Abstract
Purpose
Simultaneous cataract and glaucoma surgery has traditionally been challenging for the anterior segment surgeon. The introduction of minimally invasive glaucoma surgery (MIGS) in conjunction with cataract surgery appears safe and effective in lowering intraocular pressure. Although a significant visual impact leading from the combined procedure is unexpected, we aim to describe the refractive outcomes in a cohort of patients undergoing simultaneous cataract removal and iStent inject and discuss the potential implications of combined surgery in patients with co-existent glaucoma.
Patients and Methods
This is a retrospective consecutive case series inclusive of patients undergoing combined femtosecond laser-assisted cataract surgery and the insertion of two trabecular micro-bypass stents (iStent inject). Visual acuity, refraction and astigmatic vector analysis were collated and analysed from the preoperative and 4 weeks postoperative visits.
Results
One hundred and six eyes of 89 patients from 2 surgeons were included in the original cohort. The mean absolute difference from target refraction was 0.36 ± 0.25D. 73.9% of eyes were within ± 0.5D of the refractive target and 98.9% of eyes were within ± 1.00D. 73.8% of eyes had 0.5D or less residual refractive astigmatism following the procedure.
Conclusion
We present a novel cohort of glaucoma patients undergoing combined trabecular micro-bypass stents (iStent inject) and cataract surgery achieving excellent refractive outcomes. The results of this study indicate that this second-generation device is refractively neutral.
Keywords: glaucoma, cataract surgery, trabecular micro-bypass stents, astigmatism, intraocular lenses
Introduction
Simultaneous cataract and glaucoma surgery represents a potential challenge for the anterior segment surgeon.1 An option to reduce the surgical, anaesthetic and recovery load, however, must be weighed against an elevated risk of intraoperative complications and sub-optimal refractive outcomes in patients with concomitant ocular disease.1–3 With recent developments in intraocular lens (IOL) and biometry technology, stand-alone cataract surgery now provides a substantial opportunity to increase optical independence and vision-related quality of life.4 Given this, patient expectations have similarly increased. Despite often long-term ophthalmic care, glaucoma patients would appear to hold similar expectations of an improved range of vision following cataract surgery.5,6
Trabeculectomy has long been considered the gold standard for glaucoma patients where medical and laser treatments have been unable to control intraocular pressure (IOP).7 Although trabeculectomy and other invasive glaucoma surgical approaches provide a proven track record in reducing and maintaining IOP, successful visual rehabilitation may be compromised by surgically induced anatomical changes leading to residual defocus and astigmatic errors.7–9 Alternative IOP-lowering procedures have become commonplace for glaucoma, and increasingly anterior segment surgeons.10 Minimally invasive glaucoma surgery (MIGS) techniques and similar non-penetrating options now represent the first surgical procedure of choice for patients with mild- to- moderate glaucoma requiring additional treatment.11 With minimal or no additional need for conjunctival suturing or the application of anti-metabolite treatment, it would be expected that routine MIGS surgery should have a nominal impact on refractive outcomes. A number of studies have highlighted the safety and effectiveness of MIGS procedures in reducing IOP however few have indicated the refractive outcomes, particularly astigmatic effects.8,12,13 We present a refractive cohort of patients undergoing simultaneous glaucoma and cataract procedures and discuss the potential implications of cataract surgery and IOL implantation in patients with co-existent glaucoma.
Materials and Methods
This is a retrospective consecutive case series inclusive of patients undergoing combined femtosecond laser-assisted cataract surgery and the insertion of a minimally invasive glaucoma implant (iStent inject, Glaukos, San Clemente, CA, USA). One hundred and six eyes of 89 patients undergoing combined surgery were included in the initial cohort. To assist internal validity, only one eye of a patient was included in the final data analysis. The first eye undergoing the procedure was chosen in the 17 bilateral patients.
Inclusion criteria required patients having undergone successful cataract removal and insertion of an intraocular lens (IOL) with a concomitant implant of iStent inject. Patients were excluded from analysis in the presence of intraoperative complications including but not limited to; anterior or posterior capsular tear or the need for additional intraoperative manipulation, e.g. use of pupil expander devices. Patients who had previously undertaken glaucoma or corneal refractive surgical procedures were removed from data analysis. All patients were diagnosed prior to surgery with primary open-angle glaucoma requiring additional IOP reduction on the advice of the principal surgeon (AI/MTH).
