 4 hours until 21 days after SAH | |
| Outcomes | Clinical outcomes: independent state, dependent state, or death at 6 months and good outcome, moderate disability, severe disability, or death at 1 to 3 years after aneurysmal SAH and surgery Additional measurements: platelet function | |
| Notes | Exclusion criteria: patients who had used non‐steroidal anti‐inflammatory drugs during the 2 weeks before admission or any other calcium antagonist or investigative drug, patients with an associated intracerebral haematoma and rapidly decreased level of consciousness, pregnancy, hepatic or renal insufficiency, severe cardiac failure, cardiac arrhythmia Follow‐up duration: 6 months and 1 to 3 years Adverse effects: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Petruk 1988.
| Methods | Method of randomisation: sequentially numbered sealed opaque envelopes Blinding: double‐blinded treatment, blinded radiographic assessment (it is not stated whether clinical outcome assessment was blinded) Analysis: after exclusion of protocol violations (34 of 188 patients) Losses to follow up: 18 of 91 patients in treatment group and 14 of 97 patients in control group; and 2 unspecified patients Definition of outcome events: stated | |
| Participants | Location: 17 Canadian hospitals Rx: 91 (male to female ratio 0.6) Controls: 97 (male to female ratio 0.4) Age range: 18 years or older (mean age for Rx 53.8 years, for controls 56.1 years) Entry criteria: non‐pregnant poor grade patients (Hunt and Hess grade III or IV) with aneurysmal SAH documented by CT scan or CSF examination, and angiography within 96 hours after SAH Timing of surgery: surgery was performed on 46 nimodipine treated patients and 47 controls (71% were operated on within days 0 to 3 after SAH) Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: gelatin capsules containing nimodipine (90 mg) every 4 hours for 21 days after SAH Controls: gelatin capsules containing placebo every 4 hours for 21 days after SAH | |
| Outcomes | Clinical outcomes: according to GOS on day 21 and at 3 months after SAH Additional measurements: radiographic studies (CT scan and cerebral angiography) | |
| Notes | Exclusion criteria: proven SAH within the previous month, no angiography or aneurysm, calcium antagonist treatment prior to study entry, study drug treatment less than 21 days Follow‐up duration: 21 days and 3 months Adverse effects (usually hypotension) reported in 19 of 91 nimodipine treated patients and in 24 of 97 placebo treated patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Philippon 1986.
| Methods | Method of randomisation: not specified Blinding: double‐blind treatment, blinding of outcome assessment is not specified Analysis: after exclusion of protocol violations (11 of 81 patients) Excluded from follow up: 8 of 39 patients in treatment group and 3 of 42 patients in control group Definition of outcome events: stated | |
| Participants | Location: Hospital de la Salpêtrière, Paris, France Rx: 39 (male to female ratio 0.8) Controls: 42 (male to female ratio 0.7) Age range: 15 to 65 years (mean age for Rx 44.3 years, for controls 45.6 years) Entry criteria: patients with aneurysmal SAH documented by CT scan and angiography within 72 hours after aneurysm rupture who had Hunt and Hess grades I‐III on admission and did not present an early complication of SAH (hydrocephalus, significant intracerebral haematoma) Timing of surgery: 38.7% of nimodipine treated patients and 33.3% of placebo treated patients were operated on between days 4 and 10 after SAH; 32.3% and 33.3%, later than day 10 after SAH; and 29.0% and 33.3% were not operated on, respectively Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: nimodipine orally (60 mg every 4 hours) for 21 days after SAH Controls: placebo orally every 4 hours for 21 days after SAH | |
| Outcomes | Clinical outcomes were classified according to the GOS at 21 day after SAH Additional measurements for the subset of patients with secondary ischaemia: severity of angiographic vasospasm | |
| Notes | Exclusion criteria: patients who were operated on prior to day 4 after SAH; patients with vasospasm at the first diagnostic angiography; patients with arterial hypertension or with cardiac, liver or kidney insufficiency Follow‐up duration: 21 days Adverse effects: not observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pickard 1989.
| Methods | Method of randomisation: separate randomisation lists for each centre balanced in blocks of four Blinding: double‐blinded treatment, blinded assessment of all outcomes (including radiographic assessments) Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcome events: stated | |
| Participants | Location: 5 centres in UK Rx: 278 (male to female ratio 0.7) Controls: 276 (male to female ratio 0.6) Age range: 18 years or older (mean age for Rx 46 years, for controls 48 years) Entry criteria: documented by lumbar puncture or CT scan SAH within 96 hours of the start of nimodipine treatment (SAH due to aneurysm rupture was documented by angiography in 368 (66%) of all randomised patients) Timing of surgery: mean 10.8 days in nimodipine treated patients and 11.3 days in controls; 59.4% of cases and 55.8% of controls were not operated on Comparability of treatment groups: good for major prognostic factors 59 (11%) of patients in poor clinical condition (WFNS IV or V) | |
| Interventions | Rx: nimodipine (two 30 mg tablets) orally every 4 hours for 21 days Controls: placebo tablets orally every 4 hours for 21 days | |
| Outcomes | Clinical outcomes: frequency of cerebral infarction and ischaemic neurologic deficit, death, severe disability, and good recovery at 3 months after entry Additional measurements: plasma nimodipine concentration, severity of SAH grading | |
| Notes | Exclusion criteria: pregnancy; major renal, hepatic, or pulmonary disease; a SAH that produced a coma in the week preceding the most recent SAH Follow‐up duration: 3 months Adverse effects: mild reduction in blood pressure particularly noticeable in the hypertensive patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shibuya 1992.
