Abstract
Culex pipiens Linnaeus and Culex quinquefasciatus Say are the primary vectors of West Nile and St. Louis encephalitis viruses in California. Pyrethrins and pyrethroids (synthetic pyrethrins) are the most widely used insecticides to control adult stage mosquitoes to prevent disease transmission. The most abundant and widespread mutation associated with pyrethroid resistance is the L1014F mutation of the voltage-sensitive sodium channel gene. Statewide, based on the testing of almost 2,000 mosquitoes from 14 counties, the resistant allele frequency was 71%. Although the L1014F mutation was found in all counties assessed, the resistance allele profiles differed between regions of California. The highest resistant allele frequency occurred in the Central region and lowest frequencies were from the Northern and Southern regions. Resistance allele frequencies observed in 2014–2016 are nearly 1.5 times higher than those from pre-2012, indicating that resistance profiles can change over time. Regular monitoring of the L1014F kdr mutation will help aid in operational decisions.
Keywords: Culex pipiens, Culex quinquefasciatus, pyrethroid resistance, knockdown resistance, kdr
In California, Culex pipiens Linnaeus and Culex quinquefasciatus Say are the primary urban vectors of West Nile (WNV) and St. Louis encephalitis (SLEV) viruses. There have been over 6,500 symptomatic human infections of WNV since its introduction to California in 2003 (CDPH 2018a), and 12 human cases of SLEV since its re-emergence in California in 2015 (Feiszli et al. 2016, 2017; CDPH 2018b; Diaz et al. 2018). There are no human vaccines nor specific treatments for these viruses; therefore, personal protective measures and direct mosquito control are currently the best methods of protecting people from infection. Integrated vector management includes methods that can target immature or adult mosquito life stages through environmental manipulation, biological control, chemical control, and public education. In situations where disease transmission needs to be halted, chemical control of adult mosquitoes is the most effective method and often is the only option to quickly interrupt the virus transmission cycle. The insecticide resistance profiles of mosquito populations can directly affect the operational approach and effectiveness of an adult mosquito control campaign.
The most commonly used pesticides used for the control of adult mosquitoes belong to the pyrethrin and organophosphate classes (Howard et al. 2010), with pyrethroid (synthetic pyrethrin) use peaking in the years immediately after WNV was introduced to California (https://calpip.cdpr.ca.gov/main.cfm; California Department of Pesticide Regulation, California Pesticide Information Portal v 2018.04). The insect voltage-sensitive sodium channel (Vssc) is the target of the pyrethroid class insecticides. Certain mutations in the Vssc gene are associated with resistance development. The genetic mutation of the nucleotide base from A to T (L1014F) that converts wild-type amino acid Leucine (L) to phenylalanine (F) at codon 1014 is the most prevalent pyrethroid-associated resistance mutation (Scott et al. 2015). This mutant gene is termed knockdown resistance (kdr) and was originally discovered in houseflies (Busvine 1951). The L1014F mutation is the most widely distributed mutation around the world conferring kdr resistance in multiple insect species, including members of the Culex pipiens complex (Scott et al. 2015).
Materials and Methods
From 2014 to 2016, adult Cx. pipiens and Cx. quinquefasciatus mosquitoes were collected by local California mosquito control agencies and submitted to the California Department of Public Health Vector-Borne Disease Laboratory (CDPH-VBDL) as part of an insecticide resistance testing program. Mosquitoes were collected using CO2-baited encephalitis virus surveillance traps, identified to species by agency personnel, placed individually in 1.5-ml Eppendorf tubes with 70% ethanol, and shipped at room temperature to CDPH-VBDL. Processing commenced with the complete removal of ethanol, and DNA was extracted per manufacturer’s instructions using Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hercules, CA).
