Abstract
We report a 25-year-old woman with persistent dyspnea and wheezes that had been unsuccessfully treated with inhaled beta 2-agonists and steroids for about one year. Spirometry demonstrated a restrictive pattern. Chest CT demonstrated polypoidal lesion in left main bronchus. The lesion was excised via rigid bronchoscopy. Pathology showed a picture of typical bronchial carcinoid. In this patient, due to the lack of awareness, diagnosis of carcinoid was delayed for one year.
1. Introduction
According to the 2015 World Health organization Classification of Lung Tumors; carcinoid tumors are neuroendocrine tumors. Neuroendocrine tumors (NTs) are classified into the well differentiated [pulmonary carcinoids], (low-grade typical carcinoid, and intermediate – grade atypical carcinoid) and the poorly differentiated (high-grade large cell neuroendocrine carcinoma and small cell carcinoma) [1].
Most common site of carcinoid tumors is GI tract (64%), next is respiratory tract (28%) [2]. Bronchial carcinoids accounts for 1–2% of all lung malignancies. Male and female are equally affected with mean age of presentation is 40 years [3].
It is a very slow growing tumor hence called “cancer in slow motion.” Bronchial carcinoids are frequently discovered as a lesion on a chest radiograph and 31% of the patients are asymptomatic. Mitosis counting methods were detailed in the 2015 WHO Classification. Mitoses should be counted in the areas of highest activity and per 2 mm2 rather than 10 high – power fields.
Typical carcinoids have fewer than 2 mitoses/2 mm2 and absence of necrosis [1].
Based on microscopic appearance they are called carcinoid when less than two mitosis present ten high power field examination and without areas of necrosis.
Atypical carcinoids are diagnosed when more than ten mitosis detected after ten high power field examinations or by the presence of necrosis [4]. WHO classification includes four types: typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma and small cell carcinoma. Typical and atypical categories are not related to tobacco use but not the other two. Prognosis is excellent for, typical carcinoids and poor for small cell carcinoma [1].
2. Case presentation
A 25-year old lady complained of Dyspnea with wheezes for about one year that started during her 2nd trimester and attributed to her pregnancy. Her dyspnea persisted after delivery. Wheezes had no relation to physical exertion. She was treated as bronchial asthma with bronchodilators and steroids, both oral and inhaled but without any improvement.
On Physical examination patient was found to be of average built and well nourished. Blood pressure was 100/60 mm Hg, pulse rate was 92 per minute. Respiratory rate was 22 per minute. Bilateral widespread wheezes were heard on chest auscultation.
Hematological investigation revealed haemoglobin 11 gm/dl, total leucocyte count (12,000) platelet count 350,000, ESR 8 mm at the end of 1st hour. Urea 23 mg, creatinine 0.9mg/dl, random blood sugar 112 mg/dl. 5HIAA (end product of serotonin in 24h urine) increased 26 mg/day (ref. range 2–6mg/day).
Chest x-ray was unremarkable (Fig. 1). CT chest revealed sessile polypoidal lesion involving left main bronchus 1.5*1 cm, 3.6 cm from the carina with no mediastinal nor hilar lymphadenopathy identified (Fig. 2).
Fig. 1.
Plain chest x-ray.
Fig. 2.
CT chest revealed sessile polypoidal lesion involving left main bronchus and Virtual bronchoscopy showing endobronchial lesion.