| Methods | Method of randomisation: numbered or coded containers administered sequentially to enrolled participants Blinding: double‐blinded treatment, outcome assessments were not blinded Analysis: after exclusion of protocol violations (9 of 276 patients) Excluded from follow up: 5 of 136 patients in treatment group and 4 of 140 patients in control group Definition of outcome events: stated | |
| Participants | Location: 60 neurosurgical centres in Japan Rx: 136 (male to female ratio 0.7) Controls: 140 (male to female ratio 0.9) Age range: 24 to 75 years (mean age 55 years in the both groups) Entry criteria: patients with aneurysmal SAH documented by CT scan and angiography who underwent aneurysm surgery within 3 days after SAH Timing of surgery: surgery within first 3 days after SAH in all patients Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: AT877 iv (30 mg dissolved in 100 ml of saline over 30 minutes 3 times a day) started within 24 hours of aneurysm surgery and continued for 14 consecutive days Control: 100 ml saline iv (over 30 minutes 3 times a day) started within 24 hours after aneurysm surgery and continued for 14 consecutive days | |
| Outcomes | Primary endpoints: frequency and severity of angiographic vasospasm, frequency and size of low‐density area due to vasospasm on the postoperative CT scans, frequency of symptomatic vasospasm, poor outcome (moderate or severe disability, persistent vegetative state, or death) | |
| Notes | Exclusion criteria: history of severe cerebrovascular disease, moyamoya disease, giant aneurysm, recent previous SAH, and severe cardiopulmonary, hepato‐renal, or metabolic diseases (such as diabetes mellitus) Follow‐up duration: 1 month Adverse effects: AT877 slightly lowered systemic blood pressure by 2 mmHg over the first 15 minutes of infusion, but it resolved | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
van den Bergh 2005.
| Methods | Method of randomisation: numbered identical medication boxes administered sequentially to participants Blinding: double blinding of treatment, blind assessment of outcome Analysis: on‐treatment analysis of primary outcome (43 of 283 patients excluded because of discontinuing study medication), intention‐to‐treat analysis of secondary outcomes Losses to follow up: none Definition of outcome: stated. | |
| Participants | Location: 4 centres in the Netherlands Rx: 139 (male to female ratio 0.6) Controls: 144 (male to female ratio 0.5) Age range: 18 years or older, mean age 54.6 years Entry criteria: diagnosis of aneurysmal SAH based on typical aneurysmal pattern on CT scan or xantochromia of CSF in combination with an aneurysm on angiography. Randomisation possible within 4 days after SAH Treatment of aneurysm: By surgery in 52% of magnesium treated patients and in 58% of placebo treated patients, by endovascular treatment in 28% of magnesium treated patients and in 24% of placebo treated patients and none in 20% of magnesium treated patients and in 18% of placebo treated patients Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: continuous iv administration of 64 mmol magnesium sulphate per day starting immediately after randomisation and continued until 14 days after occlusion of the aneurysm or for a maximum of 18 days after onset of SAH Controls: 50 ml of normal saline administered in same conditions as Rx Both groups received nimodipine 360 mg/day | |
| Outcomes | Clinical outcomes: the occurrence of DCI within 3 months after onset of SAH Additional measurements: (1) occurrence of any new hypodensity on brain CT regardless of its cause; (2) death or disability defined as a Rankin score of 4 or worse; (3) non‐excellent outcome defined as the proportion of patients with a Rankin score of 1 or worse | |
| Notes | Exclusion criteria: non‐aneurysmal causes of SAH, renal failure, age less than 18 years, no informed consent, imminent death Follow‐up duration: 3 months Adverse effects: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Veyna 2002.