Biometry was performed using an IOLMaster 500 or an IOLMaster 700 (Zeiss, Jena, Germany). Based on surgeon choice, either the Barrett Universal II or Haigis IOL power calculation formulae were used to determine the postoperative refraction target in each case. Surgical pre-treatment (capsulotomy and phacofragmentation) was performed with a femtosecond laser (LenSx Alcon, Ft. Worth, TX, USA) or a manual, curvilinear capsulorhexis was performed followed by divide-and-conquer cataract surgery. Both temporal and superior clear corneal incision sites were utilised with a 2.4mm keratome. A single-focus IOL was inserted in each case. The insertion of the iStent inject device was undertaken following the cataract procedure. The iStent inject is comprised of two heparin-coated biocompatible implant-grade titanium stents preloaded in a single-use injector. Each stent is inserted ab internally through the nasal trabecular meshwork into Schlemm’s canal. In all patients, a second stent was implanted in the same manner approximately 2–3 clock hours away from the first stent which is the norm.
The study was retrospective in nature and did not require external human ethics approval for the Australian center however was approved for the Swiss center by the Cantonal Ethics Committee of the Kanton of Zurich (KEK ZH 2019-00423). All patient data were anonymised prior to statistical analysis. Additionally, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) or with the Helsinki Declaration of 1975, as revised in 1983.
Refractive data from the preoperative screening and both 1 day and 4 weeks post-surgery visits were collected and analysed. Refraction was performed at pre and postoperative evaluations by experienced technicians to ensure accuracy. Refractive data were collated into a database (Excel, Microsoft, Redmond, WA, USA), converted from diopters into vectors and transferred to the statistics program for final data analysis (V20.0, SPSS IBM, Chicago, IL, USA). This includes the determination of J0 (the vector at Jackson cross-cylinder axis 0°) and J45 (the vector at Jackson cross-cylinder axis 45°). The Cartesian coordinates could then be used for statistical comparison.14
Results
Eighty-nine eyes of 89 patients were included in the final analysis. The mean age of the cohort was 73.3 ± 8.5 years (range 57 to 90 years). Fifty-five of the patients were female (61.8%). Almost two-thirds (59.1%) of procedures were undertaken in the right eye. The mean axial length for the cohort was 23.79 ± 1.32mm (range 20.76 to 27.22mm) and mean anterior chamber depth 3.21 ± 0.47mm (range 1.82 to 4.57mm). Mean corneal astigmatism was 0.91 ± 0.76D (range 0.00 to 4.25D). Mean medicated intraocular pressure (IOP) prior to surgery 16.16 ± 5.29mmHg (range 8 to 37mmHg).
Refraction and Visual Acuity
Refractive parameters are detailed in Table 1. The mean arithmetic difference from spherical equivalent (SE) target was −0.12 ± 0.42D (range −1.00 to 0.90D) whilst the mean absolute difference from SE target refraction was 0.36 ± 0.25D (range 0.00 to 1.00D). There was no difference between outcomes based on the location of the surgical incision (e.g. superior vs temporal) for either mean or absolute difference from SE target (p = 0.425, 0.735, respectively). As indicated in Figure 1, 73.9% of eyes were within ±0.5D of the refractive target with 98.9 eyes within ±1.00D of the predicted refractive target.
Table 1.
Pre and Postoperative Refraction
| n = 89 | Preop Mean ± SD | Range | Postop Mean ± SD | Range | p value |
|---|---|---|---|---|---|
| Sphere | −0.03 ± 2.63 | −11.00 to 5.50D | −0.13 ± 0.50 | −1.25 to 1.75D | 0.508 |
| Cylinder | −0.91 ± 0.76 | −4.25 to 0.00D | −0.41 ± 0.48 | −1.50 to 0.00D | <0.001 |
| Spherical Equivalent (SE) | −0.49 ± 2.64 | −11.25 to 5.00D | −0.31 ± 0.46 | −1.25 to 1.63D | 0.900 |
Abbreviations: D, diopters; SD, standard deviation.
Figure 1.
Attempted SE versus achieved SE for the cohort.