Identification of the L1014F kdr-type Vssc mutation in Cx. pipiens and Cx. quinquefasciatus was conducted using a TaqMan assay based on Chen et al. (2010) and Hardstone et al. (2007). The 25-µl reaction mixture contained 10 µl of 2× SsoFast Probe Supermix (Bio-Rad), 8-µl molecular-grade deionized water, 900 nM each of primers (forward: 5′-GTGTCCTGCATTCCGTTCTT-3′; reverse: 5′-TTCGTTCCCACCTTTTCTTG-3′), 200 nM each of probes (Probe Wildtype: 5′-VIC-CTCACGACTAAATTTC-MGB-3′; Probe L1014F: 5′-FAM-CACGACAAAATTTC-MGB-3′), and 5 ng of genomic DNA. Thermal cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. Assay results were determined using the CFX Manager Software (version 3.1, Bio-Rad) where homozygous wildtype (SS) had an increase in VIC fluorescence, homozygous L1014F kdr mutant (RR) had increase in FAM fluorescence, and heterozygotes (RS) had signals from both VIC and FAM.
Results and Discussion
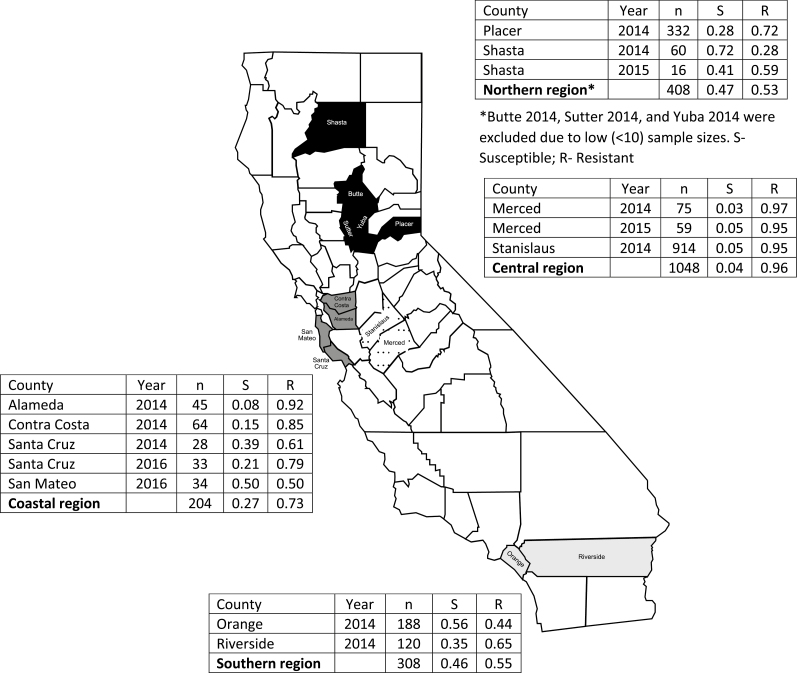
In total, 1,989 Cx. pipiens and Cx. quinquefasciatus adults were submitted from 13 California vector control agencies covering 14 counties. Three counties, Butte, Sutter, and Yuba, submitted low sample sizes (10 or fewer mosquitoes) and were excluded from statewide and regional-level analyses. The kdr allele was detected in all counties tested, representing nearly all regions of the state, with a statewide average resistant allele frequency of 71%.