| Methods | Method of randomisation: unknown Outcome assessment: not blinded | |
| Participants | Location: 1 centre in US Rx : 20 Controls: 20 Inclusion criteria: (1) aneurysmal SAH confirmed by CT and angiography; (2) admission less than 72 hours; (3) Hunt & Hess II to IV; (4) age more than 18 years; (5) informed consent Standard care included administration of nimodipine, phenytoin, triple‐H therapy, and mannitol if needed for ICP control Timing of treatment aneurysm: all patients were treated by 'early' securement of aneurysm by means of surgery or endovascular technique | |
| Interventions | Magnesium sulphate iv loading dose 6 g in 30 minutes followed by continuous infusion at 2 g per hour. Subsequent dosage adjustments to maintain serum Mg++ level between 4 and 5.5 mg/dl (between 1.7 and 2.3 mmol/L) (normal values at institution 1.5 to 2 mg/dl). If serum Mg++ was less than 4 mg/dl or between 5.5 and 7.1 mg/dl, the infusion was increased or decreased, respectively, by 0.5 g/hour (12 ml/hour). If the serum level of Mg++ was greater than 7.1 mg/dl, the infusion was suspended until the level decreased below 7 mg/ dl, after which the infusion was restarted at 1 g less per hour (25 ml/hour). The patient's serum Mg++ level was checked 6 hours after a dose change and every 12 hours thereafter Patients in the placebo group received routine infusions of magnesium sulphate if serum Mg was less than 2 mg/dl (0.8 mmol/L) Duration of treatment 10 days, or until discharge from ICU | |
| Outcomes | (1) GOS recorded at 3 months, with GOS 4 and 5 (independence) as good outcome (2) Clinical vasospasm defined as new focal neurological deficit that could not be explained by other causes | |
| Notes | Exclusion criteria: pregnancy, congestive heart failure, renal insufficiency with a calculated creatinine clearance rate of less than 30 ml/minute, known neuromuscular disease, use of neuromuscular blocking agents, serum K level greater than 5.5 mg/dl, serum Ca level lower than 0.9 mg/dl, hypotension Follow‐up duration: 3 months Adverse events: significant adverse events did not occur In the publication the outcome is given as mean GOS per group. Letter sent to trialists asking (1) proportions of patients with good or poor outcome per group, (2) outcome of patients excluded from control group; (3) method of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Wong 2006.
| Methods | Method of randomisation: numbered sealed opaque envelopes Blinding: double‐blinded treatment, blinding for outcome assessment blinded Analysis: intention‐to‐treat analysis Losses to follow up: none Definitions of outcome: stated | |
| Participants | Location: 1 university centre in China Rx: 30 (male to female ratio 0.2) Controls: 30 (male to female ratio 0.1) Age range: mean age 58 years (Rx), 62 years (controls) Entry criteria: aneurysmal SAH proven CT and cerebral angiography (CTA or DSA), randomisation within 48 hours Aneurysm treatment: endovascular treatment in 16 patients (Rx 8/23, controls 8/22), surgical clipping in 40 patients. Median time from SAH to aneurysm treatment 2 days in both groups Comparability of treatment groups: good for major prognostic factors | |
| Interventions | Rx: bolus infusion of 20 mmol magnesium sulphate in 30 minutes followed by infusion of 80 mmol magnesium sulphate per day for 14 days Controls: bolus infusion of normal saline in 30 minutes followed by infusion of 80 mmol saline per day for 14 days Both groups received nimodipine infusion 0.5 to 2 mg/hour | |
| Outcomes | Primary outcome measure: GOS, dichotomised into favourable (good and moderate disability) and unfavourable outcome Secondary outcome: clinical vasospasm ‐ reduction of 2 or more on Glasgow Coma Score, or focal deficits, for more than 6 hours when rebleed, progressive hydrocephalus and electrolyte disturbances are excluded | |
| Notes | Exclusion criteria: not stated Follow‐up duration: 6 months Adverse effects: not reported A letter to the authors was sent regarding the randomisation method and blinding of outcome assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zhu 2001.