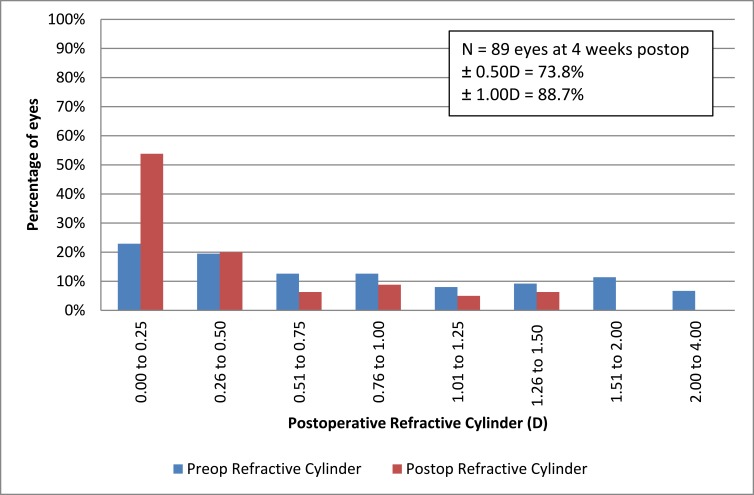
There was no significant difference between pre and postoperative spheres or SE however the mean reduction in the refractive cylinder was statistically significant following surgery (p = 0.000). There was no significant difference between right and left eyes for all measures (p > 0.05). Approximately three-quarters (73.8%) of patients achieved refractive astigmatism of 0.50D or less following surgery (Figure 2). Cumulative visual acuity is represented in Figure 3. Over ninety percent (95.4%) of eyes were able to reach 20/40 uncorrected following surgeries.
Figure 2.
Cumulative refractive astigmatism at the final visit.
Figure 3.
Cumulative snellen visual acuity.
Abbreviations: CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity.
Vector Analysis
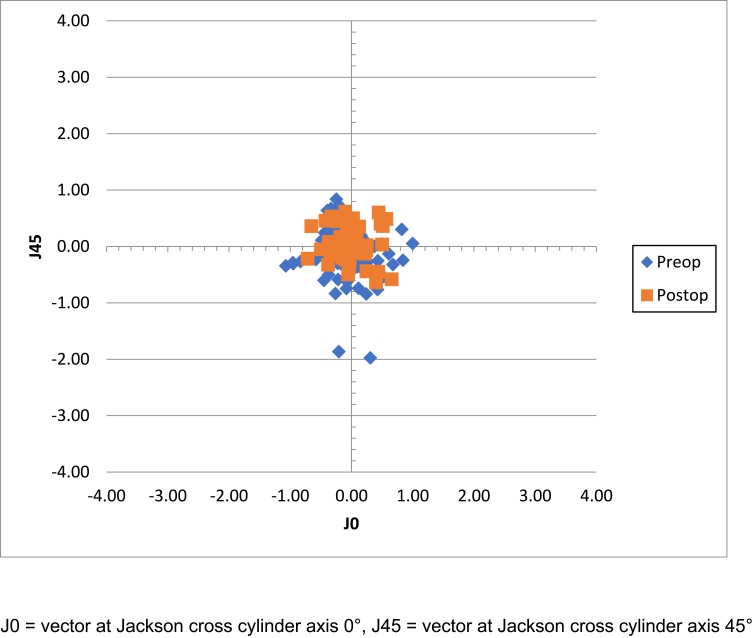
Vector-based variables are included in Table 2. There was a significant reduction in both J45 and B values only following surgery. There was no difference in parameters between right and left eyes for all variables. Similarly, there was no statistically significant difference between the change in J0 and J45 values with respect to main incision location (e.g. superior vs temporal, p = 0.634, 0.296 for J0 and J45, respectively). Manifest astigmatism represented by the astigmatism component of a power vector (referenced to the spectacle plane) for before and after surgery is provided in Figure 4.
Table 2.
Mean Vector-Based Variables
| n = 55 | Preoperative (Mean ± SD) | Postoperative (Mean ± SD) | p value |
|---|---|---|---|
| J0 | −0.04 ± 0.41 | −0.01 ± 0.22 | 0.433 |
| J45 | −0.10 ± 0.43 | 0.03 ± 0.22 | 0.005 |
| B | 1.99 ± 2.09 | 0.62 ± 0.96 | <0.001 |
Figure 4.
Manifest astigmatism before and after surgery.
Abbreviations: J0, vector at Jackson cross-cylinder axis 0°; J45, vector at Jackson cross-cylinder axis 45°.