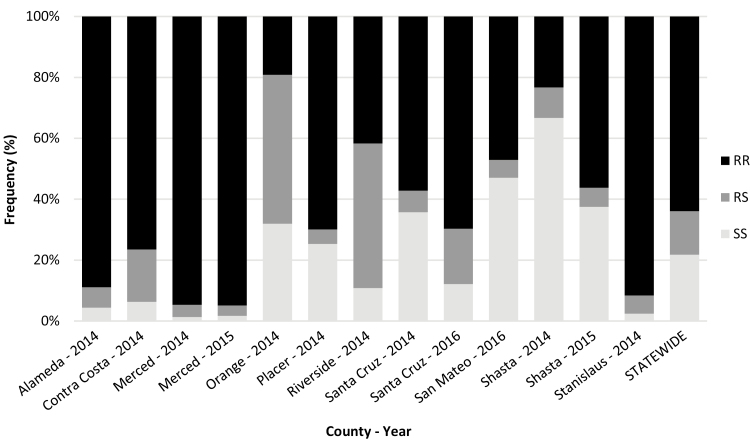
The resistance allele profiles differed between the regions of California. The resistance allele accounted for 96% of the alleles present in the Central region, whereas it accounted for nearly half that (53% Northern region, 55% Southern region) in other parts of the state (Fig. 1). The Coastal region counties exhibited wide variation by county with Alameda having a resistance allele prevalence of 92% and San Mateo at 50% (Fig. 1). The majority of the counties exhibited homozygous resistant genotypes at greater than 50%. Only four counties had homozygous resistant genotypes representing less than 50% of the genotypes in the tested population: Orange 2014, Riverside 2014, San Mateo 2016, and Shasta 2014 (Fig. 2). In addition, in the Southern region in Orange and Riverside counties, nearly 50% of the tested populations had heterozygous genotypes (Fig. 2).
Fig. 1.
Allele frequencies of L1014F kdr mutation in Culex pipiens and Culex quinquefasciatus from California, 2014–2016. Counties in the Northern region are dark gray, Coastal region counties are medium gray, Central region counties are hashed pattern, and Southern region counties are light gray.
Fig. 2.
Genotype frequencies of L1014F kdr mutation in Culex pipiens and Culex quinquefasciatus from California, 2014–2016. Black indicates homozygous resistant (RR), medium gray indicates heterozygotes (RS), and light gray indicates homozygous susceptible (SS) genotypes.
Three counties (Merced, Santa Cruz, and Shasta) submitted mosquito samples in two of the 3 yr of the testing program. In Merced County, resistance allele frequency and homozygous resistant genotype frequency held steady and are nearing fixation (Fig. 1). Although Santa Cruz County had a 2-yr interval between measurements, resistance allele frequencies increased over time as did homozygous resistant and heterozygous genotypes, indicating an overall steady increase of kdr resistance in the county. In the Northern region, Shasta underwent a nearly doubling of the resistance allele frequency (from 28 to 59%) and a near mirror swap of the genotype frequencies (Fig. 2), though these considerable changes could be due to the difference in sample sizes between the 2 yr.
The kdr allele was previously detected in Cx. pipiens populations collected before 2012 from Northern (Butte County), Central (Fresno and Tulare counties), and Southern (Los Angeles and Riverside counties) regions of California (Ahmed et al. 2012). The highest resistant allele frequency for the L1014F mutation site occurred in the Central region (Fresno, Fresno County, 63.64%), and lowest frequency was from the Southern region (Santa Fe Springs, Los Angeles County, 35%). Although the overall pattern of which region exhibited highest and lowest resistance allele frequencies matched our findings, allele frequencies observed in 2014–2016 are nearly 1.5 times higher than those from pre-2012. In addition, all of the mosquito populations tested by Ahmed et al. (2012) had less than 50% of the genotypes represented by the homozygous resistant genotype, which was more rarely observed in the 2014–2016 populations. A 2001 population from Marin County (Coastal region) had a resistance allele frequency of 70% and genotype frequencies of 12% (homozygous susceptible), 36% (heterozygous), and 52% (homozygous resistant; McAbee et al. 2004), which were all similar to the frequencies determined for the Coastal region in the present study.
Results of this multiyear pesticide resistance testing program show that the L1014F kdr mutation is widespread in Cx. pipiens and Cx. quinquefasciatus populations throughout California and that allelic and genotypic frequencies can change over time. These changes could be influenced by applications of pyrethroids and pyrethrins that target insects other than Culex mosquitoes. While noting that public health pesticide applications account for less than 1% of statewide use (Howard et al. 2010), the use of alternative active ingredients such as organophosphates may be more effective for adult mosquito control in some regions and will not contribute to kdr resistance. In areas that exhibit high-resistant allele frequencies (i.e., Central region), evaluations for potential control failures should be included as part of operational assessments. Prudent use of pyrethroid insecticides and rotation with other active ingredients are warranted for adulticiding throughout the state, due to the observed selection of resistant alleles over time. Routine monitoring for the L1014F kdr mutation is necessary to help understand the current pesticide resistance profile in a given mosquito population.