| Methods | Method of randomisation: not specified Blinding: not mentioned Analysis: intention‐to‐treat analysis Losses to follow up: none Definition of outcome: stated | |
| Participants | Location: Lin Xiang City Hospital, China Rx: 70 (male to female ratio1.3) Controls: 50 (male:female ratio not mentioned) Age range: mean age 50 +/‐ 6 years Entry criteria: admission within 96 hours after SAH | |
| Interventions | Rx: nimodipine 10 mg/50 ml glucose intravenously, 5 to 8 drops per minute in 8 to 10 hours per day for 15 days, followed by 3 drops per day 30 mg orally for 15 days Controls: regular treatment | |
| Outcomes | (1) Cerebral vasospasm as defined by either (a) fluctuating consciousness, (b) high fever with unknown cause, (c) localising signs, in combination with no fresh blood on CT scan (2) Cerebral infarction on CT scan (3) Rebleeding (4) Death | |
| Notes | Exclusion criteria: not stated Follow‐up duration: 1 month Adverse effects: not mentioned NB: Outcome 'cerebral vasospasm' not used in analysis because high fever with unknown cause was also considered to be vasospasm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
CBF: cerebral blood flow CSF: cerebrospinal fluid CT: computed tomography DCI: Delayed cerebral ischaemia GOS: Glasgow Outcome Scale ICP: intracranial pressure ICU: intensive care unit iv: intravenous, intravenously Mg: magnesium Rx: patients treated with a study drug SAH: subarachnoid haemorrhage WFNS: World Federation of Neurological Surgeons subarachnoid hemorrhage grading scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cabral 1991 | This is a double‐blind, randomised trial (method of randomisation unknown) of 15 patients receiving nimodipine (oral or nasogastric tube; 60 mg every 4 hours for 21 days) versus 15 patients receiving placebo. Data are given only for the subset of patients who developed 'symptomatic vasospasm' (placebo n = 6, resulting in death (n = 4) and persisting deficits (n = 2). Nimodipine n = 3 resulting in death (n = 1) and persisting deficits (n = 2)). No data on overall outcome. Authors did not respond to letters asking for additional information. |
| Cadoux‐Hudson 1999 | Study of magnesium sulphate. After personal contact with a close colleague of Dr Cadoux‐Hudson we found out this study was not performed as a randomised trial. Number of patients unknown. |
| Islekel 1999 | Between 1980 and 1995, 374 patients with subarachnoid haemorrhage were treated by the trialists in their hospital. Of those, 42 received nimodipine. These patients were randomly selected; the method of randomisation has not been given. Since the allocation does not seem to have occurred in a prospectively randomised way, the trial was not included in the review. |
| Jan 1988 | Treatment with nimodipine was started within 25 days of subarachnoid haemorrhage onset in patients already suffering from secondary ischaemia. Total number of patients: 188 |
| Maldonado 1990 | Controlled study of nimodipine in 58 subarachnoid haemorrhage patients. Allocation to treatment or control group was not randomised. |
| Prevedello 2006 | Study of magnesium sulphate in addition to nimodipine as standard treatment. Randomisation method not concealed (patients were placed in groups in alternate order). Not blinded. |
| Wang 1995 | Controlled study of nimodipine in subarachnoid haemorrhage patients. Allocation to treatment or control group was not randomised. |
| Xie 1994 | Randomised trial comparing 4 groups (placebo, nimodipine, nimodipine and betahistine, and betahistine) with regard to pre‐defined neurological features such as hemiparesis with a follow up of only 14 days. Excluded because there were no data on poor outcome, secondary ischemia or death. |
| Zhu 1996 | Randomised trial comparing nimodipine with magnesium sulphate with regard to pre‐defined neurological features such as hemiparesis. Excluded because patients were randomised only after development of vasospasm. |
Characteristics of ongoing studies [ordered by study ID]
IMASH.
| Trial name or title | Intravenous Magnesium sulphate in Aneurysmal Subarachnoid Hemorrhage |
| Methods | |
| Participants | Inclusion criteria: (1) ASAH (as indicated by CT scan or lumbar puncture and an intracranial aneurysm confirmed by computer tomographic or conventional angiography) (2) within 48 hours of ictus (haemorrhage event) Exclusion criteria: (1) pregnancy; (2) major renal, hepatic or pulmonary disease; (3) major cardiac disease or recent myocardial infarct (less than 6 months); (4) age less than 18 years; (5) moribund condition on admission (defined as a patient that is in such a poor clinical condition that further active neurosurgical management would not be anticipated) |
| Interventions | (1) Start magnesium sulphate 20 mmol over 30 minutes, followed by infusion of 80 mmol/day or equivalent volume of saline within 48 hours after onset of symptom (2) Study drug to be infused for 14 days from the day of haemorrhage (regarded as day 0) (3) Measure plasma magnesium concentration daily and perform transcranial Doppler to monitor blood flow velocities of both middle cerebral arteries and extracranial segment of the internal carotid arteries (4) Plasma magnesium concentration in the iv magnesium sulphate group should be raised to 2.0 to 2.5 mmol/L or twice the serum baseline level Patients that are randomised to saline infusion will only have their magnesium levels normalised if there is a clinical indication to do so |
| Outcomes | Primary outcome: extended Glasgow Outcome Scale at six months Secondary outcome: incidence of clinical vasospasm, Barthel Index, modified Rankin score, modified National Institute of Health Stroke Score, MCA velocities, other major complications |
| Starting date | Aim is to include 800 patients in 3 years with interim analyses at n = 250 and 500 patients |
| Contact information | Study co‐ordinating group and central address: Project Office Division of Neurosurgery, Department of Surgery, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, NT, Hong Kong Tel: +852 2632 2624; Fax: +852 2637 7974; Datafax: +852 2646 9296; Website: www.surgery.cuhk.edu.hk/imash‐trial Email: imash@surgery.cuhk.edu.hk (Dr. George Wong) |
| Notes |
MASH‐II.