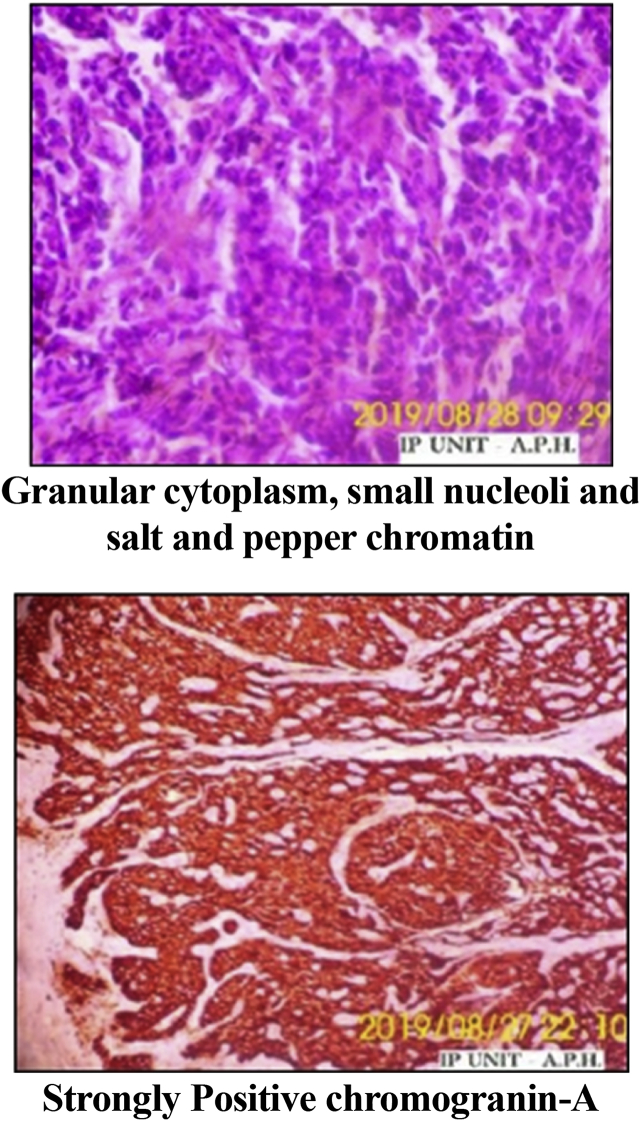
Pulmonary function tests revealed a restrictive pattern; FEV1/FVC 86, FVC 47%, FEV1 42%. Bronchoscopy revealed left sided endobronchial lesion (Fig. 3). Lesion was excised under General Anesthesia, using Rigid Bronchoscopy Universal tube 7.5 mm (Karl Storz, Germany) and electrosurgical monopolar unit for normal coagulation without cutting by using loop snare on the base of the tumor to reduce its base followed by mechanical removal with rigid grasping forceps. On histopathological examination of the excised lesion; the findings were consistent with typical carcinoid tumor (Neoplastic growth formed of sheets of monotonous small round cells having moderate finely granular cytoplasm, small nucleoli and salt and pepper chromatin and strong diffuse positive chromogranin) (Fig. 4). CT abdomen was free with no distant metastasis.
Fig. 3.
Total Resection of Endobronchial lesion using Rigid Bronchoscopy.
Fig. 4.
Histopathological examination of the excised lesion.
It was noted that surgical intervention would require either bronchial sleeve resections or pneumonectomy. Options were discussed with the patient and she preferred endoscopic removal rather than surgery. figure [3].
3. Discussion
Our patient was a lady and presented with dyspnea. From a large case series managed in a tertiary referral center; Pericleous et al. reported that well differentiated NTs were commoner in females and the commonest presenting symptoms were cough and dyspnea [5] Surgery is the treatment of choice for carcinoid tumors with loco -regional disease. [6].
Several case series showed outcome with surgery. Filosso et [7]. al reported their experience with 126 patients underwen pneumonectomy, lobectomy, or bronchial sleeve resections with adequate outcome with surgery as presented in Table 1.
Table 1.
5ys and 10ys of cases with typical or atypical carcinoid.
| Presentation | No cases | 5ys Survival | 10 ys survival |
|---|---|---|---|
| Typical carcinoid | 72 | 97 | 93 |
| Atypical carcinoid | 54 | 77 | 52 |
Cerfolio et al. reported case seriesof carcinoid and muco-epidermoid tumor in which patients underwent bronchial sleeve resection with lung preservation surgery, with relief of symptoms and good long-term outcome, similar to the other reported series [8]. Similar long-term outcomes for typical carcinoids treated with standard surgery versus lung-sparing surgery or local resection suggest that minimal treatment optionsgive similar clinical outcomes with less morbidity [[9], [10], [11]]. Theoutcomes with minimally invasive surgical techniques also suggest that local regional treatment with endoscopic ablation could potentially be a first-line alternative [[9], [10], [11]]. There are different endobronchial interventional modalities available that have been used in the published literature for endobronchial carcinoids. These include neodymium-doped yttrium aluminum garnet laser resection, cryotherapy, electrocautery, photodynamic therapy, and APC. Several small case series using endoscopic technology in patients unable to undergo traditional surgical resection have reported minimal side effects [[9], [10], [11]]. Recently, Orino et al reported a patient with recurrent endobronchial carcinoid tumor that was successfully treated by APC [12]. Recently, Reuling et al concluded after reviewing a cohort of one hundred and twenty-five patients with a diagnosis of bronchial carcinoid that patients with purely intraluminal bronchial carcinoids with a diameter <20 mm can be treated successfully with endo –bronchial treatment with a success rate of 72%.