Safety and Efficacy
There were no intraoperative complications requiring additional procedures. The mean treated intraocular pressure (IOP) prior to surgery was 16.16 ± 5.29mmHg. The change in treated IOP at the final visit was 1.86 ± 4.89mmHg (range −12 to +5mmHg) with 26.7% of patients achieving a reduction in IOP of 20% or greater from preop levels. Almost 60% percent (59.3%) of patients maintained or reduced their preop IOP value at the final visit. Forty-six (83.6%) patients reduced their medication usage following surgery, of these 7(12.7%) reduced multiple medications.
The reduction in IOP was significantly correlated with the preoperative value (r = 0.603, p = 0.000). There was no significant correlation between preoperative IOP, change in IOP and difference from postoperative refractive target (p > 0.05).
Discussion
Invasive glaucoma surgical procedures such as trabeculectomy and drainage device implantation have been shown to impact the axial length and keratometry measurements following stand-alone surgery.2 Although research has proposed the significant reduction of IOP as the main contributing factor to biometry changes; induction of corneal changes through the creation of the scleral flap, use of sutures and wound gape have also been indicated in several studies.9,15,16 Hypotony is usually temporary with the eye achieving structural consistency within a relatively short period in the majority of trabeculectomy patients.17 This suggests that optimal refractive outcomes may be achieved through sequential cataract and glaucoma procedures and once biometry values are stable. Phacotrabeculectomy remains an appropriate option for some patients however refractive outcome data is limited. Despite biometrical changes, Law et al found no statistical difference in the difference from the intended refractive outcome in a small comparative cohort suggesting that structural surgical changes may not clinically impact the refractive outcome.2 Of note, the authors described a decrease in axial length and converse increase in keratometry, which may have served to balance the refractive changes. Increasing with-the-rule (WTR) astigmatism following both trabeculectomy and other invasive glaucoma procedures has been variably described suggesting caution is still advisable.2 Given the recent increase in toric intraocular lenses usage, this may increase the risk profile for patients undergoing a combined approach.18
Minimally Invasive Glaucoma Surgery (MIGS) has been shown to be both safe and effective in reducing IOP and the need for multiple topical medications in patients with mild to moderate disease.10 Although capable as a stand-alone procedure, MIGS is often incorporated within cataract surgery through regulatory requirements. This does provide an opportunity for the surgeon to reduce the burden of additional surgical procedures and minimise recovery whilst providing the additive effect in lowering IOP. Several publications have recently shown that combined iStent inject implantation and cataract surgery significantly increased the success rate and reduced the number of medications in glaucoma patients compared to cataract surgery alone.19,20
The refractive impact of MIGS upon associated cataract surgery is expected to be minimal however few, if any studies have focused on visual outcomes.8 Manoharan and co-authors found an increase in refractive surprises in a subset of glaucoma patients undergoing cataract-MIGS surgery against a non-glaucoma cataract-only comparative cohort. The analysis did, however, include multiple MIGS devices limiting a direct comparison to the current study.21 Arriola-Villalobos et al showed a significant increase of mean LogMAR best corrected visual acuity from 0.42 ± 0.16 to 0.18 ± 0.16 following combined iStent inject and cataract surgery at 3 years post-surgery.12 Using a single trabecular micro-bypass stent implanted through the same temporal, limbal incision used for cataract surgery, Neuhann achieved best corrected of 20/40 or greater in 38 of 41 eyes (93%) at 3 years.13 In comparison, we found 95.4% of eyes greater or equal to 20/40 uncorrected distance visual acuity. Although these reports indicate visual improvement, refractive correction was not identified. Scott et al compared a cohort of 76 eyes undergoing combined cataract and iStent procedures with a cataract-only group. The authors showed no difference between groups suggesting the initial iStent device remained refractively neutral. Scott et al found 80% and 95% of eyes were within ± 0.5D and ±1.00D, respectively, highlighting excellent possible refractive outcomes with the earlier device.22 We found a mean absolute difference from SE target refraction was 0.36 ± 0.25D with 73.9% of eyes within 0.5D of the refractive target with 98.9 eyes within 1.00D of the predicted refractive target. This would appear to be similar to large cohort registry findings in standard cataract surgery. National Health Service data suggested that 62.3% of patients within 0.5D of the refractive target should represent a minimum level of efficiency following cataract surgery.23 Although this possibly represents the lower end of achievable accuracy, we compare favourably despite the higher potential risk profile of cataract surgery in cases with long-standing glaucoma. Vector astigmatic analysis suggested minor improvements although this may have been ameliorated by the relatively low preoperative refractive astigmatic values of our cohort (mean −0.91 ± 0.76D). Analysis of keratometric astigmatism will provide additional evidence of the potential impact of iStent inject insertion upon surgical-induced astigmatism.