| Trial name or title | Magnesium in Aneurysmal Subarachnoid Haemorrhage |
| Methods | |
| Participants | Patients with spontaneous SAH of presumed aneurysmal origin (defined as aneurysmal pattern of haemorrhage on CT or, if CT is negative, by xanthochromia of the cerebrospinal fluid and an aneurysm on CTA, MRA or conventional angiography), admitted to one of the participating centers within 4 days after SAH onset Exclusion criteria: (1) renal failure (serum creatinin > 150 mmol/L); (2) body weight < 50 kg; (3) death is imminent; (4) no informed consent |
| Interventions | Magnesium sulphate 64 mmol/d iv (versus placebo) is started less than 96 hours after onset and continued for 20 days after onset of the SAH, or until discharge when this is planned before day 20 |
| Outcomes | Primary outcome is poor outcome defined by the modified Rankin Scale Other outcome measurements are: patients with Rankin score of 1, global change in Rankin score |
| Starting date | Recruitment started in January 2004 Aim is to include 1200 patients |
| Contact information | (1) SM Dorhout Mees, MD, Department of Neurology G03.227, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands Tel: +31 30 2507975; Fax: +31 30 2522782; E‐mail: s.m.dorhoutmees@umcutrecht.nl (2) Gabriel JE Rinkel, MD, Department of Neurology G03.228, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands. Tel: +31 30 2508600; E‐mail: g.j.e.rinkel @umcutrecht.nl |
| Notes | Funded by the Netherlands Heart Foundation (grant number: 2005B016) |
ASAH: aneurysmal subarachnoid haemorrhage CT: computed tomography CTA: computer tomography angiography iv: intravenous MCA: middle cerebral artery MRA: magnetic resonance angiography SAH: subarachnoid haemorrhage
Contributions of authors
VL Feigin: participated in developing the protocol, retrieving papers, data extraction, appraising the quality of studies, data management, data analysis, data interpretation, and writing the review.
GJE Rinkel: participated in writing the grant application, developing the protocol, data extraction, appraising the quality of studies, data analysis, data interpretation, writing the review, and entering the review into RevMan. Dr Rinkel prepared the updates in 2001 and 2003 and is the guarantor for this review.
A Algra: participated in writing the grant application, developing the protocol, appraising the quality of studies, data analysis, data interpretation, and writing the review and updates of the review.
WM van den Bergh: participated in data extraction of the magnesium trials and writing the updates of 2003 and 2006.
M Vermeulen: participated in writing the grant application, developing the protocol, appraising the quality of studies, data interpretation, and writing the review.
J van Gijn: participated in writing the grant application, developing the protocol, appraising the quality of studies, data interpretation, writing the review and preparing the update in 1999.
SM Dorhout Mees: participated in developing the 2006 update, retrieving papers, data extraction, appraising the quality of new studies for the 2006 update, and writing the 2006 update.
Sources of support
Internal sources
University Medical Center, Utrecht, Netherlands.
External sources
The Netherlands Heart Foundation, Netherlands.
Declarations of interest
The authors are currently conducting a randomised trial with magnesium sulphate (MASH‐II).
Edited (no change to conclusions)
References
References to studies included in this review
Allen 1983 {published data only}
- Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Chou SN, et al. Cerebral arterial spasm ‐ a controlled trial of nimodipine in patients with subarachnoid hemorrhage. New England Journal of Medicine 1983;308:619‐24. [DOI] [PubMed] [Google Scholar]
Ferro 1990 {unpublished data only}
- Ferro JM, Melo TP, Oliveira V, Trindade AM, Lobo‐Antunes J, Campos JC. Prevention of vasospasm in subarachnoid hemorrhage with oral nicardipine. Unpublished study (personal communication) 1990.