Acknowledgments
We thank participating California mosquito control agencies: Alameda County Mosquito Abatement District (MAD), Butte County Mosquito and Vector Control District (MVCD), Coachella Valley MVCD, Contra Costa MVCD, Merced County MAD, Orange County MVCD, Placer MVCD, San Mateo County MVCD, Santa Clara County MVCD, Santa Cruz County MVCD, Shasta MVCD, Sutter-Yuba MVCD, and Turlock MAD. We thank CDPH-VBDS laboratorians Robert Payne, Mary Joyce Pakingan, and Ian Rose for assistance in processing samples.
References Cited
- Ahmed M. A. I., Cornel A., and Hammock B.. . 2012. Monitoring of insecticide resistance of Culex pipiens (Diptera: Culicidae) colonies-collected from California. Int. J. Environ. Sci. Dev. 3: 346–349. [Google Scholar]
- Busvine J. R. 1951. Mechanism of resistance to insecticide in houseflies. Nature 168: 193–195. [DOI] [PubMed] [Google Scholar]
- ( CDPH) California Department of Public Health. 2018a. Human West Nile virus activity, California, 2003–2018 – reported as of December 13, 2018. (http://www.westnile.ca.gov/).
- ( CDPH) California Department of Public Health. 2018b. 2018 SLEV activity by county (2018-12-18) (http://www.westnile.ca.gov/web_reports.php?report=sle&option=print&year=2018).
- Chen L., Zhong D., Zhang D., Shi L., Zhou G., Gong M., Zhou H., Sun Y., Ma L., He J., . et al. 2010. Molecular ecology of pyrethroid knockdown resistance in Culex pipiens pallens mosquitoes. PLoS One 5: e11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A., Coffey L. L., Burkett-Cadena N., and Day J. F.. . 2018. Reemergence of St. Louis Encephalitis Virus in the Americas. Emerg. Infect. Dis. 24: 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiszli T., Wong J., Porse C., and Metzger M.. . 2016. Chapter 4: mosquito-borne diseases, pp. 14–21. InKjemtrup A. M. and Kramer V. (eds.), Vector-borne disease section annual report. California Department of Public Health, Sacramento, CA. [Google Scholar]
- Feiszli T., Snyder R., Porse C., and Metzger M.. . 2017. Chapter 4: mosquito-borne diseases, pp. 13–20. InKjemtrup A. M. and Kramer V. (eds.), Vector-borne disease section annual report. California Department of Public Health, Sacramento, CA. [Google Scholar]
- Hardstone M. C., Leichter C., Harrington L. C., Kasai S., Tomita T., and Scott J. G.. . 2007. Cytochrome P450 monooxygenase-mediated permethrin resistance confers limited and larval specific cross-resistance in the southern house mosquito, Culex pipiens quiquefasciatus. Pestic. Biochem. Physiol. 89: 175–184. [Google Scholar]
- Howard T. S., Novak M. G., Kramer V. L., and Bronson L. R.. . 2010. Public health pesticide use in California: a comparative summary. J. Am. Mosq. Control Assoc. 26: 349–353. [DOI] [PubMed] [Google Scholar]
- McAbee R. D., Kang K. D., Stanich M. A., Christiansen J. A., Wheelock C. E., Inman A. D., Hammock B. D., and Cornel A. J.. . 2004. Pyrethroid tolerance in Culex pipiens pipiens var molestus from Marin County, California. Pest Manag. Sci. 60: 359–368. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Yoshimizu M. H., and Kasai S.. . 2015. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 120: 68–76. [DOI] [PubMed] [Google Scholar]