Trabecular micro-bypass stenting has some inherent advantages over other glaucoma surgical procedures. Insertion of the iStent inject stents does not require the use of additional incisions and wound stretch during the stent insertion process is nominal, minimising the risk of increasing surgically induced corneal change.10 With an excellent safety profile, significant malposition of the stent is unlikely and injection of the device into the supraciliary space is rare, limited within the literature to a single case report only24,25 Furthermore, the risk of iatrogenic damage to the zonules with subsequent impact upon IOL positioning remains largely theoretical.24
The potential impact of the disease upon IOL power calculations and outcomes may be generalised to all glaucoma patients. Preoperatively, reduced vision due to glaucomatous changes may impact the patient’s ability to accurately fixate through biometry and related assessments. With patients’ often on more than one topical medication, subtle corneal surface sequelae due to the ongoing preservative use are likely.26 Epitropoulos and co-authors found that sub-clinical corneal changes could impact the repeatability of keratometry readings potentially affecting postoperative refractive outcomes.27 Although it is beyond the scope of this paper, consideration of tear film and corneal surface optimisation prior to biometry in glaucoma patients remains a plausible option to further refine outcomes. Zonule laxity and IOL dislocation in patients with pseudoexfoliation have been described previously.28 The potential risk of subluxation or excessive IOL movement is thereby increased. As significant IOL tilt or decentration can impact both low and higher-order aberrations affecting the potential refractive outcomes, a combined surgical approach may heighten the risk if excessive manipulation is required. Although unlikely to impact refractive endpoints, reduced contrast sensitivity and pupillary dysfunction which remain common in long-term glaucoma patients represent a potential contraindication to the optimal use of multifocal or trifocal intraocular lenses.29,30 This represents a further consideration during the preoperative counselling process as patients become more aware of available IOL options.
Conclusion
We present a novel cohort of glaucoma patients undergoing combined trabecular micro-bypass stents (iStent inject) and cataract surgery achieving excellent refractive outcomes. Results suggest a minimal impact of the combined approach or from the intrinsic risk profiles of patients with mild to moderate co-existing disease. The findings of this study suggest the device does not compromise refractive outcomes and can safely be combined with cataract surgery. Future prospective case–control studies may provide additional evidence for refractive impact with the iStent inject and other glaucoma devices.
Funding Statement
Glaukos have provided support for publication fees.
Disclosure
Dr Alexandros S Ioannidis reports non-financial support from Glaukos, during the conduct of the study and is a consultant for Glaukos, outside the submitted work. Dr Marc Töteberg-Harms reports lecture fees and travel reimbursement from Iridex, travel reimbursement from ELT Sight, lecture fees from Novartis/Alcon and Heidelberg Engineering, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Tzu JH, Shah CT, Galor A, et al. Refractive outcomes of combined cataract and glaucoma surgery. J Glaucoma. 2015;24(2):161–164. [DOI] [PubMed] [Google Scholar]
- 2.Law SK, Mansury AM, Vasudev D, Caprioli J. Effects of combined cataract surgery and trabeculectomy with mitomycin C on ocular dimensions. Br J Ophthalmol. 2005;89(8):1021–1025. doi: 10.1136/bjo.2004.060053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database Syst Rev. 2015;14(7):CD008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SY, Stem MS, Oren G, et al. Patient-centered and visual quality outcomes of premium cataract surgery: a systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi: 10.5301/ejo.5000978 [DOI] [PubMed] [Google Scholar]
- 5.Ichhpujani P, Bhartiya S, Sharma A. Premium IOLs in glaucoma. J Curr Glaucoma Pract. 2013;7(2):54–57. doi: 10.5005/jp-journals-10008-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teichman JC, Vold SD, Ahmed IIK. Top 5 pearls for implanting premium IOLs in patients with glaucoma. Int Ophthalmol Clin. 2012;52(2):65–71. doi: 10.1097/IIO.0b013e31824b3ec0 [DOI] [PubMed] [Google Scholar]