Haley 1993 {published data only}
- Haley EC, Kassell NF, Torner JC. A randomised controlled trial of high‐dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 1993;78:537‐47. [DOI] [PubMed] [Google Scholar]
- Haley EC, Kassell NF, Torner JC. A randomized trial of nicardipine in subarachnoid hemorrhage: angiographic and transcranial Doppler ultrasound results. Journal of Neurosurgery 1993;78:548‐53. [DOI] [PubMed] [Google Scholar]
- Haley EC, Kassell NF, Torner JC, Kongable G. Nicardipine ameliorates angiographic vasospasm following subarachnoid hemorrhage. Neurology 1991;41(Suppl 1):346. [Google Scholar]
Han 1993 {published and unpublished data}
- Han DH, Lee SH, Lee SH. Effect of nimodipine treatment on outcome in surgical cases of aneurysmal SAH. Journal of Neurosurgery 1993;78:346A. [Google Scholar]
Luo 1996 {published data only}
- Luo W, Qiu S, MA W. Clinical study of magnesium sulphate on delayed cerebral vasospasm after subarachnoid haemorrhage. Journal of Clinical Neurology 1996;9(4):244‐5. [Google Scholar]
Messeter 1987 {published data only}
- Messeter K, Brandt L, Ljunggren B, Svendgaard NA, Algotsson L, Romner B, et al. Prediction and prevention of delayed ischemic dysfunction after aneurysmal subarachnoid hemorrhage and early operation. Neurosurgery 1987;20:548‐53. [DOI] [PubMed] [Google Scholar]
Neil‐Dwyer 1987 {published data only}
- Mee E, Dorrance D, Lowe D, Neil‐Dwyer G. Controlled study of nimodipine in aneurysm patients treated early after subarachnoid hemorrhage. Neurosurgery 1988;22:484‐91. [DOI] [PubMed] [Google Scholar]
- Mee EW, Dorrance DE, Low D, Neil‐Dwyer G. Cerebral blood flow and neurological outcome: a controlled study of nimodipine in patients with subarachnoid haemorrhage. Journal of Neurology and Psychiatry 1986;49:469. [Google Scholar]
- Neil‐Dwyer G, Mee E, Dorrance D, Lowe D. Early intervention with nimodipine in subarachnoid haemorrhage. European Heart Journal 1987;8:41‐7. [DOI] [PubMed] [Google Scholar]
Ohman 1991 {published data only}
- Juvela S, Kaste M, Hillbom M. Effect of nimodipine on platelet function in patients with subarachnoid hemorrhage. Stroke 1990;21:1283‐8. [DOI] [PubMed] [Google Scholar]
- Ohman J. Surgery for ruptured intracranial aneurysms: defining the present optimal conditions. Finland: Helsingin Yliopisto, 1991. [Google Scholar]
- Ohman J, Heiskanen O. Effect of nimodipine on the outcome of patients after aneurysmal subarachnoid hemorrhage and surgery. Journal of Neurosurgery 1988;69(5):683‐6. [DOI] [PubMed] [Google Scholar]
- Ohman J, Servo A, Heiskanen O. Long‐term effects of nimodipine on cerebral infarcts and outcome after aneurysmal subarachnoid hemorrhage and surgery. Journal of Neurosurgery 1991;74:8‐13. [DOI] [PubMed] [Google Scholar]
Petruk 1988 {published data only}
- Disney LB. Cerebral vasospasm following subarachnoid hemorrhage: clinical investigation of oral nimodipine and factors influencing outcome (hemorrhage). Dissertation Abstracts International 1990; Vol. Volume 52/11‐B:221 pages.
- Petruk KC, West M, Mohr G, Weir BKA, Benoit BG, Gentili F, et al. Nimodipine treatment in poor‐grade aneurysm patients. Results of a multicentre double‐blind placebo‐controlled trial. Journal of Neurosurgery 1988;68:505‐17. [DOI] [PubMed] [Google Scholar]
Philippon 1986 {published data only}
- Philippon J, Grob R, Dagreou F, Guggiari M, Rivierez M, Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta Neurochirurgica 1986;82:110‐4. [DOI] [PubMed] [Google Scholar]
Pickard 1989 {published data only}
- Pickard JD, Murray GD, Illingworth R, Shaw MDM, Teasdale GM, Foy PM, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989;298:636‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shibuya 1992 {published data only}
- Shibuya M, Suzuki Y. Treatment of cerebral vasospasm by a protein kinase inhibitor AT877 [Japanese]. No to Shinkei ‐ Brain & Nerve 1993;45:819‐24. [PubMed] [Google Scholar]
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo controlled double blind trial. Journal of Neurosurgery 1992;76:571‐7. [DOI] [PubMed] [Google Scholar]
van den Bergh 2005 {published data only}
- Bergh WM, Algra A, Kooten F, Dirven CM, Gijn J, Vermeulen M, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke 2005;36:1011‐5. [DOI] [PubMed] [Google Scholar]
Veyna 2002 {published data only}
- Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, et al. Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 2002;96:510‐4. [DOI] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Boet R, Chan MTV, Poon WS, Wong GKC, Wong HT, Gin T. Intravenous magnesium sulfate to improve outcome after aneurysmal subarchnoid hemorrhage: interim report from a pilot study. Acta Neurochirurgica 2005;95(Suppl):263‐4. [DOI] [PubMed] [Google Scholar]
- Wong GKC, Chan MTV, Boet R, Poon WS, Gin T. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. Journal of Neurosurgery and Anesthesiology 2006;18(2):142‐8. [DOI] [PubMed] [Google Scholar]
Zhu 2001 {published data only}
- Zhu Y. Clinical study of nimodipine in preventing and treating cerebral vasospasm after subarachnoid hemorrhage. Chinese Journal of Primary Medicine and Pharmacy 2001;8:256‐7. [Google Scholar]
References to studies excluded from this review
Cabral 1991 {published data only}
- Cabral ND, Scaff M. Use of nimodipine in spontaneous meningeal haemorrhage through aneurysm rupture [Uso da nimodipina em hemorragia meníngea espontânea por rotura de aneurisma]. Revista Brasileira de Neurologia 1991;27(1):21‐2. [Google Scholar]
Cadoux‐Hudson 1999 {published data only}
- Cadoux‐Hudson TAD. A randomised, double‐blind placebo controlled pilot trial of intravenous magnesium sulphate in subarachnoid haemorrhage. National Research Register, Issue 1, 2001.