Our patient preferred an endobronchial interventional modality rather than surgical resection so rigid bronchoscopy under general anesthesia was performed. Using a snare electrocautery the tumor was hooked and electrocoagulation was then applied via the grasping forceps allowing complete resection of the tumor. Histopathological examination revealed typical bronchial carcinoid.
4. Follow up
Completely improvement of the patient symptoms as regard dyspnea and wheezes. CT free, 5HIAA decreased to 2.6 mg/day with normal pulmonary function (Fig. 5).
Fig. 5.
6 Months follow up (CT chest and Virtual Bronchoscopy).
5. Conclusion
This patient is a good example of misdiagnosis of a rare disease as carcinoid by a common one as bronchial asthma. extensive investigation as PFT, CT and FOB in any asyhmatic patient unresponsive to conventional treatment might lead to diagnose an unexpected culprit and excellent example of avoidance of huge surgery as pneumonectomy or bronchial sleeve (resection&anastomosis) and using instead endobronchial excision by a snare electrocautry through rigid bronchoscopy.
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial.
References
- 1.Travis W.D., Brambilla E., Nicholson A.G. The 2015 World Health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 2.Santra A., Dutta P., Pothal S., Manjhi R. Misdiagnosed case of bronchial carcinoid presenting with refractory dyspnea and wheeze: a rare case report and review of literature Malays. J. Med. Sci. 2013;20:78–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Robby B.B., Drehner D., Sidman J.D. Pediatric tracheal and endobronchial tumors: an institutional experience. Arch. Otolaryngol. Head Neck Surg. 2011;137:925–929. doi: 10.1001/archoto.2011.153. [DOI] [PubMed] [Google Scholar]
- 4.Andersen J.B., Mortensen J., Damgaard K., Skov M., Sparup J., Petersen B.L., Rechnitzer C., Borgwardt L. Fourteen-year- old girl with endobronchial carcinoid tumour presenting with asthma and lobar emphysema. Clin. Res. J. 2010;4:120–124. doi: 10.1111/j.1752-699X.2009.00149.x. [DOI] [PubMed] [Google Scholar]
- 5.Pericleous M., Karpathakis A., Toumpanakis C. Well-differentiated bronchial neuroendocrine tumors: clinical management and outcomes in 105 patients. Clin. Res. J. 2018;12(3):904–914. doi: 10.1111/crj.12603. [DOI] [PubMed] [Google Scholar]
- 6.Pusceddu S., Lo Russo G., Macerelli M. Diagnosis and management of typical and atypical lung carcinoids. Crit. Rev. Oncol. Hematol. 2016;100:167–176. doi: 10.1016/j.critrevonc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Filosso P.L., Rena O., Donati G. Bronchial carcinoid tumors: surgical management and long-term outcome. J. Thorac. Cardiovasc. Surg. 2002;123:303. doi: 10.1067/mtc.2002.119886. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio R.J., Deschamps C., Allen M.S. Mainstem bronchial sleeve resection with pulmonary preservation. Ann. Thorac. Surg. 1996;61:1458–1462. doi: 10.1016/0003-4975(96)00078-1. [DOI] [PubMed] [Google Scholar]
- 9.Sutedja T.G., Schreurs A.J., Vanderschueren R.G. Bronchoscopic therapy in patients with intraluminal typical bronchial carcinoid. Chest. 1995;107:556–558. doi: 10.1378/chest.107.2.556. [DOI] [PubMed] [Google Scholar]
- 10.van Boxem A.J., Westerga J., Venmans B.J. Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Canc. 2001;31:31–36. doi: 10.1016/s0169-5002(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 11.Santos R.S., Raftopoulos Y., Keenan R.J. Bronchoscopic palliation of primary lung cancer. Single or. 2004;18:931–936. doi: 10.1007/s00464-003-9202-x. [DOI] [PubMed] [Google Scholar]
- 12.Orino K., Kawai H., Oquawa J. Bronchoscopic treatment with argon plasma coagulation for recurrent typical carcinoids: report of a case. Anticancer Res. 2004;24:4073–4077. [PubMed] [Google Scholar]