- 7.Tanito M, Matsuzaki Y, Ikeda Y, Fujihara E. Comparison of surgically induced astigmatism following different glaucoma operations. Clin Ophthalmol Auckl NZ. 2017;11:2113–2120. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan HHL, Kong YXG. Glaucoma surgery and induced astigmatism: a systematic review. Eye Vis Lond Engl. 2017;4:27. doi: 10.1186/s40662-017-0090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugkulstone CE. Changes in keratometry following trabeculectomy. Br J Ophthalmol. 1991;75(4):217–218. doi: 10.1136/bjo.75.4.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen CL, Samuelson TW. Managing coexistent cataract and glaucoma with iStent. Surv Ophthalmol. 2017;62(5):706–711. doi: 10.1016/j.survophthal.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Glaukos iStent inject(R) trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41(12):2664–2671. doi: 10.1016/j.jcrs.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 14.Thibos LN, Horner D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg. 2001;27(1):80–85. doi: 10.1016/S0886-3350(00)00797-5 [DOI] [PubMed] [Google Scholar]
- 15.Muallem MS, Nelson GA, Osmanovic S, et al. Predicted refraction versus refraction outcome in cataract surgery after trabeculectomy. J Glaucoma. 2009;18(4):284–287. doi: 10.1097/IJG.0b013e318184567b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietze PJ, Oram O, Kohnen T, et al. Visual function following trabeculectomy: effect on corneal topography and contrast sensitivity. J Glaucoma. 1997;6(2):99–103. doi: 10.1097/00061198-199704000-00005 [DOI] [PubMed] [Google Scholar]
- 17.Panarelli JF, Nayak NV, Sidoti PA. Postoperative management of trabeculectomy and glaucoma drainage implant surgery. Curr Opin Ophthalmol. 2016;27(2):170–176. doi: 10.1097/ICU.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 18.Visser N, Bauer NJC, Nuijts RMMA. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–637. doi: 10.1016/j.jcrs.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 19.Chansangpetch S, Lau K, Perez CI, et al. Efficacy of cataract surgery with trabecular microbypass stent implantation in combined-mechanism angle closure glaucoma patients. Am J Ophthalmol. 2018;195:191–198. doi: 10.1016/j.ajo.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 20.Clement CI, Howes F, Ioannidis AS, et al. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019;13:491–499. doi: 10.2147/OPTH.S187272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoharan N, Patnaik JL, Bonnell LN, et al. Refractive outcomes of phacoemulsification cataract surgery in glaucoma patients. J Cataract Refract Surg. 2018;44:348–354. doi: 10.1016/j.jcrs.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 22.Scott RA, Ferguson TJ, Stephens JD, Berdahl JP. Refractive outcomes after trabecular microbypass stent with cataract extraction in open-angle glaucoma. Clin Ophthalmol. 2019;13:1331–1340. doi: 10.2147/OPTH.S206619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brogan K, Diaper CJM, Rotchford AP. Cataract surgery refractive outcomes: representative standards in a National Health Service setting. Br J Ophthalmol. 2019;103(4):539–543. doi: 10.1136/bjophthalmol-2018-312209 [DOI] [PubMed] [Google Scholar]
- 24.Mantravadi AV, Lin C, Kinariwala B, Waisbourd M. Inadvertent implantation of an iStent in the supraciliary space identified by ultrasound biomicroscopy. Can J Ophthalmol J Can Ophthalmol. 2016;51(6):e167–e168. doi: 10.1016/j.jcjo.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 25.Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. doi: 10.1097/ICU.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11–18. doi: 10.1097/ICL.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–1677. doi: 10.1016/j.jcrs.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 28.Pueringer SL, Hodge DO, Erie JC. Risk of late intraocular lens dislocation after cataract surgery, 1980-2009: a population-based study. Am J Ophthalmol. 2011;152(4):618–623. doi: 10.1016/j.ajo.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierings RAJM, de Boer MH, Jansonius NM. Visual performance as a function of luminance in glaucoma: the de vries-rose, Weber’s, and Ferry-Porter’s law. Invest Ophthalmol Vis Sci. 2018;59(8):3416–3423. doi: 10.1167/iovs.17-22497 [DOI] [PubMed] [Google Scholar]
- 30.Lawlor M, Quartilho A, Bunce C, et al. Patients with normal tension glaucoma have relative sparing of the relative afferent pupillary defect compared to those with open angle glaucoma and elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2017;58(12):5237–5241. doi: 10.1167/iovs.17-21688 [DOI] [PubMed] [Google Scholar]