Islekel 1999 {published data only}
- Islekel S, Oktar N, Yurtseven T, Ozdamar N. Effect of nimodipine on the outcome of patients after aneurysm surgery. Journal of Neurological Sciences 1999;16(1):4. [Google Scholar]
Jan 1988 {published data only}
- Desbordes JM, Ades PE, Guggiari M. Intravenous nimodipine in curative treatment of cerebral vasospasm after subarachnoid haemorrhage caused by rupture of intra‐cranial aneurysms: a comparative multicenter study. Agressologie 1989;30:438‐40. [PubMed] [Google Scholar]
- Frerebeau Ph, Janny P, Taquoi G, Besson G, Buchheit F, Childs M, et al. Curative intravenous nimodipine treatment of subarachnoidal hemorrhage of vasospasm origine intracranial‐aneurysms. Neurochirurgie 1988;34:383‐8. [PubMed] [Google Scholar]
- Jan M, Buchheit F, Tremoulet M. Therapeutic trial of intravenous nimodipine in patients with established cerebral vasospasm after rupture of intracranial aneurysms. Neurosurgery 1988;23:154‐7. [DOI] [PubMed] [Google Scholar]
Maldonado 1990 {published data only}
- Maldonado JA, Milchorena G, Lopez‐Camacho O, Madrazo I. Management with nimodipine of the cerebral vasospasm secondary to subarachnoid hemorrhage due to the rupture of an intracranial aneurysm [Manejo con nimodipina del vaso‐espasmo cerebral secundario a hemorragia subaracnoidea por ruptura de aneuisma intracraneano]. Archivos de Investigacion Medica 1990;21:179‐87. [PubMed] [Google Scholar]
Prevedello 2006 {published data only}
- Prevedello DMS, Cordeiro JG, Morais AL, Saucedo NS, Chen IB, Araujo JC. Magnesium sulfate: role as possible attenuating factor in vasospasm morbidity. Surgical Neurology 2006;65(S1):14‐21. [DOI] [PubMed] [Google Scholar]
Wang 1995 {published data only}
- Wang H, Song D, Lu J, Jia S. Preventive effect of nimodipine on SAH and CVS. Journal of Apoplexy and Nervous Diseases 1995;12(4):237. [Google Scholar]
Xie 1994 {published data only}
- Xie R, Yao J. Observation on the therapeutic effect of nimodipine versus kang‐xuan‐ding versus placebo for 14 days. Journal of Clinical Neurology 1994;7(1):48‐9. [Google Scholar]
Zhu 1996 {published data only}
- Zhu S, Liu X, Li Y, Hou Q, Cai P. Observation of therapeutic effect of combined nimodipine and magnesium sulphate after subarachnoid haemorrhage. Stroke and Nervous Diseases 1996;3(3):163. [Google Scholar]
References to ongoing studies
IMASH {unpublished data only}
- Intravenous Magnesium sulfate in Aneurysmal Subarachnoid Hemorrhage. http://www.surgery.cuhk.edu.hk/imash‐trial/.
MASH‐II {unpublished data only}
- Major ongoing trials. Stroke 2006; Vol. 37, issue 10:e36‐44. [PubMed]
Additional references
ACROSS 2000
- The ACROSS Group. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2000;31(8):1843‐50. [DOI] [PubMed] [Google Scholar]
Barker 1996
- Barker II FG, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. Journal of Neurosurgery 1996;84:405‐14. [DOI] [PubMed] [Google Scholar]
Brilstra 2000
- Brilstra EH, Rinkel GJE, Algra A, Gijn J. Rebleeding, secondary ischemia and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000;55:1656‐60. [DOI] [PubMed] [Google Scholar]
de Rooij 2006
- Rooij NK, Linn FHH, Plas JAP, Algra A, Rinkel GJE. Incidence of subarachnoid hemorrhage: a ten years' update of a systematic review with emphasis on role of region, gender, age and time trend. In preparation.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ, 2001. [Google Scholar]
Gaab 1985
- Gaab MR, Haubitz I, Brawanski A, Korn A, Czech T. Acute effects of nimodipine on the cerebral blood flow and intracranial pressure. Neurochirurgia 1985;28(Suppl 1):93‐9. [DOI] [PubMed] [Google Scholar]
Haley 1994
- Haley EC Jr, Kassell NF, Torner JC, Truskowski LL, Germanson TP. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Journal of Neurosurgery 1994;80:788‐96. [DOI] [PubMed] [Google Scholar]
Hop 1997
- Hop JW, Rinkel GJE, Algra A, Gijn J. Case fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660‐4. [DOI] [PubMed] [Google Scholar]
Ljunggren 1984
- Ljunggren B, Brandt L, Saveland H, Nilsson PE, Cronqvist S, Andersson KE, et al. Outcome in 60 consecutive patients treated with early aneurysm operation and intravenous nimodipine. Journal of Neurosurgery 1984;61:864‐73. [DOI] [PubMed] [Google Scholar]
Mascio 1994
- Mascio RD, Marchioli R, Tognoni G. Meta‐analysis. From pharmacological promises to controlled clinical trials to meta‐analysis and back: the case of nimodipine in cerebrovascular disorders. Clinical Trials and Meta‐Analysis 1994;29:57‐79. [PubMed] [Google Scholar]
Massiou 1992
- Massiou H, Chaumet‐Riffaud P, Bourdeix I. Nicardipine in the prevention of spasm‐induced neurological deficits after subarachnoid hemorrhage: a dose‐ranging study. Surgical Neurology 1992;38:7‐11. [DOI] [PubMed] [Google Scholar]
McCalden 1989
- McCalden TA, Nath RG. Cerebrovascular autoregulation is resistant to calcium channel blockage with nimodipine. Experientia 1989;45:305‐6. [DOI] [PubMed] [Google Scholar]
Pickard 1990
- Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, et al. Oral nimodipine and cerebral ischaemia following subarachnoid haemorrhage. British Journal of Clinical Practice 1990;44(2):66‐7. [PubMed] [Google Scholar]
Ram 1991
- Ram Z, Sadeh M, Shacked I, Sahar A, Hadani M. Magnesium sulfate reverses experimental delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 1991;22(7):922‐7. [DOI] [PubMed] [Google Scholar]
Robinson 1990
- Robinson MJ, Teasdale GM. Calcium antagonists in the management of subarachnoid haemorrhage. Cerebrovascular & Brain Metabolism Reviews 1990;2:205‐26. [PubMed] [Google Scholar]
Roos 2000
- Roos YB, Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. Journal of Neurology Neurosurgery and Psychiatry 2000;68(3):337‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Satoh 1992
- Satoh S, Suzuki Y, Harada T, Ikegaki I, Asano T, Shibuya M, et al. Possible prophylactic potential of HA1077, a Ca+2 channel antagonist and vasodilator, on chronic cerebral vasospasm. European Journal of Pharmacology 1992;220:243‐8. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz K, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Seto 1995
- Seto M, Shindo K, Ito K, Sasaki Y. Selective inhibition of myosin phosphorylation and tension of hyperplastic arteries by the kinase inhibitor HA1077. European Journal of Pharmacology 1995;276:27‐33. [DOI] [PubMed] [Google Scholar]
Shibuya 1990
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, et al. Dose escalation trial of a novel calcium antagonist, AT877, in patients with aneurysmal subarachnoid haemorrhage. Acta Neurochirurgica 1990;107:11‐5. [DOI] [PubMed] [Google Scholar]
Takizawa 1993
- Takizawa S, Hori M, Ozaki H, Karaki H. Effects of isoquinoline derivatives, HA1077 and H‐7, on cytosolic Ca+2 level and contraction in vascular smooth muscle. European Journal of Pharmacology 1993;250:431‐7. [DOI] [PubMed] [Google Scholar]
Tettenborn 1990
- Tettenborn D, Dycka J. Prevention and treatment of delayed ischemic dysfunction in patients with aneurysmal subarachnoid hemorrhage. Stroke 1990;21(Suppl IV):IV85‐IV89. [PubMed] [Google Scholar]
van den Bergh 2002
- Bergh WM, Zuur JK, Kamerling NA, Asseldonk JT, Rinkel GJ, Tulleken CA, et al. Role of magnesium in the reduction of ischemic depolarization and lesion volume after experimental subarachnoid hemorrhage. Journal of Neurosurgery 2002;97(2):416‐22. [DOI] [PubMed] [Google Scholar]
van den Bergh 2003
- Bergh WM, Algra A, Sprenkel JW, Tulleken CA, Rinkel GJ. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery 2003;52(2):276‐82. [DOI] [PubMed] [Google Scholar]
van den Bergh 2004
- Bergh WM, Dijkhuizen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnesium Research 2004;17(4):301‐13. [PubMed] [Google Scholar]
van der Schaaf 2002
- Schaaf IC, Ruigrok YM, Rinkel GJE, Algra A, Gijn J. Study design and outcome measures in studies on aneurysmal subarachnoid hemorrhage. Stroke 2002;33:2043‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Feigin 1998
- Feigin VF, Rinkel GJE, Algra A, Vermeulen M, Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 1998;50:876‐83. [DOI] [PubMed] [Google Scholar]
Rinkel 2005
- Rinkel GJE, Feigin VL, Algra A, Bergh WM, Vermeulen M, Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2005, Issue 1. [Art. No.: CD000277. DOI: 10.1002/14651858.CD000277.pub3] [Google Scholar]


